Abstract
In this study the tonB2 gene was cloned from Actinobacillus pleuropneumoniae JL01 (serovar 1) and expressed as a glutathione-S-transferase (GST) fusion protein in Escherichia coli BL21(DE3). The GST fusion protein was recognized by antibodies in serum positive for A. pleuropneumoniae by Western blot analysis. Purified soluble GST-TonB2 was assessed for its ability to protect BALB/c mice against A. pleuropneumoniae infection. Mice were vaccinated with GST-TonB2 subcutaneously and challenged intraperitoneally with either ~4.0 × 105 colony-forming units (CFU) or ~1.0 × 106 CFU of A. pleuropneumoniae 4074. They were examined daily for 7 d after challenge. The survival rate of the TonB2-vaccinated mice was significant higher than that of the mice given recombinant GST or adjuvant alone. These results demonstrate that A. pleuropneumoniae TonB2 is immunogenic in mice and should be further assessed as a potential candidate for a vaccine against A. pleuropneumoniae infection. In addition, an indirect enzyme-linked immunosorbent assay (ELISA) based on the GST-TonB2 recombinant protein was developed. Compared with the ApxIVA ELISA, the TonB2 ELISA provided earlier detection of antibodies in pigs at various times after vaccination with A. pleuropneumoniae live attenuated vaccine. When compared with an indirect hemagglutination test, the sensitivity and specificity of the TonB2 ELISA were 95% and 88%, respectively. The TonB2 ELISA provides an alternative method for rapid serologic diagnosis of A. pleuropneumoniae infection through antibody screening, which would be especially useful when the infection status or serovar is unknown.
Résumé
Dans la présente étude le gène tonB2 a été cloné à partir d’Actinobacillus pleuropneumoniae JL01 (sérovar 1) et exprimé en tant que protéine de fusion de la glutathione-S-transférase (GST) dans la souche BL21(DE3) d’Escherichia coli. La protéine de fusion GST a été reconnue par les anticorps dans les sérums positifs pour A. pleuropneumoniae par analyse par immunobuvardage. Le complexe GST-TonB2 a été évalué pour sa capacité à protéger les souris BALB/c contre une infection par A. pleuropneumoniae. Les souris ont été vaccinées avec GST-TonB2 par voie sous-cutanée et inoculées par voie intra-péritonéale avec ~4,0 × 105 unités formatrices de colonies (UFC) ou ~1,0 × 106 UFC d’A. pleuropneumoniae 4074. Elles ont été examinées quotidiennement pendant 7 j après l’infection défi. Le taux de survie des souris TonB2 vaccinées était significativement plus élevé que celui des souris qui avaient reçu uniquement la GST recombinante ou l’adjuvant. Les résultats ont démontré que TonB2 d’A. pleuropneumoniae est immunogène chez les souris et devrait être évalué de manière plus approfondie comme candidat potentiel pour un vaccin contre l’infection par A. pleuropneumoniae. De plus, une épreuve immuno-enzymatique (ELISA) utilisant la protéine recombinante GST-TonB2 a été développée. Comparativement à un ELISA utilisant ApxIVA, l’ELISA TonB2 a permis une détection plus rapide des anticorps chez les porcs à différents temps après la vaccination contre A. pleuropneumoniae à l’aide d’un vaccin vivant atténué. Lorsque comparé à une épreuve d’hémagglutiantion indirecte, la sensibilité et la spécificité de l’ELISA TonB2 étaient respectivement de 95 % et 88 %. L’ELISA TonB2 fournit une méthode alternative rapide pour le diagnostic sérologique d’infection par A. pleuropneumoniae au moyen d’une méthode de tamisage des anticorps, ce qui serait spécialement utile lorsque le statut de l’infection ou le sérovar infectant sont inconnus.
(Traduit par Docteur Serge Messier)
Introduction
Actinobacillus pleuropneumoniae is the causative agent of porcine contagious pleuropneumonia (PCP), a highly contagious and often fatal disease that causes great economic losses in industrialized pig production worldwide (1). Vaccination is potentially an effective tool for the prevention of PCP. Exploration of potential immunogens is a primary step in developing effective vaccines. Previous studies of A. pleuropneumoniae immunogens were focused on surface-exposed proteins such as RTX toxins (2), lipopolysaccharides (3), outer membrane lipoprotein A (OmlA) (4), transferrin-binding protein A (5), and outer membrane proteins (6).
TonB2, the periplasm protein of the 2nd tonB system that functions in iron acquisition by transporting protons from the cytoplasmic membrane to outer membrane receptors, has been found in Pseudomonas aeruginosa, Vibrio cholerae, Vibrio anguillarum, Vibrio alginolyticus, and Photobacterium damselae (7–11). This system was first reported in A. pleuropneumoniae by Beddek et al (12). The tonB2 system is important for bacterial growth in vitro and in vivo and plays an important role in A. pleuropneumoniae virulence (12). In the current study the tonB2 gene of A. pleuropneumoniae was cloned and expressed in Escherichia coli. The immunogenicity of the recombinant protein was tested in a murine vaccination/challenge model. An indirect enzyme-linked immunosorbent assay (ELISA) based on this protein was developed. This ELISA can be used for surveillance of antibodies against A. pleuropneumoniae.
Materials and methods
Bacterial strains, primers, plasmids, and growth conditions
The bacterial strains, primers, and plasmids used in this work are listed in Table I. The A. pleuropneumoniae strains were cultured in tryptic soy broth or tryptic soy agar (Becton, Dickinson and Company, Baltimore, USA) supplemented with nicotinamide adenine dinucleotide (NAD, Sigma-Aldrich, St. Louis, Missouri, USA), 10 μg/mL. The E. coli strains were cultured in Luria–Bertani broth supplemented with ampicillin (50 μg/mL) as required.
Table I.
Bacterial strains, primers, and plasmids used in this study, in which the tonB2 gene was cloned from Actinobacillus pleuropneumoniae JL01 (serovar 1) and expressed as a glutathione-S-transferase (GST) fusion protein in Escherichia coli BL21(DE3)
| Strain, plasmid, and primer | Relevant characteristics | Source |
|---|---|---|
| A. pleuropneumoniae | ||
| JL01 | Serovar 1 | Isolated from Jilin province, China |
| 4074 | Serovar 1 | Pat Blackall |
| SLW03 | Serovar 1 | Lin et al (12) |
| E. coli | ||
| DH5a | Cloning vehicle: supE44 □lacU169 (ϕ80 lacZ□M15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Takara (Dalian, China) |
| BL21(DE3) | Expression host: F− ompT r−B m−B; DE3 is a λ derivative carrying lacI and T7 RNA polymerase genes under placUV5 control | Takara |
| Primers | ||
| P1 | 5′ GGG GGA TCC ATG AAG AAA AAA CAT TCT CG 3′: upstream primer with BamHI site (underlined) comprising positions 1 to 20 of tonB2 coding sequence | This work |
| P2 | 5′ GGG GTC GAC TTA TTC AAT CGA GAA TTT CAC C 3′: downstream primer with SalI site (underlined) comprising positions 837 to 858 of tonB2 coding sequence | |
| P3 | 5′ GGC TGT CTG TTA GTG GTT CG 3′: upstream primer comprising positions 953 to 972 of apxIVA coding sequence | Liu et al (13) |
| P4 | 5′ CCG TGT GCA GAA ATA CTG CC 3′: downstream primer comprising positions 2125 to 2144 of apxIVA coding sequence | |
| Plasmids | ||
| pMD18-T | E. coli cloning vector carrying an ampicillin resistance determinant | Takara |
| pMD-tonB2 | pMD18-T carrying the tonB2 gene open reading frame (ORF) of A. pleuropneumoniae JL01, for sequence analysis | This work |
| pGEX-KG | N-terminal glutathione-S-transferase (GST) fusion expression vector: pBR322 ori, Ampr | Guan et al (14) |
| pKG-tonB2 | pGEX-KG carrying the tonB2 gene ORF of A. pleuropneumoniae JL01, for GST-TonB2 expression | This work |
Cloning of A. pleuropneumoniae tonB2
Isolation of genomic DNA from A. pleuropneumoniae JL01 (serovar 1) was performed as described previously (16). The tonB2 open reading frame (ORF) was cloned from the genomic DNA by polymerase chain reaction (PCR) with the use of primers P1 and P2 (Table I), synthesized by Sangon Biotech, Shanghai, China. The PCR product was cloned into the A/T cloning vector pMD18-T (Takara, Dalian, China) to form pMD-tonB2, which was transformed into E. coli DH5α. Plasmids were extracted by alkaline lysis and sequenced in both directions with the use of primers M13–47 and RV-M (synthesized by Sangon Biotech, Shanghai, China). Multisequence alignment with published A. pleuropneumoniae TonB2 sequences (4074, JL03, L20, and AP76) was done by means of clustalW. The antigenic property of TonB2 was predicted with the bioinformatics method of EMBOSS explorer (http://emboss.bioinformatics.nl/cgi-bin/emboss/antigenic) and expressed as an antigenic site, defined as the occurrence of hydrophobic residues Cys, Leu, and Val on the surface of a protein.
Expression of TonB2 in E. coli
The TonB2 ORF was released from pMD-tonB2 by digestion with BamHI/SalI and ligated into prokaryotic expression vector pGEX-KG, in-frame with the glutathione-S-transferase (GST) coding region (15), which resulted in plasmid pKG-TonB2. The GST-TonB2 fusion protein was produced in E. coli BL21(DE3) (Takara) containing the recombinant plasmid pKG-TonB2. As a negative control, GST was also produced in E. coli BL21(DE3) containing the empty plasmid pGEX-KG. After induction with isopropyl-β-D-thiogalactoside for 3 h, the bacteria containing the plasmids were collected and disrupted by sonication. The 2 proteins were purified by means of a glutathione–Sepharose 4B column (Amersham Biosciences, Buckinghamshire, England), and the immunogenic activity was confirmed by Western blot analysis with use of A. pleuropneumoniae-positive pig serum as the primary antibody and a standard procedure (17). Protein samples were loaded onto two sodium dodecyl sulfate (SDS)-12% polyacrylamide gel and separated by electrophoresis, then one gel was stained with Coomassie brilliant blue and the other gel was blotted onto nitrocellulose membranes (Bio-Rad Laboratories, Berkeley, California, USA). The membrane was incubated with the primary antibody, to which was added horseradish peroxidase (HRP)-conjugated goat IgG against porcine antigen (Southern Biotechnology Associates, Birmingham, Alabama, USA). Color was developed with a 3,3′-diaminobenzidine kit (Tiangen Biotech, Beijing, China).
Protection assay in the mouse
The protective efficacy of TonB2 was tested in a mouse vaccination/ challenge model. The animal experiments were carried out according to the International Guiding Principles for Biomedical Research Involving Animals (18). Thirty-six 6-wk-old BALB/c mice, bred under satisfactory conditions, were divided into 6 groups randomly, with 6 mice in each group. The mice were anesthetized before vaccination and blood collection. Groups 1 and 2 were vaccinated subcutaneously on day 0 with 100 μg of GST-TonB2 emulsified with Freund’s complete adjuvant (Sigma-Aldrich), 0.2 mL, 0 and given a booster dose on day 14 of the same concentration of protein emulsified with Freund’s incomplete adjuvant. Groups 3 and 4 were vaccinated with the same dose of GST and the same regimen as the first 2 groups. The last 2 groups were vaccinated subcutaneously with adjuvant at the same time as the other groups. Blood samples were collected through the caudal vein for determination of serum antibody titers by ELISA on days 0, 14, and 28.
On day 28, 2 weeks after the booster vaccination, the mice were challenged intraperitoneally with A. pleuropneumoniae standard strain 4074 (serovar 1). Preparation of the challenge strain was as previously described (14). The mice in groups 1, 3, and 5 were challenged with ~4.0 × 105 colony-forming units (CFU), or about twice the median lethal dose (2 LD50); those in groups 2, 4, and 6 were challenged with ~1.0 × 106 CFU (about 5 LD50). The mice were observed daily for 7 d after challenge for clinical signs. Those seriously diseased, as determined by severe dyspnea or lethargy, or both, were euthanized immediately. After 7 days the remainder of the mice were euthanized. Bacteriologic study was performed. Lung tissue homogenates were serially diluted and used as templates directly in PCR, with the apxIVA-gene specific primers P3 and P4 (Table I). Student’s t-test was used for comparison of the specific antibody levels and survival rate in the different groups.
Development and assessment of the TonB2 ELISA
Preparation of rabbit antiserum
The antiserum was prepared as described previously (19). Briefly, fresh cultures of A. pleuropneumoniae standard strains, including serovars 1 to 12 and 15, were diluted with phosphate-buffered saline (PBS), pH 7.2, to the desired concentration, then inactivated by formaldehyde (0.3% v/v). Three rabbits were vaccinated subcutaneously on day 1 with ~1.0 × 109 CFU of each serovar in 1 mL of PBS. Boosters were given on days 14 and 28 with ~4.0 × 109 CFU of the same bacteria in the same regimen as for the initial vaccination. On day 42 the rabbits were injected intravenously with ~4.0 × 109 CFU of the same bacteria. On day 49 the rabbits were anesthetized and blood was drawn from the jugular vein. Three rabbits injected with PBS were used as negative controls.
Serum samples
Blood negative for antibodies against A. pleuropneumoniae (on the basis of vaccination and ApxIVA ELISA status) was collected from 47 pigs. In addition, 80 blood samples were collected from pigs experimentally vaccinated with A. pleuropneumoniae live attenuated strain SLW03 (serovar 1) at 4 times (13): before vaccination (day 0; n = 20), 2 wk after primary vaccination (day 14; n = 20), 2 wk after booster vaccination (day 28; n = 20), and 1 wk after challenge (day 35; n = 20). Another 223 blood samples were collected from different commercial pig farms in China between 2007 and 2008; data about disease history and vaccination status of the herds were not available. Serum samples positive for Streptococcus suis serovar 2 (n = 5), Haemophilus parasuis (n = 15), Pasteurella multocida (n = 4), and Bordetella bronchiseptica (n = 5) were kindly provided by Zhang Wei (Animal Hospital of Huazhong Agricultural University, Wuhan, China).
Checkerboard titration
An indirect microplate ELISA for detection of A. pleuropneumoniae-specific antibodies in serum was standardized on the basis of checkerboard titration of each antigen pool and serum pool. The antigen concentrations were 20, 10, 5, 2.5, 1.25, and 0.6 μg/mL. The serum dilutions were 1:10, 1:20, 1:40, 1:80, 1:160, and 1:320. On the basis of checkerboard titration the optimum antigen concentration was 5 μg/mL and the optimum serum dilution was 1:40; these were used in the subsequent assays.
Procedure for TonB2 ELISA
Flat-bottomed 96-well polystyrene ELISA plates (Haimen Shengbang Lab Consumable Instrument Company, Jiangsu, China) were coated at 37°C for 1 h and 4°C overnight with 0.5 μg of purified GST-TonB2 diluted in 100 μL of coating buffer (50 mM sodium carbonate, pH 9.6). The coated plates were washed 3 times with PBS plus 0.05% Tween-20 (PBST) and blocked at 37°C for 1 h with blocking buffer (5% skimmed milk in PBST), then washed 3 times with PBST. Serum samples (2.5-μL aliquots) were added into 97.5 μL of PBST in each well and incubated at 37°C for 30 min. After 4 washes, 100 μL of HRP-conjugated secondary antibodies (Southern Biotechnology Associates) diluted to 1:5000 in PBST was added to each well and the plates were incubated at 37°C for 30 min. After 5 washes, 100 μL of 3,3′,5,5′-tetramethyl-benzidine HRP color development solution (Biotime Institute of Biotechnology, Haimen, Jiangsu, China) was added to each well. The plates were incubated at room temperature in the dark for approximately 10 min, and the catalytic reaction was then stopped by 50 μL of 1% SDS. The optical density was read at 630 nm in an ELISA reader (PowerWave XS; Bio-Tek Instruments, Winooski, Vermont, USA).
TonB2 ELISA positive threshold
The TonB2 ELISA plate was coated with the optimum TonB2 antigen concentration, and serum at the optimum dilution was added. The ELISA was performed as described earlier, in triplicate. The end-point cut-off was established by titration as the mean OD630 value of the 47 serum samples negative for A. pleuropneumoniae plus 3 standard deviations. The antibody titer was expressed as the reciprocal of the highest serum dilution giving an OD630 value over the positive threshold.
ApxIVA ELISA and IHA test
The ApxIVA ELISA was developed in a previous study (20). In brief, the purified E. coli-expressed 6 × His-ApxIVA (amino acid positions 150 to 958 was used as a solid antigen. The optimum coating concentration (0.3 μg/well) and serum dilution (1:40) were determined by checkerboard titration. The procedure for ApxIVA ELISA was similar to that for TonB2 ELISA described earlier. The indirect hemagglutination (IHA) test was developed by Lu et al (21). In brief, alcohol-precipitated saline extracts of A. pleuropneumoniae strains (serovars 1, 3, and 7), prepared according to the methods described by Mittal et al (22), were used as the coating antigen. The optimum antigen concentration for sensitization of glutaraldehyde-treated sheep red blood cells (SRBC) was determined by checkerboard titration. The IHA test was performed with a 96-well “V”-type microtiter plate (Haimen Shengbang Lab Consumable Instrument Company). Serum samples were inactivated by heating at 56°C for 30 min, and serial 2-fold dilutions from 1:2 to 1:1024 were made in a 25-μL volume. The same volume of sensitized SRBC suspension was added to each well. The plate was shaken gently and incubated at 37°C for 2 h before the reading. The IHA titer was read as the highest dilution of serum giving half-maximal agglutination of SRBC. A titer greater than 1:16 was considered positive.
Results
Cloning of A. pleuropneumoniae TonB2
The tonB2 gene cloned from A. pleuropneumoniae JL01 genomic DNA and ligated into an A/T cloning vector was 858 base pairs in length. Its amino acid sequence exhibited a high degree of similarity (> 98%) with published A. pleuropneumoniae TonB2. From the 285 deduced amino acids 11 antigenic sites were characterized. These sites were not affected after fusion to GST.
Expression of TonB2 in E. coli
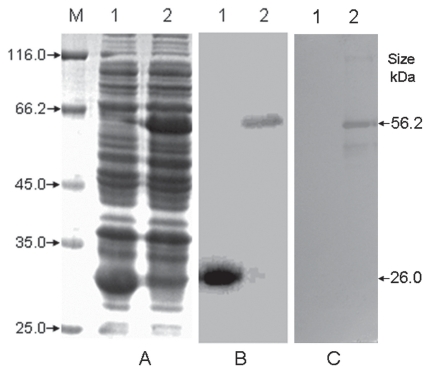
The expressed and purified GST-TonB2 protein was as displayed in Figure 1. With Western blot analysis a hybridization band with a molecular weight of approximately 56 kD was observed for GST-TonB2, whereas no hybridization signal was detectable for GST. This result confirmed that the A. pleuropneumoniae antibodies recognized and bound to the recombinant TonB2 protein.
Figure 1.
Expression (A), purification (B), and Western blot analysis (C) of the recombinant TonB2 fusion protein. Protein samples were separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis, blotted onto nitrocellulose membranes, and stained with Coomassie brilliant blue. The membranes were incubated with the primary antibody (porcine antibody against Actinobacillus pleuropneumoniae), to which was added horseradish peroxidase-conjugated goat IgG against porcine antigen. A 3,3′-diaminobenzidine kit was used for color development. M — protein marker with low molecular weight (Fermentas, Vilnius, Lithuania); 1 — glutathione-S-transferase (GST); 2 — GST-TonB2. The recombinant TonB2 protein exhibited a band with a molecular weight of approximately 56 kD, whereas no signal was detectable for the recombinant GST control.
Protective efficacy of TonB2 in the mouse
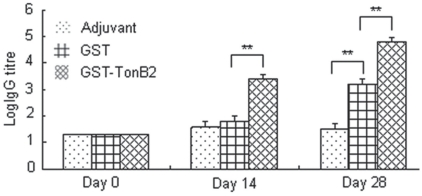
As shown in Figure 2, the mice vaccinated with GST-TonB2 were positive by ELISA 2 wk after primary vaccination, and the antibody titers were higher 2 wk after the booster vaccination. Although the mice vaccinated with recombinant GST were also positive with the TonB2 ELISA, the antibody titers were significant lower than those of the mice vaccinated with GST-TonB2 (P < 0.01). The mice vaccinated with adjuvant alone had no detectable titer with the TonB2 ELISA during the entire study.
Figure 2.
Antibody responses of mice as detected in the TonB2 enzyme-linked immunosorbent assay (n = 12). Blood was collected on days 0 (day of initial vaccination), 14 (day of booster vaccination), and 28 (day of A. pleuropneumoniae challenge). The vertical bars represent 1 standard deviation. The double asterisks indicate a highly statistically significant difference (P < 0.01; Student’s t-test) between the groups.
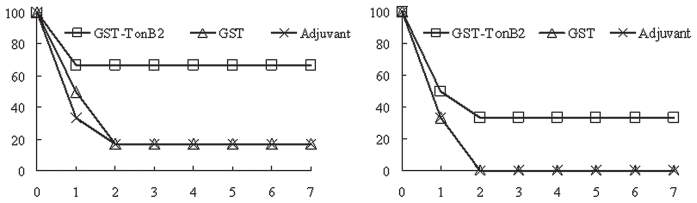
The mice were challenged 2 wk after booster vaccination and observed for 7 d. As shown in Figure 3, 4 of the 6 mice vaccinated with GST-TonB2 survived after challenge with ~4.0 × 105 CFU of A. pleuropneumoniae 4074, but only 1 mouse of the 6 mice vaccinated with GST or adjuvant alone survived, a significant difference (P < 0.01). When challenged with ~1.0 × 106 CFU, all the mice vaccinated with either GST or adjuvant alone were dead within 48 h. Of those vaccinated with GST-TonB2, 2 of 6 were protected from the virulent challenge, a significant difference (P < 0.01) when compared with the results for groups 4 and 6. These results suggest that TonB2 (not GST) could provide significant protection against A. pleuropneumoniae. All the mice that died from the challenge, as well as those in groups 3 and 5 that survived, were positive by bacteriologic examination, whereas the mice in groups 1 and 2 that survived were negative.
Figure 3.
Protection of mice (n = 6) against A. pleuropneumoniae by GST-TonB2 vaccination. Two weeks after booster vaccination the mice were challenged by intraperitoneal inoculation of either ~4.0 × 105 colony-forming units (CFU) (A) or ~1.0 × 106 CFU (B) of A. pleuropneumoniae 4074 (serovar 1). The number of surviving mice was recorded for 7 d thereafter. The protective efficacy of GST-TonB2 was significantly greater than that of GST or adjuvant alone (P < 0.01; Student’s t-test).
Evaluation of the indirect ELISA
As the TonB2 protein is immunogenic and conserved in A. pleuropneumoniae, it was evaluated as a diagnostic antigen for indirect ELISA. The mean OD630 value of the 47 pig serum samples that were negative for A. pleuropneumoniae antibody in the TonB2 ELISA was 0.133 ± 0.037 (standard deviation). Thus, a suitable cut-off OD630 value was calculated to be 0.245 (mean and 3 standard deviations). Using this cut-off value the TonB2 ELISA had 1 false-positive reaction (2%) among the 47 samples.
The detection sensitivity and specificity of the TonB2 ELISA were compared with those of the ApxIVA ELISA with use of 80 serum samples collected successively from pigs experimentally vaccinated with A. pleuropneumoniae live attenuated strain SLW03 (serovar 1). As shown in Table II, the TonB2 ELISA demonstrated better sensitivity than the ApxIVA ELISA: seroconversion on days 14 and 28 was shown in 18 of 20 and 20 of 20 samples, respectively, with the TonB2 ELISA but only 2 of 20 and 19 of 20, respectively, with the ApxIVA ELISA.
Table II.
Comparison of 2 enzyme-linked immunosorbent assays (ELISAs) in detecting antibody to A. pleuropneumoniae in the serum of 80 pigs vaccinated with live vaccine
| Day after vaccination | Number of samples positive/total number
|
|
|---|---|---|
| TonB2 ELISA | ApxIVA ELISA | |
| 0 | 0/20 | 0/20 |
| 14 | 18/20 | 2/20 |
| 28 | 20/20 | 19/20 |
| 35 | 20/20 | 20/20 |
The 223 clinical serum samples from herds whose A. pleuropneumoniae infection and vaccination status was unknown were also evaluated by these 2 ELISA methods. The TonB2 ELISA showed 178 samples to be positive and 45 to be negative, whereas the ApxIVA ELISA showed 37 samples to be positive and 186 to be negative; the correlation of the 2 methods was 37%. These samples were further evaluated by the IHA test; the results are shown in Table III. A chi-squared test found no significant difference between the IHA and the TonB2 ELISA results (P > 0.05). When the IHA test was used as a reference, the specificity and sensitivity of the TonB2 ELISA were calculated as 95% and 88%.
Table III.
Comparison of an indirect hemagglutination (IHA) test and the TonB2 ELISA in detecting antibody to A. pleuropneumoniae in 223 clinical serum samples from pig herds in which the infection and vaccination status were unknown
| Number of samples
|
|||
|---|---|---|---|
| TonB2 ELISA resulta |
|||
| IHA test result | Positive | Negative | Total |
| Positive | 173 | 9 | 182 |
| Negative | 5 | 36 | 41 |
| Total | 178 | 45 | 223 |
Relative sensitivity of TonB2 ELISA, 95% (173/182); relative specificity, 88% (36/41); correlation, 94% (173 + 36)/223.
No cross-reaction was observed in the TonB2 ELISA with pig antiserum specific for other important bacterial respiratory pathogens, such as S. suis serovar 2, H. parasuis, P. multocida, and B. bronchiseptica (data not shown).
To assess the detection range of the TonB2 ELISA, serum samples from 3 rabbits vaccinated with A. pleuropneumoniae standard strains, 13 serovars of biovar I, were examined. All the samples were positive, the antibody titer ranging from 1:2560 to 1:40 960 (Table IV), indicating that the TonB2 ELISA detects antibodies over a wide range. It can be used to detect, at least, the antibodies against biovar I of A. pleuropneumoniae.
Table IV.
Assessment of the detection range of the TonB2 ELISA in 3 rabbits vaccinated with standard strains of A. pleuropneumoniae biovar I
| Titer of antibodies against each serovara |
||||
|---|---|---|---|---|
| Serovar | A | B | C | Mean ± standard deviation |
| 1 | 1:5120 | 1:2560 | 1:5120 | 4267 ± 1478 |
| 2 | 1:5120 | 1:5120 | 1:5120 | 8533 ± 2956 |
| 3 | 1:5120 | 1:5120 | 1:2560 | 4267 ± 1478 |
| 4 | 1:5120 | 1:5120 | 1:5120 | 5120 ± 0 |
| 5 | 1:10240 | 1:20480 | 1:20480 | 17066 ± 5912 |
| 6 | 1:10240 | 1:10240 | 1:5120 | 8533 ± 2956 |
| 7 | 1:10240 | 1:5120 | 1:5120 | 6826 ± 2956 |
| 8 | 1:20480 | 1:40960 | 1:20480 | 27307 ± 11824 |
| 9 | 1:10240 | 1:10240 | 1:20480 | 13653 ± 5912 |
| 10 | 1:10240 | 1:20480 | 1:20480 | 17066 ± 5912 |
| 11 | 1:20480 | 1:20480 | 1:40960 | 27307 ± 11824 |
| 12 | 1:2560 | 1:5120 | 1:5120 | 4267 ± 1478 |
| 15 | 1:20480 | 1:40960 | 1:40960 | 34133 ± 11824 |
| Negative control | 1:40 | 1:40 | 1:20 | 33 ± 12 |
A, B, and C represent 3 samples of each serovar of A. pleuropneumoniae. Titers greater than 1:50 were considered positive.
Discussion
Porcine contagious pleuropneumonia continues to cause severe economic losses for the pig industry world wide. Fifteen serovars, based on capsular polysaccharides, have been identified (23), and the existence of so many serovars complicates vaccine development (24). Various studies have been carried out to find vaccine candidates for efficient cross-protection, such as killed bacterin vaccines, live attenuated vaccines, and subunit vaccines. However, further efforts need to be made for development of more efficient vaccines (25).
Though a ubiquitous concept that the surface-exposed proteins and secreted virulence factors (such as the Apx toxins) are good vaccine candidates, TbpB and OmlA of A. pleuropneumoniae have been proven immunogenic and could protect animals from virulent A. pleuropneumoniae infection. However, there is growing evidence that even intracellular proteins, such as ribosomal proteins and translation initiation factor, could be good vaccine candidates (26,27). TonB2, a periplasm protein upregulated under iron restriction (28), is required for growth in vitro when hemin, porcine hemoglobin, or ferrichrome is the sole source of iron and is important for A. pleuropneumoniae survival in vivo (12).
In this study we investigated the immunologic characteristics of TonB2 to see whether A. pleuropneumoniae antibodies would recognize and bind to the recombinant protein and whether TonB2 would be conserved among A. pleuropneumoniae serovars. On the basis of these considerations, we attempted to explore TonB2 as a constituent of effective vaccines. The protein elicited a systemic immune response after vaccination, and mice could be protected from challenge with a virulent A. pleuropneumoniae. The survival rate was significantly greater in the vaccinated group than in the control groups (P < 0.01).
The results suggest that TonB2 is an immunogenic vaccine candidate, although an explanation for the protective efficacy is not available. A. pleuropneumoniae could survive for more than 90 min within pulmonary macrophages then lead to the degradation of macrophages and persistent infection (29), clearance of infected bacteria is of importance to animals. It was observed that clearance of the challenge bacteria is more efficient in TonB2-vaccinated mice: the challenge strain cannot be isolated 7 d after challenge, possibly because factors secreted by the activated TonB2-specific memory lymphocytes promote the killing efficacy of macrophages. However, more evidence is needed to support this hypothesis.
Effective monitoring of specific antibodies plays an important role in controlling disease outbreaks. Numerous serologic assays are used in laboratories for a wide range of routine microbiologic diagnoses. The antigen used for antibody detection is key. The prevalent serovars of A. pleuropneumoniae differ from country to country (30). Therefore, it is essential to develop an efficient and simple method to monitor antibodies against all A. pleuropneumoniae serovars. ApxIV is species-specific and is expressed in vivo. The ApxIVA ELISA is widely used for the evaluation of A. pleuropneumoniae infection. The applicability of ApxIV for differentiating infected from vaccinated animals is not affected by the appearance of natural ApxIVA inactivation at low frequency (31). However, the generation of ApxIVA antibody is slow (32), and in our study the serum of only 2 animals out of 20 was weakly positive in the ApxIVA ELISA 2 wk after primary vaccination with A. pleuropneumoniae live vaccine.
In the present study we cloned and characterized TonB2 as an immunodominant antigen of A. pleuropneumoniae and developed an ELISA based on the recombinant TonB2 that is suitable for use in serum samples from animals infected with A. pleuropneumoniae. More important, compared with the ApxIVA ELISA, the time needed for seroconversion is short: 18 of 20 animals were positive in the TonB2 ELISA 2 wk after primary vaccination. This might be due to earlier exposure of TonB2 in vivo. To further evaluate the diagnostic applicability of the ELISA, clinical serum samples were evaluated. A low correlation between the 2 ELISA methods was observed, which might be due to the inability of animals vaccinated with inactivated bacterin to produce ApxIVA antibodies. Serum samples were then evaluated by the IHA test, which was established on the basis of 3 A. pleuropneumoniae strains (serovars 1, 3, and 7) prevalent in China. Correlation analysis showed an agreement of 94% between the TonB2 ELISA and the IHA test. However, the antigens used in the IHA test are somewhat serovar-specific (22). It would not possible to use a single IHA test method for all the serovars.
The TonB2 ELISA developed in this study could detect 13 serovars of A. pleuropneumoniae biovar I, and we believe that it can detect A. pleuropneumoniae antibodies to all serovars owing to the conservation property of TonB2. This belief can be validated in future. Our results suggest that TonB2 is an immunogenic, specific, and relatively conserved antigen that is useful for serologic diagnosis of A. pleuropneumoniae.
In conclusion, the results of the present study indicate that the TonB2 protein is an important antigen of A. pleuropneumoniae that can confer protective immunity. It may contribute to future efforts to develop efficient vaccines. The ELISA method developed here provides an alternative diagnostic tool for monitoring antibodies to A. pleuropneumoniae, especially when the infection status or serovar is unknown.
Acknowledgments
The authors thank Dr. Pat Blackall and Dr. Ross Bowles, Queensland Department of Primary Industries, Agency for Food and Fibre Science, Animal Research Institute, Brisbane, Australia, for generously donating the A. pleuropneumoniae standard strains. This study was supported by the National Nature Science Foundation of China (grant 30970109) and the Innovation Teams of the Ministry of Education (grant IRT0726).
References
- 1.Fenwick B, Henry S. Porcine pleuropneumonia. J Am Vet Med Assoc. 1994;204:1334–1340. [PubMed] [Google Scholar]
- 2.Devenish J, Rosendal S, Bossé JT. Humoral antibody response and protective immunity in swine following immunisation with the 104-kilodalton hemolysin of Actinobacillus pleuropneumoniae. Infect Immun. 1990;58:3829–3832. doi: 10.1128/iai.58.12.3829-3832.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rioux S, Girard C, Dubreuil JD, Jacques M. Evaluation of the protective efficacy of Actinobacillus pleuropneumoniae serotype 1 detoxified lipopolysaccharides or O-polysaccharide–protein conjugate in pigs. Res Vet Sci. 1998;65:165–167. doi: 10.1016/s0034-5288(98)90170-9. [DOI] [PubMed] [Google Scholar]
- 4.Gerlach GF, Anderson C, Klashinsky S, Rossi-Campos A, Potter AA, Willson PJ. Molecular characterization of a protective outer membrane lipoprotein (OmlA) from Actinobacillus pleuropneumoniae serotype 1. Infect Immun. 1993;61:565–572. doi: 10.1128/iai.61.2.565-572.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossi-Campos A, Anderson C, Gerlach GF, Klashinsky S, Potter AA, Willson PJ. Immunization of pigs against Actinobacillus pleuropneumoniae with two recombinant protein preparations. Vaccine. 1992;10:512–518. doi: 10.1016/0264-410x(92)90349-o. [DOI] [PubMed] [Google Scholar]
- 6.Oldfield NJ, Donovan EA, Worrall KE, Worrall KE, Wooldridge KE, Wooldridge KG, et al. Identification and characterization of novel antigenic vaccine candidates of Actinobacillus pleuropneumoniae. Vaccine. 2008;26:1942–1954. doi: 10.1016/j.vaccine.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Q, Poole K. A second tonB gene in Pseudomonas aeruginosa is linked to the exbB and exbD genes. FEMS Microbiol Lett. 2000;184:127–132. doi: 10.1111/j.1574-6968.2000.tb09002.x. [DOI] [PubMed] [Google Scholar]
- 8.Seliger SS, Mey AR, Valle AM, Payne SM. The two TonB systems of Vibrio cholerae: Redundant and specific functions. Mol Microbiol. 2001;39:801–812. doi: 10.1046/j.1365-2958.2001.02273.x. [DOI] [PubMed] [Google Scholar]
- 9.Stork M, Di Lorenzo M, Mouriño S, Osorio CR, Lemos ML, Crosa JH. Two tonB systems function in iron transport in Vibrio anguillarum, but only one is essential for virulence. Infect Immun. 2004;72:7326–7329. doi: 10.1128/IAI.72.12.7326-7329.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q, Liu Q, Cao X, Yang M, Zhang Y. Characterization of two TonB systems in marine fish pathogen Vibrio alginolyticus: their roles in iron utilization and virulence. Arch Microbiol. 2008;190:595–603. doi: 10.1007/s00203-008-0407-1. [DOI] [PubMed] [Google Scholar]
- 11.Naka H, Hirono I, Aoki T. Cloning and characterization of two types of tonB genes, tonB1 and tonB2, and ferric uptake regulator gene, fur from Photobacterium damselase subsp. piscicida. Japan Soc Fish Pathol. 2005;40:73–79. [Google Scholar]
- 12.Beddek AJ, Sheehan BJ, Bossé JT, Rycroft AN, Kroll JS, Langford PR. Two TonB systems in Actinobacillus pleuropneumoniae: Their roles in iron acquisition and virulence. Infect Immun. 2004;72:701–708. doi: 10.1128/IAI.72.2.701-708.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin L, Bei W, Sha Y, Liu J, Guo Y, Liu W, et al. Construction and immunogenicity of a DeltaapxIC/DeltaapxIIC double mutant of Actinobacillus pleuropneumoniae serovar 1. FEMS Microbiol Lett. 2007;274:55–62. doi: 10.1111/j.1574-6968.2007.00813.x. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Chen X, Lin L, Tan C, Chen Y, Guo Y, et al. Potential use of an Actinobacillus pleuropneumoniae double mutant strain DeltaapxIICDeltaapxIVA as live vaccine that allows serological differentiation between vaccinated and infected animals. Vaccine. 2007;25:7696–7705. doi: 10.1016/j.vaccine.2007.07.053. [DOI] [PubMed] [Google Scholar]
- 15.Guan KL, Dixon JE. Eukaryotic proteins expressed in Escherichia coli: An improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- 16.Prideaux CT, Pierce L, Krywult J, Hodgson AL. Protection of mice against challenge with homologous and heterologous serovars of Actinobacillus pleuropneumoniae after live vaccination. Curr Microbiol. 1998;37:324–332. doi: 10.1007/s002849900386. [DOI] [PubMed] [Google Scholar]
- 17.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor, New York: Cold Spring Harbor Lab Pr; 1989. [Google Scholar]
- 18.Bankowski Z, Howard-Jones N, editors. International Guiding Principles for Biomedical Research Involving Animals. Geneva, Switzerland: Council for International Organizations of Medical Sciences; 1986. [Last accessed April 12, 2011]. Available from www.cioms.ch/publications/guidelines/frame_guidelines.htm. [Google Scholar]
- 19.Chen F, He Q, Chen H, Liu Z, Zhang Z, Zhang Y, et al. The preparation of biovar I antisera of Actinobacillus pleuropneumoniae and its clinical application. Chin J Prev Vet Med. 2004;26:458–461. Chinese. [Google Scholar]
- 20.Huang H, Zhou R, Chen M, Liu J, Xu X, Chen H, et al. Cloning and expression of the apxIVA gene of Actinobacillus pleuropneumoniae and development of an indirect ApxIVA-ELISA. Chin J Biotechnol. 2005;21:131–136. Chinese. [Google Scholar]
- 21.Lu Z, Liu J, Zhao P, Li B. Detection of serum antibodies against Actinobacillus pleuropneumoniae. Chin J Vet Sci Technol. 2001;31:16–17. Chinese. [Google Scholar]
- 22.Mittal KR, Higgins R, Lariviere S. Determination of antigenic specificity and relationship among Haemophilus pleuropneumoniae serotypes by an indirect hemagglutination test. J Clin Microbiol. 1983;17:787–790. doi: 10.1128/jcm.17.5.787-790.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blackall PJ, Klaasen HL, van den Bosch H, Kuhnert P, Frey J. Proposal of a new serovar of Actinobacillus pleuropneumoniae: Serovar 15. Vet Microbiol. 2002;84:47–52. doi: 10.1016/s0378-1135(01)00428-x. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen R. Haemophilus pleuropneumoniae serotypes — cross protection experiments. Nord Vet Med. 1984;36:221–234. [PubMed] [Google Scholar]
- 25.Ramjeet M, Deslandes V, Gouré J, Jacques M. Actinobacillus pleuropneumoniae vaccines: From bacterins to new insights into vaccination strategies. Anim Health Res Rev. 2008;9:25–45. doi: 10.1017/S1466252307001338. [DOI] [PubMed] [Google Scholar]
- 26.Luo D, Ni B, Li P, Shi W, Zhang S, Han Y, et al. Protective immunity elicited by a divalent DNA vaccine encoding both the L7/L12 and Omp16 genes of Brucella abortus in BALB/c mice. Infect Immun. 2006;74:2734–2741. doi: 10.1128/IAI.74.5.2734-2741.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.González M, Andrews E, Folch H, Sáez D, Cabrera A, Salgado P, et al. Cloning, expression and immunogenicity of the translation initiation factor 3 homologue of Brucella abortus. Immunobiology. 2009;214:113–120. doi: 10.1016/j.imbio.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Deslandes V, Nash JH, Harel J, Coulton JW, Jacques M. Transcriptional profiling of Actinobacillus pleuropneumoniae under iron-restricted conditions. BMC Genomics. 2007;8:72. doi: 10.1186/1471-2164-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cruijsen TL, Van Leengoed LA, Dekker-Nooren TC, Schoevers EJ, Verheijden JH. Phagocytosis and killing of Actinobacillus pleuropneumoniae by alveolar macrophages and polymorphonuclear leukocytes isolated from pigs. Infect Immun. 1992;60:4867–4871. doi: 10.1128/iai.60.11.4867-4871.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubreuil JD, Jacques M, Mittal KR, Gottschalk M. Actinobacillus pleuropneumoniae surface polysaccharides: Their role in diagnosis and immunogenicity. Anim Health Res Rev. 2000;1:73–93. doi: 10.1017/s1466252300000074. [DOI] [PubMed] [Google Scholar]
- 31.O’Neill C, Jones SC, Bossé JT, Watson CM, Williamson SM, Rycroft AN, et al. Population-based analysis of Actinobacillus pleuropneumoniae ApxIVA for use as a DIVA antigen. Vaccine. 2010;28:4871–4874. doi: 10.1016/j.vaccine.2010.04.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dreyfus A, Schaller A, Nivollet S, Segers RP, Kobisch M, Mieli L, et al. Use of recombinant ApxIV in serodiagnosis of Actinobacillus pleuropneumoniae infections; development and prevalidation of the ApxIV ELISA. Vet Microbiol. 2004;99:227–238. doi: 10.1016/j.vetmic.2004.01.004. [DOI] [PubMed] [Google Scholar]