Abstract
This is one of few published population-based studies describing breed specific rates of canine primary bone tumors. Incidence rates related to dog breeds could help clarify the impact of etiological factors such as birth weight, growth rate, and adult body weight/height on development of these tumors. The study population consisted of dogs within 4 large/giant breeds; Irish wolfhound (IW), Leonberger (LB), Newfoundland (NF), and Labrador retriever (LR), born between January 1st 1989 and December 31st 1998. Questionnaires distributed to owners of randomly selected dogs — fulfilling the criteria of breed, year of birth, and registration in the Norwegian Kennel Club — constituted the basis for this retrospective, population-based survey. Of the 3748 questionnaires received by owners, 1915 were completed, giving a response rate of 51%. Forty-three dogs had been diagnosed with primary bone tumors, based upon clinical examination and x-rays. The breeds IW and LB, with 126 and 72 cases per 10 000 dog years at risk (DYAR), respectively, had significantly higher incidence rates of primary bone tumors than NF and LR (P < 0.0001). Incidence rates for the latter were 11 and 2 cases per 10 000 DYAR, respectively. Pursuing a search for risk factors other than body size/weight is supported by the significantly different risks of developing primary bone tumors between similarly statured dogs, like NF and LB, observed in this study. Defining these breed-specific incidence rates enables subsequent case control studies, ultimately aiming to identify specific etiological factors for developing primary bone tumors.
Résumé
Cette étude est l’une des rares publiées décrivant les taux de tumeur osseuse primaire canine spécifiques de race. Les taux d’incidence relatifs aux races de chien pourraient aider à clarifier l’impact de facteurs étiologiques tels que le poids à la naissance, le taux de croissance et le ratio poids corporel/taille à l’âge adulte sur le développement de ces tumeurs. La population à l’étude était composée de chiens parmi les 4 races de chien grandes/géantes; le lévrier irlandais (IW), le Leonberger (LB), le Terre-Neuve (NF) et le Labrador (LR), né entre le 1er janvier 1989 et le 31 décembre 1998. Des questionnaires distribués aux propriétaires de chiens sélectionnés au hasard — répondant aux critères de race, année de naissance, et enregistrement au Club Canin Norvégien — ont constitué les éléments pour cette étude rétrospective. Sur les 3748 questionnaires soumis aux propriétaires, 1915 ont été complétés, donnant un taux de réponse de 51 %. Quarante-trois chiens ont été diagnostiqués avec des tumeurs osseuses primaires, en fonction de l’examen clinique et des examens radiologiques. Les races IW et LB, avec respectivement 126 et 72 cas par 10 000 années-chien à risque (DYAR), avaient des taux d’incidence de tumeurs osseuses primaires significativement plus élevés que les races NF et LR (P < 0,0001). Les taux d’incidence pour ces derniers étaient respectivement de 11 et 2 cas par 10 000 DYAR. La recherche de facteurs de risque autres que le ratio taille/poids est supportée par les risques significativement différents observés dans la présente étude de développer des tumeurs osseuses primaires parmi les chiens de statures similaires tels les NF et LB. La définition de ces taux d’incidence spécifiques de race permettra des études cas-témoins ultérieures visant à identifier les facteurs étiologiques spécifiques pour le développement des tumeurs osseuses primaires.
(Traduit par Docteur Serge Messier)
Introduction
Osteosarcoma (OS) is the most common histological subtype of primary bone cancer both in humans and dogs (1–3). Although multi-agent chemotherapy has greatly improved the outcome among human patients, mortality is still high (2,4). Five year overall survival rates range from about 15% to 70% for patients with and without visible metastases at the time of diagnosis, respectively (2,5). Adding to the severity of this disease, it typically affects children and adolescents, constituting about 5% of pediatric cancers (6).
Osteocarcoma accounts for 80% to 90% of canine primary bone tumors (7,8). Although rare in the canine population, the rate outnumbers that of the human population, with a lifetime incidence risk about 30 to 50 times higher within the overall canine population (3,9). Breed-specific incidence rates of OS differ largely, and estimates within certain breeds even show a lifetime risk exceeding 10%, thereby affecting a substantial number of these dogs (9,10). Median survival time for dogs with primary bone cancer of the appendicular skeleton, treated with surgery and chemotherapy, ranges from 5 to 13 mo provided there are no visible metastasis at the time of diagnosis, in which case median survival time drops to about 2 mo (8,11).
Most commonly, OS is diagnosed in middle-aged to older dogs, with a median age of 7 y (8). A smaller peak in age incidence at 18 to 24 mo corresponds with the human peak incidence at late puberty, which has led to the hypothesis of skeletal growth parameters representing some of the possible etiological factors for developing this disease (8,12–14). It is well-recognized that giant and large breed dogs are at increased risk of developing OS (8); however, body size alone cannot explain the variation in incidence between different breeds of dogs, as the risk appears to differ extensively among certain breeds of similar body size (1,13,14). Epidemiological studies on human OS have also failed to show a strong correlation between body weight or height and risk of developing OS (12,14,15).
Spontaneous OS in dogs resembles that of human OS in several aspects. Both species develop these tumors most commonly in the metaphysis of long bones, with micro metastases at the time of diagnosis, and overt lung metastases as the main cause of mortality (3,16). Similar response to chemotherapy makes diseased dogs valuable contributors to the process of developing new anti-cancer therapy (3,17–20). As the biological behavior is similar in dogs and humans, common risk factors for developing the disease can be expected. Hence, information on incidence related to specific breeds of dogs might help clarify the supposed correlation between birth weight, growth rate, adult body weight or height, and the development of OS. In this context, recognizing breeds of similar stature having significantly different incidence rates of OS is of particular interest.
Ten years of litter registrations of 4 large and giant dog breeds in Norway constituted the basis for this survey, aiming to describe the incidence rate of primary bone tumors within each breed and to estimate possible differences between these breeds. To understand the implication of inherent and environmental risk factors for primary bone tumors, one should be familiar with the natural occurrence of the disease in the particular reference population.
The few studies on the occurrence of primary bone tumors and breed-specific lifetime risks and/or incidence rates in the canine population, have been based upon insurance data or pathology registers (9,10). To our knowledge, there are no previously published population-based studies describing breed specific rates of canine primary bone tumors. Estimating the incidence rate of such tumors within these 4 breeds (in Norway) will thus be of importance for further studies of this population, with the ultimate goal of identifying specific risk factors in disease development.
Materials and methods
Study population
The study population consisted of purebred dogs registered in the Norwegian Kennel Club (NKC), born between January 1st 1989 and December 31st 1998. Breeds enrolled in the study were the Irish wolfhound (IW), Leonberger (LB), Newfoundland (NF), and Labrador retriever (LR). At the initiation of the survey, none of the dogs included would have been younger than 10 y.
Sample size
In estimating the appropriate sample size, the main criterion was attaining a high probability of detecting a difference in breed specific incidence rates, provided it was substantial enough to be clinically relevant. A second criterion was to enable an expectation of at least 10 dogs diagnosed with primary bone tumors within each breed. Power was set at 0.80 and calculations were conducted using a statistical program (Stata 10.0; StataCorp, College Station, Texas, USA).
Based upon estimates from previous publications, a lifetime risk within the IW population of 8% to 10% was presumed (9,10). For sample size calculations, the lifetime risk in this breed was set to 8%, as this would require the largest sample. Further, a maximum lifetime risk within the LB and NF of 3%, and about 1% for the LR, was assumed (9,10).
All registered IW were included in the sample population, and sample sizes of NF and LB were calculated accordingly. Anticipating a response rate from owners of sampled dogs of about 50%, computed sufficient sample sizes were multiplied by 2. Hence, about 1/2 the total number of IW; 300, was used as a fixed sample size of IW. Power calculations show that a sample size of 450 dogs from NF and LB yields a power of 0.81 provided a lifetime risk of 3% within these breeds. The study population consisting of a limited number of dogs was accounted for using the formula for the finite population factor (FPC):
| Equation 1 |
where: n′ = the final size of the sample population, n = the number of dogs needed from an infinite population (in this case 450), and N = the number of dogs in the study population (21). Performing this calculation, n′ equalled 367 and 382 for LB and NF, respectively. As the expected lifetime risk in the LR population was 1%, a large sample size to prove a difference in lifetime risks was not needed; however, 1000 dogs were included so that a minimum of 10 LR positive of primary bone tumors could be expected. Considering the expected response rate of 50%, the computed sample sizes of LB, NF, and LR were multiplied by 2, while the total number of IW was 577.
Sampling of study population
The total number of registered dogs from each breed forming the study population is shown in Table I. Stratified by year of birth, the minimum number of dogs providing the desired power was calculated for each of the 10 y included. Within each breed, the largest number of dogs required from any of these years was then sampled by computerized random sampling; that is, the same number of dogs was sampled from each year within one specific breed, constituting a sample population of 4868 dogs. However, a number of dogs had to be excluded: 149 dogs were excluded as their owners were no longer alive, 34 dogs were excluded due to their owners living outside of Scandinavia, and 305 LR were registered guide dogs whose owners could not be traced. The final number of dogs in the study population was 4380; 2119 males and 2261 females.
Table I.
Total number of dogs from each breed studied that were registered in the Norwegian Kennel Club
| Year of birth
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Breed | 1989 | 1990 | 1991 | 1992 | 1993 | 1994 | 1995 | 1996 | 1997 | 1998 | Total |
| Newfoundland | 326 | 364 | 286 | 288 | 177 | 183 | 242 | 217 | 216 | 224 | 2523 |
| Leonberger | 133 | 181 | 277 | 277 | 105 | 140 | 236 | 180 | 204 | 245 | 1978 |
| Labrador retriever | 688 | 720 | 780 | 523 | 586 | 550 | 644 | 546 | 591 | 644 | 6272 |
| Irish wolfhound | 67 | 53 | 52 | 58 | 37 | 58 | 58 | 72 | 64 | 58 | 577 |
| Total | 1214 | 1318 | 1395 | 1146 | 905 | 931 | 1180 | 1015 | 1075 | 1171 | 11 350 |
Study design
This study was designed as a retrospective, descriptive survey based upon questionnaires distributed to owners, or previous owners, of dogs from the 4 actual breeds. Recipients not responding to the request received one reminder. Owners whose dog was no longer alive were asked to describe cause of death or euthanasia. All recipients were asked specifically whether their dog had suffered from any cancer- and/or tumor-related disease, as the main objective was identifying dogs euthanized due to primary bone tumors. They were also requested to state general health information regarding vaccination intervals, neutering status, hormone treatments, breeding history, and occurrence of any chronic diseases. History of skeletal diseases such as fractures, arthroses, and osteochondrosis was also included in the forms. Owners whose dog had suffered from primary bone tumors were questioned further on the diagnosis, histological classification, whether or not metastases were detected, location of the primary tumor, and what kind of treatment their dog had received, if any. As the owners were also asked to state the name of the veterinarian or veterinary clinic that diagnosed the dog, the diagnosis for a primary bone tumor could be confirmed by contacting each veterinarian regarding the basis for their diagnoses. For a dog to be included as positive of primary bone tumor, a description of typical clinical signs in addition to coinciding radiographic findings were considered to be sufficient. Cases where the diagnosis could not be confirmed by the veterinarian were not included as positive for the disease.
Ethical issues
In this observational study, no interventions affecting animal health were conducted. The only ethical issue of concern was that of confidentiality, as responses to the questionnaires distributed to dog owners might reveal sensitive information on the health status of their dog. All information obtained on individual dogs has been kept confidential and when results are presented, no information exposing the dogs’ or their owners’ identity is revealed. The NKC approved access to their registry of dogs, thus enabling contact with the dog owners.
Statistical analyses
Incidence rates are reported as number of cases per 10 000 dog years at risk (DYAR), and lifetime risks as the proportion of dogs with primary bone tumors, with 95% confidence intervals (CI) based on the Poisson distribution. Other proportions, such as response rates and localizations, are given with 95% CI based on the binomial distribution. When calculating the response rate, the number of forms returned as undeliverable plus the number of dogs whose registered owners did not possess any knowledge of the dogs in question was first subtracted from the denominator. This takes into account that these owners had no opportunity to respond, i.e. they were ineligible for the study and thereby this calculation probably serves as the best measure of the response rate (22). Age at time of diagnosis within each breed is given as median with range. A chi-squared (X2) test was performed to test the hypotheses of differences in lifetime risks between subgroups of the study population, such as breed and gender; P < 0.05 was considered significant.
Results
Study population
Of the 4380 questionnaires initially distributed to previous or current dog owners, 534 were untraceable by the Norwegian phone and address registry and 98 were excluded, mostly due to uncertainty as to who took care of the dog after leaving its breeder, or after relocation at an early age. This resulted in 3748 forms received by dog owners; representing 1778 male and 1970 female dogs.
A total of 1915 questionnaires were completed and returned to the Norwegian School of Veterinary Science (NSVS), constituting an overall response rate of 51% (50% to 53%). A significant difference was observed between the response rate of the owners of LR, which had the highest proportion of responders, 53% (51% to 56%), and that of IW, displaying the lowest, 47% (42% to 53%), (P = 0.03). With respect to gender, the proportions of male and female dogs whose forms were returned were also significantly different (P = 0.04), owners of female dog showing a response rate of 53% (50% to 55%), whereas the corresponding ratio for the male dogs was 49% (47% to 52%). At the end of the study period, 291 dogs were still alive; 1, 38, 15, and 237 of the IW, NF, LB and LR, respectively. Average age at the time of death/euthanasia was 8.9 y (range: 8.7 to 9.0 y) for all breeds; 7.0 y (range: 6.6 to 7.4 y) for IW, 8.2 y (range: 7.8 to 8.5 y) for NF, 8.0 y (range: 7.7 to 8.3 y) for LB, and 10.2 y (9.9 to 10.5 y) for LR. For 197 dogs age at time of death was not reported.
Lifetime risks and incidence rates
Forty-three dogs fulfilled the criteria for being included as positive of primary bone tumors; the diagnosis based upon clinical examination and x-rays, yielding an overall lifetime risk of 2.3% (1.6% to 3.0%). Of these, the tumors of only 6 dogs were biopsied, from which the results of 4 dogs could be obtained. Three of these biopsies yielded OS, and one, observed in the frontal bone of a male NF, was diagnosed as a multilobular osteochondrosarcoma.
Of the 1385 dogs whose owners responded to the first request, 29 dogs, 2.1% (1.4% to 3.0%), had suffered from primary bone tumors. The number of affected dogs among the 530 responses to the reminder, was 14; 2.6% (1.4% to 4.4%).
The highest incidence rates of primary bone tumors were found in IW and LB; incidence rates within these breeds estimated to be approximately 11 and 7 times higher, respectively, than in NF, and about 60 and 35 times higher, respectively, compared to LR (Table II). Thus, incidence rates of primary bone tumors among IW and LB were found to be significantly higher than those of NF and LR (P < 0.0001).
Table II.
Incidence rates of primary bone tumors as proportion of the total number of dogs and as number of cases per 10 000 dog years at risk (DYAR) within 4 breeds of dogs born between 1989 and 1998, and registered in the Norwegian Kennel Club
| Breed | Number of dogs (DYAR) among the responders | Number of dogs with primary bone tumors | Rate (%) (95% CI) | Rate per DYAR (95% CI) | Median (range) age (years) at diagnosis |
|---|---|---|---|---|---|
| Irish wolfhound | 169 (1187) | 15 | 8.9 (5.0–14.6) | 126 (71–208) | 5.5 (3.3–8.4) |
| Leonberger | 381 (3074) | 22 | 5.8 (3.6–8.7) | 72 (45–108) | 7.2 (1.6–10.1) |
| Newfoundland | 427 (3574) | 4 | 0.9 (0.3–2.4) | 11 (3–29) | 8.8 (4–11.5) |
| Labrador retriever | 938 (9798) | 2 | 0.2 (0.03–0.8) | 2 (0.3–7) | 11.6 (11.6) |
CI — confidence interval.
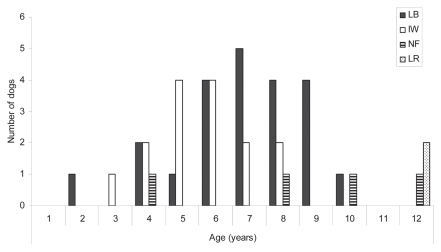
No significant gender differences could be found (χ2 = 0.16, P = 0.69), as 21 male, 2.4% (1.5% to 3.7%), and 22 female, 2.1% (1.3% to 3.2%), dogs among the responders suffered from primary bone tumors. Median age at time of diagnosis was 6.7 y (range: 1.6 to 11.6 y). This was similar among male and female dogs; 6.7 y (range: 3.7 to 11.6 y) and 6.6 y (range: 1.6 to 11.6 y), respectively, and there were no significant differences between the breeds (Figure 1).
Figure 1.
Age at time of diagnosis of primary bone tumors in 4 breeds of dogs: Leonberger (LB), Irish wolfhound (IW), Newfoundland (NF), and Labrador retriever (LR), born between 1989 and 1998, and registered in the Norwegian Kennel Club.
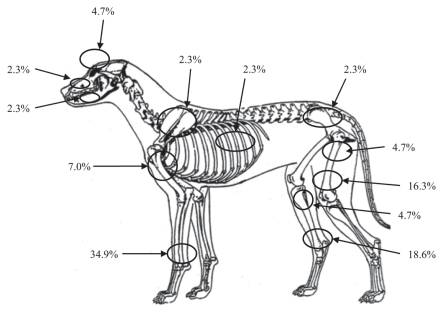
Distal radius/ulna, distal tibia and distal femur were the most common sites of the primary tumor, encompassing 35% (21% to 51%), 19% (8.4% to 33%), and 16% (6.8% to 31%) of the tumors, respectively. Most of the tumors, 86% (72% to 95%), originated in the appendicular skeleton. Only 12% (3.9% to 25%) occurred in the axial skeleton, including scapula, and 2.3% (0.1% to 12%) in the pelvis (Figure 2). In this study, no correlation was found between primary bone tumors and health-related aspects such as vaccination status, hormone treatments, chronic diseases, cancers (with primary tumor unrelated to bone) or orthopedic injuries (data not shown). The proportion of neutered dogs was similar among those diagnosed with primary bone tumors, 16.3% (7/43), and those without this diagnosis, 13.1% (245/1872). Median and mean age at time of neutering was between 5 and 6 y for both groups.
Figure 2.
Anatomical location of primary bone tumors among 4 breeds of dogs born between 1989 and 1998, and registered in the Norwegian Kennel Club.
Discussion
The overall response rate of about half the sample population corresponds with our expectations, as there has been a decline in survey response rates during the past decades (22). Response rate has traditionally been used as a measure of the quality of surveys; higher response rates indicating more reliable results (23). Identifying bias, that is, a difference in responders versus non-responders, is difficult — resulting in the use of response rate as an easily obtained measure of quality (23). However, there is not necessarily a direct correlation between response rate and bias of the results (23). Aiming to evaluate the amount of bias, one can compare early versus late responders, assuming late responders to be representative of the non-responders (24). Applying this to the present study, the proportion of dogs suffering from primary bone tumors within each of these 2 groups was found to be similar; supporting the assumption of response bias being low in this survey.
Responders could be more concerned with their dog’s overall health than non-responders. Moreover, it would be reasonable to expect owners whose dog had suffered from primary bone tumors to take a personal interest in research related to this disease and consequently being more likely to respond. If so, this would lead to an overestimation of the incidence rate(s). A slightly higher, but significant, response rate was found among the owners of the breed least affected by primary bone tumors, LR, than among those of the most commonly affected breed, IW. Consequently, the estimated incidence rates are probably not overestimated.
The principal finding of this population-based study of breed specific incidence rates of canine primary bone tumors, is that there is a large variation in rates between the different breeds IW, LB, NF, and LR. The incidence rate in the largest breed (IW) is some sixtyfold greater than in the smallest one (LR); however, factors other than a large body size or weight also pose an increased risk for developing the disease. This is evidenced by a significantly higher incidence rate found in LB compared with NF, 2 breeds of similar size and stature. Growth rate is one possible factor contributing to the observed difference between these breeds. It has been shown that NF, when accounting for differences in adult body weight, has the slowest growth rate among the 4 breeds included in this study, while the LB reaches adult body weight after approximately the same number of days as the LR — a considerably smaller breed (25). Irish wolfhounds, having the highest adult body weight, also reaches this point in a short period of time, compared with the NF and LR (25). Thus, of the 4 breeds validated, the 2 breeds showing the lowest incidence rates of OS have the slowest growth rate.
Previous estimates of incidence risk correspond with the result of the present study, as IW is one of the breeds commonly reported to be at high risk of developing bone tumors (9,10,13). Although Egenvall et al (10) found a somewhat lower incidence rate for IW and LB, and slightly higher for NF than in the present study, the confidence intervals for these estimates are largely overlapping when comparing the 2 studies. Not surprisingly, LR had a significantly lower risk of primary bone tumors than IW and LB (13), although this breed has also been found to be well-represented among bone tumor patients (11,26).
Some breeds, such as the great dane, St. Bernard, and greyhound, are also observed to be at high risk for developing primary bone tumors (1,10,11,13). However, breed specific incidence rates are often not known (27) and, with a few exceptions (28), most estimates are not population-based. Previous studies aiming to describe the epidemiology of this disease have mostly been based upon insurance, and clinical or pathology records (1,10,26,29).
Epidemiologic canine cancer studies have typically employed clinical records, mainly from larger referral clinics or veterinary teaching hospitals (1,26). Several studies have been founded on the Veterinary Medical Data Program, established in 1964 by the National Cancer Institute (US), collecting data from participating veterinary teaching hospitals (13,30). Despite the advantage of good clinical data, often with an accurate diagnosis including biopsy and staging, this method suffers from not being based on an unselected population sample (29). This is because dogs referred to these clinics are more likely to suffer from severe disease and that some of these centers have specialized in oncology. Also, dogs with cancerous disease in which radical treatment is warranted, such as primary bone cancer, may be overrepresented at oncology referral centers, compared with dogs suffering from cancers that respond well to more conventional therapy, such as lymphoma. Studies based upon pathology records also encounter the problem of defining the reference population, as most pathology registers only include dogs in which biopsy and/or autopsy were performed.
Egenvall’s study on canine primary bone tumor epidemiology utilized the database of Sweden’s largest companion animal insurance company, Agria (10). This company serves about 30% of the Swedish dog population, providing a relatively representative sample (31). However, some discrepancies between this sample population and the reference population exist. Insured dogs tend to be somewhat younger than the total canine population (32). By only including dogs with life-insurance, which does not apply after the dog is 10 years old, Egenvall’s study probably underestimated the rate of primary bone tumors in breeds developing the disease later in life. This is a possible explanation for the higher incidence rate in LR observed in the present study, although the 2 cases identified in this study are far too few to convincingly estimate median age at diagnosis. Further, diagnoses obtained from insurance records are based upon the treating veterinarian’s evaluation, regardless of the extent of diagnostic aids, such as radiography and biopsy.
In most dogs diagnosed with primary bone tumors in this study a histological diagnosis was lacking, and the diagnosis was based upon evaluation of the presenting clinical signs and radiography. Histopathology would have strengthened the results presented, but the retrospective design of the study precludes the possibility of obtaining biopsy specimens. However, it can be argued that the clinical signs, including rapid progression of the disease along with typical radiographic findings, strongly favor the probability of OS, which is well known to be the most common canine primary bone tumor; accounting for up to 85% of these tumors (1,8,33). Three out of 4 cases, 75%, being histologically confirmed as OS in this study can probably be explained by the low number of histological diagnoses.
Most cases of primary bone tumors in this study were seen in middle aged to older dogs, the only non-giant breed included (LR) being affected at an older age than the 3 giant breeds (IW, LB, and NF). This corresponds with previous observations of giant breeds being diagnosed with this disease at an earlier age than large breeds, such as the LR (1,29,34,35). Also, the LR is eligible for developing disease at higher ages, simply due to its longer life span. Having a bimodal occurrence in humans, the second, smaller peak after the age of 60 corresponds to this peak incidence seen in middle-aged to older dogs (36). A small increase in age-specific incidence rates has also been seen in dogs of 1 to 2 years of age (34), consistent with the peak incidence among humans. This was not observed in the present study; probably due to a low number of cases.
Distal radius/ulna is the most common site of primary bone tumors according to the literature (1,10,27,34). With > 1/3 of the primary tumors occurring at this site, the present study found a stronger predilection for the distal forelimb than the latter reports — apart from Brodey, who found a similar (1,33), or an even higher (37), proportion of these tumors originating in radius/ulna (distal not specified). The higher prevalence of tumors of the front limbs, especially in large- and giant-breed dogs, has been related to the hypothesis of weight bearing stress as one possible etiological factor. Brodey (1) and Brodey and Riser (33) also reported a strong predilection for this site in giant-breed dogs. Distal femur and distal tibia are previously described as 2 frequently affected locations, while affection of the proximal humerus was found in a lower proportion of cases than generally reported (1,10,27,29,37,38). Interestingly, the primary tumor of both diseased LR was located in the axial skeleton, coinciding with axial involvement being more common among smaller breeds — considering LR to be “smaller” in this context (1,26,29,33,34). However, the number of LR diagnosed with primary bone tumors included in this study is too low to elaborate on this observation.
The results obtained in this survey correspond to previous studies with respect to gender, location of the primary tumor, and median age at the time of diagnosis. In agreement with prior observations, no gender predisposition could be found (13,35). Although several studies have concluded that male dogs are more often affected than their female counterparts (1,33,37,38), this is not a consistent observation, as it is for human OS; Brodey and Riser (33) reporting female St. Bernards to be affected more frequently than male, and Heyman et al (26) observing twice as many females as males when studying pathology records of axial OS. Some studies have shown an increased risk of primary bone tumors in neutered dogs, especially when this procedure is performed at an early age (13,39). Due to the Norwegian animal protection law, prohibiting neutering except for health related purposes, most dogs in Norway are intact, and the mean age at neutering is relatively high — which was also observed in the present survey. As expected, this study therefore could not support this hypothesis. This study estimates lifetime risks and incidence rates for canine primary bone tumors within IW, LB, LR, and NF. As one of few population-based surveys, it provides a valuable contribution to the knowledge on each of these breeds’ risks of developing such tumors. Further pursuing the search of risk factors other than body size or weight is encouraged by the observation of similarly statured dogs, NF, and LB, displaying significantly different risks. Defining these breed specific risks enables subsequent case control studies to be conducted, ultimately aiming to identify specific risk factors for this disease.
Acknowledgments
The authors would like to thank all dog owners who participated in this study. Financial support was provided by “Torsteds legat,” “Dyrlege Smidts stiftelse,” and “Raagholtstiftelsen.” Support from the Norwegian Kennel Club and each of the breed specific associations is also gratefully acknowledged.
References
- 1.Brodey RS, Sauer RM, Medway W. Canine bone neoplasms. J Am Vet Med Assoc. 1963;143:471–495. [PubMed] [Google Scholar]
- 2.Bruland OS, Bauer H, Alvegaard T, Smeland S. Treatment of osteosarcoma. The Scandinavian Sarcoma Group Experience. In: Jaffe N, Bruland OS, Bielack S, editors. Pediatric and Adolescent Osteosarcoma. 1st ed. New York: Springer; 2010. pp. 309–318. [DOI] [PubMed] [Google Scholar]
- 3.Withrow SJ, Powers BE, Straw RC, Wilkins RM. Comparative aspects of osteosarcoma. Dog versus man. Clin Orthop Relat Res. 1991;270:159–568. [PubMed] [Google Scholar]
- 4.Bruland OS, Pihl A. On the current management of osteosarcoma. A critical evaluation and a proposal for a modified treatment strategy. Eur J Cancer. 1997;33:1725–1731. doi: 10.1016/s0959-8049(97)00252-9. [DOI] [PubMed] [Google Scholar]
- 5.Bacci G, Rocca M, Salone M, et al. High grade osteosarcoma of the extremities with lung metastases at presentation: Treatment with neoadjuvant chemotherapy and simultaneous resection of primary and metastatic lesions. J Surg Oncol. 2008;98:415–420. doi: 10.1002/jso.21140. [DOI] [PubMed] [Google Scholar]
- 6.Parkin DM, Stiller CA, Nectoux J. International variations in the incidence of childhood bone tumours. Int J Cancer. 1993;53:371–376. doi: 10.1002/ijc.2910530305. [DOI] [PubMed] [Google Scholar]
- 7.Arnesen K, Gamlem H, Glattre E, Moe L, Nordstoga K. Registration of canine cancer. Tidsskr Nor Laegeforen. 1995;115:714–717. [PubMed] [Google Scholar]
- 8.Dernell WS, Ehrhart NP, Straw RC, Vail DM. Tumors of the skeletal system. In: Withrow S, Vail DM, editors. Withrow & MacEwen’s Small Animal Clinical Oncology. 4 ed. St Louis, Missouri: Saunders Elsevier; 2007. pp. 540–582. [Google Scholar]
- 9.Prestrud KW, Moe L, Gamlem H. Primary bone tumours in dogs in Norway. Norsk Veterinaertidsskrift. 2002;114:15–20. [Google Scholar]
- 10.Egenvall A, Nodtvedt A, von Euler H. Bone tumors in a population of 400 000 insured Swedish dogs up to 10 y of age: Incidence and survival. Can J Vet Res. 2007;71:292–299. [PMC free article] [PubMed] [Google Scholar]
- 11.Boston SE, Ehrhart NP, Dernell WS, Lafferty M, Withrow SJ. Evaluation of survival time in dogs with stage III osteosarcoma that undergo treatment: 90 cases (1985–2004) J Am Vet Med Assoc. 2006;228:1905–1908. doi: 10.2460/javma.228.12.1905. [DOI] [PubMed] [Google Scholar]
- 12.Fraumeni JF., Jr Stature and malignant tumors of bone in childhood and adolescence. Cancer. 1967;20:967–973. doi: 10.1002/1097-0142(196706)20:6<967::aid-cncr2820200606>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 13.Ru G, Terracini B, Glickman LT. Host related risk factors for canine osteosarcoma. Vet J. 1998;156:31–39. doi: 10.1016/s1090-0233(98)80059-2. [DOI] [PubMed] [Google Scholar]
- 14.Troisi R, Masters MN, Joshipura K, Douglass C, Cole BF, Hoover RN. Perinatal factors, growth and development, and osteosarcoma risk. Br J Cancer. 2006;95:1603–1607. doi: 10.1038/sj.bjc.6603474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pui CH, Dodge RK, George SL, Green AA. Height at diagnosis of malignancies. Arch Dis Child. 1987;62:495–499. doi: 10.1136/adc.62.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruland OS, Hoifodt H, Saeter G, Smeland S, Fodstad O. Hematogenous micrometastases in osteosarcoma patients. Clin Cancer Res. 2005;11:4666–4673. doi: 10.1158/1078-0432.CCR-05-0165. [DOI] [PubMed] [Google Scholar]
- 17.Hansen K, Khanna C. Spontaneous and genetically engineered animal models; Use in preclinical cancer drug development. Eur J Cancer. 2004;40:858–880. doi: 10.1016/j.ejca.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 18.Khanna C, Vail DM. Targeting the lung: Preclinical and comparative evaluation of anticancer aerosols in dogs with naturally occurring cancers. Curr Cancer Drug Targets. 2003;3:265–273. doi: 10.2174/1568009033481903. [DOI] [PubMed] [Google Scholar]
- 19.Paoloni M, Khanna C. Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer. 2008;8:147–156. doi: 10.1038/nrc2273. [DOI] [PubMed] [Google Scholar]
- 20.Paoloni MC, Khanna C. Comparative oncology today. Vet Clin North Am Small Anim Pract. 2007;37:1023–1032. doi: 10.1016/j.cvsm.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dohoo I, Martin W, Stryhn H. Sampling. In: Dohoo I, Martin W, Stryhn H, editors. Veterinary Emidemiologic Research. Charlottetown: AVC; 2003. pp. 27–52. [Google Scholar]
- 22.Curtin R, Presser S, Singer E. Changes in telephone survey non-response over the past quarter century. Public Opinion Quarterly. 2005;69:87–98. [Google Scholar]
- 23.Asch DA, Jedrziewski MK, Christakis NA. Response rates to mail surveys published in medical journals. J Clin Epid. 1997;50:1129–1136. doi: 10.1016/s0895-4356(97)00126-1. [DOI] [PubMed] [Google Scholar]
- 24.Merkle D, Edelman M. Nonresponse in exit polls: A comprehensive analysis. In: Groves R, Dillman D, Eltinge J, Little R, editors. Survey Nonresponse. New York: John Wiley & Sons; 2002. pp. 243–257. [Google Scholar]
- 25.Trangerud C, Grondalen J, Indrebo A, Tverdal A, Ropstad E, Moe L. A longitudinal study on growth and growth variables in dogs of four large breeds raised in domestic environments. J Anim Sci. 2007;85:76–83. doi: 10.2527/jas.2006-354. [DOI] [PubMed] [Google Scholar]
- 26.Heyman SJ, Diefenderfer DL, Goldschmidt MH, Newton CD. Canine axial skeletal osteosarcoma. A retrospective study of 116 cases (1986 to 1989) Vet Surg. 1992;21:304–310. doi: 10.1111/j.1532-950x.1992.tb00069.x. [DOI] [PubMed] [Google Scholar]
- 27.Wolke RE, Nielsen SW. Site incidence of canine osteosarcoma. J Small Anim Pract. 1966;7:489–492. doi: 10.1111/j.1748-5827.1966.tb04475.x. [DOI] [PubMed] [Google Scholar]
- 28.Lord LK, Yaissle JE, Marin L, Couto CG. Results of a web-based health survey of retired racing Greyhounds. J Vet Intern Med. 2007;21:1243–1250. doi: 10.1892/07-063.1. [DOI] [PubMed] [Google Scholar]
- 29.Tjalma RA. Canine bone sarcoma: Estimation of relative risk as a function of body size. J Natl Cancer Inst. 1966;36:1137–1150. [PubMed] [Google Scholar]
- 30.Kelsey JL, Moore AS, Glickman LT. Epidemiologic studies of risk factors for cancer in pet dogs. Epidemiol Rev. 1998;20:204–217. doi: 10.1093/oxfordjournals.epirev.a017981. [DOI] [PubMed] [Google Scholar]
- 31.Sallander M, Hedhammar A, Rundgren M, Lindberg JE. Demographic data of a population of insured Swedish dogs measured in a questionnaire study. Acta Vet Scand. 2001;42:71–80. doi: 10.1186/1751-0147-42-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Egenvall A, Hedhammar A, Bonnett BN, Olson P. Survey of the Swedish dog population: Age, gender, breed, location and enrollment in animal insurance. Acta Vet Scand. 1999;40:231–240. doi: 10.1186/BF03547021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brodey RS, Riser WH. Canine osteosarcoma. A clinicopathologic study of 194 cases. Clin Orthop Relat Res. 1969;62:54–64. [PubMed] [Google Scholar]
- 34.Misdorp W, Hart AA. Some prognostic and epidemiologic factors in canine osteosarcoma. J Natl Cancer Inst. 1979;62:537–545. doi: 10.1093/jnci/62.3.537. [DOI] [PubMed] [Google Scholar]
- 35.Rosenberger JA, Pablo NV, Crawford PC. Prevalence of and intrinsic risk factors for appendicular osteosarcoma in dogs: 179 cases (1996–2005) J Am Vet Med Assoc. 2007;231:1076–1080. doi: 10.2460/javma.231.7.1076. [DOI] [PubMed] [Google Scholar]
- 36.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brodey RS, Abt DA. Results of surgical treatment in 65 dogs with osteosarcoma. J Am Vet Med Assoc. 1976;168:1032–1035. [PubMed] [Google Scholar]
- 38.Misdorp W. Skeletal osteosarcoma. Animal model: Canine osteosarcoma. Am J Pathol. 1980;98:285–288. [PMC free article] [PubMed] [Google Scholar]
- 39.Cooley DM, Beranek BC, Schlittler DL, Glickman NW, Glickman LT, Waters DJ. Endogenous gonadal hormone exposure and bone sarcoma risk. Cancer Epidemiol Biomarkers Prev. 2002;11:1434–1440. [PubMed] [Google Scholar]