Abstract
Although innate immune signals shape the activation of naive T cells, it is unclear how innate signals influence effector T-cell function. This study determined the effects of stimulating the NKG2D receptor in conjunction with the TCR on human effector CD8+ T cells. Stimulation of CD8+ T cells through CD3 and NKG2D simultaneously or through a chimeric NKG2D receptor, which consists of NKG2D fused to the intracellular region of CD3ζ, activated β-catenin and increased expression of β-catenin–induced genes, whereas T cells stimulated through the TCR or a combination of the TCR and CD28 did not. Activation by TCR and NKG2D prevented expression and production of anti-inflammatory cytokines IL-10, IL-9, IL-13, and VEGF-α in a β-catenin– and PPARγ- dependent manner. NKG2D stimulation also modulated the cytokine secretion of T cells activated simultaneously through CD3 and CD28. These data indicate that activating CD8+ T cells through the NKG2D receptor along with the TCR modulates signal transduction and the production of anti-inflammatory cytokines. Thus, human effector T cells alter their function depending on which innate receptors are engaged in conjunction with the TCR complex.
Introduction
Proper activation of naive CD8+ T cells requires stimulation through the TCR and costimulatory receptors. Costimulatory receptors bind to ligands on APCs, and these ligands are often expressed on triggering of innate pattern recognition receptors on APCs. The nature of costimulation and other innate signals during APC–T-cell interaction shapes the generation of the adaptive effector T-cell response. Once an effector T cell is recruited to peripheral tissues, it can continue to be stimulated through various innate and other non-TCR receptors. How these other receptors alter the T-cell effector response may determine the outcome of the immune response to tumors or infections. Therefore, it is imperative to study how triggering of innate and other non-TCR receptors on CD8+ effector T cells alters the functions of these cells.
NKG2D is an innate receptor expressed on human CD8+ T cells. The NKG2D receptor binds to ligands (MICA, MICB, ULBP1-6) that are expressed on many types of tumor cells, in autoimmune diseases, and during some infections, but they are absent or expressed in low amounts on healthy tissues.1–5 Previously, we created a chimeric NKG2D receptor (chNKG2D) consisting of the NKG2D receptor fused to the intracellular region of the CD3ζ chain.6,7 chNKG2D-expressing T cells lyse NKG2D ligand–positive tumor cells and secrete proinflammatory cytokines and chemokines.6–9 Although NKG2D provides a primary activation signal in natural killer (NK) cells, in T cells NKG2D associates with DAP10 and provides a “costimulation-type” of signal.10–12 In T cells, both the wild-type (wt) NKG2D and chNKG2D receptors require association with DAP10 for cell-surface expression, and DAP10 is responsible for the activation of intracellular signaling of the NKG2D receptor.6,13 The cytoplasmic domain of DAP10 has one known signaling motif, a YINM sequence, which is similar to the YMNM signaling domain found in CD28.11–15 When this tyrosine is phosphorylated, PI3K and a Grb2-Vav1 complex are subsequently activated.13 Compared with CD28, human DAP10 lacks other signaling domains, including 2 proline-rich domains responsible for the binding of other downstream signaling molecules, including Itk, Tec, and Lck.16 Previous studies have shown that NKG2D has similar but not identical effects as CD28 in naive and effector human T cells, suggesting that the activation of intracellular signaling may not be identical between the 2 receptors.10,12,14,17 It is unknown whether activation of NKG2D alters the function of effector CD8+ T cells and how this might affect the immune response.
Activation of the WNT/β-catenin pathway is important for regulating T-cell development and has been recently implicated in the development of CD8+ T-cell memory.18 In the absence of stimulation, intracellular β-catenin is hyperphosphorylated, leading to ubiquitination and degradation by the proteasome.19 Activation of β-catenin, either through WNT proteins binding to a Frizzled receptor or through alternative mechanisms such as activation of AKT, leads to phosphorylation and inhibition of GSK3β and accumulation of β-catenin, which then translocates to the nucleus, activates transcription factors, and increases the expression of target genes.18–20 Recent studies have shown that the WNT/β-catenin pathway is regulated in naive and effector T cells, can be modulated by signaling through the TCR, and may be involved in the generation of central memory T cells.21–25 It remains unknown what leads to the activation of this pathway in T cells or how this may alter their effector functions.
This study shows how NKG2D signaling alters the function of human effector CD8+ T cells. We determined how simultaneous signals from the TCR complex and NKG2D altered signal transduction pathways in human CD8+ T cells. The data link changes in specific cytokine expression to the activation of β-catenin, which was induced by NKG2D activation. Importantly, NKG2D ligands are expressed in tumor microenvironments, in autoimmune diseases, and during infections, so these data have implications for regulation of an ongoing effector T-cell response because of local expression of NKG2D ligands.
Methods
Cell lines
Blood cells from healthy donors were obtained from Dartmouth Medical Center after informed consent. Human cell line U937 and murine cell lines P815 and P815-MICA were cultured in RPMI supplemented with 10% heat-inactivated FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, 1mM pyruvate, 10mM HEPES, 0.1mM nonessential amino acids, and 50μM 2-mercaptoethanol.
Transduction and stimulation of CD8+ T cells
Human PBMCs were isolated from 6 healthy donors and from cell cones from 8 healthy donors as previously described.7,9 All procedures were approved by the Institutional Review Board at Dartmouth College. PBMCs were stimulated with anti-CD3 Abs (OKT3, 40 ng/mL) and 50 U/mL recombinant human IL-2 (rHuIL-2; National Cancer Institute) for 3 days. Retroviral transduction of PBMCs was performed with human wtNKG2D and chNKG2D retroviral vectors as previously described.7 Two days after transduction, T cells were selected in complete RPMI media containing G418 (0.5 mg/mL) and 50 U/mL rHuIL-2 for an additional 3 days. CD8+ cells were isolated with MACS columns and were cultured for an additional 2 days in 50 U/mL rHuIL-2 before use. For activated, nontransduced cells, PBMCs were stimulated with OKT3 and IL-2, expanded, and isolated in a similar manner but without retroviral transduction or addition of G418. For stimulation with Ab-coated beads, CD8+ cells (4 × 105) were stimulated in triplicate with human T-cell–activator CD3/CD28 beads (Dynabeads; Invitrogen) or Biotin Binder beads (Dynabeads; Invitrogen) coated with biotin–anti-CD3 (OKT3; eBioscience) or biotin-anti NKG2D (1D11; eBioscience) Abs (1 μg total Ab/107 beads) or both Abs in 96-well plates. For stimulation with tumor cells, CFSE-labeled CD8+ cells (2 × 105) were cultured in triplicate with P815, P815-MICA, or U937 cells (2 × 105) in 96-well plates. Anti-CD3 (OKT3; eBioscience) or isotype (mouse IgG2a; eBioscience) Abs (40 ng/mL) were also added to the wells. For experiments with inhibitors, AKT inhibitor API-2 (0.25μM, 2.5μM; Tocris Bioscience), β-catenin inhibitor XAV 939 (1nM, 10nM; Tocris Bioscience), or PPARγ inhibitor GW9662 (0.1μM, 1.0μM, 10μM; Sigma) were added to the cells at the time of stimulation with Ab-coated beads. All inhibitors were dissolved in DMSO, and vehicle controls contained equal concentrations of DMSO specific for each inhibitor.
RT-PCR
Total RNA was extracted from stimulated T cells with the use of the RNeasy Mini Kit (QIAGEN). Synthesis of complementary DNA with the use of random hexamer primers (Fermentas) was performed according to the manufacturer's instructions. Complementary DNA (5 ng) was used as a template for RT-PCR amplification with the use of SYBR Green Master Mix (Applied Biosystems) in a total volume of 25 μL according to the manufacturer's instructions. Sequences of specific primers (500nM) used for amplification are shown in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Primer pairs for IFNγ, TNFα, and GM-CSF were obtained from a human NF-κB PCR array (RealTimePrimers.com).
AKT in-cell ELISA
chNKG2D T cells (2 × 105) were cultured in a 96-well plate with Biotin Binder beads coated with anti-CD3 or anti-NKG2D Abs. After 5, 20, and 60 minutes, cells were fixed with 4% paraformaldehyde, and phosphorylation of AKT was measured with the Pierce AKT Colorimetric In-Cell ELISA kit (Thermo Scientific) according to the manufacturer's instructions. Total AKT and phosphorylated AKT were detected, and the optical density values were normalized to cell number with the use of the Janus Whole-Cell stain as per the manufacturer's instructions.
Flow cytometry
For flow cytometric analysis of β-catenin or phosphorylated (Serine 9) GSK3β, transduced T cells (2 × 105) were stimulated with Ab-coated beads or tumor cells. Cells were then fixed with 1% paraformaldehyde, permeabilized with 0.1% saponin, and stained with Alexa Fluor 647–conjugated anti–β-catenin (clone 15B8; eBioscience), FITC-conjugated anti-Phospho(Ser9)–GSK3β (R&D Systems), or isotype controls. For CD44, NKG2D, and CD28 staining, T cells were incubated with Cohn fraction to block FcR and stained with PE-conjugated anti-CD44 (clone IM7; BioLegend), PE-conjugated anti-NKG2D (clone 1D11; BioLegend), APC-conjugated anti-CD28 (clone CD28.2; eBioscience), or isotype controls. All isotype controls were purchased from eBioscience. Cell fluorescence was monitored with an Accuri C6 cytometer (Accuri Cytometers).
Cytokine production by transduced T cells
Culture of T cells (4 × 105) and Ab-coated beads was performed in 96-well plates. Cell-free conditioned medium were collected after 24 hours and were assayed for IFNγ by ELISA with the use of human Duoset ELISA kits (R&D Systems) based on the manufacturer's protocol or were analyzed after 24 or 72 hours for additional cytokines with the use of Luminex kits (Bio-Rad). Luminex analysis was performed by the Immune Monitoring Laboratory of the Norris Cotton Cancer Center.
Statistical analysis
Differences between groups were analyzed with a 2-tailed Student t test, an ANOVA comparing multiple groups, or a Wilcoxon 2-group test to compare δCt values for the RT-PCR experiments.26 Values of P < .05 were considered significant.
Results
Stimulation through a chNKG2D receptor activates AKT and β-catenin
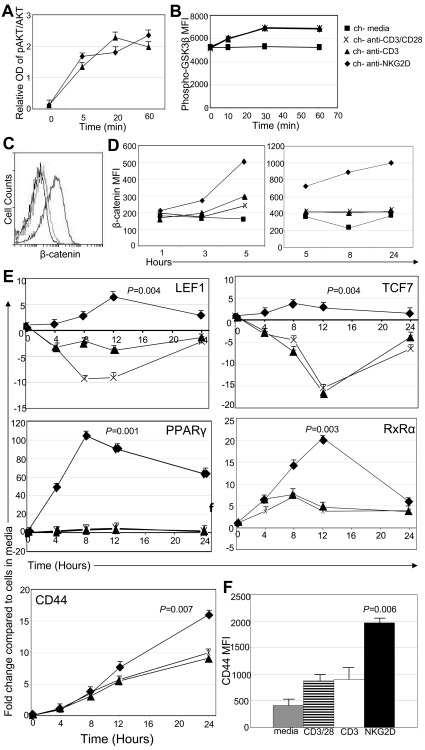
Previous studies have shown that human T cells expressing a chNKG2D receptor lyse human tumor cells and secrete proinflammatory cytokines, leading to long-term tumor-free survival in several tumor models.7–9 However, it is unclear how this chNKG2D receptor alters T-cell signaling, ultimately leading to efficient tumor elimination and long-term survival. Stimulation of NKG2D/DAP10 activates PI3K and AKT (also known as protein kinase B, PKB), which can further affect many downstream signaling pathways.13 Anti-NKG2D mAbs should stimulate both NKG2D/DAP10 and CD3ζ simultaneously in chNKG2D T cells. To test whether activation of T cells through the chNKG2D receptor also activated AKT, chNKG2D T cells were stimulated with beads coated with anti-CD3 Abs to stimulate cells through the TCR or anti-NKG2D Abs to stimulate cells through the chNKG2D receptor (Figure 1A). On stimulation with either anti-CD3 or anti-NKG2D beads, AKT phosphorylation increased over time, indicating that stimulation through the TCR or the chNKG2D receptor activated AKT.
Figure 1.
Signaling through the chNKG2D receptor activates AKT and β-catenin. (A) chNKG2D T cells were stimulated with anti-CD3 or anti-NKG2D Ab-coated beads. Phosphorylated and total AKT were measured with In-Cell ELISA. Data are shown as mean ± SD from 1 donor and are representative of data from 2 donors. (B) Phosphorylated (Ser9) GSK3β expression in chNKG2D T cells after stimulation with media, anti-CD3/CD28–, anti-CD3–, or anti-NKG2D–coated beads. Data shown were obtained from 1 donor and are representative of data from 5 donors. (C) Representative histogram of chNKG2D T cells stained with Abs specific for β-catenin 24 hours after stimulation with media (black), anti-CD3–coated (light gray), or anti-NKG2D–coated (dark gray) beads. (D) β-Catenin expression in chNKG2D T cells after stimulation with media, anti-CD3/CD28–, anti-CD3–, or anti-NKG2D–coated beads. Data shown were obtained from 1 donor and are representative of data from ≥ 6 donors. (E) chNKG2D T cells were stimulated with beads coated with anti-CD3/CD28, anti-CD3, or anti-NKG2D Abs, and gene expression was determined by RT-PCR. Data are shown as the fold change in expression compared with T cells cultured in media at each time point. Data are shown as mean ± SD from 1 donor and are representative of data from ≥ 3 donors. (F) chNKG2D T cells were stimulated with media, anti-CD3/CD28–, anti-CD3–, or anti-NKG2D–coated beads. After 24 hours, CD44 cell surface expression was determined by FACS. Data are shown as an average ± SD of 4 donors. Stimulating chNKG2D T cells with anti-NKG2D–coated beads significantly increased expression of β-catenin–induced genes compared with stimulating T cells through CD3 or CD3/CD28.
One downstream mediator possibly affected by AKT is GSK3β. When AKT is activated, it can phosphorylate and inhibit GSK3β, allowing for the accumulation and translocation of β-catenin to the nucleus where it alters the transcription of numerous genes.27 To test whether signaling through the chNKG2D receptor phosphorylated GSK3β and activated β-catenin, intracellular phospho-GSK3β and β-catenin were measured by flow cytometry after stimulation with beads coated with Abs against NKG2D or CD3. GSK3β phosphorylation at serine 9 was increased after stimulation through CD3, CD3 and CD28, or the chNKG2D receptor (Figure 1B). This shows that similar to AKT, stimulation through CD3 alone is sufficient to activate GSK3β in activated CD8+ T cells. However, intracellular β-catenin increased in chNKG2D T cells only after stimulation with anti-NKG2D–coated beads, with a significant increase beginning after 3 hours and maintained for 24 hours (Figure 1C-D). In contrast, chNKG2D T cells stimulated through CD3 or a combination of CD3 and CD28 did not increase β-catenin.
After translocation to the nucleus, β-catenin interacts with DNA-binding proteins and regulates the expression of many genes. When chNKG2D T cells were stimulated through the chNKG2D receptor, expression of transcription factors activated by β-catenin, including LEF1, TCF7, PPARγ, and RXRα, increased, with peak expression 8 hours after stimulation (Figure 1E). This increase was restricted to signaling through the chNKG2D receptor, because triggering T cells through CD3 or CD3/CD28 did not lead to similar changes in gene expression. CD44 is also induced by β-catenin. Although CD44 expression increased in T cells after all stimulations, the increase in CD44 expression was highest when cells were activated through chNKG2D (Figure 1F). Expression of Bcl-2 and Bcl-xL, which are also regulated by β-catenin, were increased by NKG2D stimulation, but there were no differences in the survival or proliferation of T cells stimulated through NKG2D compared with CD3 (data not shown). These data indicate that activating T cells through the chNKG2D receptor, which is a combination of CD3ζ and DAP10, but not CD3 or CD3/CD28, activated β-catenin and increased the expression of β-catenin–regulated genes.
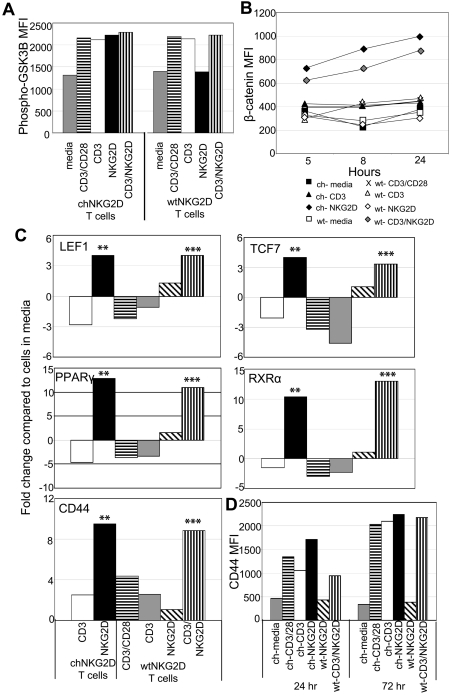
Activation of T cells through a wtNKG2D receptor increases expression of β-catenin–regulated genes
Human CD8+ T cells express the NKG2D receptor, so it is possible that stimulating through a combination of the TCR and NKG2D receptor, similar to activation through the chNKG2D receptor, would also activate β-catenin. T cells used in this study were stimulated with anti-CD3 Abs and expanded for 10 days in IL-2 and were considered early effector T cells. These T cells expressed CD45RO, CD27, CCR7, L-selectin, and little CD57.9 Compared with CD8+ T cells isolated directly from PBMCs, these activated effector T cells maintained expression of NKG2D and had decreased expression of CD28 (supplemental Figure 1).17 T cells expressing wtNKG2D receptors were stimulated with anti-CD3–coated beads or beads coated with both anti-CD3 and anti-NKG2D Abs. Although phosphorylation of GSK3β increased after all stimulations, intracellular β-catenin and expression of β-catenin–induced genes increased in wtNKG2D T cells stimulated only through both CD3 and NKG2D but not CD3 or NKG2D alone, indicating that the combination of TCR and NKG2D stimulation in CD8+ T cells was required for β-catenin activation (Figure 2). Similarly activated, untransduced T cells stimulated with beads coated with both anti-CD3 and anti-NKG2D Abs also increased intracellular β-catenin and expression of β-catenin downstream genes (supplemental Figure 2). These data show that, unlike activation through the TCR (with or without CD28), stimulation of human CD8+ effector T cells either through the chNKG2D receptor or through a combination of the TCR and NKG2D led to accumulation of β-catenin and increased expression of genes regulated by β-catenin.
Figure 2.
Activation of T cells through NKG2D and CD3 activates β-catenin. chNKG2D (ch) and wtNKG2D (wt) T cells were stimulated with media, anti-CD3/CD28–, anti-CD3–, anti-NKG2D–coated beads, or beads coated with both anti-CD3 and anti-NKG2D Abs. (A) Phosphorylated (Ser9) GSK3β expression in chNKG2D or wtNKG2D T cells was measured 30 minutes after stimulation. Data shown were obtained from 1 donor and are representative of data from 3 to 5 donors. (B) β-Catenin was measured with anti–β-catenin Abs at the indicated time points. Data shown were obtained from 1 donor and are representative of data from ≥ 3 donors. (D) Gene expression by chNKG2D and wtNKG2D T cells was determined by RT-PCR 8 hours after stimulation. Data are shown as the fold change in gene expression compared with T cells cultured in media. Stimulation of chNKG2D T cells through NKG2D significantly changed gene expression compared with CD3 stimulation (**P < .05), and stimulation through CD3 and NKG2D significantly changed gene expression compared with CD3 stimulation alone (***P < .05). Data shown were obtained from 1 donor and are representative of data from ≥ 4 donors. (D) ChNKG2D and wtNKG2D T cells were stimulated, and CD44 cell surface expression was determined by FACS after 24 or 72 hours. Data shown were obtained from 1 donor and are representative of data from ≥ 3 donors.
Stimulation through the chNKG2D receptor decreases expression of anti-inflammatory cytokines
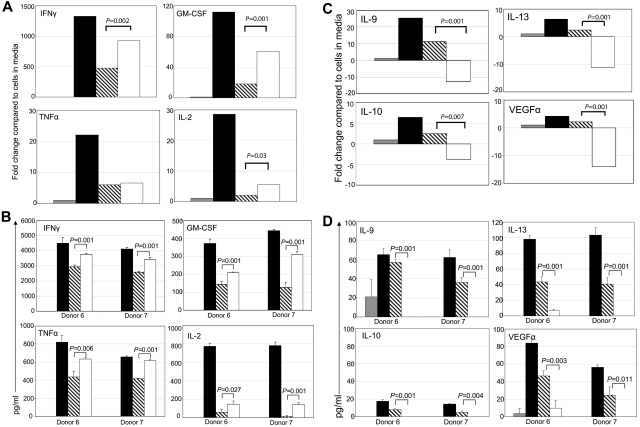
Effector CD8+ T cells can be a significant source of cytokines when activated by their target Ag. Stimulation of T cells through the chNKG2D receptor led to an increased production of IFNγ, GM-CSF, TNFα, and IL-2, compared with signaling through the TCR alone (Figure 3A-B). Stimulation through CD3 and CD28 also increased secretion of proinflammatory cytokines, particularly IL-2, indicating that CD28 on the effector T cells was functional (supplemental Figure 1). The most striking finding was that activating through the chNKG2D receptor decreased production of anti-inflammatory cytokines IL-10, IL-9, IL-13, and the proangiogenic factor VEGFα to baseline amounts (Figure 3C-D). In contrast, activation through CD3/CD28 led to an increase in the secretion of both proinflammatory and anti-inflammatory cytokines. A decrease in anti-inflammatory cytokines was also observed when wtNKG2D T cells or activated, nontransduced T cells were stimulated through both CD3 and NKG2D, compared with an induction of these cytokines when T cells were stimulated through CD3 (data not shown). Thus, stimulation of T cells through the combination of NKG2D and the TCR complex is unique in its ability to induce proinflammatory cytokines while simultaneously preventing expression of anti-inflammatory cytokines.
Figure 3.
Signaling through the chNKG2D receptor decreases expression of anti-inflammatory cytokines and a proangiogenic molecule, VEGFα. chNKG2D T cells were stimulated with media (gray bars), anti-CD3/CD28–coated (filled bars), anti-CD3– (hashed bars), or anti-NKG2D–coated (open bars) beads for 24 hours. Expression of GM-CSF, IFNγ, TNFα, and IL-2 (A-B) or IL-9, IL-13, IL-10, and VEGFα (C-D) was determined by RT-PCR (A,C) or by measuring levels in cell-free supernatants by Luminex analysis (B,D). RT-PCR data are shown as the fold change in expression compared with T cells cultured in media and are representative of data from ≥ 8 donors. Cytokine secretion data are presented as mean ± SD and are from 2 donors (as indicated by donors 6 and 7). Similar cytokine secretion data were obtained when 72-hour supernatant fluids were analyzed from 2 additional donors (data not shown). When stimulated through NKG2D, chNKG2D T cells produced more inflammatory cytokines and less anti-inflammatory cytokines and VEGFα than when stimulated through CD3 alone.
β-Catenin and AKT are both required for decreased expression of anti-inflammatory cytokine genes
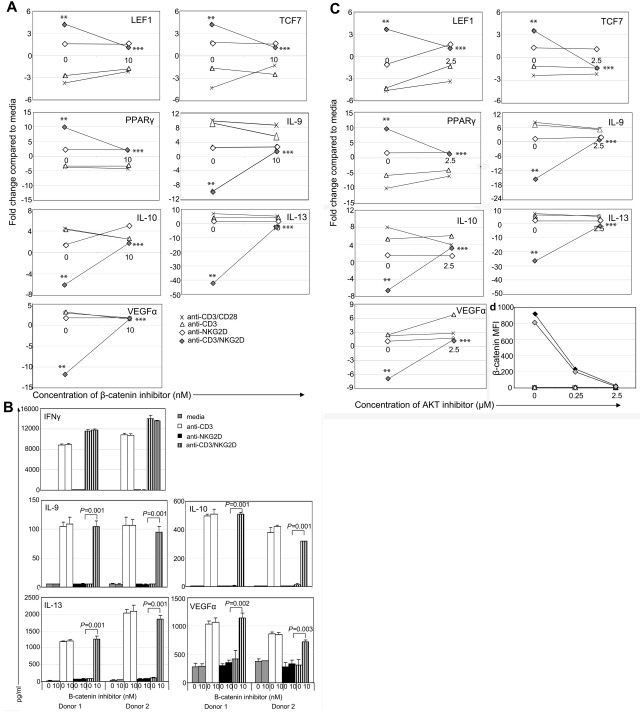
To show the role of β-catenin in decreased cytokine expression after NKG2D activation, wtNKG2D or activated, nontransduced T cells were stimulated in the presence of a β-catenin inhibitor, XAV939. The changes in gene expression induced by the combination of CD3 and NKG2D were diminished when β-catenin was inhibited, indicating that β-catenin contributed to the increased gene expression of LEF1, TCF7, and PPARγ, and the decreased gene expression and protein secretion of IL-10, IL-9, IL-13, and VEGFα (Figure 4A-B). In T cells stimulated with anti-CD3 with or without anti-CD28 Abs, expression of these genes was not significantly altered in the presence of a β-catenin inhibitor, indicating that β-catenin's role in the expression of these genes was restricted to cells stimulated through the NKG2D/DAP10 pathway. Inhibiting β-catenin in chNKG2D T cells stimulated through NKG2D had a similar effect of inhibiting alterations in gene expression (supplemental Figure 3). However the β-catenin inhibitor did not globally affect gene expression in the T cells because IFNγ secretion did not change in the presence of the inhibitor (Figure 4B; supplemental Figure 3).
Figure 4.
AKT and β-catenin are required for activation of β-catenin–induced genes and inhibition of anti-inflammatory genes. wtNKG2D T cells were stimulated with anti-CD3/CD28–, anti-CD3–, anti-NKG2D–, or anti-CD3– and anti-NKG2D–coated beads in the presence of (A) β-catenin inhibitor XAV939 (10nM) or DMSO control (0nM) or (C,D) AKT inhibitor API-2 (2.5μM) or DMSO vehicle control (0μM). (A,C) After 8 hours, gene expression was determined by RT-PCR. Data are shown as the fold change in gene expression compared with T cells cultured in media at each concentration of inhibitor. Stimulation of T cells through NKG2D significantly changed gene expression compared with CD3 stimulation (**P < .05), and the presence of the inhibitor significantly changed gene expression compared with NKG2D stimulation in the presence of vehicle control (***P < .05). (B) Activated, nontransduced T cells were stimulated with media, anti-CD3–, anti-NKG2D–, or anti-CD3– and anti-NKG2D–coated beads in the presence of β-catenin inhibitor XAV939 (10nM) or DMSO vehicle control (0nM). After 24 hours, secretion of IFNγ was determined by ELISA, or after 72 hours production of IL-9, IL-10, IL-13, and VEGFα was determined by Luminex analysis. Cytokine secretion data are presented as mean ± SD and are from 2 donors (as indicated by donors 1 and 2). In the presence of the β-catenin inhibitor, T cells stimulated through CD3 and NKG2D secreted more anti-inflammatory cytokines and VEGFα than in the presence of the vehicle control. (D) chNKG2D (closed symbols) and wtNKG2D (open symbols) T cells were stimulated with media (squares), anti-CD3–coated (triangles), anti-NKG2D–coated (diamonds), or anti-CD3 and anti-NKG2D–coated (wtNKG2D T cells only; gray diamonds) beads in the presence of AKT inhibitor or vehicle DMSO. β-Catenin was measured by staining cells with anti–β-catenin Abs after 5 hours. Data shown were obtained from 1 donor and are representative of data from 3 donors.
When AKT was inhibited in stimulated T cells, there were similar effects on gene expression as with the β-catenin inhibitor, indicating that AKT was also required for the expression of β-catenin–regulated genes (Figure 4C; supplemental Figure 3). Accumulation of β-catenin was significantly decreased when AKT was inhibited in both chNKG2D- and CD3/NKG2D-stimulated wtNKG2D T cells compared with the DMSO vehicle, which showed that AKT was upstream of β-catenin activation (Figure 4D).
PPARγ contributes to the decreased expression of anti-inflammatory cytokines and VEGFα
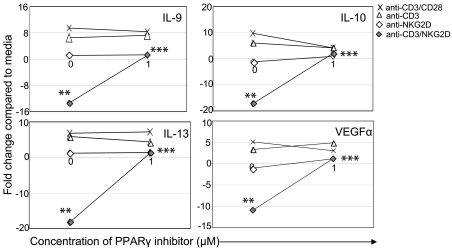
One of the genes that increased after activation through NKG2D was PPARγ, a nuclear receptor that, when activated, binds to RXRα and can either repress or activate the expression of multiple genes, including TNFα, IFNγ, and IL-10.28,29 To determine whether this increased expression of PPARγ contributed to the altered cytokine expression after stimulation through the NKG2D receptor, an inhibitor of PPARγ was added to cells during stimulation. Inhibition of PPARγ in wtNKG2D T cells activated through CD3 and NKG2D increased expression of IL-10, IL-9, IL-13, and VEGFα, suggesting that the increased PPARγ expression after NKG2D stimulation was required for the decrease in cytokine expression (Figure 5). Inhibiting PPARγ in chNKG2D T cells also increased expression of anti-inflammatory cytokines but did not have a significant effect on production of IFNγ, TNFα, or IL-2 (supplemental Figure 4). Thus, in T cells stimulated through the combination of CD3 and NKG2D receptors, PPARγ was required to inhibit the expression of anti-inflammatory cytokines.
Figure 5.
PPARγ is required to decrease expression of IL-9, IL-10, IL-13, and VEGFα. wtNKG2D T cells were stimulated with anti-CD3/CD28–, anti-CD3–, anti-NKG2D–, or anti-CD3 and anti-NKG2D–coated beads in the presence of PPARγ inhibitor GW9662 (1.0μM) or DMSO vehicle control (0μM). After 8 hours, gene expression was determined by RT-PCR. Data are shown as the fold change in gene expression compared with T cells cultured in media at each concentration of inhibitor. Data shown were obtained from 1 donor and are representative of data from 3 donors. Stimulation of T cells through NKG2D significantly changed gene expression compared with CD3 stimulation (**P < .05), and the presence of the inhibitor significantly changed gene expression compared with NKG2D stimulation in the presence of vehicle control (***P < .05).
Stimulation of T cells with NKG2D ligand–positive tumor cells activates β-catenin and decreases anti-inflammatory cytokines
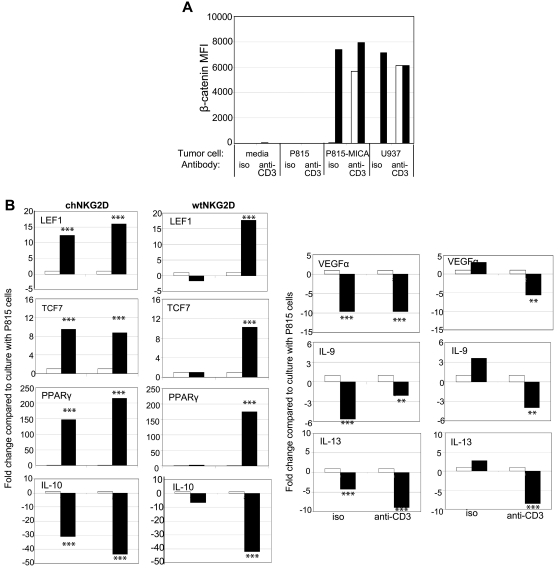
To test whether similar results occurred when human T cells encountered NKG2D ligand–expressing cells rather than Ab-coated beads, T cells were cultured with tumor cells, P815 cells, a murine cell line that will stimulate neither the TCR nor NKG2D receptor, P815-MICA cells, to stimulate the human NKG2D receptor only, or U937 cells, a human tumor cell line that naturally expresses NKG2D ligands. The tumor cells, which express Fc receptors and can be used to trigger specific receptors, were incubated with anti-CD3 mAbs or isotype control Abs to induce CD3/TCR stimulation. Incubation with Ab-coated beads stimulated chNKG2D T cells in a similar manner as when cultured with NKG2D ligand–positive tumor cells, as shown by comparable IFNγ secretion (supplemental Figure 5). β-Catenin increased when chNKG2D T cells were stimulated by NKG2D ligand–positive tumor cells with or without additional stimulation through the TCR complex (Figure 6A). wtNKG2D T cells also increased β-catenin expression but only when stimulated with tumor cells that triggered both the TCR and NKG2D receptors. Expression of β-catenin–induced genes increased and anti-inflammatory cytokines decreased when chNKG2D T cells were cultured with P815-MICA cells but not P815 cells, or when wtNKG2D T cells were stimulated with P815-MICA cells and anti-CD3 Abs (Figure 6B). Thus, similar to stimulation by Ab-coated beads, stimulation by NKG2D ligand–positive cells and the TCR complex led to the accumulation of β-catenin and decreased expression of anti-inflammatory cytokines and proangiogenic factor VEGFα in CD8+ T cells.
Figure 6.
Stimulation of T cells with NKG2D ligand–positive tumor cells activates β-catenin and decreases anti-inflammatory genes. (A) chNKG2D (closed bars) and wtNKG2D (open bars) T cells were cultured with media, P815, P815-MICA, or U937 cells in the presence of anti-CD3 Abs or isotype (iso) control Abs. After 5 hours, β-catenin was measured by staining cells with anti–β-catenin Abs. (B) chNKG2D T cells and wtNKG2D T cells cultured with P815 (open bars) or P815-MICA (closed bars) cells in the presence of anti-CD3 Abs or isotype control Abs. After 8 hours, gene expression was determined by RT-PCR. Data are shown as the fold change in gene expression compared with T cells cultured with P815 cells for each Ab. Data shown were obtained from 1 donor and are representative of data from 3 donors. Stimulation of T cells with P815-MICA cells significantly changed gene expression compared with stimulation with P815 cells (**P < .01 and ***P < .001).
Stimulation through NKG2D modulates CD3/CD28 signaling
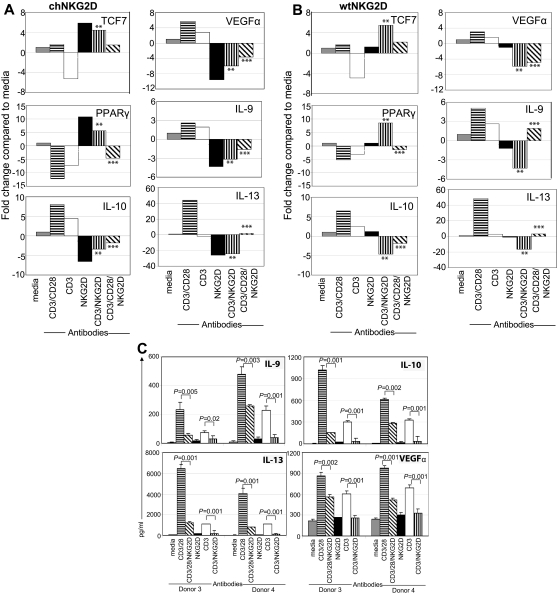
In vivo, it is possible that effector CD8+ T cells may simultaneously encounter ligands for many different cell surface receptors. In addition, many chimeric Ag receptors currently being developed include multiple signaling domains to increase T-cell efficacy.30,31 To determine the effect of signaling through the NKG2D receptor, TCR, and CD28 simultaneously, T cells were activated with beads coated with a combination of anti-NKG2D, anti-CD3, and anti-CD28 Abs. The addition of NKG2D to CD3/CD28 stimulation modulated the outcome in chNKG2D, wtNKG2D, and activated, nontransduced T cells (Figure 7; data not shown). The presence of NKG2D led to an increase in TCF7 expression and a decrease in the gene expression and protein secretion of IL-10, IL-9, IL-13, and VEGFα compared with cells stimulated through CD3/CD28. Together, these data show that activation of the NKG2D receptor on effector T cells altered the function of T cells by activating β-catenin and PPARγ, resulting in a decreased expression of anti-inflammatory cytokines. Thus, human effector T cells alter their function, depending on which innate receptors are engaged in conjunction with the TCR complex.
Figure 7.
Activation of T cells through NKG2D modulates CD3/CD28 signal. (A) chNKG2D or (B) wtNKG2D T cells were stimulated with media, anti-CD3/28–, anti-CD3–, anti-NKG2D–, anti-CD3– and anti-NKG2D–, or anti-CD3–, anti-CD28–, and anti-NKG2D–coated beads. After 8 hours gene expression was determined by RT-PCR. Data are shown as the fold change in gene expression compared with T cells cultured in media. Data shown were obtained from 1 donor and are representative of data from ≥ 3 donors. Stimulation of T cells through CD3/NKG2D significantly changed gene expression compared with CD3 stimulation (**P < .05), and stimulation through CD3/CD28/NKG2D significantly changed gene expression compared with CD3/CD28 stimulation (***P < .05). (C) Activated, nontransduced T cells were stimulated with media, anti-CD3/CD28–, anti-CD3–, anti-NKG2D–, anti-CD3– and anti-NKG2D–, or anti-CD3–, anti-CD28–, and anti-NKG2D–coated beads. After 72 hours, IL-9, IL-10, IL-13, and VEGFα in conditioned medium were determined by Luminex analysis. Cytokine secretion data are presented as mean ± SD and are from 2 donors (as indicated by donors 3 and 4). Stimulation of T cells through CD3/NKG2D significantly decreased cytokine secretion compared with CD3 stimulation, and stimulation through CD3/CD28/NKG2D significantly decreased cytokine secretion compared with CD3/CD28 stimulation.
Discussion
Engagement of the NKG2D receptor in conjunction with the TCR complex, either through the chNKG2D receptor or the combination of CD3 and wtNKG2D, altered signal transduction cascades and gene transcription in human effector CD8+ T cells. Stimulation of NKG2D and CD3 specifically activated β-catenin and led to an increase in proinflammatory cytokines and a decrease in anti-inflammatory cytokines compared with T cells stimulated through CD3 or CD3 and CD28. NKG2D also modulated the production of cytokines by T cells when concurrently activated through CD3, CD28, and NKG2D. Thus, triggering of innate immune receptors alters effector functions of TCR-activated CD8+ T cells.
Activation of costimulatory receptors is essential for naive T cells to become fully activated effector cells, but costimulatory receptors also have important roles in effector and memory T cells. After activation by a professional APC, the effector T cell will travel to a site of inflammation, such as a tumor or site of infection. At this site, the TCR may engage its MHC-peptide ligand, but the function of the T cell can also be altered by cytokines or other cellular interactions. To enhance efficacy of effector CD8+ T cells for therapy, many approaches for adoptive T-cell therapy use chimeric Ag receptors (CARs) that combine a molecule specific for an Ag with ≥ 1 intracellular signaling domains from costimulatory receptors.30,31 Although many first-generation CARs used solely the signaling domains of CD3ζ or Fc receptor γ, second-generation receptors include costimulation domains to enhance T-cell efficacy and survival.30,31 These costimulatory domains have included sequences from CD28, OX40, 41BB, or ICOS. The chNKG2D receptor falls into this category of a second-generation receptor because it will activate both CD3ζ and DAP10. The current study found that inclusion of the NKG2D/DAP10 signal in a CAR provides a unique way to alter the effector functions of T cells to increase secretion of proinflammatory cytokines and to decrease anti-inflammatory cytokines. Many CARs use CD3/CD28 signaling domains, and it is possible that T cells expressing these CARs may encounter NKG2D ligands in the tumor microenvironment. As the data in this study showed, activation of NKG2D may alter the effector functions of the CD3/CD28 CAR-expressing T cells. Thus, it will be important to study how combining and integrating various receptor signals may affect signal transduction and subsequent effector functions of T cells used for therapies.
The NKG2D receptor induces signaling in CD8+ T cells through the associated adaptor molecule DAP10. Previous studies have shown that in human naive CD8+ T cells, stimulation with NKG2D was able to enhance proliferation, cytotoxicity, and production of IFNγ, TNFα, and IL-2.10,32 The current study illustrates that activation of effector CD8+ T cells through NKG2D in conjunction with the TCR activates AKT, which then leads to activation of β-catenin and downstream genes. Activation of β-catenin was because of NKG2D, as stimulation through CD28 did not trigger this pathway, showing that DAP10 induces different signal transduction cascades than CD28. One possible explanation is that, although both molecules activate PI3K and AKT, recruitment of additional signaling molecules to the intracellular domain of CD28, such as Itk, Tec, and Lck, act in concert with the AKT-signaling cascade to activate alternative pathways.
In addition to WNT being a key regulator of T-cell development and thymocyte differentiation, recent work has shown the importance of WNT/β-catenin signaling in mature T cells.18,20 It has been shown that naive CD8+ T cells express Lef1 and Tcf1, and these proteins decrease after stimulation through the TCR.25,33 Other studies have shown that accumulation of β-catenin during T-cell activation arrested the differentiation of CD8+ T cells and supported the development of central memory T cells.21–23,34 Although host T cells develop memory responses to tumor Ags after chNKG2D T-cell treatment, murine chNKG2D T cells themselves do not live long term in vivo, indicating that activation of β-catenin may not always lead to the development of memory T cells.35,36 The data in the current study showed that NKG2D activation of β-catenin modulates the expression of cytokines, leading to a decrease in anti-inflammatory/Th2 cytokines IL-10, IL-13, and IL-9. This decrease depended not only on β-catenin but also on PPARγ, a transcription factor downstream of β-catenin activation. One possible mechanism through which β-catenin modulates the expression of cytokines is through the nuclear receptor PPARγ, which associates with binding partner RXRα, binds to the DNA, and regulates expression of many genes. When PPARγ is bound to DNA in the presence of its ligand, PPARγ recruits coactivator complexes and activates expression of its target genes. Ligands for PPARγ include various fatty acids such as 15-HETE, 13-hydroxyoctadecadienoic acid, and 15-deoxy-Δ12,14-prostaglandin J2.37 However, in the absence of PPARγ ligands, PPARγ has been shown to recruit corepressor complexes and to inhibit gene expression. Stimulation of NKG2D and CD3 increases expression of PPARγ but may not concurrently induce the expression of PPARγ ligands. This may lead to the presence of ligand-free PPARγ bound to the DNA-recruiting corepressor complexes and result in the inhibition of downstream target genes, such as IL-10, IL-9, and IL-13.37,38 Another possible mechanism may involve PPARγ directly inhibiting transcription factors that control cytokine secretion, including NF-κB, nuclear factor of activated T cells (NFAT), or extracellular signal-regulated kinase (ERK).37,38 PPARγ has also been shown to decrease angiogenesis, and these data indicate that NKG2D-induced PPARγ decreased expression of VEGFα compared with T cells stimulated through the TCR or TCR/CD28, suggesting a possible mechanism for PPARγ's role in inhibiting angiogenesis.39 Together, these data show that activation of β-catenin by NKG2D signaling promoted proinflammatory cytokines and inhibited anti-inflammatory cytokines compared with TCR/CD28 signaling in CD8+ effector T cells.
Several studies have investigated the role of WNT/β-catenin signaling in T cells with the use of chemical activators of the β-catenin pathway, by adding WNT proteins, or with the use of T cells deficient in or overexpressing molecules involved in WNT and β-catenin signaling.18,21–23,40 However, it remains unclear how β-catenin is activated in effector T cells during a physiologic immune response. There are ≥ 19 known WNT proteins and 10 Frizzled receptors,19,20 and it cannot be ruled out that CD8+ T cells stimulated through NKG2D up-regulate expression of Frizzled receptors or secretion of WNT proteins. Alternatively, the data in this study show activation of AKT by NKG2D triggering, and that this was required for stabilization of β-catenin and changes in downstream gene expression. Among its myriad of functions, AKT is able to phosphorylate and inhibit GSK3β, and thus simulate signaling along the WNT pathway by activating β-catenin during T-cell activation.27 However, stimulation through CD3 alone also activated AKT and GSK3β, indicating that NKG2D-specific activation of β-catenin may be downstream of GSK3β. GSK3β is known to phosphorylate > 40 different protein substrates in the cell, including TSC2 of the mammalian target of rapamycin-2 complex, NF-κB, and cAMP response element binding.41 Therefore, differences in the activation of these additional pathways in CD3 versus CD3/NKG2D-activated T cells may also play a role as to whether GSK3β is associated with the β-catenin destruction complex, and thus involved in regulating β-catenin. Indeed, preliminary work shows that in addition to β-catenin, there are differences in the activation of NF-κB and CCAAT/enhancer binding protein–signaling pathways when signaling through CD3 alone or CD3 and NKG2D together in effector CD8+ T cells (data not shown).
CD8+ T cells are best known for their ability to lyse target cells; however, they also secrete numerous cytokines that contribute to the development and regulation of immune responses. In addition to secreting proinflammatory cytokines such as IFNγ and TNFα, effector CD8+ T cells have been shown to regulate inflammation through secretion of IL-10, a cytokine that can act on multiple cell types to dampen inflammatory responses.42–44 Similar to CD4+ T cells, CD8+ T cells can be classified into effector subsets, with Tc1 cells secreting IFNγ, and Tc2 cells secreting Th2-associated cytokines IL-4, IL-9, and IL-13.45,46 The factors that drive the development of these CD8+ T-cell subsets are still unclear. The data in this study showed that NKG2D activation of WNT/β-catenin pathway inhibits expression of Tc2-associated cytokines, whereas it did not significantly affect IFNγ expression. This supports the idea that NKG2D may contribute to CD8+ T-cell differentiation at tissue sites.
The ligands for the NKG2D receptor are known to be up-regulated on cell stress or DNA damage.5,47 They are expressed on many types of tumors and after infection with some viruses, including HIV and influenza virus.5,47–49 APCs, such as macrophages and dendritic cells, can also transiently express NKG2D ligands after TLR activation.5 In addition, they are expressed at low levels on some healthy tissues, including differentiated intestinal epithelial cells, where they do not induce an immune response in healthy persons, but are up-regulated and may be involved in Crohn and celiac diseases.5,50 Because NKG2D can alter effector CD8+ T-cell cytokines, this may be one mechanism that regulates CD8+ T cells at sites of infection or tumor growth. Because activated CD8+ T cells expressing NKG2D encounter NKG2D ligands and their cognate MHC-peptide complexes on infected target cells or activated APCs, NKG2D may induce CD8+ T cells to secrete proinflammatory cytokines, such as IFNγ, while at the same time preventing production of anti-inflammatory cytokines. This may induce the development of Tc1 cells at that tissue site, and it will promote inflammation to eradicate the pathogen or to attack a tumor. As NKG2D ligand expression decreases, CD8+ T cells no longer receive the signal through NKG2D, so they may begin to produce anti-inflammatory cytokines, such as IL-10 and VEGFα, which can limit inflammation and tissue damage and promote wound healing. However, continued expression of NKG2D ligands, which can occur during chronic inflammation, may result in a continual local differentiation of Tc1 CD8+ T cells, leading to long-term tissue damage and promoting autoimmunity. Thus, the expression of NKG2D ligands may fine-tune the T-cell response to induce proinflammatory cytokines at the initiation of an immune response and subsequently begin resolution of inflammation once NKG2D ligand expression is down-regulated.
In summary, the data in this study show that activation of human CD8+ T cells through the chNKG2D receptor alone or NKG2D and the TCR activate β-catenin and downstream genes, and this led to a decreased expression of anti-inflammatory cytokines. These data show that the downstream signaling cascades triggered by various non-TCR receptors may differentially induce and modulate the TCR signal and the functions of effector T cells. In addition, activation of β-catenin in T cells during vaccination or T-cell therapy may have beneficial effects through reducing the expression of anti-inflammatory cytokines and thus promoting the development of immune responses to infections or tumors. These non–TCR-induced signals should be considered when designing new receptors for immunotherapy and for designing strategies for manipulating T-cell effector functions in cancer, transplantation, autoimmunity, and infection.
Supplementary Material
Acknowledgments
We thank the Immune Monitoring Laboratory for assistance in Luminex analysis (Norris Cotton Cancer Center, Lebanon, NH), Anastasia Hyrina for technical assistance, and Adam Schmucker for the helpful discussions on PPARγ. We thank Dr Ed Usherwood and Dr Alan Eastman for helpful comments on this manuscript.
This study was supported in part by grants from the National Institutes of Health (T32 AI07363, CA130911).
The contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.B. designed and performed experiments, analyzed data, and wrote the paper; and C.L.S. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The chimeric NKG2D technology used in this study is licensed by Celdara Medical LLC. C.L.S. and Celdara are developing the technology for clinical use. If they are successful, C.L.S. will receive compensation. This arrangement is under compliance with the policies of Dartmouth College. A.B. declares no competing financial interests.
Correspondence: Charles L. Sentman, Department of Microbiology and Immunology, Dartmouth Medical School, 6W Borwell Bldg, One Medical Center Dr, Lebanon, NH 03756; e-mail: charles.sentman@dartmouth.edu.
References
- 1.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3(10):781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 2.Conejo-Garcia JR, Benencia F, Courreges MC, et al. Letal, a tumor-associated NKG2D immunoreceptor ligand, induces activation and expansion of effector immune cells. Cancer Biol Ther. 2003;2(4):446–451. doi: 10.4161/cbt.2.4.479. [DOI] [PubMed] [Google Scholar]
- 3.Groh V, Bahram S, Bauer S, Herman A, Beauchamp M, Spies T. Cell stress-regulated human major histocompatibility complex class I gene expressed in gastrointestinal epithelium. Proc Natl Acad Sci U S A. 1996;93(22):12445–12450. doi: 10.1073/pnas.93.22.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sentman CL, Barber MA, Barber A, Zhang T. NK cell receptors as tools for cancer immunotherapy. Adv Cancer Res. 2006;95:249–292. doi: 10.1016/S0065-230X(06)95007-6. [DOI] [PubMed] [Google Scholar]
- 5.Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunol Rev. 2010;235(1):267–285. doi: 10.1111/j.0105-2896.2010.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang T, Lemoi BA, Sentman CL. Chimeric NK-receptor-bearing T cells mediate antitumor immunotherapy. Blood. 2005;106(5):1544–1551. doi: 10.1182/blood-2004-11-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang T, Barber A, Sentman CL. Generation of antitumor responses by genetic modification of primary human T cells with a chimeric NKG2D receptor. Cancer Res. 2006;66(11):5927–5933. doi: 10.1158/0008-5472.CAN-06-0130. [DOI] [PubMed] [Google Scholar]
- 8.Barber A, Zhang T, DeMars LR, Conejo-Garcia J, Roby KF, Sentman CL. Chimeric NKG2D receptor-bearing T cells as immunotherapy for ovarian cancer. Cancer Res. 2007;67(10):5003–5008. doi: 10.1158/0008-5472.CAN-06-4047. [DOI] [PubMed] [Google Scholar]
- 9.Barber A, Zhang T, Megli CJ, Wu J, Meehan KR, Sentman CL. Chimeric NKG2D receptor-expressing T cells as an immunotherapy for multiple myeloma. Exp Hematol. 2008;36(10):1318–1328. doi: 10.1016/j.exphem.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, Spies T. Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol. 2001;2(3):255–260. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- 11.Maasho K, Opoku-Anane J, Marusina AI, Coligan JE, Borrego F. NKG2D is a costimulatory receptor for human naive CD8+ T cells. J Immunol. 2005;174(8):4480–4484. doi: 10.4049/jimmunol.174.8.4480. [DOI] [PubMed] [Google Scholar]
- 12.Markiewicz MA, Carayannopoulos LN, Naidenko OV, et al. Costimulation through NKG2D enhances murine CD8+ CTL function: similarities and differences between NKG2D and CD28 costimulation. J Immunol. 2005;175(5):2825–2833. doi: 10.4049/jimmunol.175.5.2825. [DOI] [PubMed] [Google Scholar]
- 13.Lanier LL. DAP10- and DAP12-associated receptors in innate immunity. Immunol Rev. 2009;227(1):150–160. doi: 10.1111/j.1600-065X.2008.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehrlich LI, Ogasawara K, Hamerman JA, et al. Engagement of NKG2D by cognate ligand or antibody alone is insufficient to mediate costimulation of human and mouse CD8+ T cells. J Immunol. 2005;174(4):1922–1931. doi: 10.4049/jimmunol.174.4.1922. [DOI] [PubMed] [Google Scholar]
- 15.Harada Y, Tanabe E, Watanabe R, et al. Novel role of phosphatidylinositol 3-kinase in CD28-mediated costimulation. J Biol Chem. 2001;276(12):9003–9008. doi: 10.1074/jbc.M005051200. [DOI] [PubMed] [Google Scholar]
- 16.Upshaw JL, Leibson PJ. NKG2D-mediated activation of cytotoxic lymphocytes: unique signaling pathways and distinct functional outcomes. Semin Immunol. 2006;18(3):167–175. doi: 10.1016/j.smim.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Rajasekaran K, Xiong V, Fong L, Gorski J, Malarkannan S. Functional dichotomy between NKG2D and CD28-mediated co-stimulation in human CD8+ T cells. PLoS ONE. 2010;5(9):e12635. doi: 10.1371/journal.pone.0012635. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gattinoni L, Ji Y, Restifo NP. Wnt/{beta}-catenin signaling in T-cell immunity and cancer immunotherapy. Clin Cancer Res. 2010;16(19):4695–4701. doi: 10.1158/1078-0432.CCR-10-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol. 2008;8(8):581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- 21.Jeannet G, Boudousquie C, Gardiol N, Kang J, Huelsken J, Held W. Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Proc Natl Acad Sci U S A. 2010;107(21):9777–9782. doi: 10.1073/pnas.0914127107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gattinoni L, Zhong XS, Palmer DC, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15(7):808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao DM, Yu S, Zhou X, et al. Constitutive activation of Wnt signaling favors generation of memory CD8 T cells. J Immunol. 2010;184(3):1191–1199. doi: 10.4049/jimmunol.0901199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu B, Crampton SP, Hughes CC. Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity. 2007;26(2):227–239. doi: 10.1016/j.immuni.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willinger T, Freeman T, Herbert M, Hasegawa H, McMichael AJ, Callan MF. Human naive CD8 T cells down-regulate expression of the WNT pathway transcription factors lymphoid enhancer binding factor 1 and transcription factor 7 (T cell factor-1) following antigen encounter in vitro and in vivo. J Immunol. 2006;176(3):1439–1446. doi: 10.4049/jimmunol.176.3.1439. [DOI] [PubMed] [Google Scholar]
- 26.Yuan JS, Reed A, Chen F, Stewart CN., Jr Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang D, Hawke D, Zheng Y, et al. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282(15):11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Straus DS, Glass CK. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 2007;28(12):551–558. doi: 10.1016/j.it.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Clark RB. The role of PPARs in inflammation and immunity. J Leukoc Biol. 2002;71(3):388–400. [PubMed] [Google Scholar]
- 30.Sadelain M, Brentjens R, Riviere I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21:215–223. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cartellieri M, Bachmann M, Feldmann A, et al. Chimeric antigen receptor-engineered T cells for immunotherapy of cancer. J Biomed Biotechnol. 2010:956304. doi: 10.1155/2010/956304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maccalli C, Pende D, Castelli C, Mingari MC, Robbins PF, Parmiani G. NKG2D engagement of colorectal cancer-specific T cells strengthens TCR-mediated antigen stimulation and elicits TCR independent anti-tumor activity. Eur J Immunol. 2003;33(7):2033–2043. doi: 10.1002/eji.200323909. [DOI] [PubMed] [Google Scholar]
- 33.Schilham MW, Wilson A, Moerer P, Benaissa-Trouw BJ, Cumano A, Clevers HC. Critical involvement of Tcf-1 in expansion of thymocytes. J Immunol. 1998;161(8):3984–3991. [PubMed] [Google Scholar]
- 34.Zhou X, Yu S, Zhao DM, Harty JT, Badovinac VP, Xue HH. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity. 2010;33(3):229–240. doi: 10.1016/j.immuni.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang T, Barber A, Sentman CL. Chimeric NKG2D modified T cells inhibit systemic T-cell lymphoma growth in a manner involving multiple cytokines and cytotoxic pathways. Cancer Res. 2007;67(22):11029–11036. doi: 10.1158/0008-5472.CAN-07-2251. [DOI] [PubMed] [Google Scholar]
- 36.Barber A, Zhang T, Sentman CL. Immunotherapy with chimeric NKG2D receptors leads to long-term tumor-free survival and development of host antitumor immunity in murine ovarian cancer. J Immunol. 2008;180(1):72–78. doi: 10.4049/jimmunol.180.1.72. [DOI] [PubMed] [Google Scholar]
- 37.Glass CK, Ogawa S. Combinatorial roles of nuclear receptors in inflammation and immunity. Nat Rev Immunol. 2006;6(1):44–55. doi: 10.1038/nri1748. [DOI] [PubMed] [Google Scholar]
- 38.Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002;2(10):748–759. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- 39.Xin X, Yang S, Kowalski J, Gerritsen ME. Peroxisome proliferator-activated receptor gamma ligands are potent inhibitors of angiogenesis in vitro and in vivo. J Biol Chem. 1999;274(13):9116–9121. doi: 10.1074/jbc.274.13.9116. [DOI] [PubMed] [Google Scholar]
- 40.Driessens G, Zheng Y, Locke F, Cannon JL, Gounari F, Gajewski TF. Beta-catenin inhibits T cell activation by selective interference with linker for activation of T cells-phospholipase C-gamma1 phosphorylation. J Immunol. 2011;186(2):784–790. doi: 10.4049/jimmunol.1001562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci. 2010;35(3):161–168. doi: 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15(3):277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Endharti AT, Rifa IM, Shi Z, et al. Cutting edge: CD8+CD122+ regulatory T cells produce IL-10 to suppress IFN-gamma production and proliferation of CD8+ T cells. J Immunol. 2005;175(11):7093–7097. doi: 10.4049/jimmunol.175.11.7093. [DOI] [PubMed] [Google Scholar]
- 44.Stumhofer JS, Silver JS, Laurence A, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8(12):1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 45.Miyahara N, Swanson BJ, Takeda K, et al. Effector CD8+ T cells mediate inflammation and airway hyper-responsiveness. Nat Med. 2004;10(8):865–869. doi: 10.1038/nm1081. [DOI] [PubMed] [Google Scholar]
- 46.Shrikant PA, Rao R, Li Q, et al. Regulating functional cell fates in CD8 T cells. Immunol Res. 2010;46(1–3):12–22. doi: 10.1007/s12026-009-8130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stern-Ginossar N, Mandelboim O. An integrated view of the regulation of NKG2D ligands. Immunology. 2009;128(1):1–6. doi: 10.1111/j.1365-2567.2009.03147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richard J, Sindhu S, Pham TN, Belzile JP, Cohen EA. HIV-1 Vpr up-regulates expression of ligands for the activating NKG2D receptor and promotes NK cell-mediated killing. Blood. 2010;115(7):1354–1363. doi: 10.1182/blood-2009-08-237370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Draghi M, Pashine A, Sanjanwala B, et al. NKp46 and NKG2D recognition of infected dendritic cells is necessary for NK cell activation in the human response to influenza infection. J Immunol. 2007;178(5):2688–2698. doi: 10.4049/jimmunol.178.5.2688. [DOI] [PubMed] [Google Scholar]
- 50.Burgess SJ, Maasho K, Masilamani M, Narayanan S, Borrego F, Coligan JE. The NKG2D receptor: immunobiology and clinical implications. Immunol Res. 2008;40(1):18–34. doi: 10.1007/s12026-007-0060-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.