Abstract
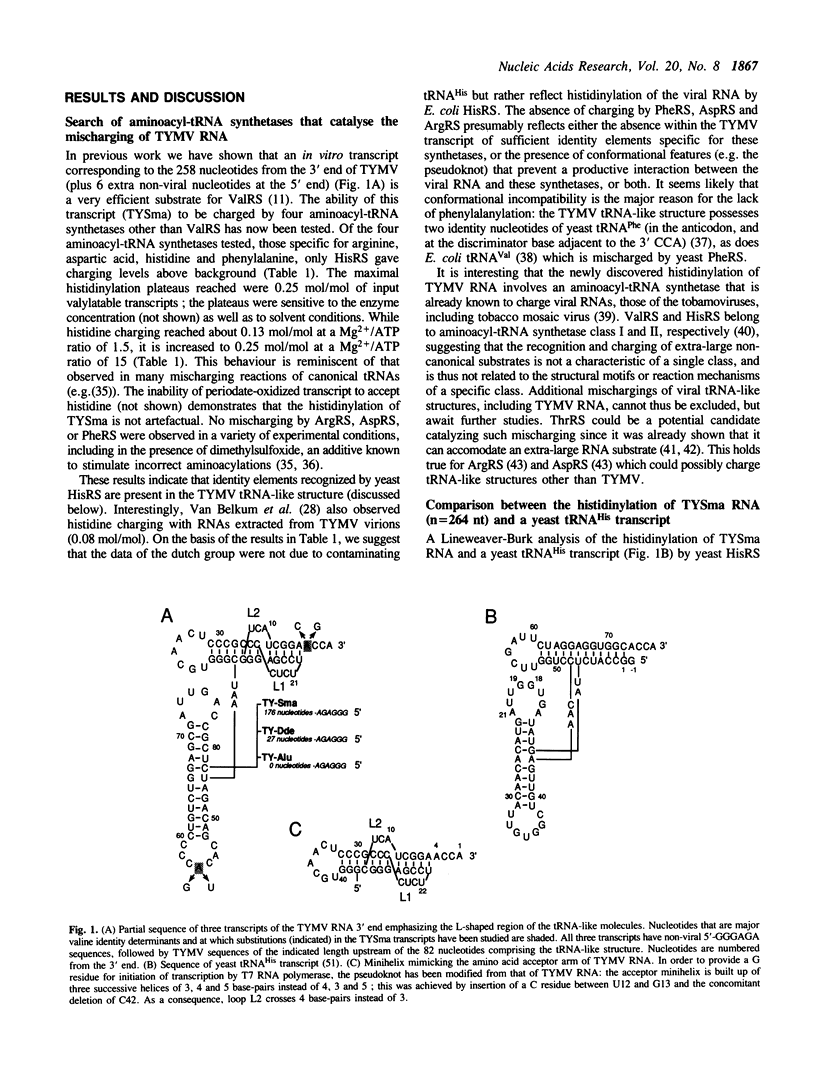
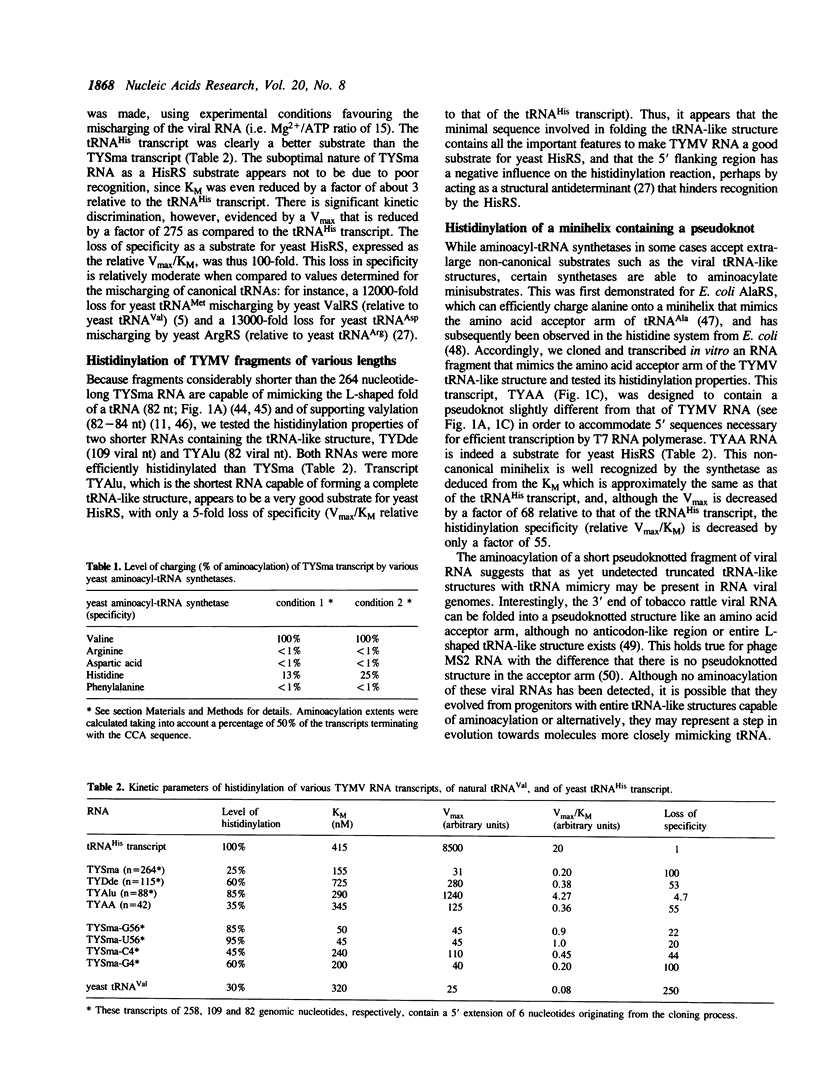
Mischarging of the valine specific tRNA-like structure of turnip yellow mosaic virus (TYMV) RNA has been tested in the presence of purified arginyl-, aspartyl-, histidinyl-, and phenylalanyl-tRNA synthetases from bakers' yeast. Important mischarging of a 264 nucleotide-long transcript was found with histidinyl-tRNA synthetase which can acylate this fragment up to a level of 25% with a loss of specificity (expressed as Vmax/KM ratios) of only 100 fold as compared to a yeast tRNA(His) transcript. Experiments on transcripts of various lengths indicate that the minimal valylatable fragment (n = 88) is the most efficient substrate for histidinyl-tRNA synthetase, with kinetic characteristics similar to those found for the control tRNA(His) transcript. Mutations in the anticodon or adjacent to the 3' CCA that severely affect the valylation capacity of the 264 nucleotide long TYMV fragment are without negative effect on its mischarging, and for some cases even improve its efficiency. A short fragment (n = 42) of the viral RNA containing the pseudoknot and corresponding to the amino acid accepting branch of the molecule is an efficient histidine acceptor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachy R. N., Zaitlin M., Bruening G., Israel H. W. A genetic map for the cowpea strain on TMV. Virology. 1976 Sep;73(2):498–507. doi: 10.1016/0042-6822(76)90411-6. [DOI] [PubMed] [Google Scholar]
- Dreher T. W., Bujarski J. J., Hall T. C. Mutant viral RNAs synthesized in vitro show altered aminoacylation and replicase template activities. Nature. 1984 Sep 13;311(5982):171–175. doi: 10.1038/311171a0. [DOI] [PubMed] [Google Scholar]
- Dreher T. W., Florentz C., Giege R. Valylation of tRNA-like transcripts from cloned cDNA of turnip yellow mosaic virus RNA demonstrate that the L-shaped region at the 3' end of the viral RNA is not sufficient for optimal aminoacylation. Biochimie. 1988 Dec;70(12):1719–1727. doi: 10.1016/0300-9084(88)90030-2. [DOI] [PubMed] [Google Scholar]
- Dreher T. W., Hall T. C. Mutational analysis of the tRNA mimicry of brome mosaic virus RNA. Sequence and structural requirements for aminoacylation and 3'-adenylation. J Mol Biol. 1988 May 5;201(1):41–55. doi: 10.1016/0022-2836(88)90437-8. [DOI] [PubMed] [Google Scholar]
- Dreher T. W., Rao A. L., Hall T. C. Replication in vivo of mutant brome mosaic virus RNAs defective in aminoacylation. J Mol Biol. 1989 Apr 5;206(3):425–438. doi: 10.1016/0022-2836(89)90491-9. [DOI] [PubMed] [Google Scholar]
- Dumas P., Moras D., Florentz C., Giegé R., Verlaan P., Van Belkum A., Pleij C. W. 3-D graphics modelling of the tRNA-like 3'-end of turnip yellow mosaic virus RNA: structural and functional implications. J Biomol Struct Dyn. 1987 Apr;4(5):707–728. doi: 10.1080/07391102.1987.10507674. [DOI] [PubMed] [Google Scholar]
- Ebel J. P., Giegé R., Bonnet J., Kern D., Befort N., Bollack C., Fasiolo F., Gangloff J., Dirheimer G. Factors determining the specificity of the tRNA aminoacylation reaction. Non-absolute specificity of tRNA-aminoacyl-tRNA synthetase recognition and particular importance of the maximal velocity. Biochimie. 1973 May;55(5):547–557. doi: 10.1016/s0300-9084(73)80415-8. [DOI] [PubMed] [Google Scholar]
- Eriani G., Delarue M., Poch O., Gangloff J., Moras D. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature. 1990 Sep 13;347(6289):203–206. doi: 10.1038/347203a0. [DOI] [PubMed] [Google Scholar]
- Florentz C., Dreher T. W., Rudinger J., Giege R. Specific valylation identity of turnip yellow mosaic virus RNA by yeast valyl-tRNA synthetase is directed by the anticodon in a kinetic rather than affinity-based discrimination. Eur J Biochem. 1991 Jan 1;195(1):229–234. doi: 10.1111/j.1432-1033.1991.tb15698.x. [DOI] [PubMed] [Google Scholar]
- Francklyn C., Schimmel P. Aminoacylation of RNA minihelices with alanine. Nature. 1989 Feb 2;337(6206):478–481. doi: 10.1038/337478a0. [DOI] [PubMed] [Google Scholar]
- Francklyn C., Schimmel P. Enzymatic aminoacylation of an eight-base-pair microhelix with histidine. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8655–8659. doi: 10.1073/pnas.87.21.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie D. R., Feder J. N., Schimke R. T., Walbot V. Functional analysis of the tobacco mosaic virus tRNA-like structure in cytoplasmic gene regulation. Nucleic Acids Res. 1991 Sep 25;19(18):5031–5036. doi: 10.1093/nar/19.18.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangloff J., Schutz A., Dirheimer G. Arginyl-tRNA synthetase from baker's yeast. Purification and some properties. Eur J Biochem. 1976 May 17;65(1):177–182. doi: 10.1111/j.1432-1033.1976.tb10403.x. [DOI] [PubMed] [Google Scholar]
- Giegé R., Briand J. P., Mengual R., Ebel J. P., Hirth L. Valylation of the two RNA components of turnip-yellow mosaic virus and specificity of the tRNA aminoacylation reaction. Eur J Biochem. 1978 Mar;84(1):251–256. doi: 10.1111/j.1432-1033.1978.tb12163.x. [DOI] [PubMed] [Google Scholar]
- Giegé R., Kern D., Ebel J. P., Grosjean H., de Henau S., Chantrenne H. Incorrect aminoacylations involving tRNAs or valyl-tRNA synthetase from Bacillus stearothermophilus. Eur J Biochem. 1974 Jun 15;45(2):351–362. doi: 10.1111/j.1432-1033.1974.tb03560.x. [DOI] [PubMed] [Google Scholar]
- Giegé R., Kern D., Ebel J. P. Incorrect aminoacylations catalysed by E. coli valyl-tRNA synthetase. Biochimie. 1972;54(10):1245–1255. doi: 10.1016/s0300-9084(72)80065-8. [DOI] [PubMed] [Google Scholar]
- Grosjean H., Charlier J., Darte C., Dirheimer G., Giege R., de Henau S., Keith G., Parfait R., Takada V. Purification, characterization and mechanism of action of several aminoacyl-tRNA synthetases from Bacillus stearothermophilus. Experientia Suppl. 1976;26:347–362. doi: 10.1007/978-3-0348-7675-9_28. [DOI] [PubMed] [Google Scholar]
- Haenni A. L., Joshi S., Chapeville F. tRNA-like structures in the genomes of RNA viruses. Prog Nucleic Acid Res Mol Biol. 1982;27:85–104. doi: 10.1016/s0079-6603(08)60598-x. [DOI] [PubMed] [Google Scholar]
- Hall T. C., Shih D. S., Kaesberg P. Enzyme-mediated binding of tyrosine to brome-mosaic-virus ribonucleic acid. Biochem J. 1972 Oct;129(4):969–976. doi: 10.1042/bj1290969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himeno H., Hasegawa T., Ueda T., Watanabe K., Miura K., Shimizu M. Role of the extra G-C pair at the end of the acceptor stem of tRNA(His) in aminoacylation. Nucleic Acids Res. 1989 Oct 11;17(19):7855–7863. doi: 10.1093/nar/17.19.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S., Chapeville F., Haenni A. L. Length requirements for tRNA-specific enzymes and cleavage specificity at the 3' end of turnip yellow mosaic virus RNA. Nucleic Acids Res. 1982 Mar 25;10(6):1947–1962. doi: 10.1093/nar/10.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S., Chapeville F., Haenni A. L. Turnip yellow mosaic virus RNA is aminoacylated in vivo in Chinese cabbage leaves. EMBO J. 1982;1(8):935–938. doi: 10.1002/j.1460-2075.1982.tb01274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S., Haenni A. L., Hubert E., Huez G., Marbaix G. In vivo aminoacylation and 'processing' of turnip yellow mosaic virus RNA in Xenopus laevis oocytes. Nature. 1978 Sep 28;275(5678):339–341. doi: 10.1038/275339a0. [DOI] [PubMed] [Google Scholar]
- Kern D., Giegé R., Ebel J. P. Incorrect aminoacylatins catalysed by the phenylalanyl-and valyl-tRNA synthetases from yeast. Eur J Biochem. 1972 Nov 21;31(1):148–155. doi: 10.1111/j.1432-1033.1972.tb02513.x. [DOI] [PubMed] [Google Scholar]
- Kern D., Giegé R., Robre-Saul S., Boulanger Y., Ebel J. P. Complete purification and studies on the structural and kinetic properties of two forms of yeast valyl-tRNA synthetase. Biochimie. 1975;57(10):1167–1176. doi: 10.1016/s0300-9084(76)80579-2. [DOI] [PubMed] [Google Scholar]
- Kohl R. J., Hall T. C. Aminoacylation of RNA from several viruses: amino acid specificity and differential activity of plant, yeast and bacterial synthetases. J Gen Virol. 1974 Nov;25(2):257–261. doi: 10.1099/0022-1317-25-2-257. [DOI] [PubMed] [Google Scholar]
- Lorber B., Kern D., Dietrich A., Gangloff J., Ebel J. P., Giegé R. Large scale purification and structural properties of yeast aspartyl-tRNA synthetase. Biochem Biophys Res Commun. 1983 Nov 30;117(1):259–267. doi: 10.1016/0006-291x(83)91569-3. [DOI] [PubMed] [Google Scholar]
- Mans R. M., Pleij C. W., Bosch L. tRNA-like structures. Structure, function and evolutionary significance. Eur J Biochem. 1991 Oct 15;201(2):303–324. doi: 10.1111/j.1432-1033.1991.tb16288.x. [DOI] [PubMed] [Google Scholar]
- Mertes M., Peters M. A., Mahoney W., Yarus M. Isoleucylation of transfer RNA f Met (E. coli) by isoleucyl-transfer RNA synthetase from Escherichia coli. J Mol Biol. 1972 Nov 28;71(3):671–685. doi: 10.1016/s0022-2836(72)80031-7. [DOI] [PubMed] [Google Scholar]
- Miller W. A., Bujarski J. J., Dreher T. W., Hall T. C. Minus-strand initiation by brome mosaic virus replicase within the 3' tRNA-like structure of native and modified RNA templates. J Mol Biol. 1986 Feb 20;187(4):537–546. doi: 10.1016/0022-2836(86)90332-3. [DOI] [PubMed] [Google Scholar]
- Moine H., Romby P., Springer M., Grunberg-Manago M., Ebel J. P., Ehresmann C., Ehresmann B. Messenger RNA structure and gene regulation at the translational level in Escherichia coli: the case of threonine:tRNAThr ligase. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7892–7896. doi: 10.1073/pnas.85.21.7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg B., Philipson L. Binding of histidine to tobacco mosaic virus RNA. Biochem Biophys Res Commun. 1972 Aug 21;48(4):927–932. doi: 10.1016/0006-291x(72)90697-3. [DOI] [PubMed] [Google Scholar]
- Perret V., Florentz C., Giegé R. Efficient aminoacylation of a yeast tRNA(Asp) transcript with a 5' extension. FEBS Lett. 1990 Sep 17;270(1-2):4–8. doi: 10.1016/0014-5793(90)81221-9. [DOI] [PubMed] [Google Scholar]
- Perret V., Garcia A., Grosjean H., Ebel J. P., Florentz C., Giegé R. Relaxation of a transfer RNA specificity by removal of modified nucleotides. Nature. 1990 Apr 19;344(6268):787–789. doi: 10.1038/344787a0. [DOI] [PubMed] [Google Scholar]
- Perret V., Garcia A., Puglisi J., Grosjean H., Ebel J. P., Florentz C., Giegé R. Conformation in solution of yeast tRNA(Asp) transcripts deprived of modified nucleotides. Biochimie. 1990 Oct;72(10):735–743. doi: 10.1016/0300-9084(90)90158-d. [DOI] [PubMed] [Google Scholar]
- Pinck M., Yot P., Chapeville F., Duranton H. M. Enzymatic binding of valine to the 3' end of TYMV-RNA. Nature. 1970 Jun 6;226(5249):954–956. doi: 10.1038/226954a0. [DOI] [PubMed] [Google Scholar]
- Quivy J. P., Chroboczek J. The interaction of wheat germ tyrosyl-tRNA synthetase and the tRNA-like end of brome mosaic virus RNA has no effect on in vitro viral protein synthesis and on in vitro encapsidation. Biochimie. 1991 Oct;73(10):1269–1273. doi: 10.1016/0300-9084(91)90087-h. [DOI] [PubMed] [Google Scholar]
- Rao A. L., Dreher T. W., Marsh L. E., Hall T. C. Telomeric function of the tRNA-like structure of brome mosaic virus RNA. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5335–5339. doi: 10.1073/pnas.86.14.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietveld K., Van Poelgeest R., Pleij C. W., Van Boom J. H., Bosch L. The tRNA-like structure at the 3' terminus of turnip yellow mosaic virus RNA. Differences and similarities with canonical tRNA. Nucleic Acids Res. 1982 Mar 25;10(6):1929–1946. doi: 10.1093/nar/10.6.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson J. R., DiRenzo A. B., Behlen L. S., Uhlenbeck O. C. Nucleotides in yeast tRNAPhe required for the specific recognition by its cognate synthetase. Science. 1989 Mar 10;243(4896):1363–1366. doi: 10.1126/science.2646717. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Dank N., Nock S., Schön A. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1991 Apr 25;19 (Suppl):2127–2171. doi: 10.1093/nar/19.suppl.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. H., Dreher T. W. Turnip yellow mosaic virus RNAs with anticodon loop substitutions that result in decreased valylation fail to replicate efficiently. J Virol. 1991 Jun;65(6):3060–3067. doi: 10.1128/jvi.65.6.3060-3067.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A. M., Maizels N. tRNA-like structures tag the 3' ends of genomic RNA molecules for replication: implications for the origin of protein synthesis. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7383–7387. doi: 10.1073/pnas.84.21.7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt J. R., Chastain M., Puglisi J. D. Synthesis and purification of large amounts of RNA oligonucleotides. Biotechniques. 1991 Dec;11(6):764–769. [PubMed] [Google Scholar]
- Yarus M., Mertes M. The variety of intraspecific misacylations carried out by isoleucyl transfer ribonucleic acid synthetase of Escherichia coli. J Biol Chem. 1973 Oct 10;248(19):6744–6749. [PubMed] [Google Scholar]
- van Belkum A., Cornelissen B., Linthorst H., Bol J., Pley C., Bosch L. tRNA-like properties of tobacco rattle virus RNA. Nucleic Acids Res. 1987 Apr 10;15(7):2837–2850. doi: 10.1093/nar/15.7.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]


