Abstract
NAADP is a potent second messenger that mobilizes Ca2+ from acidic organelles such as endosomes and lysosomes. The molecular basis for Ca2+ release by NAADP, however, is uncertain. TRP mucolipins (TRPMLs) and two-pore channels (TPCs) are Ca2+-permeable ion channels present within the endolysosomal system. Both have been proposed as targets for NAADP. In the present study, we probed possible physical and functional association of these ion channels. Exogenously expressed TRPML1 showed near complete colocalization with TPC2 and partial colocalization with TPC1. TRPML3 overlap with TPC2 was more modest. TRPML1 and to some extent TRPML3 co-immunoprecipitated with TPC2 but less so with TPC1. Current recording, however, showed that TPC1 and TPC2 did not affect the activity of wild-type TRPML1 or constitutively active TRPML1(V432P). N-terminally truncated TPC2 (TPC2delN), which is targeted to the plasma membrane, also failed to affect TRPML1 and TRPML1(V432P) channel function or TRPML1(V432P)-mediated Ca2+ influx. Whereas overexpression of TPCs enhanced NAADP-mediated Ca2+ signals, overexpression of TRPML1 did not, and the dominant negative TRPML1(D471K) was without affect on endogenous NAADP-mediated Ca2+ signals. Furthermore, the single channel properties of NAADP-activated TPC2delN were not affected by TRPML1. Finally, NAADP-evoked Ca2+ oscillations in pancreatic acinar cells were identical in wild-type and TRPML1−/− cells. We conclude that although TRPML1 and TPCs are present in the same complex, they function as two independent organellar ion channels and that TPCs, not TRPMLs, are the targets for NAADP.
Keywords: Calcium, Calcium Channels, Calcium Intracellular Release, Calcium Transport, TRP Channels, NAADP, TPC2
Introduction
Ca2+ plays a major role in the function of intracellular organelles including biosynthesis and membrane trafficking (1, 2). Although the mechanism controlling Ca2+ in the endoplasmic reticulum has been studied extensively, very little is known about Ca2+ homeostasis by other organelles. Furthermore, although accumulating evidence indicates that acidic organelles such as endosomes and lysosomes are dynamic Ca2+ stores, the molecular basis for Ca2+ release from these so-called “acidic Ca2+ stores” (3) is at present defined poorly.
By far, the best characterized route for mobilization of acidic Ca2+ stores is through the production of the potent Ca2+-releasing second messenger, nicotinic acid adenine dinucleotide phosphate (NAADP)5 (4). The Ca2+-mobilizing properties of NAADP were discovered in sea urchin eggs (5), in which NAADP was shown to mobilize Ca2+ not from the endoplasmic reticulum but instead from lysosome-related organelles (6). Its effects have subsequently been extended to a variety of cell types, including pancreatic acinar and β cells, smooth muscle cells, neurons, and breast cancer cells (4). NAADP is produced by several extracellular stimuli in an agonist-specific manner and implicated in a number of physiological responses, including fertilization, glucose sensing, exocytosis, and neuronal growth (4). Deregulated lysosomal Ca2+ signaling may also result in disease (2, 7, 8). Despite the physiological and potential pathophysiological importance of NAADP signaling, the molecular identity of the NAADP receptor is not entirely clear (9). Recently, members of the transient receptor potential mucolipin (TRPML) (10, 11) and two-pore channel (TPC) (12–16) families, which are all present in the endo/lysosomal system, have been proposed as candidates.
TRPMLs form a subfamily of the superfamily of the TRP channels. The founding member is TRPML1, which was identified as the protein mutated in the lysosomal storage disease mucolipidosis type IV (17). TRPML2 was found by database searches (18), and TRPML3 is mutated in the mouse to cause the varitint-waddler phenotype (18). The function of TRPMLs is known to a limited extent. TRPML1 is expressed largely in late endosomes and lysosomes (19–22), where it appears to regulate lysosomal pH (23–25). Deletion of TRPML1 in mammalian cells (24, 26, 27) and Caenorhabditis elegans (21, 28) indicate that TRPML1 is required for membrane trafficking. TRPML3 is expressed in multiple intracellular organelles and shuttles between the plasma membrane and these organelles (29). TRPML3 functions as Ca2+ channel (30–32) that is regulated by extracytoplasmic Na+ and pH (30, 31, 33) and by Ca2+ trapping within the TRPML3 pore (33). Like TRPML1, TRPML3 is also involved in membrane trafficking (29, 34) and regulates endocytosis and autophagy (29). The TRPML3(A419P) mutation in varitint-waddler mice is due to a gain of function resulting in increased extracellular Ca2+ influx and cell death (30, 35, 36) upon expansion of the TRPML3 pore (33).
All available clones of wild-type TRPML1 do not result in detectable spontaneously active current upon overexpression. Insertion of the gain-of-function mutation A419P in TRPML3 into the corresponding position in TRPML1(V432P), however, resulted in a constitutively active Ca2+-permeable channel (35, 36). TRPML1 was suggested as the NAADP receptor based on the findings that NAADP-activated channel activity in a lipid bilayer reconstituted with lysosomal membranes was inhibited by an anti-TRPML1 antibody (10, 11) by immunodepletion with a second anti-TRPML1 antibody or by treating the cells with TRPML1 siRNA prior to isolation of lysosomal membranes for reconstitution (11). These results, however, have yet to be independently verified and whether overexpression of TRPML1 potentiated NAADP responses was not tested.
Although TPCs were first identified in the rat, most is known about these channels in plants. In Arabidopsis, TPC1 localizes in the vacuole where it mediates the slow vacuolar current (37). Transgenic approaches have identified many physiological processes that are regulated by plant TPC1, including germination and stomatal movement (38). In animals, the TPC gene has undergone multiplication and divergence, giving rise to a family of three genes in most deuterostomes, including sea urchins, whereas, interestingly, only two isoforms (TPC1 and TPC2) are present in rats, mice, and humans (14, 16, 39). All TPC isoforms analyzed thus far localize to acidic organelles. TPC1 is expressed in endosomes, lysosomes (14, 16), and other yet undefined organelles, whereas TPC2 is expressed in late endosomes and lysosomes (14, 16). Importantly, overexpression of human TPC1 (16) or human/mouse TPC2 (13, 14, 40) potentiates NAADP-mediated Ca2+ increase. Similar results were obtained with sea urchin TPCs (40, 41). Knockdown of human TPC1 and overexpression of TPC1 mutated in a predicted pore region (L273P) inhibits endogenous NAADP response in SKBR3 cells (16). NAADP responses are also abolished in pancreatic β cells from mice lacking the TPC2 gene (14). These studies provide strong evidence that TPCs are NAADP-sensitive Ca2+ channels.
Multiple lines of evidence thus suggest that TPCs are essential for NAADP-mediated Ca2+ release, but the reported involvement of TRPML1 in the NAADP response (10, 11) raises the possibility that TRPMLs and TPCs may interact. Here, we examined this issue by comparing their subcellular distribution and ability to form protein complexes. We also used several established experimental paradigms for probing TRPML and TPC/NAADP function, including TRPML1−/− mice. We report that although TRPMLs and TPCs are present in the same organelles and physically interact with each other, they function as independent organellar ion channels. We find no evidence for TRPML1 in regulating NAADP responses.
MATERIALS AND METHODS
Constructs
N-terminally enhanced GFP (EGFP)-tagged human TRPML1 or TRPML3 were constructed in the pEGFPC1 vector (Clontech) as detailed previously (22, 30). The TRPML1(V432P) and TRPML1(D471K) mutants were prepared by site-directed mutagenesis as described previously (22). mCherry was inserted at the N terminus of TRPML1 or TRPML3 in pCMV-HA vector (Clontech) (22, 30). The lysosomal targeting motifs in the N terminus (amino acids 11–16) and C terminus (amino acids 573–578) of TRPML1 were truncated by subcloning using the primers: 5′-GCCCAAGCTTCGATGACCCCCAACCCCGGGTATGGGAC-3′ (HindIII site +ATG +nucleotides corresponding amino acids 17–24) and 5′-CCGGAATTCTACGAGGGGTCCCTTCCGCAGC-3′ (EcoRI site +stop codon +nucleotide corresponding amino acids 572–576). The PCR product was inserted between HindIII and EcoRI site of the pEGFPC1 vector. Human TPC1 and TPC2 tagged at their C termini with GFP or monomeric red fluorescent protein in pCS2+ were described previously (16). C-terminally GFP-tagged TPC2 lacking amino acids 3–25 (TPC2delN) was described in Ref. 42. pCS2+ constructs encoding for C-terminally Myc-tagged TPC1 and TPC2 were generated by replacing the GFP tag of the corresponding C-terminally GFP-tagged constructs with five Myc tags. The Myc tags were amplified by PCR using pCS2+ MT as the template: the forward primers, 5′-GAATTCATCCCATCGATTTAAAGCTATGG-3′ (for TPC1) and 5′-CTCGAGGCATCCCATCGATTTAAAGCTATGG-3′ (for TPC2) and a common reverse primer (5′-GCTTGGGCGACCTCACCTAATCTAGAGC-3′). The products were cloned at the EcoRI and XbaI or XhoI and XbaI sites of pCS2+ TPC1-GFP and pCS2+ TPC2-GFP, respectively. Myc-tagged SpARC2 was described previously (43). All constructs were confirmed by sequencing.
Cell Culture and Transfection
HEK293T cells and HeLa cells were maintained in DMEM and 10% fetal bovine serum (Thermo scientific, South Logan, UT). SKBR3 human breast carcinoma cells were maintained in McCoy's 5A modified media and 10% fetal bovine serum (Invitrogen). All media were supplemented with penicillin (100 units/ml)/streptomycin (100 μg/ml), and the cells were maintained in a water-saturated 5% CO2, 95% air atmosphere. Cells were transiently transfected using Lipofectamine 2000 (Invitrogen) and were used for experiments 24–36 h post-transfection. In the case of Ca2+ measurements with cells transfected with TRPML1(V432P), to reduce Ca2+ toxicity, the cells were maintained in media supplemented with 1.5 mm EGTA to reduce the medium-free Ca2+ concentration to 0.4 mm.
Confocal Microscopy
Transfected cells grown on glass coverslips were washed once in PBS and fixed in 4% paraformaldehyde for 15 min at room temperature. The fluorescence of EGFP, mCherry, or mRFP was acquired with Bio-Rad MRC 1000 or Olympus Fluoview FV1000 confocal microscopes with a ×40 objective in a sequential scan mode to avoid bleeding of the EGFP signal into the red channel. The overlap of vesicles expressing EGFP and mCherry was counted using the RGB2 co-localization plugin and the automated particle counting function of NIH ImageJ software. Overlap was calculated as the number of organelles expressing TRPML channels relative to organelles expressing TPC channels. SpARC2 expression was determined using an anti-Myc antibody as described previously (43).
Co-immunoprecipitation
HEK cells transfected with Myc-tagged TPCs and/or EGFP-tagged TRPMLs were washed once with chilled PBS. The cells were scraped in lysis buffer (350-μl/35-mm dish) composed of PBS, 10 mm sodium pyrophosphate, 50 mm NaF, 1 mm sodium orthovanadate, 1% Triton X-100, complete mini protease inhibitor mixture tablet (Roche Applied Science) and sonicated on ice. The homogenized suspension was centrifuged at 13,000 × g for 20 min at 4 °C to remove debris, and the supernatant was transferred to fresh microfuge tubes. A sample of the lysate comprising ∼5% of the total lysate used for immunoprecipitation was mixed with 4× Laemmli sample buffer with 5% β-mercaptoethanol, heated at 50 °C for 15 min, and used as inputs. For the co-immunoprecipitation, 100 μl of lysate was incubated with 1 μg mouse anti-c-Myc antibody (Santa Cruz Biotechnology, Santa Cruz, CA) or 2 μg of rabbit anti-GFP antibody (Invitrogen) for 1 h at 4 °C on a rocking platform. A protein G (for mouse antibody, GE Healthcare) or protein A (for rabbit antibody) Sepharose bead suspension (50 μl) was then added to the lysate and incubated overnight at 4 °C on a rocking platform. The complexes were collected by centrifugation at 270 × g for 30 s at 4 °C. After three washes with lysis buffer, immunoprecipitated proteins were eluted with 4× Laemmli sample buffer supplemented with 5% β-mercaptoethanol by heating to 50 °C for 15 min, analyzed by SDS-PAGE and subjected to Western blotting.
Current Recording
For HEK293T cells, transfected cells were plated on 35-mm Petri dishes on the day of experiment and incubated with culture media for at least 4 h to allow attachment to the dish. Current recordings were made using standard whole-cell current recordings. Patch clamp pipettes were pulled from glass capillaries (G120F-3, Warner Instruments, Hamden, CT) using a vertical puller (Model PP-830, Narishige, Tokyo, Japan) and had a resistance of 3–5 megohms when filled with the pipette solution. The tip of the pipette was heat-polished. A reference Ag-AgCl electrode was connected to the bath solution via an agar bridge filled with 3 m KCl. An Axopatch 200B patch clamp amplifier (Axon Instruments, Union City, CA) was used to measure the whole-cell currents. In whole-cell experiments, the amplifier was controlled by pCLAMP9 software (Clampex, Axon Instruments) to allow the delivery of voltage-step protocols with concomitant digitization of the current. The whole-cell currents were filtered at 1 kHz with an internal four-pole Bessel filter, sampled at 5 kHz, and stored directly to hard drive by Digidata 1322 (Axon Instruments). Expanded channel openings were filtered at 0.5 kHz. Amplitude histograms were determined for all openings except opening smaller than 700 microseconds. Current-voltage relationships were obtained by voltage ramps with command voltage from −100 to +100 mV over 400 ms (or 200 ms in some experiments) every 5 s from a holding potential of 0 mV. The capacitance transient current was compensated for using the Axopatch 200B amplifier. The cell capacitance was 10.0 ± 0.6 pF (n = 49). The average series resistance (Rs), which was 7.8 ± 0.4 megohms (n = 49), was not compensated for. The bath solution A contained (pH 7.4 with NaOH) 140 mm NaCl, 5 mm KCl, 10 mm HEPES, 10 mm glucose, 1 mm MgCl2, and 1 mm CaCl2. Divalent cation-free solution contained 140 mm NaCl, 5 mm KCl, 10 mm HEPES, and 0.5 mm EGTA. The pipette solution (pH 7.3 with CsOH) contained 120 mm cesium methanesulfonate, 10 mm HEPES, 10 mm EGTA, 5 mm MgCl2, and 5 mm ATP-Na2. All experiments were performed at room temperature.
Single channel recordings were performed in an excised inside-out patch configuration at 23 °C from HEK cells transiently transfected with TPC2delN essentially as detailed previously (42). In brief, both bath and pipette solutions contained 200 mm CsSO3CH3, 2 mm CsCl, and 10 mm HEPES (pH 7.2 with CsOH). The pipettes were made from borosilicate capillaries and coated with Sylgard (Dow Corning, Midland, MI). The average pipette resistance was 10 megohms. Single channel currents were recorded using an Axopatch 200B amplifier. The currents were low pass-filtered at 2 kHz and digitized at a sampling frequency of 10 KHz. Recordings and analyses were done using Clampfit software. For this, currents were analyzed using the 50% threshold crossing criterion. Openings briefer than 700 μs (twice the filter rise time) were excluded from the analysis. Because it is difficult to establish the number of active channels when Po (the single channel open probability) is low, we used NPo to report the channel activity.
For current recording in pancreatic acinar cells, single pancreatic acinar cells were freshly dissociated from the pancreas of wild type mice or TRPML1−/− mice as described previously (44). The TRPML1−/− mice were generated by replacement of exons 1, 2, and a portion of exon 3 with a tetracycline-responsive transcriptional activator that resulted in deletion of TRPML1. Deletion of TRPML1 was verified by PCR and Western blot, and the detailed generation of the mice are described elsewhere (45). Whole-cell Ca2+-activated Cl− current as a reporter of [Ca2+]i was recorded at a holding potential of −60 mV as detailed previously (44). Pipette solution contained 140 mm KCl, 1.13 mm MgCl2, 10 mm HEPES, 5 mm Na2ATP, and 0.2 mm EGTA, and 50 nm NAADP was added before use.
Measurement of [Ca2+]i
Transfected HEK293T cells were loaded with Fura-2/AM (12 μm) in bath solution with no added Ca2+ at 37 °C for 50 min to minimize cell toxicity. [Ca2+]i was measured at the 340/380 nm excitation ratio. The emitted light was collected by a digital camera with a cut-off filter at 510 nm and analyzed with Metafluor (Universal Imaging). After a 5-min recording, the bath solution was supplemented with 2 mm Ca2+. [Ca2+]i in SKBR3 cells was also measured using Fura-2 exactly as described in Ref. 16 for the microinjection experiments or in Ref. 8 for the NAADP-AM experiments. Unless otherwise indicated, all chemicals used were from Sigma Aldrich.
RESULTS AND DISCUSSION
Localization and Assembly of TRPML1, TRPML3, TPC1, and TPC2 Complexes
TRPMLs and TPCs both localize to the endolysosomal system (12, 22, 29). To determine their relative localization, we evaluated the co-localization of TRPML1 and TRPML3 with TPC1 and TPC2. mCherry-tagged TRPMLs and EGFP-tagged TPCs were co-expressed in HeLa cells (Fig. 1). Fig. 1, A and C, show that TRPML1 and TPC2 localize in the same organelles with TRPML1/TPC2 overlap of 90.2 ± 4.4%, (n = 16). TRPML3 also showed substantial co-localization with TPC2 with TRPML3/TPC2 overlap of 64.9 ± 6.0% (n = 16) (Fig. 1, B and C). Co-localization of TRPML1 and TRPML3 with TPC1, however, was more limited, with TRPML1/TPC1 overlap of 20.3 ± 3.1% (n = 15) and TRPML3/TPC1 overlap of and 33.9 ± 5.2% (n = 11) (Fig. 1). We verified that the TPCs do not affect expression pattern of TRPML1, and their expression pattern is not altered by TRPML1. This is shown in supplemental Fig. S1, in which Myc-tagged TPC1 and TPC2 had no effect on the lysosomal targeting of EGFP-TRPML1 reported by co-localization with LysoTracker. Similarly, Myc-tagged TRPML1 had no effect on the targeting of GFP-TPC2 and GFP-TPC1.
FIGURE 1.
Localization of TRPMLs and TPCs. A and B, confocal images showing expression of mCherry-tagged TRPML1 (A) or TRPML3 (B) and GFP-tagged TPC1 (a) or TPC2 (b) in HeLa cells. Red and green images were acquired sequentially. C, the % overlaps (mean ± S.E.) were calculated as organelles expressing TRPML1 or TRPML3 relative to the total organelles expressing TPC1 or TPC2. In C, a–d summarize the combinations indicated in the figure.
We also examined the co-localization in HEK and SKBR3 cells. The images in Fig. 2 show the co-localization of TRPML1 and TRPML3 with TPC1 and TPC2 in HEK cells and those in Fig. 4 below in SKBR3 cells. The localization is similar in all cell types examined, indicating that targeting of the channels is likely similar in all cells.
FIGURE 2.
co-IP of TRPMLs and TPCs. A–D, confocal images showing expression of mCherry-tagged TRPML1 (A and B) or TRPML3 (C and D) and GFP-tagged TPC1 (A and C) or TPC2 (B and D) in HEK293T. E and F, cells were transfected with empty vectors (controls), EGFP-tagged TRPML1, or TRPML3 and Myc-tagged TPC1 or TPC2. Cell lysates were immunoprecipitated with either anti-Myc (IP: myc shown in E) or anti-GFP (IP: GFP shown in F) and were probed with anti-GFP (E) or anti-Myc antibodies (F) to detect TRPML1/TRPML3 or TPC1/2, respectively. The migration of full length (ML1) and cleaved (*ML1) TRPML1 is highlighted. An overexposed version of the blots in F is provided in supplemental Fig. 2A to show the modest co-IP of TPC1 with TRPML1 and TRPML3.
FIGURE 4.
TRPML1 does not affect NAADP-mediated Ca2+ signals in SKBR3 cells. A and B, SKBR3 cells were transfected with TRPML1 (red) and either TPC1 (A) or TPC2 (B) to show the partial (A) and extensive (B) co-localization of the channels. C–F, cytosolic Ca2+ responses of individual Fura-2 loaded SKBR3 cells stimulated with NAADP. C, responses of mock-transfected cells or cells expressing TRPML1 upon microinjection of 10 nm NAADP (pipette concentration). The inset shows the response of cells expressing TPC2. D and E, responses of mock-transfected cells or cells expressing TRPML1, TPC1, or TPC2 and stimulated with a cell-permeable NAADP analog (NAADP-AM, 1 μm). 20 randomly selected cells from a typical field of view (∼50 cells) are shown in each panel. F, responses of mock-transfected cells or cells expressing the channel dead mutant TRPML1(D471K) upon microinjection of 10 μm NAADP (pipette concentration). Summary data are presented as mean ± S.E. Where appropriate, only cells that expressed the protein of interest as judged by fluorescence of fused GFP were injected with NAADP.
The co-expression analysis was extended by demonstrating that TPCs and TRPMLs co-immunoprecipitate. Fig. 2 shows the mutual co-immunoprecipitation (co-IP) of TPC2 with TRPML1 and TRPML3. TPC1 also showed modest co-IP with TRPML1 and TRPML3 (Fig. 2). The co-IP of TRPML3 with the TPCs can be seen better in supplemental Fig. 2A, which shows longer exposure of the blots in Fig. 2F. TRPML1 is cleaved in the lysosome by cathepsin B at Arg-200, in the large loop between transmembranes 1 and 2 located in the lysosome lumen. The cleavage inactivates the channel and may function to regulate the duration of channel activity once in the lysosome (22). Both expressed and native TRPML1 undergo cleavage in the lysosome (22). Interestingly, the cleaved TRPML1 fragment (the lowest band ∼58 kDa marked as *ML1) showed minimal co-precipitation with TPC1 (Fig. 2E, top blot). This may indicate that either TPC1 interacts with the nontagged C-terminal of TRPML1 or that TPC1 interacts with TRPML1 in late endosomes before TRPML1 is trafficked and cleaved in the lysosome. This raises the possibility that TRPML1 activity is somewhat different when in late endosomes and lysosomes.
TPCs Do Not Affect TRPML1 Function
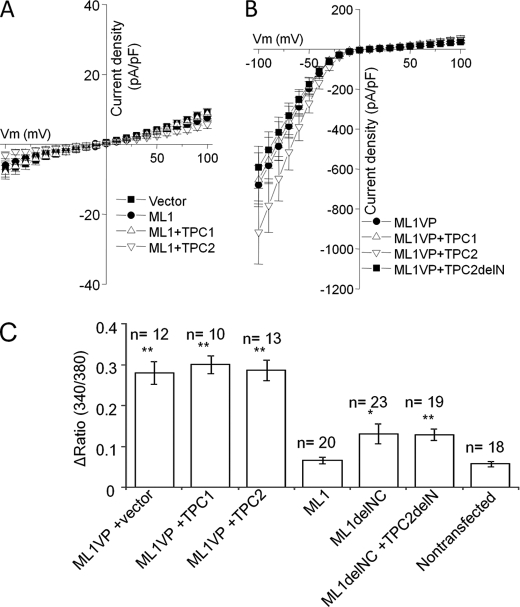
To determine the potential functional consequences of the co-localization of TRPML1 and TPC1 and TPC2, we measured the whole-cell currents in HEK293T cells expressing wild-type TRPML1 and TPC1 or TPC2. Cells expressing wild-type TRPML1 showed no current above that measured in nontransfected cells (Fig. 3A). Co-expressing TRPML1 and TPC1 or TPC2 gave similar results (Fig. 3A). To further examine the potential effect of the TPCs on TRPML1, we measured the whole-cell current mediated by the gain-of-function mutant TRPML1(V432P). TRPML1(V432P) mediates robust inward rectifying monovalent cation currents. The current mediated by TRPML1(V432P) was not affected by co-expression of TPC1 or TPC2 (Fig. 3B). Similarly, the TRPML1(V432P) Ca2+ currents measured at extracellular (105 mm) Ca2+ was also not affected by the TPCs (data not shown). Thus, the TPCs do not appear to regulate TRPML1 activity.
FIGURE 3.
TPC1 and TPC2 do not affect TRPML1 function. A and B, whole-cell current density evoked by ramp pulse protocol from −100 mV to 100 mV is shown at 10-mV intervals. HEK293T cells transfected with the indicated channels were perfused with divalent cation free NaCl-rich solution. A, cells were transfected with wild-type TRPML1 alone (●) or with TPC1 (△) or TPC2 (▿). B, cells were transfected with TRPML1(V432P) alone (●) or with TPC1 (△), TPC2 (▿), or the N-terminal truncated TPC2 (TPC2delN, ■). A (n = 3–7) and B (n = 7–8) show the mean ± S.E. C, the Ca2+ influx (340/380 ratio) elicited in cells expressing the indicated channels and bathed in Ca2+-free media when exposed to a solution containing 2 mm Ca2+. The difference of the mean between the group of TRPML1 and the other conditions were analyzed by Student's t test or Welch's test. *, p < 0.05; **, p < 0.01. ML1VP, TRPML1(V432P); ML1, TRPML1; ML1delNC, TRPML1 mutant with truncated N and C termini; TPC2delN, TPC2 mutant with truncated N terminus-targeting motif.
Overexpressed TRPML1 was shown to be targeted in part to the plasma membrane (22). However, it is possible that the TPCs are not targeted to the plasma membrane even when overexpressed, thereby explaining the lack of affect on TRPML activity. The N terminus of TPC2 contains a lysosomal targeting di-leucine motif (42). In a recent study, we showed that N-terminally truncated TPC2 (TPC2delN) is targeted to the plasma membrane. This is confirmed in supplemental Fig. S2B showing overlap of TPC2delN with a plasma membrane marker. The whole-cell current obtained from cells expressing TRPML1(V432P) and TPC2delN, however, was similar to the current observed with TRPML1(V432P) alone (Fig. 3B). Similar results were obtained with wild-type TRPML1 and TPC2delN (data not shown). These data further suggest that TRPML1 activity is not regulated by TPCs.
To evaluate TRPML1 function under more physiological conditions, we measured intracellular Ca2+ concentration ([Ca2+]i) with Fura-2 and evaluated Ca2+ influx across the plasma membrane. Ca2+ influx through TRPML1(V432P) was not affected by co-expression of TPC1 or TPC2 (Fig. 3C). Because Ca2+ influx was not observed in cells expressing wild type TRPML1 (Fig. 3C), we attempted to increase the expression of TRPML1 at the plasma membrane by using a plasma membrane targeting mutant of TRPML1, in which both the N and C terminus lysosomal targeting motifs were truncated (TRPML1delNC) (20). We confirmed the plasma membrane targeting of TRPML1delNC (supplemental Fig. S2B) and its co-localization with TPC2delN (supplemental Fig. S2C). Overexpression of TRPML1delNC induced slight but significant increase in [Ca2+]i influx (Fig. 3C). However, co-expression of TPC2delN with TRPML1delNC did not affect Ca2+ influx induced by TRPML1delNC (Fig. 3C). The [Ca2+]i measurements confirm the results obtained with the current measurements, suggesting that the TPCs do not regulate TRPML1.
TPCs, but Not TRPML1, Mediate NAADP-mediated Ca2+ Release
In a converse set of experiments, we examined the potential role of TRPML1 in regulating NAADP-mediated Ca2+ signals in SKBR3 cells in which we have previously characterized the function of TPCs (16, 40). Fig. 4, A and B, shows the partial and marked colocalization, respectively, of TPC1 and TPC2 with TRPML1. Microinjection of a low concentration of NAADP failed to evoke a significant Ca2+ signal in mock-transfected cells (Fig. 4C). Similar results were obtained in cells overexpressing TRPML1 (Fig. 4C). In contrast, robust responses were recorded from parallel experiments using cells expressing TPC1 (data not shown) or TPC2 (Fig. 4C, inset) consistent with our previous analyses (16, 40).
We also performed experiments using cell-permeable NAADP-AM (46) to affect intracellular delivery of NAADP. Stimulation of mock-transfected cells with 1 μm NAADP-AM evoked only modest Ca2+ signals (Fig. 4D). Again, as with the microinjection experiments, no response was recorded in similar experiments using cells expressing TRPML1 (Fig. 4D). However, cells expressing TPC1 or TPC2 responded robustly (Fig. 4E). That similar results were obtained by microinjection and NAADP-AM is inconsistent with the suggestion that enhanced responses to NAADP in TPC-expressing cells using the former method is due to heightened mechanosensitivity (41). Taken together, these data suggest that in contrast to TPCs, TRPML1 is not sufficient to confer NAADP sensitivity.
Endogenous NAADP-sensitive Ca2+ channels in SKBR3 cells can be readily activated by microninjection with higher concentrations of NAADP (10 μm) (16). These signals are blocked by overexpression of inactive TPC1 mutated within a putative pore consistent with the mutant acting in a dominant negative manner (16). To further probe the potential role of TRPML1 in mediating endogenous NAADP responses in SKBR3 cells, we examined the effects of TRPML(D471K), a channel-dead, dominant negative inhibitor of TRPML1 (35). As shown in Fig. 4F, NAADP-mediated Ca2+ signals were similar in mock-transfected cells and cells overexpressing TRPML(D471K). These data suggest that NAADP responses in these cells do not require TRPML1.
We also recorded single channel activity of TRPML1 and TPC2 in response to NAADP (Fig. 5). For these experiments, we expressed plasma membrane-targeted TRPML1delNC and TPC2delN and recorded currents in excised inside-out patches. NAADP did not induce single channel activity in patches excised from cells transfected with either GFP (Fig. 5A) or TRPML1delNC (Fig. 5B). In marked contrast, NAADP induced robust single channel activity in cells expressing TPC2delN without (Fig. 5C) or with TRPML1delNC (Fig. 5D) with similar latency time of 106 ± 29 s for TPC2dN and 95 ± 16 s for TPC2dn+ML1dNC (n = 4). Notably, co-expression of TRPML1delNC had no effect on the conductance (Fig. 5E) or NPo (Fig. 5F) of TPC2delN, further negating a role for TRPML1 in NAADP action.
FIGURE 5.
TRPML1 does not affect NAADP-mediated channel activity of TPC2. Single channel currents are from excised inside-out patches from the plasma membrane of HEK cells expressing GFP (A), TRPML1delNC (B), TPC2delN (C), or TPC2delN+TRPML1delNC (D) at a holding potential of −60 mV. Where indicated, the bath solution was supplemented with 500 nm NAADP. Traces of single or multiple channel openings are shown with expanded time course for the periods marked with 1 and 2 in the main traces. The whole-point histograms show a main channel conductance of 5 pA of TPC2 in the presence and absence of TRPML1. The bar graphs show the average TPC2 Cs+ conductance (E) and NPo (F) in the presence and absence of TRPML1. Results are means ± S.E. with the number of experiments (n) shown above each bar. NPo were calculated at a −40 mV holding potential.
NAADP Responses Are Normal in TRPML1−/− Pancreatic Acinar Cells
Endogenous NAADP responses have been extensively characterized in pancreatic acinar cells (47). In these cells, NAADP-induced [Ca2+]i oscillations can be readily evaluated by measuring whole-cell Ca2+-activated Cl− current with a pipette solution containing NAADP (48). As a final test to determine the role of TRPML1 in NAADP signaling, we compared NAADP responses in freshly dissociated pancreatic acinar cells from wild type and TRPML1−/− mice (Fig. 6). The results shows that NAADP-induced [Ca2+]i oscillations are not affected upon deletion of TRPML1. These data provide further evidence that TRPML1 is not necessary for NAADP responses.
FIGURE 6.
Knock-out of TRPML1 has no effect on NAADP responses in pancreatic acinar cells. Pancreatic acinar cells were acutely isolated from the pancreas of wild type (A) or TRPML1−/− mice (B). Whole-cell current was measured at a holding membrane potential of −60 mV. NAADP response was initiated by including 50 nm NAADP in the pipette solution. As controls, the cells were stimulated with 0.1 mm carbachol at the end of each experiment. Similar results were observed in 13 control and six TRPML1−/− cells from three wild-type and two TRPML1−/− animals. In C, the amplitude of the current oscillations were averaged within each experiment and then among experiments, and D shows the average oscillation frequency. The results are the mean ± S.E., and there is no significant difference between wild-type and TRPML1−/− cells in either C or D.
CONCLUSIONS
The present results lead to three conclusions. First, TRPMLs and TPCs are present within a protein complex. Second, TPCs do not affect TRPML1 activity; and third, TRPML1 is not necessary for the NAADP response. The latter finding is particularly significant because the exact molecular nature of the NAADP receptor has been somewhat ambiguous (9). Our findings, including those using pancreatic acinar cells from TRPML1−/− mice (Fig. 6), provide firm evidence that TRPML1 is not a target for NAADP and support TPCs as NAADP-activated channels. Indeed, our conclusions are consistent with three recent studies reporting channel activity for TPC2 from isolated lysosomes, immunopurified TPC2 fused into lipid bilayers, or in cells expressing plasma membrane targeted TPC2 (42, 49, 50). Importantly, in two of these studies, currents were eliminated or modified by mutations in the putative channel pore (42, 50).
Assembly of TRPMLs and the TPCs into complexes in intracellular organelles is clearly specific, as evident from isoform specific differences in co-IP (Fig. 2), mirroring their differential localization (Figs. 1, 2, and 4). Moreover, N-terminally cleaved TRPML1 shows minimal co-IP with TPC1 (Fig. 2). The functional significance, if any, of complex assembly, however, is not known at present but may in part explain why in a previous study, NAADP-mediated channel activity was reduced following immunoprecipitation of lysosomal preparations with a TRPML1 antibody (11). We note that, unlike TPCs, evidence that TRPML1 functions as a Ca2+ channel in the lysosomes or to participate in Ca2+ release from other intracellular organelles is limited. However, TRPML1 has been shown to regulate lysosomal pH (23–25). Based on the available evidence, we thus suggest that TRPMLs and the TPCs may have different functions within the endo/lysosomal system. TRPML1 may primarily control lysosomal pH, whereas TPCs likely mediate Ca2+ release from endosomes and lysosomes in response to NAADP. In this respect, it is of interest that TPC2 is sensitive to pH, with activation or functional modification by acidic pH (49, 50). Thus, it would have been expected that through regulation of lysosomal pH TRPML1 may regulate the function of the TPCs and thus lysosomal Ca2+ content and Ca2+ release. That we could not detect such a relationship may indicate that the range of changes in lysosomal pH by TRPML1 is not sufficient to affect TPC activity. However, further work is needed to determine a more subtle relationship between the two channels in lysosomal function.
Supplementary Material
Acknowledgments
We thank Dr. Grant Churchill (Oxford University) for kindly supplying the NAADP-AM and Chi Li for useful discussions.
This work was supported by National Institutes of Health Grants DE12309 and DK38938 and the intramural NIDCR/DIR (Grant ZIA DE000735-01), National Institutes of Health (to S. M.) and HL90804 (to E. B.). This work was also supported by Biotechnology and Biological Sciences Research Council Grant BB/G013721/1 (to S. P.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- NAADP
- nicotinic acid adenine dinucleotide phosphate
- TPC
- two-pore channel
- TRPML1
- transient receptor potential mucolipin 1
- [Ca2+]i
- free cytoplasmic Ca2+
- EGFP
- enhanced GFP
- co-IP
- co-immunoprecipitation.
REFERENCES
- 1. Berridge M. J., Bootman M. D., Roderick H. L. (2003) Nat. Rev. Mol. Cell Biol. 4, 517–529 [DOI] [PubMed] [Google Scholar]
- 2. Kiselyov K., Yamaguchi S., Lyons C. W., Muallem S. (2010) Cell Calcium 47, 103–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patel S., Docampo R. (2010) Trends Cell Biol. 20, 277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guse A. H., Lee H. C. (2008) Sci. Signal 1, re10 [DOI] [PubMed] [Google Scholar]
- 5. Lee H. C., Aarhus R. (1995) J. Biol. Chem. 270, 2152–2157 [DOI] [PubMed] [Google Scholar]
- 6. Churchill G. C., Okada Y., Thomas J. M., Genazzani A. A., Patel S., Galione A. (2002) Cell 111, 703–708 [DOI] [PubMed] [Google Scholar]
- 7. Lloyd-Evans E., Morgan A. J., He X., Smith D. A., Elliot-Smith E., Sillence D. J., Churchill G. C., Schuchman E. H., Galione A., Platt F. M. (2008) Nat. Med. 14, 1247–1255 [DOI] [PubMed] [Google Scholar]
- 8. Dickinson G. D., Churchill G. C., Brailoiu E., Patel S. (2010) J. Biol. Chem. 285, 13321–13325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guse A. H. (2009) Curr. Biol. 19, R521–523 [DOI] [PubMed] [Google Scholar]
- 10. Zhang F., Li P. L. (2007) J. Biol. Chem. 282, 25259–25269 [DOI] [PubMed] [Google Scholar]
- 11. Zhang F., Jin S., Yi F., Li P. L. (2009) J. Cell Mol. Med. 13, 3174–3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu M. X., Ma J., Parrington J., Galione A., Evans A. M. (2010) FEBS Lett. 584, 1966–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zong X., Schieder M., Cuny H., Fenske S., Gruner C., Rötzer K., Griesbeck O., Harz H., Biel M., Wahl-Schott C. (2009) Pflugers Arch. 458, 891–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Calcraft P. J., Ruas M., Pan Z., Cheng X., Arredouani A., Hao X., Tang J., Rietdorf K., Teboul L., Chuang K. T., Lin P., Xiao R., Wang C., Zhu Y., Lin Y., Wyatt C. N., Parrington J., Ma J., Evans A. M., Galione A., Zhu M. X. (2009) Nature 459, 596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patel S., Marchant J. S., Brailoiu E. (2010) Cell Calcium 47, 480–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brailoiu E., Churamani D., Cai X., Schrlau M. G., Brailoiu G. C., Gao X., Hooper R., Boulware M. J., Dun N. J., Marchant J. S., Patel S. (2009) J. Cell Biol. 186, 201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bargal R., Avidan N., Ben-Asher E., Olender Z., Zeigler M., Frumkin A., Raas-Rothschild A., Glusman G., Lancet D., Bach G. (2000) Nat. Genet. 26, 118–123 [DOI] [PubMed] [Google Scholar]
- 18. Di Palma F., Belyantseva I. A., Kim H. J., Vogt T. F., Kachar B., Noben-Trauth K. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 14994–14999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thompson E. G., Schaheen L., Dang H., Fares H. (2007) BMC Cell Biol. 8, 54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vergarajauregui S., Puertollano R. (2006) Traffic 7, 337–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fares H., Greenwald I. (2001) Nat. Genet. 28, 64–68 [DOI] [PubMed] [Google Scholar]
- 22. Kiselyov K., Chen J., Rbaibi Y., Oberdick D., Tjon-Kon-Sang S., Shcheynikov N., Muallem S., Soyombo A. (2005) J. Biol. Chem. 280, 43218–43223 [DOI] [PubMed] [Google Scholar]
- 23. Miedel M. T., Rbaibi Y., Guerriero C. J., Colletti G., Weixel K. M., Weisz O. A., Kiselyov K. (2008) J. Exp. Med. 205, 1477–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Soyombo A. A., Tjon-Kon-Sang S., Rbaibi Y., Bashllari E., Bisceglia J., Muallem S., Kiselyov K. (2006) J. Biol. Chem. 281, 7294–7301 [DOI] [PubMed] [Google Scholar]
- 25. Venkatachalam K., Long A. A., Elsaesser R., Nikolaeva D., Broadie K., Montell C. (2008) Cell 135, 838–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen C. S., Bach G., Pagano R. E. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 6373–6378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pryor P. R., Reimann F., Gribble F. M., Luzio J. P. (2006) Traffic. 7, 1388–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Treusch S., Knuth S., Slaugenhaupt S. A., Goldin E., Grant B. D., Fares H. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 4483–4488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim H. J., Soyombo A. A., Tjon-Kon-Sang S., So I., Muallem S. (2009) Traffic 10, 1157–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim H. J., Li Q., Tjon-Kon-Sang S., So I., Kiselyov K., Muallem S. (2007) J. Biol. Chem. 282, 36138–36142 [DOI] [PubMed] [Google Scholar]
- 31. Kim H. J., Li Q., Tjon-Kon-Sang S., So I., Kiselyov K., Soyombo A. A., Muallem S. (2008) EMBO J. 27, 1197–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grimm C., Jors S., Saldanha S. A., Obukhov A. G., Pan B., Oshima K., Cuajungco M. P., Chase P., Hodder P., Heller S. (2010) Chem. Biol. 17, 135–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim H. J., Yamaguchi S., Li Q., So I., Muallem S. (2010) J. Biol. Chem. 285, 16513–16520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martina J. A., Lelouvier B., Puertollano R. (2009) Traffic 10, 1143–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu H., Delling M., Li L., Dong X., Clapham D. E. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 18321–18326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grimm C., Cuajungco M. P., van Aken A. F., Schnee M., Jörs S., Kros C. J., Ricci A. J., Heller S. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 19583–19588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peiter E., Maathuis F. J., Mills L. N., Knight H., Pelloux J., Hetherington A. M., Sanders D. (2005) Nature 434, 404–408 [DOI] [PubMed] [Google Scholar]
- 38. Pottosin II, Schönknecht G. (2007) J. Exp. Bot. 58, 1559–1569 [DOI] [PubMed] [Google Scholar]
- 39. Cai X., Patel S. (2010) Mol. Biol. Evol. 27, 2352–2359 [DOI] [PubMed] [Google Scholar]
- 40. Brailoiu E., Hooper R., Cai X., Brailoiu G. C., Keebler M. V., Dun N. J., Marchant J. S., Patel S. (2010) J. Biol. Chem. 285, 2897–2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ruas M., Rietdorf K., Arredouani A., Davis L. C., Lloyd-Evans E., Koegel H., Funnell T. M., Morgan A. J., Ward J. A., Watanabe K., Cheng X., Churchill G. C., Zhu M. X., Platt F. M., Wessel G. M., Parrington J., Galione A. (2010) Curr. Biol. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brailoiu E., Rahman T., Churamani D., Prole D. L., Brailoiu G. C., Hooper R., Taylor C. W., Patel S. (2010) J. Biol. Chem. 285, 38511–38516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Churamani D., Boulware M. J., Ramakrishnan L., Geach T. J., Martin A. C., Vacquier V. D., Marchant J. S., Dale L., Patel S. (2008) Cell Signal 20, 2347–2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim J. Y., Zeng W., Kiselyov K., Yuan J. P., Dehoff M. H., Mikoshiba K., Worley P. F., Muallem S. (2006) J. Biol. Chem. 281, 32540–32549 [DOI] [PubMed] [Google Scholar]
- 45. Chandra M., Zhou H., Li Q., Muallem S., Hofmann S. L., Soyombo A. A. (2011) Gastroenterology 140, 857–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Parkesh R., Lewis A. M., Aley P. K., Arredouani A., Rossi S., Tavares R., Vasudevan S. R., Rosen D., Galione A., Dowden J., Churchill G. C. (2008) Cell Calcium 43, 531–538 [DOI] [PubMed] [Google Scholar]
- 47. Petersen O. H., Tepikin A. V. (2008) Annu. Rev. Physiol. 70, 273–299 [DOI] [PubMed] [Google Scholar]
- 48. Cancela J. M., Gerasimenko O. V., Gerasimenko J. V., Tepikin A. V., Petersen O. H. (2000) EMBO J. 19, 2549–2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pitt S. J., Funnell T. M., Sitsapesan M., Venturi E., Rietdorf K., Ruas M., Ganesan A., Gosain R., Churchill G. C., Zhu M. X., Parrington J., Galione A., Sitsapesan R. (2010) J. Biol. Chem. 285, 35039–35046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schieder M., Rötzer K., Brüggemann A., Biel M., Wahl-Schott C. A. (2010) J. Biol. Chem. 285, 21219–21222 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.