Abstract
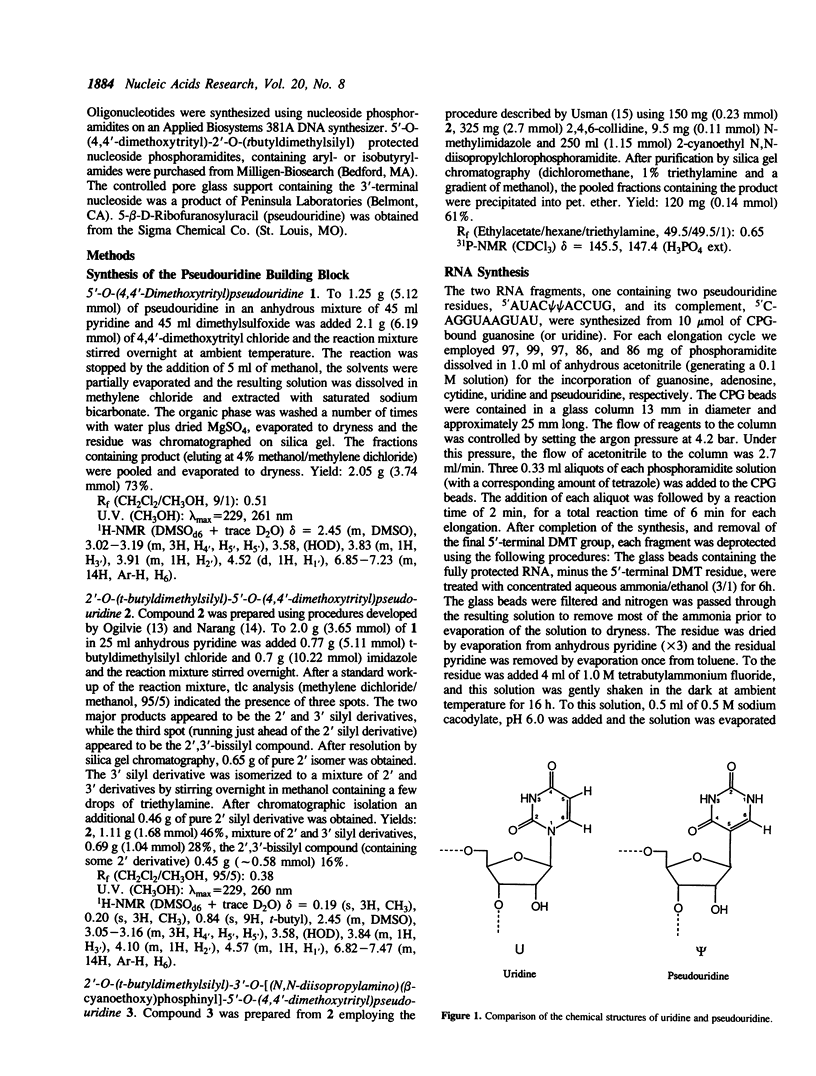
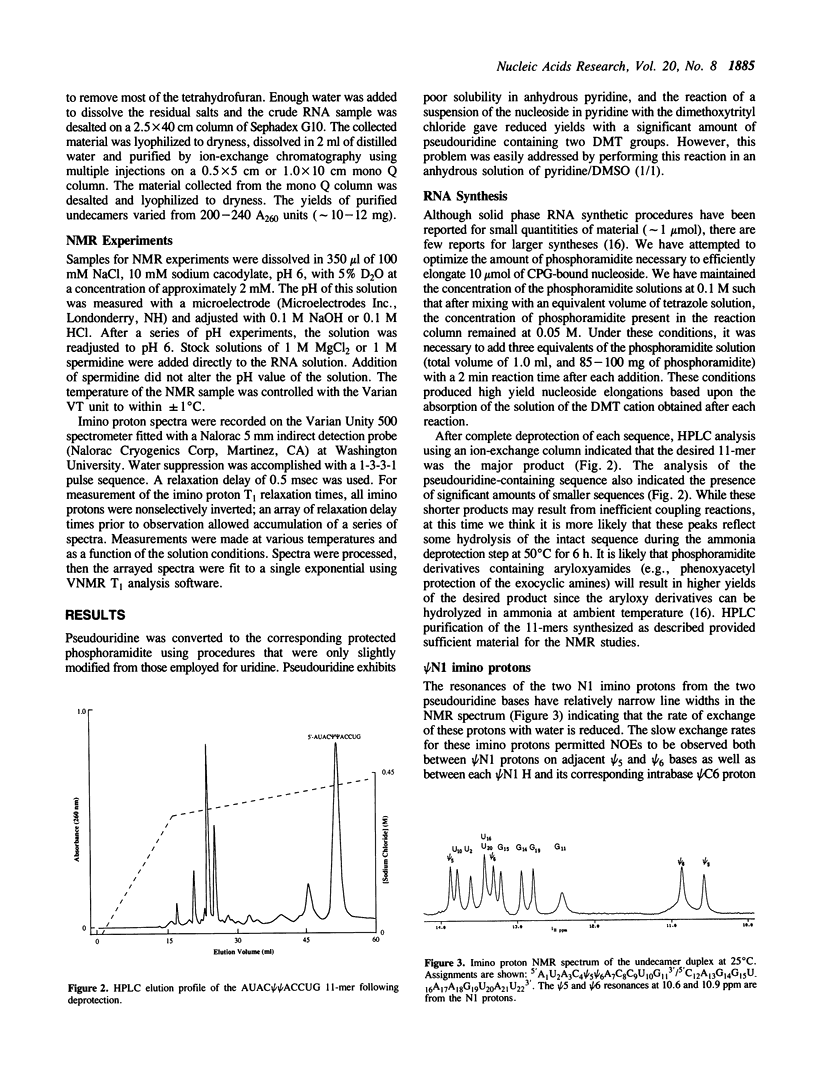
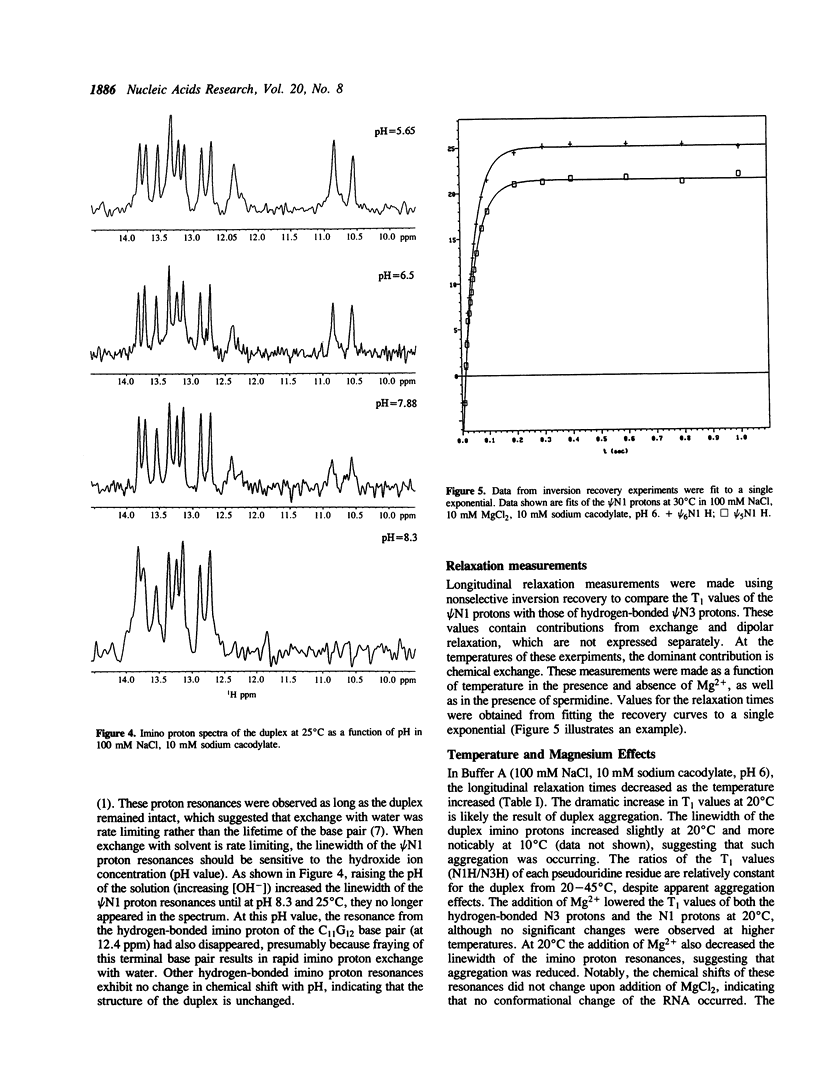
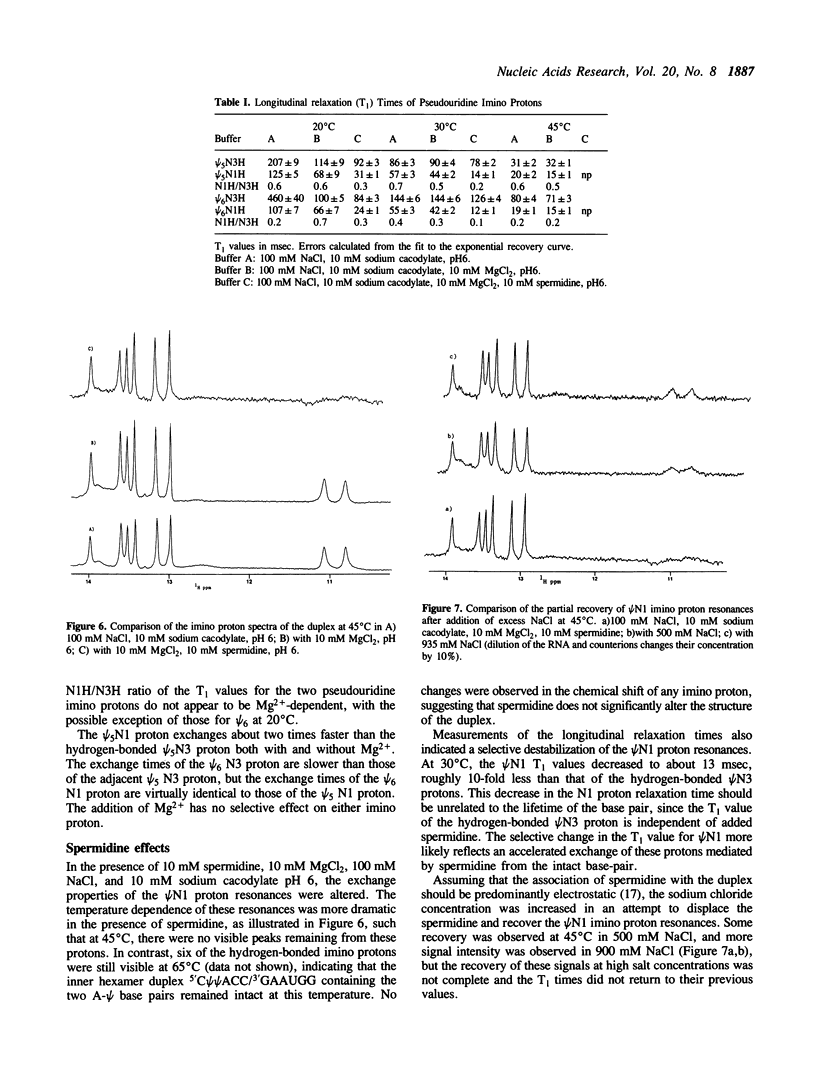
The exchangeable N1 imino protons of two pseudouridine (psi) bases located at adjacent internal positions within an undecamer RNA duplex (5'AUAC psi psi ACCUG/3'UAUGAAUGGUC) can report on the environment of the major groove of an A-form double-stranded nucleic acid. The psi N1 imino protons of these residues (which are not involved in interstrand Watson-Crick hydrogen bonding) are protected from chemical exchange with the solvent water and thus are observable in the proton NMR spectrum in H2O (1). These protons will exchange readily at increased pH values or upon thermal denaturation of the duplex. The longitudinal (T1) relaxation times of the psi N1 imino protons in 100 mM NaCl or in 10 mM MgCl2 and 100 mM NaCl are approximately two-fold faster than those of the psi N3 imino protons which are involved in Watson-Crick base pairing. With the addition of spermidine, the psi N1 imino protons become readily exchangeable at a temperature some 20 degrees C below the melting temperature of the duplex.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blommers M. J., Walters J. A., Haasnoot C. A., Aelen J. M., van der Marel G. A., van Boom J. H., Hilbers C. W. Effects of base sequence on the loop folding in DNA hairpins. Biochemistry. 1989 Sep 5;28(18):7491–7498. doi: 10.1021/bi00444a049. [DOI] [PubMed] [Google Scholar]
- Braunlin W. H., Strick T. J., Record M. T., Jr Equilibrium dialysis studies of polyamine binding to DNA. Biopolymers. 1982 Jul;21(7):1301–1314. doi: 10.1002/bip.360210704. [DOI] [PubMed] [Google Scholar]
- Calnan B. J., Biancalana S., Hudson D., Frankel A. D. Analysis of arginine-rich peptides from the HIV Tat protein reveals unusual features of RNA-protein recognition. Genes Dev. 1991 Feb;5(2):201–210. doi: 10.1101/gad.5.2.201. [DOI] [PubMed] [Google Scholar]
- Chou S. H., Flynn P., Reid B. Solid-phase synthesis and high-resolution NMR studies of two synthetic double-helical RNA dodecamers: r(CGCGAAUUCGCG) and r(CGCGUAUACGCG). Biochemistry. 1989 Mar 21;28(6):2422–2435. doi: 10.1021/bi00432a013. [DOI] [PubMed] [Google Scholar]
- Clore G. M., Gronenborn A. M., Piper E. A., McLaughlin L. W., Graeser E., van Boom J. H. The solution structure of a RNA pentadecamer comprising the anticodon loop and stem of yeast tRNAPhe. A 500 MHz 1H-n.m.r. study. Biochem J. 1984 Aug 1;221(3):737–751. doi: 10.1042/bj2210737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D. R., Poulter C. D. 1H-15N NMR studies of Escherichia coli tRNA(Phe) from hisT mutants: a structural role for pseudouridine. Biochemistry. 1991 Apr 30;30(17):4223–4231. doi: 10.1021/bi00231a017. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Dickerson R. E. Structure of a B-DNA dodecamer. III. Geometry of hydration. J Mol Biol. 1981 Sep 25;151(3):535–556. doi: 10.1016/0022-2836(81)90009-7. [DOI] [PubMed] [Google Scholar]
- Englander S. W., Kallenbach N. R. Hydrogen exchange and structural dynamics of proteins and nucleic acids. Q Rev Biophys. 1983 Nov;16(4):521–655. doi: 10.1017/s0033583500005217. [DOI] [PubMed] [Google Scholar]
- Griffey R. H., Davis D., Yamaizumi Z., Nishimura S., Bax A., Hawkins B., Poulter C. D. 15N-labeled Escherichia coli tRNAfMet, tRNAGlu, tRNATyr, and tRNAPhe. Double resonance and two-dimensional NMR of N1-labeled pseudouridine. J Biol Chem. 1985 Aug 15;260(17):9734–9741. [PubMed] [Google Scholar]
- Guéron M., Kochoyan M., Leroy J. L. A single mode of DNA base-pair opening drives imino proton exchange. Nature. 1987 Jul 2;328(6125):89–92. doi: 10.1038/328089a0. [DOI] [PubMed] [Google Scholar]
- Haasnoot C. A., de Bruin S. H., Berendsen R. G., Janssen H. G., Binnendijk T. J., Hilbers C. W., van der Marel G. A., van Boom J. H. Structure, kinetics and thermodynamics of DNA hairpin fragments in solution. J Biomol Struct Dyn. 1983 Oct;1(1):115–129. doi: 10.1080/07391102.1983.10507429. [DOI] [PubMed] [Google Scholar]
- Hall K. B., McLaughlin L. W. Properties of a U1/mRNA 5' splice site duplex containing pseudouridine as measured by thermodynamic and NMR methods. Biochemistry. 1991 Feb 19;30(7):1795–1801. doi: 10.1021/bi00221a010. [DOI] [PubMed] [Google Scholar]
- Heerschap A., Haasnoot C. A., Hilbers C. W. Nuclear magnetic resonance studies on yeast tRNAPhe. III. Assignments of the iminoproton resonances of the tertiary structure by means of nuclear Overhauser effect experiments at 500 MHz. Nucleic Acids Res. 1983 Jul 11;11(13):4501–4520. doi: 10.1093/nar/11.13.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerschap A., Walters J. A., Hilbers C. W. Influence of the polyamines spermine and spermidine on yeast tRNAPhe as revealed from its imino proton NMR spectrum. Nucleic Acids Res. 1986 Jan 24;14(2):983–998. doi: 10.1093/nar/14.2.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S., Zon G., Sundaralingam M. Base only binding of spermine in the deep groove of the A-DNA octamer d(GTGTACAC). Biochemistry. 1989 Mar 21;28(6):2360–2364. doi: 10.1021/bi00432a002. [DOI] [PubMed] [Google Scholar]
- Lavery R., Pullman B. The molecular electrostatic potential and steric accessibility of A-DNA. Nucleic Acids Res. 1981 Sep 25;9(18):4677–4688. doi: 10.1093/nar/9.18.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy J. L., Kochoyan M., Huynh-Dinh T., Guéron M. Characterization of base-pair opening in deoxynucleotide duplexes using catalyzed exchange of the imino proton. J Mol Biol. 1988 Mar 20;200(2):223–238. doi: 10.1016/0022-2836(88)90236-7. [DOI] [PubMed] [Google Scholar]
- Ofengand J., Schaefer H. On the ionization constant of 5-ribosyluracil. Biochemistry. 1965 Dec;4(12):2832–2835. doi: 10.1021/bi00888a038. [DOI] [PubMed] [Google Scholar]
- Puglisi J. D., Wyatt J. R., Tinoco I., Jr Conformation of an RNA pseudoknot. J Mol Biol. 1990 Jul 20;214(2):437–453. doi: 10.1016/0022-2836(90)90192-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R. Compilation of small nuclear RNA sequences. Methods Enzymol. 1989;180:521–532. doi: 10.1016/0076-6879(89)80121-1. [DOI] [PubMed] [Google Scholar]
- Rould M. A., Perona J. J., Steitz T. A. Structural basis of anticodon loop recognition by glutaminyl-tRNA synthetase. Nature. 1991 Jul 18;352(6332):213–218. doi: 10.1038/352213a0. [DOI] [PubMed] [Google Scholar]
- Roy S., Papastavros M. Z., Sanchez V., Redfield A. G. Nitrogen-15-labeled yeast tRNAPhe: double and two-dimensional heteronuclear NMR of guanosine and uracil ring NH groups. Biochemistry. 1984 Sep 11;23(19):4395–4400. doi: 10.1021/bi00314a024. [DOI] [PubMed] [Google Scholar]
- Scaringe S. A., Francklyn C., Usman N. Chemical synthesis of biologically active oligoribonucleotides using beta-cyanoethyl protected ribonucleoside phosphoramidites. Nucleic Acids Res. 1990 Sep 25;18(18):5433–5441. doi: 10.1093/nar/18.18.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman N. C., Rosenberg J. M., Rich A. Sequence-specific recognition of double helical nucleic acids by proteins. Proc Natl Acad Sci U S A. 1976 Mar;73(3):804–808. doi: 10.1073/pnas.73.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siliciano P. G., Guthrie C. 5' splice site selection in yeast: genetic alterations in base-pairing with U1 reveal additional requirements. Genes Dev. 1988 Oct;2(10):1258–1267. doi: 10.1101/gad.2.10.1258. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Hartmann T., Weber J., Blank J., Zeidler R. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1989;17 (Suppl):r1–172. doi: 10.1093/nar/17.suppl.r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz T. A. Structural studies of protein-nucleic acid interaction: the sources of sequence-specific binding. Q Rev Biophys. 1990 Aug;23(3):205–280. doi: 10.1017/s0033583500005552. [DOI] [PubMed] [Google Scholar]
- Séraphin B., Kretzner L., Rosbash M. A U1 snRNA:pre-mRNA base pairing interaction is required early in yeast spliceosome assembly but does not uniquely define the 5' cleavage site. EMBO J. 1988 Aug;7(8):2533–2538. doi: 10.1002/j.1460-2075.1988.tb03101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropp J. S., Redfield A. G. Proton exchange rates in transfer RNA as a function of spermidine and magnesium. Nucleic Acids Res. 1983 Apr 11;11(7):2121–2134. doi: 10.1093/nar/11.7.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks K. M., Crothers D. M. RNA recognition by Tat-derived peptides: interaction in the major groove? Cell. 1991 Aug 9;66(3):577–588. doi: 10.1016/0092-8674(81)90020-9. [DOI] [PubMed] [Google Scholar]
- Wemmer D. E., Srivenugopal K. S., Reid B. R., Morris D. R. Nuclear magnetic resonance studies of polyamine binding to a defined DNA sequence. J Mol Biol. 1985 Sep 20;185(2):457–459. doi: 10.1016/0022-2836(85)90418-8. [DOI] [PubMed] [Google Scholar]
- Zhuang Y., Weiner A. M. A compensatory base change in U1 snRNA suppresses a 5' splice site mutation. Cell. 1986 Sep 12;46(6):827–835. doi: 10.1016/0092-8674(86)90064-4. [DOI] [PubMed] [Google Scholar]



