Abstract
Protein phosphorylation is the hallmark of checkpoint activation. Hundreds of targets of checkpoint kinases have been identified recently by genome-wide investigations. However, the complete picture of a phosphorylation network required for activation of a checkpoint pathway has not been available. The DNA replication checkpoint in Schizosaccharomyces pombe contains two major protein kinases, the sensor kinase Rad3 and the effector kinase Cds1, with the latter mediating most of the checkpoint functions. We show here that when DNA replication is arrested, efficient activation of Cds1 requires five phosphorylations that cooperate in a parallel or a sequential manner. Phosphorylation of a threonine residue (Thr11) in Cds1 by Rad3 occurs at a basal level in the absence of three other parallel Rad3-dependent phosphorylations on the mediator Mrc1 and Rad9 in the checkpoint clamp complex. However, the three parallel Rad3-dependent phosphorylations are all required for efficient phosphorylation of Thr11 in Cds1 by Rad3. Phosphorylation of Thr11 has been shown previously to promote autophosphorylation of Thr328 in the kinase domain of Cds1, which directly activates the enzyme, leading to full activation of the checkpoint pathway. Interestingly, phosphorylation of Mrc1 by Rad3 does not require the phosphorylation of Rad9, suggesting that activation of the sensor kinase Rad3 in the replication checkpoint of fission yeast may involve a different mechanism.
Keywords: Cell Cycle, Checkpoint Control, DNA Replication, Protein Kinases, Protein Phosphorylation, Cds1, Rad3, Checkpoint Activation, Fission Yeast, Phosphorylation Network
Introduction
The DNA replication checkpoint is a complex signal transduction pathway that is activated when replication forks stall by nucleotide deprivation, damage to DNA template, or inhibition of polymerases (1–3). Under these circumstances, checkpoint activation is crucial for maintenance of genome integrity and cell survival. The checkpoint elicits protective cellular responses such as stabilization of stalled replisomes, suppression of firing of late origins, delay of mitosis, and stimulation of nucleotide production so that DNA replication can resume when normal conditions are restored. Consistent with its important function in the maintenance of genome stability, the replication checkpoint is highly conserved in eukaryotes. Mutations in the pathway are linked to cancer (4–6).
Conceptually, the replication checkpoint pathway contains three functional units. Sensors detect stalled replication forks for initiation of the checkpoint signaling, mediators transduce the checkpoint signal, and effectors regulate the protective cellular responses mentioned above. In the fission yeast Schizosaccharomyces pombe, six Rad proteins mediate the sensor function (7, 8). The sensor kinase Rad3 (ATR2 in human cells) forms a complex with Rad26 (ATR-interacting protein in human cells) and binds to DNA structures associated with stalled forks. A heterotrimeric ringlike complex that contains Rad9, Rad1, and Hus1 is also loaded at sites of stalled forks by the specific loader Rad17-Rfc2–5 complex (9, 10). This so-called “9-1-1” clamp is related to the replication processivity factor proliferating cell nuclear antigen (PCNA). Once the sensors are assembled at the stalled forks, Rad3 initiates checkpoint signaling by phosphorylating key targets, including the mediator Mrc1 and the effector kinase Cds1 (Claspin and CHK2 in human cells, respectively) (11–13). Rad3 also phosphorylates the mediator Crb2 (BRCA1 in human cells) and the second effector kinase Chk1 when DNA damage occurs outside the S phase (14, 15). Tel1 (ATM in human cells) is also activated under replication stress, but it is not required for cell survival (16).
Checkpoint activation is characterized by protein phosphorylation. Recent proteomics studies have identified hundreds of targets of the checkpoint kinases that mediate diverse cellular activities (17–20). Although vigorously investigated, a complete picture of the phosphorylation network required for activation of a checkpoint pathway has remained unavailable. Rad3 and Cds1 are two major kinases in the replication checkpoint of S. pombe. Several phosphorylation sites on Mrc1, Rad9, and Cds1 have been identified by large scale mutational analyses and implicated for the activation of Cds1 (13, 21–25). However, whether these sites represent all phosphorylations required for Cds1 activation has been an unsolved issue.
In this study, we extended our large scale mutational analysis to the checkpoint sensor proteins Rad3, Rad26, Rad17, Rad9, Rad1, and Hus1 and examined all potential Rad3 phosphorylation sites in the sensor proteins, the mediator Mrc1, and the effector kinase Cds1 by site-specific mutations and immunoblottings using phosphospecific antibodies. We show here, together with previous studies (13, 21–24), that five coordinated phosphorylations are required for efficient activation of the replication checkpoint. Analysis of the functional relationship between the phosphorylation events revealed a clear picture of the phosphorylation network required for activation of the replication checkpoint pathway in fission yeast. We also show that Mrc1 can be fully phosphorylated by Rad3 in the absence of Rad9 phosphorylation. Phosphorylated Rad9 can recruit Cut5 (TopBP1 in human cells), a protein with dual essential functions in DNA replication and the activation of checkpoint pathways (15, 26–29). However, our results suggest that activation of the sensor kinase Rad3 in the replication checkpoint pathway of S. pombe may involve a different mechanism that does not require the recruitment of Cut5 by phosphorylated Rad9.
EXPERIMENTAL PROCEDURES
Yeast Culture, Site-specific Mutation, and Drug Sensitivity Assay
Growth of S. pombe strains and medium preparation followed standard methods (30). Point mutations of potential phosphorylation sites were made by QuikChange mutagenesis PCR using the high fidelity thermal resistant polymerase Pfu (Agilent Technologies, Santa Clara, CA). All mutations and sequences were confirmed by DNA sequencing (Retrogen, San Diego, CA). Vectors were introduced into S. pombe cells by electroporation (Gene Pulser II, Bio-Rad). To test the drug sensitivity, 2 × 107 cells/ml logarithmically growing S. pombe were diluted in 5-fold steps and spotted onto YE6S plates containing hydroxyurea (HU) or methyl methanesulfonate (MMS) at the indicated concentrations. The plates were usually incubated at 30 °C for 3 days and then photographed.
Phosphospecific Antibodies
Phosphospecific polyclonal antibodies against phosphorylated Mrc1-Thr645, Mrc1-Ser604, and Rad9-Thr412 were generated in rabbits and affinity-purified by Bethyl Laboratories, Inc. (Montgomery, TX). To generate the phosphospecific antibodies, phosphopeptides Mrc1-T645-P (CVDSLVPpTQLDST where pT is phosphothreonine), Mrc1-S604-P (CNSQPSApSQLTIV where pS is phosphoserine), and Rad9-T412-P (DAEFGPpTQAEQS) were chemically synthesized, conjugated to keyhole limpet hemocyanin, and used as the immunogens in rabbits. The reactivity ratios of phosphorylated titer versus nonphosphorylated titer for all antibodies are ≥99:1 and were confirmed by immunoblotting (supplemental Fig. S2). The phosphospecific antibodies for phosphorylated Rad9-Ser423 and Rad9-Thr225, also prepared by Bethyl Laboratories, Inc., were kindly provided by Furuya et al. (22). The antibodies against phosphorylated Cds1-Thr11 and Cds1-Thr328 have been described previously (31).
Western Blotting
The phosphorylation of Rad9 and Cds1, tagged with a hemagglutinin (HA) epitope, was assayed in proteins immunoprecipitated with anti-HA antibody (Santa Cruz Biotechnology). Loadings of Cds1-HA and HA-Rad9 were revealed by immunoblotting using anti-HA antibody. The same blot was stripped and reprobed with phosphospecific antibodies overnight at 1:3000–1:5000 dilutions. Immunoblotting signal was revealed with electrochemiluminescence and photographed by an Image Reader LAS-3000 (Fujifilm). Band intensities were analyzed by ImageGauge (Fujifilm) and are shown as percentages relative to wild-type proteins. Phosphorylation of Mrc1-Thr645 or Mrc1-Ser604 was examined in whole cell lysate prepared by the trichloroacetic acid method as described before (13). The same membrane was stripped and blotted with polyclonal antibody against Mrc1 for the loading.
Cds1 Kinase Assay
The kinase activity of Cds1 was examined using the substrate GST-Wee1 (amino acids 11–152) as described previously (32). Briefly, 1 × 108 cells were homogenized in a minibead beater in lysis buffer (50 mm Tris/HCl, 150 mm NaCl, 2 mm EDTA, 1 mm Na3VO4, 10 mm pyrophosphate, 50 mm NaF, 60 mm β-glycerophosphate, 0.1% Nonidet P-40, pH 7.6, and protease inhibitors). After clarification by centrifuging at 4 °C at 14,000 rpm for 5 min, the cell extract was added to glutathione beads with bound GST-Wee1 and rotated at 4 °C for 1 h. The beads were washed twice with ice-cold PBS-Tween 20 buffer mixed with 25 μl of kinase reaction buffer (20 mm Tris/HCl, pH 7.5, 5 mm MgCl2, 1 mm dithiothreitol, 75 mm KCl, 50 μm [γ-32P]ATP) and incubated at 30 °C for 20 min. After separation by SDS-PAGE, the gel was stained with Coomassie Blue to reveal GST-Wee1, and incorporation of 32P was measured by phosphorimaging.
RESULTS
Only Four Potential Rad3 Phosphorylation Sites in Sensor Proteins Are Important
By systematic mutational analysis of Cds1, we have shown previously that activation of Cds1 requires the phosphorylation of a single residue, Thr328, in the kinase domain (31). Phosphorylation of Cds1-Thr328 is achieved by trans-autophosphorylation and promoted by Rad3-dependent phosphorylation of Thr11 in Cds1 and two functionally redundant residues (Thr645 and Thr653) in the Cds1-docking repeats on Mrc1 (13). To obtain a clear picture of the phosphorylation network required for Cds1 activation, we extended the mutational analysis to the sensor proteins in the pathway.
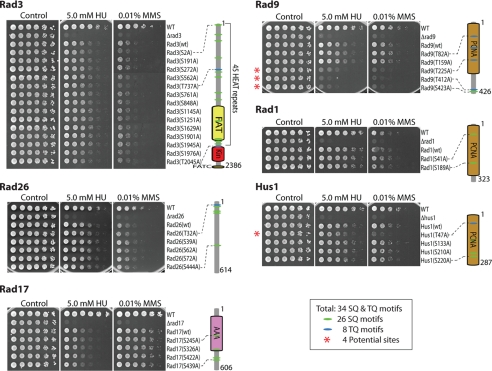
Like other phosphoinositide 3-kinase-related protein kinases, Rad3 phosphorylates target proteins preferentially at the SQ or TQ motifs (33). There are 34 SQ/TQ motifs in the sensor proteins (Fig. 1; 26 SQ and eight TQ motifs). Cut5, the replication protein with an essential checkpoint function (34), does not contain any SQ/TQ motif. To identify the potential Rad3 phosphorylation sites, serines or threonines in all SQ/TQ motifs of the sensor proteins were individually mutated to alanine. The resulting mutant proteins were each expressed in their corresponding null mutant cells under the control of their own promoters and tested for sensitivity to HU, the inhibitor of ribonucleotide reductase, or the DNA-damaging agent MMS.
FIGURE 1.
Four potential phosphorylation sites in sensor proteins are important for checkpoint functions. Molecular architectures with the indicated domains for each checkpoint sensor protein are shown on the right. 34 potential Rad3 phosphorylation sites (26 SQ and eight TQ) and their relative locations are marked by green and blue ovals, respectively. Serines or threonines of the SQ/TQ motifs were individually mutated to alanines. Wild-type or mutant proteins were expressed from plasmids under the control of their own promoters in their corresponding gene deletion mutant cells. 5-fold dilutions of the cells were spotted on YE6S plates (Control) or YE6S plates containing 5 mm HU or 0.01% MMS. The plates were incubated at 30 °C for 3 days and then photographed. Red asterisks indicate the four potential phosphorylation sites on Rad9 (Thr225, Thr412, and Ser423) and Hus1 (Thr47) that are important for checkpoint function. The essential replication protein Cut5 with a critical checkpoint function does not contain any SQ/TQ motifs and is therefore not included in this experiment. The FAT(FRAP-ATM-TRRAP) domain and the kinase domain in the sensor kinase Rad3 are shown in yellow and red, respectively. The AAA (ATPases associated with a wide variety of cellular activities) domain in Rad17 is shown in purple. PCNA indicates the PCNA-like domain conserved in the three subunits of the 9-1-1 clamp complex.
Surprisingly, only four of the 34 Rad3 phosphorylatable sites (Thr225, Thr412, and Ser423 in Rad9 and Thr47 in Hus1) are important for checkpoint function as judged by sensitivities of the mutants to HU or MMS (Fig. 1, red asterisks). The Rad9(T412A) mutant was sensitive to HU and MMS almost the same as Rad9-null cells, whereas the Rad9(T225A), Rad9(S423A), and Hus1(T47A) mutants were less sensitive (Fig. 1 and supplemental Figs. S3 and S4), indicating that a lower level of checkpoint function is retained in these mutant cells. The similar pattern of sensitivity to HU and MMS of the four mutants suggests that Rad9 and Hus1 function similarly in response to depletion of deoxyribonucleotides or DNA damage caused by MMS. The remaining mutants behave like the cells expressing wild-type proteins or are slightly sensitive, indicating that the corresponding phosphorylatable residues are not essential or functionally redundant.
To identify potential redundant phosphorylation sites, all SQ/TQ motifs in Rad26, Rad17, Rad1, or Hus1 were simultaneously mutated and then tested for drug sensitivities (supplemental Fig. S1). Simultaneous mutation of all SQ/TQ motifs in Rad17, Rad26, or Rad1 did not sensitize the cells, suggesting that there is no important redundant Rad3 phosphorylation in the three sensors, although phosphorylation occurs in vivo (35, 36). The addition of three other SQ site mutations to the Thr47 mutation in Hus1 did not further increase the sensitivity, indicating that redundant phosphorylation of Hus1, if it occurs, is not important for the checkpoint.
Rad3 is a large protein of 2386 amino acids with the kinase domain residing near the C terminus. Individual mutation of three sites at Ser2, Thr737, and Ser1976 slightly sensitized the cells to MMS or HU (Fig. 1). We have shown previously in Mrc1 that, although functionally redundant, single mutation of Thr645 or Thr653 can sensitize the cells at a detectable level (13). To see whether there is redundant autophosphorylation of Rad3, we simultaneously mutated the three sites (supplemental Fig. S1, bottom panels). Unlike Mrc1 in which simultaneous mutation of the two TQ sites completely abolished the checkpoint signaling function, combined mutation of the three sites in Rad3 did not significantly increase the drug sensitivity, indicating that the three residues are not essential for the checkpoint function. Autophosphorylation of Ser1981 in ATM has been well established (37, 38). The homologous region of Rad3 where Ser1981 is located in ATM contains four potential phosphorylation sites: Ser1901, Ser1945, Ser1976, and Thr2045. However, simultaneous mutation of these sites did not sensitize the cells, indicating that they are not important for checkpoint functions. Together, only four potential Rad3 phosphorylation sites were identified in the sensor proteins of S. pombe that are important for checkpoint function. We found no evidence for redundant Rad3 phosphorylation in the sensor proteins.
Rad9-Thr225 and Hus1-Thr47 Are Structurally Important
Earlier mutational studies have uncovered four phosphorylations that are required for Cds1 activation: the SQ cluster and the redundant TQ motifs in Mrc1 and Thr11 and Thr328 in Cds1 (13, 21, 24, 31). The experiments described above discovered four more potential Rad3 phosphorylation sites: Thr225, Thr412, and Ser423 in Rad9 and Thr47 in Hus1. Phosphorylation of Rad9 has been studied before for Chk1 activation and regulation of the choice of DNA repair pathways (22, 25, 39). All together, eight potential phosphorylations have been uncovered in the replication checkpoint pathway of S. pombe. However, it is possible that some of the residues are not true phosphorylation sites but are important in protein structure.
Thr225 in Rad9 and Thr47 in Hus1 are the only two Rad3 phosphorylatable sites found in the PCNA-like domains of the 9-1-1 clamp complex. Based on the crystal structure of the human 9-1-1 complex (40–42), the two sites are near the interface between Rad9 and Hus1, suggesting that they may be important for the complex formation. To see whether this is the case, we mutated the two residues to a structurally similar residue, cysteine (supplemental Fig. S3, A and C). Cysteine substitutions in the two proteins significantly reversed the drug sensitivity caused by the alanine mutations, especially in Hus1, which behaves almost like the wild-type protein (compare Hus1(T47C) with wild-type Hus1 and Hus1(T47A) mutant in supplemental Fig. S3C). This suggests that Rad9-Thr225 and Hus1-Thr47 may not be true phosphorylated residues but are required for the clamp structure or other functions (39). Consistent with this, mutation of the two sites to the phosphomimic residue glutamic acid made the cells even more sensitive (supplemental Fig. S3, A and C).
To provide further evidence that Rad9-Thr225 is not phosphorylated, we tried to detect the phosphorylation by a phosphospecific antibody (supplemental Fig. S3B). In response to HU treatment, a weak phosphorylation signal can be detected in Rad9 by the phosphospecific antibody. However, the signal was clearly not due to the phosphorylation of Thr225, as it was not abolished by the unphosphorylatable T225A mutation. More likely, it was caused by a cross-reactivity of the antibody to other phosphorylated residues on Rad9 because the T412A/S423A mutation completely eliminated it. Although the possibility of Thr412-dependent phosphorylation of Thr225 in Rad9 cannot be completely ruled out by this experiment (25), we found no convincing evidence for crucial Rad3 phosphorylation in the PCNA-like domain of Rad9 and probably all PCNA-like domains in the 9-1-1 complex. We propose that the two residues Rad9-Thr225 and Hus1-Thr47 are structurally important for the 9-1-1 clamp complex.
Rad9-Thr412 Is the Major Phosphorylation Site in 9-1-1 Complex
Phosphorylation of the Rad9 C terminus is highly conserved in eukaryotes (22, 43–46). In S. pombe, two Rad3 phosphorylatable sites, Thr412 and Ser423, in the Rad9 C-terminal tail are important for checkpoint function (Ref. 22 and Fig. 1). To study the functions of the two sites to have a complete picture of the phosphorylation network, we repeated the experiments of a previous study by another group (22) using the phosphospecific antibodies we generated (Fig. 2, A and B). The antibodies are specific because single mutation of each site completely abolished the binding of the antibodies. The low level phosphorylation of the two sites in untreated cells, especially Thr412, was observed before (22) and is consistent with the basal checkpoint activity under normal conditions (see below). Phosphorylation of Rad9-Thr412 is dependent on both Rad3 and Tel1 (supplemental Fig. S4A) as the single mutation of Rad3 or Tel1, including the Rad3 kinase-inactive mutation D2249E, only eliminated part of the phosphorylation, whereas the double mutation of Rad3 and Tel1 completely abolished it. Mutation in the effector kinases Cds1 and Chk1 did not affect the phosphorylation, indicating that they are not responsible for the phosphorylation. Other checkpoint sensors such as Rad17 and the proteins in the 9-1-1 complex also play an important role in promoting Thr412 phosphorylation (supplemental Fig. S4B). The protein level of Rad9 was generally lower in the replication checkpoint-deficient cells, indicating that Rad9 is more stable in checkpoint-competent cells. Phosphorylation of Ser423 in Rad9 was also confirmed by a mutational analysis (supplemental Fig. S4C). The phosphomimic S423E mutation made the cells more drug-resistant than the S423A mutant, whereas the structure mimic S423C mutant had a drug sensitivity similar to that of the S423A mutant.
FIGURE 2.
Phosphorylated Ser423 promotes phosphorylation of Thr412 in Rad9 C terminus. A, wild-type or mutant Rad9, tagged with HA at the N terminus, was expressed from the genomic rad9+ locus. Cells were cultured in medium containing no (−) or 25 mm HU (+) at 30 °C for 3 h. Rad9 was immunoprecipitated with anti-HA antibody from the cell extracts prepared from an equal number of cells. Untagged wild-type cells were used as the immunoprecipitation control. After SDS-PAGE, phosphorylated Thr412 in Rad9 was detected by Western blotting with the phosphospecific antibody (upper panels). The level of phosphorylation was analyzed by ImageGauge and is shown as percentages (below the upper panel on the right) as compared with HU-treated wild-type cells. The same blots were stripped and reprobed with anti-HA antibody to assess the loadings (lower panels). B, phosphorylation of Ser423 in Rad9 was examined by immunoblotting essentially the same as in A. C, drug sensitivity of wild-type cells or cells containing various Rad9 mutants was examined by spot assay as described under “Experimental Procedures.”
To examine the functional relationship between the two phosphorylations in the Rad9 C terminus, phosphorylation of one site was examined in the mutant background of the other (Fig. 2, A and B, right panels). We found that the S423A mutation reduced the phosphorylation of Thr412 more than 50%, especially in untreated cells. In contrast, the T412A mutation did not affect the phosphorylation of Ser423 much. This suggests that while Thr412 phosphorylation plays a major role in checkpoint function, the main function of phosphorylated Ser423 may be to facilitate the phosphorylation of Thr412. Consistent with this, the T412A mutation eliminated most of the checkpoint function of Rad9, whereas the S423A mutant was much less sensitive to the drug treatment (Fig. 2C). Addition of the S423A mutation to the T412A mutant did not further increase the drug sensitivity. In fact, the mutant cells containing the single T412A mutation behaved almost the same as the mutant with a triple T225A/T412A/S423A mutation or even the mutant that carried the C-terminal deletion. Furthermore, phosphorylation of Thr412 was slightly increased in the phosphomimic mutant S423E as compared with that in the S423A mutant (supplemental Fig. S4D), which is consistent with the drug resistance improved by the S423E mutation (supplemental Fig. S4C) and supports the notion that phosphorylated Ser423 can promote the phosphorylation of Thr412.
Taken together, these results show that Thr412 in the Rad9 C terminus is the only phosphorylated residue on the 9-1-1 complex that plays a key role in checkpoint function and cell survival under replication stress. The second phosphorylated residue, Ser423, is less important; its main function appears to be the promotion of Thr412 phosphorylation. However, other possibilities cannot be ruled out completely. For example, S423A mutation may indirectly affect the phosphorylation of Thr412 by perturbing the cell cycle.
Independent Phosphorylation of TQ Motifs and SQ Cluster in Mrc1
Phosphorylation of Mrc1 is required for checkpoint activation (13, 21). Genetic studies have uncovered two separate functions of the Mrc1 phosphorylation. One is mediated by two redundant TQ motifs (Thr645 and Thr653) in the Cds1-docking repeats, and the other is mediated by four SQ motifs (Ser572, Ser599, Ser604, and Ser614) clustered in the middle of Mrc1 near the TQ motifs (13). However, phosphorylation of these motifs has not been well established due in part to the lack of phosphospecific antibodies. To investigate the functions of Mrc1 phosphorylation, we generated phosphospecific antibodies against phosphorylated Thr645 and Ser604 to represent the two redundant TQ motifs and the SQ cluster, respectively.
After cells were treated with HU, phosphorylation of Thr645 could be readily detected in Mrc1 by the phosphospecific antibody (Fig. 3A). The weak signal observed in HU-treated T645A mutant cell was probably due to a cross-reactivity of the antibody to phosphorylated Thr653 because double mutation of T645A/T653A completely eliminated it, which verifies the specificity of the antibody and is consistent with the redundant function of the two Cds1-docking repeats (13). Phosphorylation of Thr645 is solely dependent on Rad3 as deletion or the kinase-dead D2249E mutation of Rad3 completely abolished the phosphorylation (Fig. 4B). Depletion of other checkpoint kinases Tel1, Cds1, and Chk1 had no or little effect on the phosphorylation. Unlike the phosphorylation in Rad9 (Fig. 2) and the SQ cluster in Mrc1 (see below), which occurs at a minimal level under normal conditions, phosphorylation of Mrc1-Thr645 was not detectable in untreated cells (Fig. 3, A and B).
FIGURE 3.
Independent phosphorylation of TQ motifs and SQ cluster in Mrc1. A, Mrc1-Thr645 is phosphorylated after cells are treated with HU. Wild-type or mutant Mrc1 was expressed from the leu1+ genomic locus. Whole cell extracts were prepared from cells treated with (+) or without (−) HU. After SDS-PAGE, phosphorylation of Thr645 in Mrc1 was examined by Western blotting using the phosphospecific antibody (upper panel). A nonspecific band is marked by the asterisk. The membrane was stripped and blotted with polyclonal antibody against Mrc1 for the loading (lower panel). B, phosphorylation of Mrc1-Thr645 is dependent on Rad3. Thr645 phosphorylation was examined as in A in wild-type cells or cells containing the mutations of the indicated checkpoint kinases. D2249E is the kinase-inactive mutation of Rad3. C, phosphorylation of Mrc1-Ser604 is dependent on both Rad3 and Tel1. Mrc1 was tagged with the HA epitope at the C terminus and expressed in wild-type cells or cells containing the indicated mutations. After HU treatment, Mrc1 was immunoprecipitated with anti-HA antibody, and phosphorylation of Ser604 was examined by Western blotting using the phosphospecific antibody (upper panel). Loading of Mrc1 was slightly normalized in this experiment and shown by immunoblotting with anti-HA antibody (lower panel). D, phosphorylation of Thr645 and Ser604 in Mrc1 does not absolutely require the 9-1-1 complex. Phosphorylation of Ser604 (middle panel) in immunoprecipitated Mrc1 was examined by using the phosphospecific antibody as in C. The membrane was stripped and reprobed with the phosphospecific antibody against phosphorylated Thr645 (top panel). E, phosphorylations of Thr645 and Ser604 in Mrc1 are independent events. Phosphorylation of Ser604 (top panel) was examined in wild-type cells or cells containing various Mrc1 mutations as in A. All S/TQ mut is the mutant in which serines and threonines in all SQ/TQ motifs of Mrc1 were simultaneously mutated to alanines. SQ Cluster mut contains a simultaneous mutation of all four serines in the SQ cluster. The membrane was stripped and then probed with the phosphospecific antibody for the phosphorylation of Thr645 (middle panel). Asterisks indicate cross-reactive materials. Loading is shown in the bottom panel.
FIGURE 4.
Independent phosphorylation of Rad9 and Mrc1. A, phosphorylation of Thr645 and Ser604 in Mrc1 is not dependent on Rad9 phosphorylation. Phosphorylation of Mrc1-Thr645 was examined in whole cell extracts prepared from cells expressing various Rad9 phosphorylation site mutants as in Fig. 3A and is shown in the upper three panels. Loadings of Mrc1 and the expressed Rad9 mutant proteins are shown by immunoblotting with antibodies against Mrc1 or HA epitope. Phosphorylation of Mrc1-Ser604 was examined in Mrc1 immunoprecipitated from cells expressing wild-type Rad9 or Rad9 containing the indicated mutations as described in Fig. 3C and shown in the lower two panels. B, phosphorylation of Rad9-Thr412 does not require Mrc1 or the phosphorylation of Mrc1. Thr412 phosphorylation was examined in Rad9 immunoprecipitated from wild-type cells or cells lacking Mrc1 or expressing Mrc1 with the indicated phosphorylation site mutations (mut) (top panel) as in Fig. 2A. Loadings of Rad9 and the expressed Mrc1 are shown in the lower two panels.
Phosphorylation of Ser604 in Mrc1 was also detected after HU treatment (Fig. 3C). Mutation of Ser604 to alanine completely eliminated the signal, indicating that the antibody is specific. When Ser604 was mutated to the structure mimic residue cysteine or the phosphomimic residue glutamic acid, the cells were still sensitive (supplemental Fig. S5A). However, mutation of Ser604 to the phosphorylatable residue threonine did not affect the function much, suggesting that the phosphorylation and the local structure may both be important for Ser604 and probably the SQ cluster as a whole. We also mutated three other serines in the SQ cluster to cysteines. However, unlike Rad9-Thr225 and Hus1-Thr47, cysteine substitutions of the serines in the SQ cluster in various combinations did not make the cells significantly more resistant (supplemental Fig. S5B).
A basal level of Mrc1-Ser604 phosphorylation could be detected under normal conditions (Fig. 3, C and D), which is similar to Rad9 but different from the TQ motifs in Mrc1. It is possible that the basal level of Cds1 activation (see below) under normal conditions is realized through the phosphorylation of the Rad9 C terminus and the Mrc1 SQ cluster. The TQ motifs in Mrc1 were phosphorylated by Rad3 only when cells were under replication stress (Fig. 3A). Interestingly, similar to the phosphorylation of Rad9-Thr412 (supplemental Fig. S4A), phosphorylation of Mrc1-Ser604 is also dependent on both Rad3 and Tel1 because complete removal of the phosphorylation required depletion of both Rad3 and Tel1 (Fig. 3C). However, whether the Tel1-dependent phosphorylation of the Mrc1 SQ cluster and Rad9 C terminus contributes to the basal checkpoint activation in an unperturbed cell cycle remains unclear.
We also examined the possible dependence of Mrc1 phosphorylation on other checkpoint sensor proteins (Fig. 3D). To our surprise, depletion of Rad17 and the 9-1-1 complex did not have a significant effect on the phosphorylation of Thr645 and Ser604, indicating that phosphorylation of Mrc1 by Rad3 does not absolutely require these sensors. The differences between the phosphorylation of Thr645 and Ser604 in Mrc1 were also confirmed by this experiment. Ser604 phosphorylation was dependent on both Rad3 and Tel1 and occurred at a basal level under normal conditions, whereas the phosphorylation of Thr645 was dependent only on Rad3 and specific to HU treatment.
To see whether the phosphorylation of the TQ motifs and the SQ cluster in Mrc1 are interdependent, we examined the phosphorylation in various Mrc1 phosphorylation site mutants (Fig. 3E). We found that the two essential phosphorylations in Mrc1 are independent events. Phosphorylation of Ser604 was not affected by the T645A mutation or the double mutation of Thr645 and Thr653. Similarly, phosphorylation of Thr645 could be readily observed in the S604A mutant or the mutant that contained mutations of all serines in the SQ cluster. Phosphorylation of both residues was completely eliminated in the Mrc1 all SQ/TQ mutant as expected.
Independent Phosphorylation of Mrc1 and Rad9
Having confirmed the essential phosphorylations in Rad9 and Mrc1, we then asked whether the phosphorylations of the two separate proteins are interdependent. Previous reports showed that phosphorylated Rad9 can recruit Cut5 (15, 22), and the Cut5 homolog TopBP1 in Xenopus can directly activate the Rad3 homolog ATR (29). It has also been shown in budding yeast that Dpb11 can activate Mec1 (Cut5 and Rad3 in S. pombe, respectively) (27, 28). It is possible that in fission yeast phosphorylated Rad9 can recruit Cut5 for the activation of kinase Rad3 to initiate the checkpoint signaling by phosphorylating Mrc1 and other key targets in the replication checkpoint pathway. If this is true, it would be expected that phosphorylation of Mrc1 by Rad3 is dependent on the phosphorylation of Rad9. To see whether this is the case, we examined the phosphorylation of both Thr645 and Ser604 in Mrc1 in various Rad9 phosphorylation site mutants. To our surprise, Thr645 and Ser604 in Mrc1 could be efficiently phosphorylated in all Rad9 mutants even in a mutant in which the C terminus was deleted (Fig. 4A).
To see whether phosphorylation of Rad9 is dependent on the phosphorylation of Mrc1, Rad9-Thr412 phosphorylation was assayed in various Mrc1 phosphorylation site mutants. Similar to Mrc1 phosphorylation, phosphorylation of Rad9-Thr412 does not require the phosphorylation of Mrc1 as mutations in all Mrc1 SQ/TQ motifs did not have any effect on Rad9 phosphorylation (Fig. 4B). Furthermore, phosphorylation of Rad9-Thr412 occurred normally even in the absence of Mrc1, indicating that Rad9 phosphorylation is completely independent of Mrc1.
Taken together, the three essential Rad3-dependent phosphorylations on Mrc1 (SQ cluster and TQ motifs) and Rad9 are independent events. Phosphorylation of Rad9, which recruits Cut5 (15, 22, 47), is not required for the phosphorylation of Mrc1 by Rad3, suggesting that activation of the sensor kinase Rad3 is not dependent on the recruitment of Cut5 and may involve an unknown mechanism. However, as we show below, Rad9 phosphorylation (and probably the recruitment of Cut5 by phosphorylated Rad9) is important for activation of the effector kinase Cds1.
Phosphorylation Network Required for Cds1 Activation
Because the effector kinase Cds1 mediates most checkpoint functions in S. pombe, we then asked how the three independent phosphorylations affect the activation of Cds1. In response to replication stress, Cds1 is activated by two sequential phosphorylations at Thr11 and Thr328 (31). Phosphorylation of Thr11 in Cds1 by Rad3 is essential for activation of the effector kinase in vivo that primes Cds1 for autoactivation by trans-autophosphorylation of Thr328 in the kinase domain (31).
To assay the Cds1 phosphorylation, antibodies against phosphorylated Thr11 and Thr328 of Cds1 were generated (31). After the specificity of the antibody against phosphorylated Cds1-Thr11 was confirmed by immunoblottings (supplemental Figs. S2 and S6A), we went on to see how phosphorylation of this residue in Cds1 is affected by mutations in other checkpoint proteins. That phosphorylation of Cds1-Thr11 is dependent on Rad3 has been shown by another group (23) and is confirmed here using our own antibody for the complete picture of the phosphorylation network (supplemental Fig. S6B). Consistent with the Rad3-dependent phosphorylation, phosphorylation of Cds1-Thr11 also requires Rad26, the cofactor of Rad3, as depletion of Rad26 completely eliminated the phosphorylation (Fig. 5A). However, unlike Rad26 and Rad3, depletion of Mrc1 and subunits in the 9-1-1 complex did not completely eliminate the basal phosphorylation of Cds1-Thr11 (see the longer exposure in Fig. 5A). The minimal phosphorylation observed in these mutants is not due to a cross-reaction of the antibody as deletion of Rad26 or the T11A mutation (supplemental Fig. S6A) completely eliminated the binding of the antibody. This result clearly shows that the main function of Mrc1 and the 9-1-1 complex is to facilitate, but is not absolutely required for, the phosphorylation of Cds1-Thr11 by Rad3. Deletion of Crb2 had no effect on the phosphorylation of Cds1-Thr11 (Fig. 5A) as expected because it mediates Chk1 activation in response to DNA damage (15). The minimal phosphorylation of Cds1-Thr11 observed in the absence of HU treatment is consistent with the basal checkpoint activity under normal conditions as mentioned above.
FIGURE 5.
Phosphorylation of Mrc1 and Rad9 facilitates phosphorylation and activation of Cds1. A, Cds1 was tagged with HA at the C terminus and expressed at the cds1+ genomic locus in wild-type cells or cells containing the indicated mutations. After the cells were treated with HU, Rad3-dependent phosphorylation of Thr11 was analyzed in immunoprecipitated Cds1 by the phosphospecific antibody (top two panels). Short and Long indicate different exposure times of the same blot. Loading of Cds1 is shown in the bottom panel. B, wild-type or mutant Rad9 was expressed on plasmids under the control of the rad9+ promoter in cells expressing the HA-tagged Cds1. After HU treatment, phosphorylation of Cds1-Thr11 (top panel) was examined as in A. The levels of phosphorylation are shown in percentages below the top panel. The blot was stripped and then immunoblotted with the phosphoantibody to assess the phosphorylation of Cds1-Thr328 (middle panel), the direct marker for Cds1 activation. The kinase activity of Cds1 (bottom two panels) in wild-type or Rad9 mutant cells was measured using GST-Wee1 as the substrate (32) following the protocol described under “Experimental Procedures.” After SDS-PAGE, 32P incorporation (upper) in Wee1 protein (lower) was analyzed by phosphorimaging and is shown in percentages relative to HU-treated wild-type cells. C, phosphorylation of Cds1-Thr11 in various phosphomutants (mut) of Mrc1 was examined essentially as in A and B.
We then analyzed the Cds1 phosphorylation in various Rad9 and Mrc1 phosphorylation site mutants to see how the phosphorylation of the mediator and the 9-1-1 complex affects Cds1 activation. When Cds1 phosphorylation was examined in Rad9 mutants, we found that Rad9 phosphorylation had a profound effect on the phosphorylation and activation of Cds1 (Fig. 5B). Although the single mutation of Thr412 eliminated over 60% of the phosphorylation of Cds1-Thr11, double mutation of Thr412 and Ser423 or the C-terminal deletion mutation reduced the phosphorylation by about 75% (Fig. 5B, top panel). Because autophosphorylation of Thr328 is dependent on phosphorylated Thr11, the level of phosphorylated Thr328 correlates to Thr11 phosphorylation and was similarly affected by the Rad9 phosphorylation site mutations (Fig. 5B, second panel from the top). The kinase activities of Cds1 in various Rad9 mutants was measured by using GST-Wee1 as the substrate (32). As expected, mutations in Rad9 phosphorylation sites had a significantly negative effect on Cds1 activation (Fig. 5B, bottom two panels), similar to the phosphorylation observed in Cds1.
Phosphorylation of Mrc1 also has a dramatic effect on Cds1-Thr11 phosphorylation as the mutations in the TQ motifs or SQ cluster removed most of the phosphorylation (Fig. 5C). Although both Rad9 and Mrc1 phosphorylation can promote the phosphorylation and subsequent activation of Cds1, phosphorylation of Mrc1 appears to have a more dramatic impact. Mutation of the two redundant TQ motifs Thr645 and Thr653 or the SQ cluster on Mrc1 reduced Cds1 phosphorylation to almost the basal level, whereas the double mutation of T412A/S423A in Rad9 only eliminated about 75% of the phosphorylation. Mutations of Rad9(S423A) and Rad9(T225A) had a much smaller effect on the phosphorylation of Cds1-Thr11, consistent with the results from the drug sensitivity assay (Fig. 2C). Single mutation of the two redundant TQ motifs (T645A) or the SQ cluster (S604A) in Mrc1 also had a minimal effect on Cds1 phosphorylation as expected (Fig. 5C) because they are less sensitive to HU.
DISCUSSION
The sensor kinase Rad3 and the effector kinase Cds1 are two essential kinases in the replication checkpoint pathway of S. pombe. Previous studies showed that activation of Cds1 requires two sequential phosphorylations of this kinase; one is the Rad3-dependent phosphorylation of Thr11 in the SQ/TQ domain, and the other is the autophosphorylation of Thr328 in the kinase domain (31). Phosphorylation of the two sites in Cds1 has been confirmed by mutations, phosphospecific antibodies, two-dimensional phosphopeptide mapping, and MS (23, 31). In this study, we investigated the potential Rad3 phosphorylation in the sensor proteins, confirmed the phosphorylation of the SQ cluster and TQ motifs in Mrc1 and the C terminus of Rad9, and discovered that the three phosphorylations on Mrc1 and Rad9 are probably the only Rad3-dependent phosphorylations required for efficient phosphorylation and subsequent activation of Cds1. We also examined the relationships of the three phosphorylations with one another and with the phosphorylation of Cds1. Based on the results from this study and previous reports (13, 21–23), we present in Fig. 6 a diagram of the phosphorylation network essential for activation of the replication checkpoint pathway in S. pombe.
FIGURE 6.

Phosphorylation network for efficient activation of Cds1. The four Rad3-dependent phosphorylations (marked with letter P) occur in parallel. Thr11 in Cds1 is phosphorylated by Rad3 at a basal level in the absence of Mrc1, Rad9, or the phosphorylation of the two proteins. Phosphorylation of the SQ cluster and the TQ motifs in Mrc1 and Thr412 in Rad9 can all facilitate the phosphorylation of Cds1-Thr11 by Rad3. Although phosphorylation of the Mrc1 TQ motifs recruits Cds1 for its phosphorylation at Thr11 by Rad3, it remains unclear how the phosphorylated Mrc1 SQ cluster and Rad9-Thr412 promote the phosphorylation of Cds1-Thr11 (dashed arrows). However, phosphorylation of Thr11 is known to promote the trans-autophosphorylation of Thr328 in the kinase domain of Cds1 (31), which directly activates the enzyme, leading to the full activation of the replication checkpoint pathway in S. pombe.
Individual mutation in the large scale mutational analysis of all potential Rad3 phosphorylation sites in the sensor proteins identified four potential phosphorylation sites on Rad9 (Thr225, Thr423, and Thr412) and Hus1 (Thr47) that are important for checkpoint function. However, detailed mutational and immunoblotting analyses showed that Rad9-Thr225 and Hus1-Thr47 may not be true phosphorylation sites. Although Thr412-dependent phosphorylation of Thr225 in Rad9 remains possible (25, 39), our data imply that Rad9-Thr225, together with Hus1-Thr47, is more likely involved in the complex formation or other functions. Phosphorylation of Thr412 and Ser423 in the Rad9 C terminus has been observed before for Chk1 activation (22, 25). We show here that phosphorylation of the Rad9 C terminus is also important for the activation of Cds1. We also show that although the main function of phosphorylated Ser423 appears to be the promotion of Thr412 phosphorylation, phosphorylated Thr412 in the Rad9 C terminus probably mediates most of the function of the phosphorylated 9-1-1 complex.
An earlier report showed that in human cells two serines in the C terminus of Rad17 (Ser635 and Ser645) undergo ATR/ATM-dependent phosphorylation when DNA is damaged, and phosphorylation of the two residues is a critical early event for checkpoint signaling (48). Because redundant phosphorylation occurs in Mrc1 and individual mutations of Rad17 did not identify critical phosphorylation sites, it is possible that redundant phosphorylation exists in Rad17 as well as other sensor proteins in fission yeast. However, simultaneous mutation of all SQ/TQ motifs ruled out this possibility for Rad17, Rad26, Rad1, and Hus1, indicating that redundant phosphorylation of checkpoint sensors may not be conserved among eukaryotes. Individual mutations of Ser2, Thr737, and Ser1976 in Rad3 made the cells slightly sensitive to HU and MMS. However, simultaneous mutation of the three sites as well as the four sites in front of the kinase domain did not significantly increase the drug sensitivity. We believe that autophosphorylation of Rad3, if it occurs, is not essential for the checkpoint function. Briefly, we found no evidence for critical redundant phosphorylation in the checkpoint sensor proteins of S. pombe.
The three Rad3-dependent phosphorylations essential for efficient activation of Cds1, the SQ cluster and the two TQ motifs in Mrc1, and the Rad9 C terminus were found to occur in parallel (Fig. 6). Phosphorylation of the TQ motifs in Mrc1 has been suggested to recruit Cds1 to the stalled forks for the phosphorylation of Thr11 by Rad3 (13). However, it remains unclear why the phosphorylated Mrc1 SQ cluster and Rad9 C terminus are needed for efficient Rad3-dependent phosphorylation of Cds1. Unlike that in Rad9, the phosphorylated SQ cluster does not promote the phosphorylation of TQ motifs in Mrc1. Mrc1 is a typical intrinsically unstructured protein (49) that may bind to its targets before folding into a defined structure. Phosphorylation of the SQ cluster occurs in normal cell cycle, suggesting that the phosphorylation may facilitate proper assembly of Mrc1 into replisomes. Phosphorylation of the SQ cluster may also affect the interaction of Mrc1 with components of the replisome such as polymerase ϵ as described in Saccharomyces cerevisiae (50). Phosphorylation of the SQ cluster was increased after cells were treated with HU. It is also possible that the stress-specific phosphorylation of the SQ cluster can induce a local structural change in Mrc1 for efficient recruitment of Cds1 by the phosphorylated TQ motifs or directly facilitates Cds1 activation. Further mechanistic studies are needed to delineate the important function of the Mrc1 SQ cluster.
Phosphorylation of the Rad9 C terminus can recruit Cut5 and the mediator Crb2 for the DNA damage checkpoint response (15, 22, 34). However, unlike Rad9 mutants, deletion of Crb2 had little effect on the phosphorylation of Cds1. It is still unclear how phosphorylated Rad9-Thr412 promotes the activation of Cds1, but not Chk1, during a perturbed S phase. It is possible that the recruitment of Cut5 by phosphorylated Rad9, which may not be required for the activation of the kinase Rad3, can facilitate the recruitment of Cds1 by phosphorylated TQ motifs in Mrc1. Because the Rad9 C terminus and the Cds1-docking repeats in Mrc1 share some sequence similarities, it is also possible that phosphorylated Rad9 can directly promote the recruitment of Cds1 to the stalled forks. A previous report in which the kinase activity of Cds1 was measured by using myelin basic protein as the substrate suggested that mutation of Rad9-Thr412 did not have a significant effect on the activation of Cds1 (22). However, when the kinase activity of Cds1 was measured by the GST-Wee1 method (32), we found that the activity as well as the phosphorylation of Cds1 decreased dramatically in Rad9 mutants (Fig. 5B). A plausible explanation for this discrepancy is the different methods used in the experiments. Cds1 may undergo autoactivation when the local concentration is increased by immunoprecipitation. Although both methods are reliable, the GST-Wee1 method appears to be more sensitive in assaying a small change of Cds1 activity.
During the past decades, genetic studies, especially those in yeasts, have contributed greatly to our understanding of the checkpoint mechanism (51, 52). It is believed that most, if not all, of the essential proteins involved in the main checkpoint pathways have been discovered (53, 54). Recent studies in budding yeast showed that phosphorylation of Sld3 and Dbf4 by Rad53 (Cds1 in S. pombe) function redundantly in suppression of firing of late origins (55, 56). A central question remaining in the area is how the cell recognizes and transduces diverse noncanonical structures of DNA into a checkpoint response. Recent biochemical studies suggested that replication protein A-coated single strand DNA can efficiently stimulate ATR- or Mec1-initiated checkpoint signaling (28, 57–60). Because single strand DNA can be the intermediate of diverse DNA metabolisms, it is believed that single strand DNA is the common structure that is recognized by the checkpoint in response to stalled forks or other DNA lesions (60). However, if single strand DNA is the common structure for checkpoint signaling in S. pombe, it is unclear why it is recognized by Rad3 to activate Cds1 during S phase, whereas in other phases of the cell cycle, it activates Rad3 to initiate the Chk1-mediated DNA damage checkpoint response. This is an important question because different checkpoint responses to the cell cycle and the stimulated DNA repair activities have to be coordinated properly. Temporal regulation of checkpoint responses during the cell cycle is clearly important, but the mechanism remains less investigated. In vivo genetic analysis complemented with in vitro biochemical studies is a powerful means of dissecting the molecular details of a complex biological system. The clear picture of the phosphorylation network required for efficient activation of Cds1 we described here can serve as a roadmap for in vitro biochemical studies of the replication checkpoint pathway in fission yeast.
Supplementary Material
Acknowledgments
We thank T. Carr and K. Furuya for kindly sharing the phosphospecific antibodies, yeast strains, and unpublished data. We also thank M. Leffak, G. Q. Liu, and anonymous reviewers for critical reading of the manuscript and helpful comments. T. J. Kelly is gratefully acknowledged for advice and generous encouragement.
This work was supported in part by a grant from Ohio Cancer Research Associates, Inc.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S6.
- ATR
- ATM- and Rad3-related
- ATM
- ataxia telangiectasia mutated
- PCNA
- proliferating cell nuclear antigen
- HU
- hydroxyurea
- MMS
- methyl methanesulfonate.
REFERENCES
- 1. McGowan C. H., Russell P. (2004) Curr. Opin. Cell Biol. 16, 629–633 [DOI] [PubMed] [Google Scholar]
- 2. Ciccia A., Elledge S. J. (2010) Mol. Cell 40, 179–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tsang E., Carr A. M. (2008) DNA Repair 7, 1613–1623 [DOI] [PubMed] [Google Scholar]
- 4. Gorgoulis V. G., Vassiliou L. V., Karakaidos P., Zacharatos P., Kotsinas A., Liloglou T., Venere M., Ditullio R. A., Jr., Kastrinakis N. G., Levy B., Kletsas D., Yoneta A., Herlyn M., Kittas C., Halazonetis T. D. (2005) Nature 434, 907–913 [DOI] [PubMed] [Google Scholar]
- 5. Bartkova J., Horejsí Z., Koed K., Krämer A., Tort F., Zieger K., Guldberg P., Sehested M., Nesland J. M., Lukas C., Ørntoft T., Lukas J., Bartek J. (2005) Nature 434, 864–870 [DOI] [PubMed] [Google Scholar]
- 6. Bartek J., Lukas C., Lukas J. (2004) Nat. Rev. Mol. Cell Biol. 5, 792–804 [DOI] [PubMed] [Google Scholar]
- 7. Furuya K., Carr A. M. (2003) J. Cell Sci. 116, 3847–3848 [DOI] [PubMed] [Google Scholar]
- 8. Boddy M. N., Russell P. (2001) Curr. Biol. 11, R953–956 [DOI] [PubMed] [Google Scholar]
- 9. Bermudez V. P., Lindsey-Boltz L. A., Cesare A. J., Maniwa Y., Griffith J. D., Hurwitz J., Sancar A. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 1633–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ellison V., Stillman B. (2003) PLoS Biol. 1, E33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alcasabas A. A., Osborn A. J., Bachant J., Hu F., Werler P. J., Bousset K., Furuya K., Diffley J. F., Carr A. M., Elledge S. J. (2001) Nat. Cell Biol. 3, 958–965 [DOI] [PubMed] [Google Scholar]
- 12. Tanaka K., Russell P. (2001) Nat. Cell Biol. 3, 966–972 [DOI] [PubMed] [Google Scholar]
- 13. Xu Y. J., Davenport M., Kelly T. J. (2006) Genes Dev. 20, 990–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nakamura T. M., Du L. L., Redon C., Russell P. (2004) Mol. Cell. Biol. 24, 6215–6230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saka Y., Esashi F., Matsusaka T., Mochida S., Yanagida M. (1997) Genes Dev. 11, 3387–3400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nakamura T. M., Moser B. A., Russell P. (2002) Genetics 161, 1437–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matsuoka S., Ballif B. A., Smogorzewska A., McDonald E. R., 3rd, Hurov K. E., Luo J., Bakalarski C. E., Zhao Z., Solimini N., Lerenthal Y., Shiloh Y., Gygi S. P., Elledge S. J. (2007) Science 316, 1160–1166 [DOI] [PubMed] [Google Scholar]
- 18. Mu J. J., Wang Y., Luo H., Leng M., Zhang J., Yang T., Besusso D., Jung S. Y., Qin J. (2007) J. Biol. Chem. 282, 17330–17334 [DOI] [PubMed] [Google Scholar]
- 19. Stokes M. P., Rush J., Macneill J., Ren J. M., Sprott K., Nardone J., Yang V., Beausoleil S. A., Gygi S. P., Livingstone M., Zhang H., Polakiewicz R. D., Comb M. J. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 19855–19860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smolka M. B., Albuquerque C. P., Chen S. H., Zhou H. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 10364–10369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhao H., Tanaka K., Nogochi E., Nogochi C., Russell P. (2003) Mol. Cell. Biol. 23, 8395–8403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Furuya K., Poitelea M., Guo L., Caspari T., Carr A. M. (2004) Genes Dev. 18, 1154–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tanaka K., Boddy M. N., Chen X. B., McGowan C. H., Russell P. (2001) Mol. Cell. Biol. 21, 3398–3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tanaka K., Russell P. (2004) J. Biol. Chem. 279, 32079–32086 [DOI] [PubMed] [Google Scholar]
- 25. Furuya K., Miyabe I., Tsutsui Y., Paderi F., Kakusho N., Masai H., Niki H., Carr A. M. (2010) Mol. Cell 40, 606–618 [DOI] [PubMed] [Google Scholar]
- 26. Araki H., Leem S. H., Phongdara A., Sugino A. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 11791–11795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mordes D. A., Nam E. A., Cortez D. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 18730–18734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Navadgi-Patil V. M., Burgers P. M. (2008) J. Biol. Chem. 283, 35853–35859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kumagai A., Lee J., Yoo H. Y., Dunphy W. G. (2006) Cell 124, 943–955 [DOI] [PubMed] [Google Scholar]
- 30. Moreno S., Klar A., Nurse P. (1991) Methods Enzymol. 194, 795–823 [DOI] [PubMed] [Google Scholar]
- 31. Xu Y. J., Kelly T. J. (2009) J. Biol. Chem. 284, 16016–16027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boddy M. N., Furnari B., Mondesert O., Russell P. (1998) Science 280, 909–912 [DOI] [PubMed] [Google Scholar]
- 33. Kim S. T., Lim D. S., Canman C. E., Kastan M. B. (1999) J. Biol. Chem. 274, 37538–37543 [DOI] [PubMed] [Google Scholar]
- 34. Saka Y., Yanagida M. (1993) Cell 74, 383–393 [DOI] [PubMed] [Google Scholar]
- 35. Edwards R. J., Bentley N. J., Carr A. M. (1999) Nat. Cell Biol. 1, 393–398 [DOI] [PubMed] [Google Scholar]
- 36. Caspari T., Dahlen M., Kanter-Smoler G., Lindsay H. D., Hofmann K., Papadimitriou K., Sunnerhagen P., Carr A. M. (2000) Mol. Cell. Biol. 20, 1254–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee J. H., Paull T. T. (2004) Science 304, 93–96 [DOI] [PubMed] [Google Scholar]
- 38. Bakkenist C. J., Kastan M. B. (2003) Nature 421, 499–506 [DOI] [PubMed] [Google Scholar]
- 39. Kai M., Furuya K., Paderi F., Carr A. M., Wang T. S. (2007) Nat. Cell Biol. 9, 691–697 [DOI] [PubMed] [Google Scholar]
- 40. Sohn S. Y., Cho Y. (2009) J. Mol. Biol. 390, 490–502 [DOI] [PubMed] [Google Scholar]
- 41. Xu M., Bai L., Gong Y., Xie W., Hang H., Jiang T. (2009) J. Biol. Chem. 284, 20457–20461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Doré A. S., Kilkenny M. L., Rzechorzek N. J., Pearl L. H. (2009) Mol. Cell 34, 735–745 [DOI] [PubMed] [Google Scholar]
- 43. Delacroix S., Wagner J. M., Kobayashi M., Yamamoto K., Karnitz L. M. (2007) Genes Dev. 21, 1472–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Paciotti V., Lucchini G., Plevani P., Longhese M. P. (1998) EMBO J. 17, 4199–4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roos-Mattjus P., Hopkins K. M., Oestreich A. J., Vroman B. T., Johnson K. L., Naylor S., Lieberman H. B., Karnitz L. M. (2003) J. Biol. Chem. 278, 24428–24437 [DOI] [PubMed] [Google Scholar]
- 46. Lupardus P. J., Cimprich K. A. (2006) Mol. Biol. Cell 17, 1559–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mochida S., Esashi F., Aono N., Tamai K., O'Connell M. J., Yanagida M. (2004) EMBO J. 23, 418–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bao S., Tibbetts R. S., Brumbaugh K. M., Fang Y., Richardson D. A., Ali A., Chen S. M., Abraham R. T., Wang X. F. (2001) Nature 411, 969–974 [DOI] [PubMed] [Google Scholar]
- 49. Tompa P. (2002) Trends Biochem. Sci. 27, 527–533 [DOI] [PubMed] [Google Scholar]
- 50. Lou H., Komata M., Katou Y., Guan Z., Reis C. C., Budd M., Shirahige K., Campbell J. L. (2008) Mol. Cell 32, 106–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Weinert T. A., Hartwell L. H. (1988) Science 241, 317–322 [DOI] [PubMed] [Google Scholar]
- 52. Callegari A. J., Kelly T. J. (2007) Cell Cycle 6, 660–666 [DOI] [PubMed] [Google Scholar]
- 53. Nyberg K. A., Michelson R. J., Putnam C. W., Weinert T. A. (2002) Annu. Rev. Genet. 36, 617–656 [DOI] [PubMed] [Google Scholar]
- 54. Xu Y. J., Leffak M. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 13561–13562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lopez-Mosqueda J., Maas N. L., Jonsson Z. O., Defazio-Eli L. G., Wohlschlegel J., Toczyski D. P. (2010) Nature 467, 479–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zegerman P., Diffley J. F. (2010) Nature 467, 474–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Majka J., Niedziela-Majka A., Burgers P. M. (2006) Mol. Cell 24, 891–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Navadgi-Patil V. M., Burgers P. M. (2009) Mol. Cell 36, 743–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lindsey-Boltz L. A., Serçin O., Choi J. H., Sancar A. (2009) J. Biol. Chem. 284, 33107–33114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Choi J. H., Lindsey-Boltz L. A., Kemp M., Mason A. C., Wold M. S., Sancar A. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 13660–13665 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.