Abstract
Dairy cattle suffer stress from management and production; contemporary farming tries to improve animal welfare and reduce stress. Therefore, the assessment of long-term hypothalamic-pituitary-adrenal function using non-invasive techniques is useful. The aims in this study were: to measure cortisol concentration in cow and calves hair by radioimmunoassay (RIA), to test cortisol accumulation in bovine hair after adrenocorticotropic hormone (ACTH) challenges, and determine the influence of hair color on cortisol concentrations. Fifteen Holstein heifers were allotted to 3 groups (n = 5 each): in control group (C), just the hair was sampled; in the saline solution group (SS), IV saline solution was administered on days 0, 7, and 14; and the ACTH group was challenged 3 times with ACTH (0.15 UI per kg of body weight) on days 0, 7, and 14. Serum samples from the SS and ACTH groups were obtained 0, 60 and 90 min post-injection. Serum cortisol concentration was greater 60 and 90 min after injection with ACTH. Hair was clipped on days 0, 14, 28, and 44. Hair cortisol was methanol extracted and measured by RIA. Hair cortisol was preserved for 11 mo. Hair cortisol concentrations in the ACTH group were greater than in the saline and control groups on days 14 and 28, but not on day 44. Concentrations were greater in calves than in cows and greater in white hair than in black hair. Cortisol accumulated in bovine hair after ACTH challenges, but the concentration was affected by both age and hair color. If hair color effects are taken into account, assessing cortisol concentration in hair is a potentially useful non-invasive method for assessing stress in cattle.
Résumé
Les bovins laitiers souffrent de stress provenant de la régie et de la production; l’élevage contemporain tente d’améliorer le bien-être animal et de réduire le stress. Ainsi, l’évaluation à long terme de la fonction hypothalamo-hypophyso-surrénalienne à l’aide de technique non-invasive est utile. Les objectifs de la présente étude étaient : mesurer les concentrations de cortisol dans le poil des vaches et des veaux par radio-immuno-essai (RIA), tester l’accumulation de cortisol dans les poils bovins après des challenges avec de l’hormone adrénocorticotrope (ACTH), et déterminer l’influence de la couleur des poils sur les concentrations de cortisol. Quinze génisses Holstein ont été réparties en trois groupes de 5 animaux : dans le groupe témoin (C), seulement les poils ont été échantillonnés; dans le groupe recevant de la saline (SS), une solution saline a été administrée par voie IV aux jours 0, 7 et 14; et le groupe ACTH a été challengé 3 fois avec de l’ACTH (0,15 UI par kg de poids corporel) aux jours 0, 7 et 14. Des échantillons de sérum provenant des animaux des groupes SS et ACTH ont été obtenus 0, 60 et 90 min post-injection. La concentration de cortisol sérique était plus élevée 60 et 90 min après l’injection d’ACTH. Des poils ont été récoltés aux jours 0, 14, 28 et 44. Le cortisol dans les poils a été extrait à l’aide de méthanol et mesuré par RIA. Le cortisol des poils a été conservé pendant 11 mois. La concentration de cortisol dans les poils des animaux du groupe ACTH était plus élevée que dans le poil des animaux SS et C aux jours 14 et 28, mais pas au jour 44. Les concentrations étaient plus élevées chez les veaux que chez les vaches, et plus élevées dans les poils blancs que les poils noirs. Le cortisol s’est accumulé dans les poils bovins après challenge avec l’ACTH, mais les concentrations étaient influencées par l’âge de l’animal et la couleur du poil. Si les effets de la couleur des poils sont pris en compte, la détermination de la concentration de cortisol dans les poils est potentiellement une méthode non-invasive utile pour évaluer le stress chez les bovins.
(Traduit par Docteur Serge Messier)
Introduction
Determination of hypothalamus-pituitary-adrenal (HPA) axis activity is the standard procedure to evaluate stress conditions in farm animals (1). However, blood cortisol concentrations vary due to different factors including circadian, ultradian, or annual rhythms; diet; food intake; environmental temperature or humidity; management; and physiological conditions (2–6). Consequently pulsatile and episodic release of cortisol is generally irregular and difficult to interpret in the short and, particularly, in the long term (1,2). Advancement in knowledge of long-term stress is limited because of the absence of adequate methodology to determine variations in physiological responses of animals exposed to stressful conditions during relatively long intervals. The adrenocorticotropic hormone (ACTH) challenge test has been proposed as an indicator of long term activity of the HPA axis, consisting of an intravenous administration of ACTH preceded and followed by blood samples measuring cortisol levels in serum or plasma. Cortisol peaks 30 min after the challenge (7,8). In general, under stressful conditions adrenal responsiveness in cattle increases in the short term, but decreases over the long term (1). Despite the reliability of the technique for determining intensity of chronic stress, it is of limited use for dairy farms because it is invasive and stressful for animals.
Glucocorticoids (9–15) have been found in the hair of several species, as well, synthetic glucocorticoids (10,12,16,17) and cortisol (10) have been measured in bovine hair. The authors are not aware of any studies that have measured serum and hair cortisol concentrations in cattle after an ACTH challenge. Using hair samples could be advantageous, since they are easily collected and preserved, and, more importantly, this method is safe and causes little disturbance to the animals. Moreover, cortisol concentrations accumulated in hair reflects the cortisol synthesized in the long-term, avoiding changes due to circadian and ultradian rhythms (2). However, some influencing factors exist, such as hair color (12,18) and age (19,20), that could have an effect on the final values of hair cortisol content and should be included in any study as controlled variables.
The aims in this study were to measure and compare cortisol concentrations in the hair of cows and calves by radioimmunoassay (RIA), to test if cortisol accumulates in bovine hair after ACTH challenges, and determine any relationship between cortisol levels and hair color (black or white) in Holstein cows.
Materials and methods
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Centro de Investigaciones y Estudios Avanzados del Instituto Politécnico Nacional (IACUC-CINVESTAVIPN). Animals were maintained in irrigated alfalfa pastures under an intensive rotational system. Diet was supplemented with mineral mix, alfalfa hay, and corn silage. Animals were trained the month prior to the experimental procedures to reduce stress due to handling and management and kept in a loose house type farm at La Santillana Ranch, a National University (UNAM) facility in Queretaro, Mexico.
Hair cortisol content according to hair color
Samples were collected from 12 Holstein cows in order to compare cortisol concentrations in hair of different color (black and white). Two samples, one of each color, from the same costal side of each cow were collected on the same day from 2-year-old animals. Hair samples were analyzed for cortisol content by RIA.
Hair cortisol content according to age
Hair samples of different aged animals were compared to determine the effect of age on hair cortisol. Hair samples were collected from the coastal region of 2-year-old cows (n = 6) and from 15-day-old heifers (n = 6). All hair samples were obtained the same day between 13:00 and 15:00 h. Hair samples were analyzed for cortisol content by using RIA.
The ACTH challenge effect on hair cortisol levels
Holstein heifers (n = 15) ranging between 17 and 22 mo of age and from 2 to 6 mo pregnant were used. Heifers were homogenously allotted to 3 groups, each group had 1 cow of each age and month of pregnancy (n = 5). Hair samples from the control group (Control) were obtained from animals with no further handling. Animals in the saline solution (SS) and ACTH groups received 3 injections (IV) of physiological saline solution (2.5 mL) or ACTH [0.15 UI per kg bodyweight (BW), Sinachten, Porcine Pituitary; Sigma, St. Louis Missouri, USA], respectively, on days 0, 7, and 14. In order to minimize circadian rhythm variables, all injections were administered between 12:00 and 13:00 h.
Sampling procedures
Blood samples were collected immediately before and 60 and 90 min after ACTH injections. Blood samples (7 mL) were obtained by venipuncture of the coccygeal vein using vacutainer tubes without anticoagulant and centrifuged at 1520 × g. Serum aliquots were stored in Eppendorf tubes at −25°C until analyzed for cortisol content by RIA.
Samples of black hair from an area of approximately 20 × 30 cm were harvested using an electric hair clipper (Oster model Turbo A5. 2009 Jarden Corporation New York, USA), with a blade number 5, on day 0 (basal hair sample). The clipped area was divided into 3 smaller areas that were harvested on days 14, 28, and 42. The blade of the clipper was cleaned using absolute ethanol before every hair sample collection to keep cross-contamination at a minimum. Hair samples were kept in clean plastic zip-lock bags and stored at room temperature for 30 d until processing for cortisol levels. We decided to use black hair, because on most Holstein cows the predominant color is black, only 12.9% of hair on the body (not legs or face) is white (21).
Hair cortisol extraction
Hair samples were processed as previously described (11,22), with some modifications. Hair was first cleaned of macroscopic debris and was washed once with absolute ethanol (Merk, Darmstadt, Germany) and shaken in a multitube vortexer (VWR Grant Instruments) for 1 min. The hair was recovered, dried, and cut into approximately 2-mm fragments. Three hundred milligrams were placed into borosilicate glass vials with 13 mL of HPLC-grade methanol (Merck). Samples were shaked for 2 h, maintained at 22°C for 20 h, followed by 4 additional hours in the multitube vortexer. Supernatant was decanted into borosilicate glass conical tubes and kept at rest for 2 h for sedimentation. Then, the supernatant was transferred into borosilicate glass vials and evaporated to dryness under a high purity nitrogen stream.
Determination of cortisol concentrations in serum and hair
Cortisol concentrations in bovine serum were measured without extraction, whereas cortisol in bovine hair was quantified in reconstituted hair extracts (70 μL of assay buffer: Na2HPO4, 1.39%; sodium azide, 0.1%; NaCl, 0.5%; gelatine, 0.1% diluted in deionized water). In both cases, duplicates from each sample were processed following the instructions of a commercial solid phase kit (Coat-a-Count; DPC Laboratories 125I, Los Angeles, California, USA). According to manufacturers, the cortisol specific monoclonal antibodies cross reactivity with respect to other endogen steroids (aldosterona, corticosterona, cortisone, 11-deoxicorticosterona, and tetrahidrocortisol) is < 1%. For each assay, a standard curve was made at 0, 10, 50, 100, 200, and 500 ng/mL. Measurements were done using a gamma rays counter (Cobra II Auto Gamma Counting System; Packard Institute Illinois, USA). For all assays, a pool of bovine sera was used as the external standard to determine the inter-assay coefficient of variation and a point of the standard curve (200 ng/mL of cortisol). It was also used as the internal standard to determine intra-assay coefficient of variation. The inter-assay and intra-assay coefficient of variation were 8.51% and 6.05%, respectively. Following the manufacturer specifications, detection limits of the assay were 2 to 500 ng/mL; the coefficient of correlation (R2) of the calculated linear regression was > 0.98 in all assays. Parallelism (n = 10) was demonstrated between serial dilutions (1:2, 1:4, and 1:8) of hair extracts samples and the standard curve.
Statistical analysis
The statistical analyses were done using computer software (Statistical Analysis System, version 6.12; SAS Institute, Cary, North Carolina, USA). Results are expressed as the mean ± standard error. Data relative to cortisol concentration in serum and hair were assessed by analysis of variance (ANOVA) with repeated measures in ACTH, SS, and Control groups, and time as other factor. F-test was used to test the hypothesis with 95% of significance. The statistical model performed was as follows:
| (Equation 1) |
Where: Yijk = cortisol concentration (serum ng/mL and hair pg/mg), μ = the overall mean, αi = effect of groups (ACTH, SS, control), βj = effect of sampling time, γik = effect of cortisol concentration within repeated measures, ɛijk = residual variation.
Linear regression and parallelism analysis were done using computer software (Graph Pad Inplot, version 4; Graph Pad Software, San Diego, California, USA). Differences were considered significant when P < 0.05 (23,24).
Results
Serum cortisol
Serum cortisol concentration, evaluated on days 0, 7, and 14, at 60 and 90 min after ACTH injection, was significantly higher for the ACTH group compared with the SS group (P < 0.05), while no differences were found in cortisol levels on the same days at time zero (Figure 1).
Figure 1.
Serum cortisol concentration in response to adrenocorticotropic hormone (ACTH) challenges. Three different ACTH challenges were performed on days 0, 7, and 14. Serum cortisol concentration (ng/mL mean ± standard error) was measured at time 0, 60, and 90 min after ACTH injection. The saline solution (SS) group of heifers received IV saline solution (n = 5); ACTH group was challenged with 0.15 UI/kg BW, IV, ACTH (n = 5).
ab Differences between letters show statistical differences (P < 0.05).
Hair cortisol
Parallelism was demonstrated between serially diluted hair extracts and the standard curve, R = 0.997. Accuracy was demonstrated by adding 30 μL of hair extract to each point of the standard curve (n = 10). Correlation coefficient was R = 0.99, P < 0.01. Recovery efficiency was estimated as 43.5% (25).
Stability of cortisol in hair samples was good and independent of storage methods. No differences (P > 0.05) were found between cortisol values from hair samples (n = 12) stored in zip lock plastic bags at room temperature for 30 d (11.3 ± 1.19 pg/mg) and those stored for 12 mo (10.7 ± 0.2 pg/mg) after harvesting. No differences (P > 0.05) were found between cortisol values from hair extract samples dried with nitrogen stream and stored into borosilicate glass vials (n = 6) at 2 to 4°C for 1 d (16.77 ± 2.12 pg/mg of hair) and those stored for 11 mo (14.26 ± 1.08 pg/mg of hair).
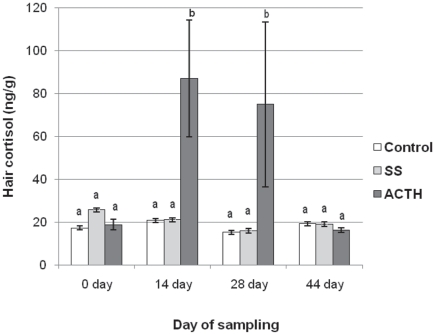
No significant differences (P > 0.05) were found among groups in cortisol concentrations in hair samples obtained on days 0 and 44. In the ACTH group, hair cortisol concentrations were significantly higher in samples obtained on days 14 and 28 (P < 0.05) compared with those obtained on days 0 and 44 (Figure 2).
Figure 2.
Adrenocorticotropic hormone (ACTH) challenge induced response in hair cortisol concentration (pg/mg, mean ± Sx̄). Hair was collected on days 0, 14, 28, and 42 of the experiment. The ACTH challenge was done on days 0, 7, and 14. The control group (n = 5) received no injections. The saline solution (SS) group of heifers received saline solution (n = 5). The ACTH group (n = 5), challenged 3 times (1 wk apart) with ACTH.
ab Differences between letters show statistical differences (P < 0.05).
Influence of animal age and hair color on hair cortisol concentrations
Cortisol concentrations in hair samples obtained from 15-day-old heifers (114.5 ± 14.43 pg/mg of hair) was significantly higher (P < 0.05) compared with those from 2-year-old cows (12.15 ± 1.85 pg/mg of hair). Hair cortisol concentration of 2-year-old cows was higher in the white hair (23.84 ± 2.51 pg/mg of hair) than in black hair (14.31 ± 1.07 pg/mg of hair, P < 0.05).
Discussion
In our study, cortisol recovery efficiency from hair is comparable with that obtained in other studies (10,17,26). We found that hair cortisol is stable for at least 11 mo. This finding is supported by others, who found that a large variety of endogenous substances, drugs, and contaminants may accumulate and can be detected in hair stored for a long time (27). It is generally accepted that drugs accumulated in hair samples remain for long intervals, for example, concentrations of several steroids in cattle hair showed little change over 100 d post-injection (27).
Additionally, hair is an excellent resource to use to identify physiological events in animals, because collecting it is less invasive than other procedures (such as venipuncture) and is an easy material to store and transport.
To our knowledge, this is the first report that relates cortisol content to hair color in cattle. We found that white hair stores higher amounts of cortisol than black hair. Previous studies on bovine hair have reported that accumulation of some substances, such as selenium (28), clenbuterol (10), testosterone in bulls (29), and metiltestosterone (30), was greater in black than in white hair. On the other hand, in cows, hormones such as estradiol and testosterone, have been found in similar proportions in black and white hair (29). In humans, some drugs were found to be more concentrated in dark than in fair colored hair (29), but cortisol and corticosterone concentrations seem not to be affected by hair color (12,14,17,18,31). This suggests that the accumulation of a given substance in hair depends upon the interactions between the substance and the different type, concentration, or both of melanin (32).
The mechanisms by which cortisol incorporates itself into shaved and growing hair were proposed by Henderson (33) using a multiple pool model including: 1 — diffusion from blood to the growing follicle; 2 — from the apocrine and sebaceous gland after shaft formation; and 3 — by absorption from the external environment.
To our knowledge this study is also the first to report the influence of age on hair cortisol concentrations in cattle. We found that heifers had greater cortisol concentrations than 2-year-old cows. These results could be explained by the high serum cortisol concentrations present late in pregnancy (6) that are stimulated by the fetal pituitary adrenal axis, the route by which parturition is initiated in cows (fetal ACTH stimulates the adrenal gland to increase cortisol secretion) (34). Thus, an increase in the cortisol concentration found on day 14 could have been the result of an accumulation of cortisol released from adrenal cortex after the first and second injections of ACTH. Hair cortisol on day 28 might be a composite of cortisol elicited by the second and third ACTH injections. The low cortisol concentration found on day 44 may be explained by dilution of cortisol content of old hair with the new one; it could also be for the elimination of old hair in the telogen phase (27).
Since ACTH increases cortisol in circulating blood (4), it was assumed that the hormone is derived from the adrenal cortex. Recently Ito et al (35), working in vitro, have demonstrated that hair follicle cells from the human scalp produce cortisol in response to corticotropin releasing hormone (CRH) or ACTH and Sharpley et al (36) found that the application of transitory local stressors increased hair cortisol in the zone.
In summary, in the present study we found that cortisol concentrations can be measured by RIA in bovine hair. This technique allowed us to demonstrate that white hair accumulated larger amounts of cortisol than black hair, that calf hair has greater cortisol concentrations than cow hair, and that hormone accumulation in bovine hair was influenced by 3 ACTH challenges.
As this study demonstrates, cow hair accumulates cortisol in response to ACTH and, therefore, it could be used as a retrospective indicator for circumstances that involve the activation of the hypothalamus-pituitary-adrenal axis.
Acknowledgments
The authors thank the UNAM for financial support via project PAPIIT-IN228003 and the Consejo Nacional de Ciencia y Tecnología CONACYT for a fellowship. The authors also thank the personnel of Experimental farm CEIEPAA as well as Dr. Felipe de Anda, owner of Agropecuaria El Gigante. Thanks to Dr. Agustín González Villanueva for assistance in statistical work and to M.C. Yolanda Gómez, Mrs. Araceli Mendoza, Mr. Armando Vargas, and Mr. Jesús Velázquez for their help at CINVESTAV laboratory.
References
- 1.Mormède P, Andanson S, Aupérin B, et al. Exploration of the hypothalamic-pituitary-adrenal function as a tool to evaluate animal welfare. Physiol Behav. 2007;92:317–339. doi: 10.1016/j.physbeh.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Lefcourt AM, Bitman J, Kahl S, Wood LD. Circadian and ultradian rhythms of peripheral cortisol concentrations in lactating dairy cows. J Dairy Sci. 1993;76:2607–2612. doi: 10.3168/jds.S0022-0302(93)77595-5. [DOI] [PubMed] [Google Scholar]
- 3.Wagonner JW, Löest CA, Turner JL, Mathis CP, Hallford DM. Effects of dietary protein and bacterial lipopolysaccharide infusion on nitrogen metabolism and hormonal responses of growing beef steers. J Anim Sci. 2009;87:3656–3668. doi: 10.2527/jas.2009-2011. [DOI] [PubMed] [Google Scholar]
- 4.Harrison RO, Young JW, Ford SP. Relationships between cortisol concentration and milk production energy balance and exhibition of estrus during early lactation in Holstein cows. J Dairy Sci. 1989;72:312–317. [Google Scholar]
- 5.Negrao JA, Porcionato AM, De Pasillé AM, Rushen J. Cortisol in saliva and plasma of cattle after ACTH administration and milking. J Dairy Sci. 2004;8:1713–1718. doi: 10.3168/jds.S0022-0302(04)73324-X. [DOI] [PubMed] [Google Scholar]
- 6.Kindahl H, Kornmatitsuk B, Königsson K, Gustafsson H. Endocrine changes in late bovine pregnancy with special emphasis on fetal well-being. Dom Anim Endocrinol. 2002;23:321–328. doi: 10.1016/s0739-7240(02)00167-4. [DOI] [PubMed] [Google Scholar]
- 7.Lay DC, Friend TH, Randel RD, et al. Adrenocorticotropic hormone dose response and some physiological effects of transportation on pregnant Brahaman cattle. J Anim Sci. 1996;74:1806–1811. doi: 10.2527/1996.7481806x. [DOI] [PubMed] [Google Scholar]
- 8.González VM, Yabuta AK, Galindo F. Behaviour and adrenal activity of first parturition and multiparous cows under a competitive situation. Appl Anim Behav Sci. 2003;83:259–266. [Google Scholar]
- 9.Bévalot F, Gaillard Y, Lhermitte MA, Pépin G. Analysis of corticosteroids in hair by liquid chromatography–electrospray ionization mass spectrometry. J Chromatogr B. 2000;740:227–236. doi: 10.1016/s0378-4347(00)00085-2. [DOI] [PubMed] [Google Scholar]
- 10.Antignac JP, Le Bizec B, Monteau F, Poulain F, François A. Multi-residue extraction-purification procedure for corticosteroids in biological samples for efficient control of their misuse in livestock production. J Chromatogr B. 2001;757:11–19. doi: 10.1016/s0378-4347(00)00626-5. [DOI] [PubMed] [Google Scholar]
- 11.Koren L, Mokady O, Karastov T, Koren G, Geffen E. A novel method using hair for determining hormonal levels in wildlife. Anim Behav. 2002;63:403–406. [Google Scholar]
- 12.Raul J-S, Cirimele V, Bertrand L, Kintz P. Detection of physiological concentrations of cortisol and cortisone in human hair. J Clinbiochem. 2004;37:1105–1111. doi: 10.1016/j.clinbiochem.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen Comp Endocrinol. 2006;147:255–261. doi: 10.1016/j.ygcen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Kalra S, Einarson A, Karaskov T, Van Uum S, Koren G. The relationship between stress and hair cortisol in healthy pregnant women. Clin Invest Med. 2007;30:E103–E107. doi: 10.25011/cim.v30i2.986. [DOI] [PubMed] [Google Scholar]
- 15.Accorsi PA, Carloni E, Valsecchi P, et al. Cortisol determination in hair and feces from domestic cats and dogs. Gen Comp Endocrinol. 2008;155:398–402. doi: 10.1016/j.ygcen.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Van den hauwe O, Dumoulin F, Elliot C, Van Peteghem C. Detection of synthetic glucocorticoid residues in cattle tissue and hair samples after a single dose administration using LC-MS/MS. Chromatogr B. 2005;817:215–223. doi: 10.1016/j.jchromb.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Cirimele V, Kintz P, Dumestre V, Goullé JP, Ludes B. Identification of ten corticosteroids in human hair by liquid chromatography–ionspray mass spectrometry. Forensic Sci Int. 2000;107:381–388. doi: 10.1016/s0379-0738(99)00180-2. [DOI] [PubMed] [Google Scholar]
- 18.Sauvé B, Koren G, Tokmakejian S, Van Uum SHM. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin Invest Med. 2007;30:E183–E191. doi: 10.25011/cim.v30i5.2894. [DOI] [PubMed] [Google Scholar]
- 19.Zhi-Yan Z, Fang-Hong L, Yi X, Yue-Rong F, Bogdan A, Touitou Y. Cortisol secretion in the elderly, influence of age, sex and cardiovascular disease in a Chinese population. Steroids. 2003;68:551–555. doi: 10.1016/s0039-128x(03)00083-7. [DOI] [PubMed] [Google Scholar]
- 20.Van Uum SHM, Sauvé B, Fraser LA, Morley-Forster P, Paul TL, Koren G. Elevated content of cortisol in hair of patients with severe chronic pain: A novel biomarker for stress. Stress. 2008;11:483–488. doi: 10.1080/10253890801887388. [DOI] [PubMed] [Google Scholar]
- 21.Becerril CE, Wilcox CJ. Determination of percentage of white coat color from registry certificate in Holsteins. J Dairy Sci. 1992;75:3582–3586. [Google Scholar]
- 22.Gaillard Y, Vaysette F, Gilbert P. Compared interest between hair analysis and urinalysis in doping controls. Results for amphetamines, corticosteroids and anabolic steroids in racing cyclists. Forensic Sci Int. 2000;107:361–379. doi: 10.1016/s0379-0738(99)00179-6. [DOI] [PubMed] [Google Scholar]
- 23.Deming WE. Some Theory of Sampling. 2th ed. New York: Dover Publications; 1966. pp. 192–193. [Google Scholar]
- 24.Spiegel RM. Estadística. 2th ed. México: McGraw-Hill; 1991. pp. 380–386. [Google Scholar]
- 25.Buchanan KL, Goldsmith AR. Noninvasive endocrine data for behavioural studies: The importance of validation. Anim Behav. 2004;67:183–185. [Google Scholar]
- 26.Hooijerik HA, Lommen A, Mulder PPJ, Van Rhijin JA, Nielen MWF. Liquid chromatography-electrospray ionisation-mass spectrometry based method for the determination of estradiol benzoate in hair of cattle. Anal Chim Acta. 2005;529:167–172. [Google Scholar]
- 27.Gratacós-Cubarsí M, Castellari M, Valero A, García-Regueiro JA. Hair analysis for veterinary drug monitoring in livestock production. J Chromatogr B. 2006;834:14–25. doi: 10.1016/j.jchromb.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Christodoulopoulos G, Roubies N, Karatzias H, Papasteriadis A. Selenium concentration in blood and hair of Holstein dairy cows. Biol Trace Elem Res. 2003;91:145–150. doi: 10.1385/BTER:91:2:145. [DOI] [PubMed] [Google Scholar]
- 29.Gleixner A, Meyer HD, Heinrich H. Detection of estradiol and testosterone in hair of cattle by HPLC/EIA. Fresenius. J Anal Chem. 1997;357:1198–1201. [Google Scholar]
- 30.Rambaud L, Bichon E, Cesbron N, Françoise A, Le Bizec B. Study of 17β-estradiol-3-benzoate, 17α-metiltestosterone and medroxiprogesterone acetate fixation in bovine hair. Anal Chim Acta. 2005;532:165–176. [Google Scholar]
- 31.Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. Hair as a retrospective calendar of cortisol production-increased cortisol incorporation into hair in the third trimester of pregnancy. Psyneuen. 2009;34:32–37. doi: 10.1016/j.psyneuen.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 32.Stout PR, Ruth JA. 3 H-Nicotine, 3-flunitazepam, and 3 H-cocaine incorporation into melanin: A model for the examination of drug-melanin interactions. J Anal Toxicol. 2001;25:607–611. doi: 10.1093/jat/25.7.607. [DOI] [PubMed] [Google Scholar]
- 33.Henderson GL. Mechanisms of drug incorporation into hair. Forensic Sci Int. 1993;63:19. doi: 10.1016/0379-0738(93)90256-a. [DOI] [PubMed] [Google Scholar]
- 34.Flint APF, Rickets AP, Craig VA. The control of placental steroid synthesis at parturition in domestic animals. Anim Reprod Sci. 1979;2:239–251. [Google Scholar]
- 35.Ito N, Ito T, Kromminga A, et al. Human hair follicles display a functional equivalent of the hypothalamic-pitutary-adrenal (HPA) axis and synthesize cortisol. FASEB J. 2005;1096:1–2. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- 36.Sharpley CF, Kauter KG, McFarlane JR. An initial exploration of in vivo hair cortisol responses to a brief pain stressor: Latency, localization and independence effects. Physiol Res. 2009;58:757–761. doi: 10.33549/physiolres.931544. [DOI] [PubMed] [Google Scholar]