Abstract
To define a role for phospholipase Cϵ (PLCϵ) signaling in cardiac myocyte hypertrophic growth, PLCϵ protein was depleted from neonatal rat ventricular myocytes (NRVMs) using siRNA. NRVMs with PLCϵ depletion were stimulated with endothelin (ET-1), norepinephrine, insulin-like growth factor-1 (IGF-1), or isoproterenol and assessed for development of hypertrophy. PLCϵ depletion dramatically reduced hypertrophic growth and gene expression induced by all agonists tested. PLCϵ catalytic activity was required for hypertrophy development, yet PLCϵ depletion did not reduce global agonist-stimulated inositol phosphate production, suggesting a requirement for localized PLC activity. PLCϵ was found to be scaffolded to a muscle-specific A kinase anchoring protein (mAKAPβ) in heart and NRVMs, and mAKAPβ localizes to the nuclear envelope in NRVMs. PLCϵ-mAKAP interaction domains were defined and overexpressed to disrupt endogenous mAKAPβ-PLCϵ complexes in NRVMs, resulting in significantly reduced ET-1-dependent NRVM hypertrophy. We propose that PLCϵ integrates multiple upstream signaling pathways to generate local signals at the nucleus that regulate hypertrophy.
Keywords: Adaptor Proteins, Adrenergic Receptor, Cardiac Hypertrophy, G Protein-coupled Receptors (GPCR), Phosphatidylinositol Signaling, Phospholipase C, Signal Transduction
Introduction
Pathologic hypertrophic growth of the myocardium, a process associated with the development of heart failure, occurs in response to various stimuli including pressure overload and chronic increases in circulating hormones that place long term stress on the heart. Many of these hormones act on receptors in cardiac myocytes including adrenergic and endothelin receptors to initiate hypertrophic signaling cascades (1–3). The exact participants in these hypertrophic signaling cascades have been the subject of intense investigation. One common signaling mechanism closely coupled to receptor activation is activation of phosphatidylinositol-specific phospholipase C (PLC)3 (4). A role for PLC activity in regulation of hypertrophic signaling has been suggested because Gq, a potent stimulator of hypertrophy (5), directly stimulates PLCβ as a major regulatory pathway (6). Additionally, growth factors such as insulin-like growth factor-1 (IGF-1) that stimulate physiological hypertrophy activate PLCγ (7, 8). Recently, it has been reported that a splice variant of PLCβ, PLCβ1b, is required for hypertrophy downstream of α1-adrenergic receptor stimulation and suggested to be a major mediator of Gq-dependent cardiac hypertrophy (9).
The PLC family consists of 12 isoforms regulated by diverse upstream signal inputs, all of which hydrolyze phosphatidylinositol 4,5-bisphosphate to diacylglycerol (DAG) and inositol trisphosphate (IP3) (10, 11). Although containing a common PLC catalytic core domain, PLCβ, PLCγ, PLCδ, and PLCϵ isoforms are very different in terms of overall domain structure and regulatory mechanisms, indicating that they are unlikely to play overlapping roles in the cells. PLCϵ is unique in that it is directly regulated by various small GTPases, including Ras, Rho, and Rap (12–16), and Gβγ subunits, but not Gαq. PLCϵ has been shown to signal downstream of G protein-coupled receptors, including β-adrenergic receptors (βARs), lysophosphatidic acid and sphingosine 1-phophate receptors (EdgRs), protease-activated receptors (13, 17), and growth factor receptor tyrosine kinases, including PDGF receptors (15) and EGF receptors (13). Thus, PLCϵ is ideally positioned to integrate multiple signaling inputs in various cell types including cardiac myocytes.
Recent studies of PLCϵ−/− mice revealed alterations in βAR-dependent regulation of cardiac ionotropy and hypertrophy (18). These mice had no hypertrophy at baseline but developed greater hypertrophy than wild type animals in response to chronic isoproterenol treatment. Thus, PLCϵ appeared to protect mice from stimulus-induced cardiac hypertrophy. In wild type mice, the levels of PLCϵ mRNA and protein increase during pressure overload or chronic isoproterenol (Iso) treatment. PLCϵ RNA levels are elevated in biopsies from human heart failure samples. To explore a mechanistic role for PLCϵ in hypertrophy, we utilized an siRNA protocol in an established cellular model of cardiac hypertrophy, the neonatal rat ventricular myocyte (NRVM) (19). Surprisingly, in this cell model, we found that PLCϵ appears to be required for development of cardiac hypertrophy downstream of multiple hypertrophic stimuli, rather than protective.
We also examined the subcellular scaffolding of PLCϵ that might lead to generation of IP3 and DAG at specific cellular sites critical for activation of hypertrophic transcriptional mechanisms. A common family of scaffolding molecules in the heart is the protein kinase A anchoring protein (AKAP) family (20, 21). One member of this family that is involved in cytokine- and adrenergic-induced hypertrophy is the alternatively spliced isoform of muscle AKAP (mAKAPβ) expressed exclusively in striated myocytes (22). mAKAPβ binds multiple proteins besides protein kinase A (PKA) including exchange factor activated by cAMP (Epac), adenylate cyclase 5 (23), cAMP-phosphodiesterase 4D3 (PDE4D3), extracellular signal-regulated kinase 5 (ERK5) (22), protein phosphatase 2A (PP2A) (21), and calcineurin (24). mAKAPβ localizes to the nuclear envelope of myocytes, where it is targeted by nesprin-1α (25). It has also been suggested that mAKAPβ scaffolds PKA to ryanodine receptor type 2 (RyR2) in the sarcoplasmic reticulum (SR) (26), but this is controversial. Here we demonstrate that PLCϵ binds directly to mAKAPβ and that the scaffolding by mAKAPβ is important for the ability of PLCϵ to regulate cardiomyocyte hypertrophy. This suggests that scaffolding of PLCϵ to the nuclear envelope by mAKAPβ regulates expression of hypertrophic genes.
EXPERIMENTAL PROCEDURES
Plasmid Constructs
mAKAPβ expression vectors are as described previously (25). All mAKAP vectors are numbered according to mAKAPα because mAKAPβ is identical to mAKAPα 245–2314 (27). PLCϵ RA1 and PLCϵ RA2 were in pFLAG3. PLCϵ ΔCT and PLCϵ ΔGEF were expressed under the CMV promoter in pCMVscript.
Adenoviral Constructs
Short interfering hairpin RNAs (siRNAs) were designed for targeting mouse, rat, or human PLCϵ: siRNAPLCϵ 5, 5′GCCAAATATTCCTACAGCA; RANsiRNA, ACTGTCACAAGTACCTACA (scrambled siRNAPLCϵ 5). siRNA sequences were subcloned into pShuttle-CMV as in (28), and recombinant viruses were generated following the manufacturer's protocol for the Q-biogene AdEasy adenoviral vector system. Adenoviruses expressing PLCϵ-RA1 and mAKAP-SR1 were prepared by PCR amplification of the appropriate cDNA fragments and were subcloned into a shuttle vector, under the control of the mouse cytomegalovirus promoter, which also expresses the YFP protein under the control of a separate mouse cytomegalovirus promoter. The shuttle vector was recombined with the parent vector in HEK293 cells to generate adenovirus expressing the PLCϵ-RA1 or mAKAP-SR1 domain. Recombined virus was amplified in HEK293 cells and purified by CsCl gradient centrifugation. Viral titers were calculated by an immunoreactivity spot assay (29).
Glutathione S-Transferase (GST) Fusion Proteins
GST fusion protein constructs were prepared as described previously (23). BL21 DE3 cells containing pGEX-4T2 plasmids with the indicated regions of mAKAP fused to the C terminus of GST were grown to an optical density (600 nm) of 0.6 followed by induction with 100 μmol/liter isopropyl-1-thio-β-d-galactopyranoside for 16 h at 18 °C in 1 liter of each. Cells were harvested by centrifugation followed by lysis in 30 ml of lysis buffer (10 mmol/liter Tris-Cl, pH 7.5, 150 mmol/liter NaCl, 2 mmol/liter EDTA, and 10 mmol/liter β-mercaptoethanol) + protease inhibitors: 133 μm phenylmethylsulfonyl fluoride, 21 μg/ml 1-chloro-3-tosylamido-7-amino-2-heptanone and l-1-tosylamido-2-phenylethyl chloromethyl ketone, 0.5 μg/ml aprotinin, 0.2 μg/ml leupeptin, 1 μg/ml pepstatin A, 42 μg/ml p-tosyl-l-arginine methyl ester, 10 μg/ml soybean trypsin inhibitor, by probe sonication. Nonidet P-40 detergent was added to a final concentration of 1% (v/v), and the samples were incubated on ice for 30 min. Lysates were cleared of insoluble material by centrifugation at 100,000 × g for 30 min. Soluble lysates were incubated with 500 μl of glutathione agarose beads (Thermo Fisher) for 2 h at 4 °C. Beads were harvested by centrifugation and washed four times in 20 ml of lysis buffer. Beads bound to GST fragments were analyzed directly by SDS-PAGE and staining with Coomassie Blue as well as by an Amido Black protein assay and stored at 4 °C.
For GST-mAKAP fragment pulldowns, 2 μg of GST fusion protein bound to 25 μl of beads was mixed with 50 ng of purified PLCϵ in 500 μl of pulldown buffer (10 mmol/liter Tris-Cl, pH 7.5, 150 mmol/liter NaCl, 2 mmol/liter EDTA+). Mixtures were rotated for 2 h at 4 °C. Beads were harvested by centrifugation at 16,000 × g at 4 °C for 2 min. After supernatant removal, beads were washed three times with 500 μl of pulldown buffer. Beads were boiled in 30 μl of sample buffer, and 10 μl was loaded on a 7.5% (w/v) SDS-polyacrylamide gel for resolving and Western blotting.
PLCϵ Purification
His6-tagged PLCϵ was purified according to previously published procedures (12).
HEK Cell Culture and Transfection
5 × 105 HEK293 cells cultured in Dulbecco's modified Eagle's medium (DMEM) + 10% (v/v) FBS were plated on poly-d-lysine-coated 6-well plates the day before transfection. 2 μg of DNA was transfected using Lipofectamine Plus reagent (Invitrogen). Proteins were expressed for 48 h prior to harvesting for immunoprecipitation.
Immunoprecipitation, Immunocytochemistry, and Western Blotting
Cells were lysed in 1% (v/v) Nonidet P-40 lysis buffer (10 mmol/liter Tris-Cl, pH 7.5, 50 mmol/liter NaCl, 30 mmol/liter sodium pyrophosphate, 50 mmol/liter NaF, 100 μmol/liter phenylmethylsulfonyl fluoride, and 1% (v/v) Nonidet P-40). After sonication and centrifugation, the supernatant was incubated overnight with anti-Myc antibody (Covance) and Protein G plus agarose beads (Santa Cruz Biotechnology Inc., Santa Cruz, CA) at 4 °C with rocking. Beads were centrifuged for 1 min at 16,000 × g, washed twice with 1.0 ml of lysis buffer, washed once with 1.0 ml of phosphate-buffered saline, boiled in 50 μl of 2× SDS sample buffer, and loaded onto a 7.5% (w/v) SDS-polyacrylamide gel. After SDS-PAGE, proteins were transferred to nitrocellulose for 16 h at 25 V followed by immunoblotting for mAKAP (anti-Myc antibody) or PLCϵ.
Neonatal Cardiac Myocyte Culture, Adenoviral Infection, and Hormone Treatment
NRVMs were isolated and cultured from hearts from 2–3-day-old Sprague-Dawley rats as has been previously described (19) with modifications. Briefly, hearts were excised, and ventricles were separated and rinsed in Hanks' balanced salt solution prior to digestion with three rounds of collagenase type II (Worthington) in Hanks' balanced salt solution without Ca2+ and Mg2+. Cells were collected by centrifugation and resuspended in DMEM containing 10% (v/v) fetal bovine serum (FBS), 100 IU/ml penicillin, 100 μg/ml streptomycin, 2 mmol/liter glutamine, and 2 μg/ml vitamin B-12. Non-myocytes were removed by preplating of the cells for 1 h at 37 °C. The NRVMs were cultured in DMEM as described above containing 10 μmol/liter cytosine arabinoside. 10 μmol/liter cytosine arabinoside was applied for the first 3 days. After overnight incubation, FBS was reduced to 1% (v/v) followed by DMEM lacking serum for at least 24 h. After NRVMs were plated for 6–8 h, adenovirus expressing PLCϵ siRNA 5, random siRNA, PLCϵ, PLCϵ(H1460L), PLCϵ-RA1, mAKAP-SR1, or YFP as control was added (multiplicity of infection 50) for 4–6 h followed by washing. NRVMs were then cultured in serum-free DMEM medium containing 0.1× insulin-transferrin-selenium. After adenovirus infection for at least 2 days and serum starvation for at least 24 h, 10 nmol/liter ET-1, 10 μmol/liter Iso, 10 μmol/liter norepinephrine (NE), or 10 nmol/liter IGF-1 was added for 48 h to induce hypertrophy.
[3H]Leucine Incorporation
NRVMs were plated in 24-well 0.2% (w/v) gelatin-coated plates at a density of 2 × 105 cells/ml and 500 μl/well. [3H]Leucine (1 μCi/ml) was used to label newly translated proteins for 48 h in the absence or presence of hypertrophic agonist as described above. Cells were washed twice with ice-cold PBS and incubated in 5% (w/v) trichloroacetic acid for 30 min at 4 °C to precipitate the protein. Precipitates were washed twice and solubilized in 0.4 n NaOH for 2 h. Trichloroacetic acid (TCA) precipitable radioactivity was measured by scintillation counting. For analysis, data were pooled from 2–3 independent experiments performed in triplicate.
Cell Area Measurements
NRVMs were plated in 6-well 0.2% (w/v) gelatin-coated plates at a density of 1.0 × 105 cells/ml and 2 ml/well. After hypertrophic agonist treatment, NRVMs were fixed in 4% (w/v) paraformaldehyde, permeabilized with 0.1% (v/v) Nonidet P-40, and immunostained with myocyte marker, sarcomeric α-actinin antibody (Sigma 1:500) in phosphate-buffered saline (PBS) (0.1% Nonidet P-40 (v/v), and bovine serum albumin (BSA) 3% (w/v)) followed by Alexa Fluor 546 (Invitrogen 1:300) in the same solution. Fluorescent images were taken at 20× magnification using an Olympus IX-71 fluorescence microscope. NRVM surface areas were calculated using the NIH ImageJ software from over 400 myocytes from each condition. Data were pooled from 2 independent experiments.
Measurement of PLC Activity in NRVMs
NRVMs were plated in 24-well 0.2% (w/v) gelatin-coated plates at a density of 2 × 105 cells/ml and 500 μl/well. After adenovirus infection for 2 days, NRVMs were labeled for 2 days with [3H]inositol (20 μCi/ml) in inositol-free DMEM supplemented as above. LiCl was added to a final concentration of 100 mm for 10 min at 37 °C. Then agonist was added for 30 min at 37 °C. Total inositol phosphates (IPs) produced were measured as in Ref. 30.
Immunocytochemical Staining
NRVMs were fixed and permeabilized and immunostained as described for cell area measurements using an mAKAPβ antibody (1:500) and an Alexa Fluor 546 secondary antibody. Cells were also treated with 4′,6-diamidino-2-phenylindole (DAPI) to stain nuclei.
Measurement of ANF Levels by Real Time PCR
NRVMs were plated in 6-well 0.2% gelatin-coated plates at a density of 5.0 × 105 cells/ml and 500 μl/well. For real time PCR, total RNA was extracted from NRVM using the RNeasy mini kit (Qiagen). 300 ng was reverse-transcribed with reverse primers to GAPDH and atrial natriuretic factor (ANF) using SuperScript III reverse transcriptase (Invitrogen). Real time PCR was performed on this product on the BioRadCFX96 real time system with a C 1000 thermal cycler using the iQ SYBR Green supermix (Bio-Rad).
The primers used were: ANF, 169-bp PCR product, ANF forward (169), 5′-ATCTGATGGATTTCAAGAACC-3′; ANF reverse (338), 5′-CTCTGAGACGGGTTGACTTC-3′; GAPDH, 260-bp PCR product, GAPDH forward (648), 5′-AAGGTCATCCCAGAGCTGAAC-3′; GAPDH reverse (908), 5′-TCATTGAGAGCAATGCC-3′. ANF values were normalized to GAPDH, and data collected were analyzed using the GraphPad Prism software. Each experiment was performed 2–3 times independently with results from each experiment giving one data point.
Isolation of Nuclei from NRVMs
The protocol was as previously reported except that the fraction containing SR, plasma membrane, and Golgi bodies was not further purified (31).
Statistical Analysis
For the majority of the [3H]leucine incorporation, cell area, real time PCR, and inositol phosphate measurements, significance was calculated by dividing the mean ligand-stimulated response by the unstimulated response for either random or PLCϵ siRNA treatment to determine fold activation. The difference in fold activation for random siRNA versus PLCϵ siRNA was analyzed for statistical significance using a one-tailed unpaired Student's t test. Selected data in Fig. 3A were analyzed by one-way analysis of variance with Bonferroni's post test.
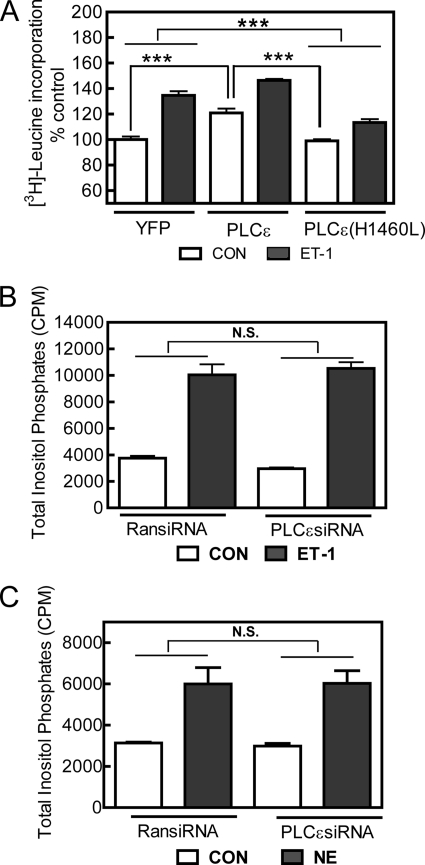
FIGURE 3.
PLCϵ hydrolytic activity promotes hypertrophy but does not contribute significantly to global IP3 production in NRVMs. A, NRVMs were infected with adenoviruses expressing either PLCϵ or catalytically inactive PLCϵ(H1460L) and treated with or without ET-1 for 48 h. [3H]Leucine incorporation was measured as described under “Experimental Procedures.” Data are pooled results from 3 separate experiments performed in triplicate, ***, p < 0.001. Error bars indicate S.E. CON, control. B and C, total inositol phosphates were measured after a 30-min treatment of NRVMs with or without (CON) 100 nm ET-1 (B) or 100 μmol/liter NE (C). Data are combined results from 2 separate experiments performed in triplicate. N.S., not significant; RansiRNA, random siRNA.
RESULTS
PLCϵ Knockdown in NRVMs Inhibits Endothelin-1-dependent Hypertrophy
We previously established an siRNA protocol for PLCϵ-specific knockdown in Rat-1 fibroblasts. This siRNA treatment did not affect expression of other PLC isoforms or expression of G proteins, and several siRNA sequences produced similar biological effects (28). We selected the most effective siRNA sequence from those tested (siRNAPLCϵ 5) that knocked down PLCϵ by 97% in Rat-1 fibroblasts and created an adenovirus for infection of NRVMs. Supplemental Fig. 1A shows RT-PCR analysis of PLCϵ RNA extracted from NRVMs infected for 3 and 6 days. PLCϵ mRNA was effectively depleted after 3 days and remained low after 6 days of viral infection. PLCϵ protein was also depleted after 3 days of viral infection (supplemental Fig. 1B), whereas the levels of other PLC isoforms were unaffected by the siRNA treatment (supplemental Fig. 1C).
First, we examined the role of PLCϵ in ET-1-dependent cardiac hypertrophy. ET-1 treatment for 48 h caused a significant increase in three markers of hypertrophy: [3H]leucine incorporation (Fig. 1A), cell area (Fig. 1, B and C), and ANF mRNA (Fig. 1D) in random siRNA infected cells, indicating stimulation of the hypertrophic response. Surprisingly, PLCϵ siRNA blocked ET-1-dependent increases in all hypertrophic markers (Fig. 1, A–D), indicating a requirement for PLCϵ for ET-1-dependent hypertrophy in NRVMs. To show that this result was not due to a nonspecific effect of the PLCϵ siRNA, we co-expressed PLCϵ with either a LacZ control or PLCϵ with a non-coding mutation that provides resistance to the siRNA (PLCϵ (res)) to replace the endogenously depleted PLCϵ protein with exogenously expressed PLCϵ. Leucine incorporation was monitored as a measure of hypertrophy (supplemental Fig. 1D). As in Fig. 1A, PLCϵ siRNA suppressed ET-1-induced [3H]leucine incorporation, but co-expression with PLCϵ (Res) prevented this effect, demonstrating rescue of the hypertrophic response upon re-expression of PLCϵ in the PLC siRNA background.
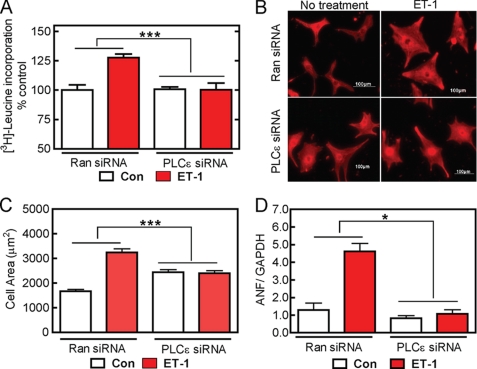
FIGURE 1.
PLCϵ is required for ET-1-dependent cardiomyocyte hypertrophy. NRVMs were infected with PLCϵ-siRNA or random siRNA (Ran siRNA) adenoviruses followed by stimulation with 10 nmol/liter ET-1 as described under “Experimental Procedures.” A, [3H]leucine incorporation was measured as described under “Experimental Procedures.” Data are combined results from four separate preparations of myocytes with each experiment performed in triplicate. Con, control. B, NRVMs were fixed and stained with α-actinin antisera as described under “Experimental Procedures.” Scale bar is 100 μm. C, NRVM surface areas were calculated using the NIH ImageJ software from over 400 myocytes for each condition. Data were pooled from 2 independent experiments. D, ANF mRNA was measured by real time PCR and normalized to GAPDH as described under “Experimental Procedures.” Data are combined from two different preparations of cells. Error bars indicate S.E. *, p < 0.05, ***, p < 0.001.
Overall, suppression of the hypertrophic response to ET-1 by PLCϵ depletion was unexpected because it is the opposite of what was observed in the PLCϵ−/− mice where the loss of PLCϵ appeared to increase hypertrophy. Regardless of this apparent contradiction, this result was also unexpected because ET-1 is thought to stimulate phosphatidyl inositol hydrolysis through a Gq-dependent mechanism that would not be expected to directly involve PLCϵ. ETA receptors can also signal via G12/13-Rho-, Gi/βγ-, and Gs-dependent pathways, and it is possible that PLCϵ is regulated through one of these signaling mechanisms. It was noted that PLCϵ knockdown alone increased cell area in the absence of stimulation by ET-1, but this appears to be unrelated to the hypertrophic response because the other markers of hypertrophy, ANF mRNA levels and protein synthesis, are not significantly altered by PLCϵ siRNA treatment in the absence of stimulation by agonist.
PLCϵ Is a Central Participant in Ligand-dependent Hypertrophic Responses in NRVMs
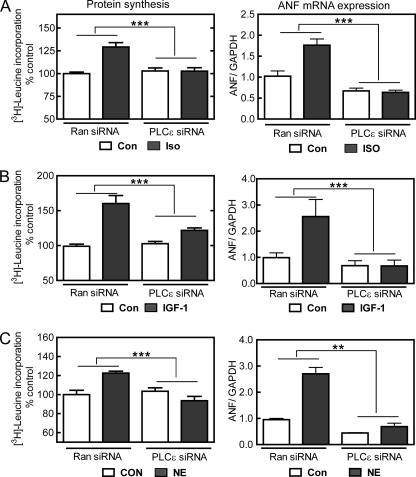
PLCϵ has the potential to be regulated by multiple upstream signaling mechanisms. We have extensively characterized a role in cAMP-Epac- and Rap-dependent signaling, and PLCϵ can respond to multiple G protein-coupled receptor-dependent and receptor tyrosine kinase-dependent pathways through Ras, Rho, Gβγ, or other potential regulatory molecules (13, 16, 17, 32). For this reason, we examined the effects of PLCϵ knockdown on stimulation of hypertrophy by agonists that signal through distinct cellular mechanisms. Iso likely works through a cAMP-dependent mechanism. NE activates Gq-dependent signaling via stimulation of α1-adrenergic receptors in neonatal myocytes (33). IGF-1 signals via tyrosine phosphorylation and couples to PLCγ (8). Again, PLCϵ knockdown almost completely inhibited ligand-dependent hypertrophy by all of these agonists (Fig. 2 and supplemental Fig. 2), indicating a central role of PLCϵ in hypertrophic signaling by diverse hormonal regulatory mechanisms.
FIGURE 2.
PLCϵ mediates cardiomyocyte hypertrophy downstream of multiple signaling pathways. Cells were treated as in Fig. 1 except with 10 μmol/liter Iso (A), 10 nmol/liter IGF-1 (B), or 10 μmol/liter NE (C). [3H]Leucine incorporation and ANF mRNA were measured as in Fig. 1. Data are pooled from three preparations of NRVMs for leucine incorporation performed in triplicate or two preparations of NRVMs for ANF measurement. Error bars indicate S.E. **, p < 0.01, ***, p < 0.001. Con, control; Ran siRNA, random siRNA.
To determine whether PLCϵ overexpression can promote hypertrophy in NRVMs, cells were infected with an adenovirus expressing PLCϵ under the control of a mouse cytomegalovirus promoter, and [3H]leucine incorporation was measured. PLCϵ expression alone significantly increased leucine incorporation (Fig. 3A), indicating that increasing PLCϵ expression was sufficient to increase hypertrophy. ET-1 was able to further increase [3H]leucine incorporation in the context of PLCϵ overexpression. To determine whether PLCϵ catalytic activity was required for the PLCϵ-dependent increase in hypertrophy, we expressed PLCϵ with a point mutation in the catalytic domain that eliminates PLC hydrolytic activity (PLCϵ(H1460L)) and measured hypertrophy. Expression of PLCϵ(H1460L) did not stimulate hypertrophy when compared with the YFP control. Expression of PLCϵ(H1460L) also suppressed hypertrophy stimulated by ET-1, likely acting in a dominant negative manner (Fig. 3A). Adenoviral expression of PLCϵ and PLCϵ(H1460L) gives equal levels of PLCϵ protein in ventricular myocytes (not shown). These data indicate that the products of PLCϵ catalytic activity are important for the hypertrophic response. To examine the role of PLCϵ in receptor-driven phosphatidylinositol 4,5-bisphosphate hydrolysis, we knocked down PLCϵ with siRNA and examined ET-1- and NE-stimulated IP production. PLCϵ knockdown had no effect on the ability of these agonists to stimulate total IP production (Fig. 3, B and C). We propose that PLCϵ activity does not significantly contribute to global cellular IP production either because it is expressed at very low levels, because its localization is restricted, or both. Possible mechanisms for subcellular localization of activity are discussed in depth below. Overall, these data suggest that PLCϵ is necessary for hypertrophy driven by multiple neurohumoral stimuli and that PLCϵ activity is sufficient to drive hypertrophic responses.
mAKAP Binds PLCϵ in Transfected Cells and in the Heart
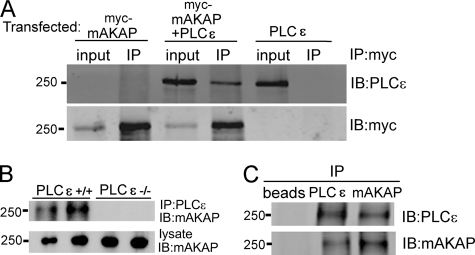
Because the data suggest that PLCϵ may be acting locally to produce its physiological effects and because one of the regulators of PLCϵ, Epac, scaffolds to mAKAPβ, we tested whether PLCϵ could interact with mAKAPβ. Myc-mAKAP was co-expressed with PLCϵ in HEK293 cells followed by immunoprecipitation of mAKAP and immunoblotting for either PLCϵ or mAKAP (Fig. 4A). PLCϵ was found in Myc-mAKAP immunoprecipitates only when both proteins were expressed. No PLCϵ immunoreactivity was detected if either PLCϵ or Myc-mAKAP was absent. To determine whether mAKAPβ and PLCϵ associate in cardiac tissue, mouse heart lysates were prepared from PLCϵ+/+ and PLCϵ−/− mice followed by PLCϵ immunoprecipitation and immunoblotting for mAKAPβ (Fig. 4B). Lysates from PLCϵ−/− mouse hearts were used as controls to confirm that bands observed from the immunoprecipitation were the result of association with immunoprecipitated PLCϵ and not nonspecific immunoprecipitation. mAKAPβ immunoreactivity was detected in immunoprecipitates from PLCϵ+/+ heart lysates but not in immunoprecipitates from PLCϵ−/− hearts, indicating that mAKAPβ associates with PLCϵ in native cardiac tissue. PLCϵ also co-precipitated with mAKAPβ when mouse heart extracts were immunoprecipitated with mAKAPβ antisera (Fig. 4C, lane 3).
FIGURE 4.
PLCϵ interacts with mAKAP in transfected HEK293 cells and in native mouse heart tissue. A, HEK293 cells were transfected with Myc-mAKAP alone, PLCϵ alone, or PLCϵ with Myc-mAKAP. Cells were lysed and immunoprecipitated (IP) with an anti-Myc specific antibody. Lysates were Western blotted (IB) for either PLCϵ or Myc. B, Nonidet P40 soluble lysates were prepared from hearts isolated from PLCϵ+/+ mice, or PLCϵ−/− mice as a control, immunoprecipitated with anti PLCϵ antibodies, and immunoblotted for mAKAPβ. C, Nonidet P40 lysates from PLCe+/+ mice as in B were immunoprecipitated with either anti-mAKAP or PLCϵ antibodies and immunoblotted as indicated. Lysates were directly blotted with mAKAPβ antisera as loading controls. Each experiment was repeated at least 3 times with similar results.
Direct Binding of PLCϵ to mAKAPβ and Mapping of the Binding Site on the Scaffold
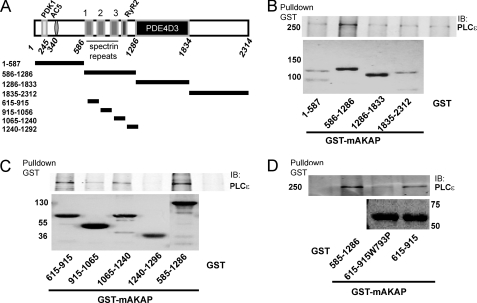
To test for a direct interaction between PLCϵ and mAKAP and to map the PLCϵ binding regions on mAKAP, GST fusion proteins corresponding to different domains of the scaffold were purified (Fig. 5A) and tested for binding to purified full-length PLCϵ. First, fragments of mAKAP indicated in Fig. 5A were fused to GST, purified, and tested for binding to purified full-length PLCϵ. A fragment corresponding to mAKAP 586–1286 consistently bound specifically to PLCϵ, demonstrating direct binding of PLCϵ to mAKAP and localization to this region of mAKAP (Fig. 5B). The 586–1286 domain contains three spectrin repeat-like domains, the third of which is involved in interactions with nesprin-1α that localizes mAKAPβ to the nuclear envelope in cardiac myocytes (25). To further dissect these domains, fragments comprising each of the different repeats were fused to GST, purified, and tested for PLCϵ binding. Of these fragments, GST-615–915 binds the strongest, with fragment GST-1065–1240 also showing some binding (Fig. 5C). The 615–915 fragment of mAKAP contains the first of the three spectrin repeat-like structures (amino acids 772–882). Spectrin repeat-like domains contain two conserved tryptophans (Trp-793 and Trp-867 in the mAKAP first spectrin repeat-like domain) that are important for thermodynamic stability. Mutation of analogous Trp residues to proline in the third spectrin repeat-like domain disrupted interactions between mAKAP and nesprin-1α. Trp-793 was mutated to proline in the mAKAP spectrin repeat-like domain 1, and binding of this mutant protein fused to GST to purified PLCϵ was examined (Fig. 5D). This mutation strongly decreased binding of PLCϵ to this domain, confirming that PLCϵ binds to the first spectrin repeat-like domain in mAKAP.
FIGURE 5.
Direct binding of PLCϵ to mAKAP subdomains. A, diagram of mAKAP showing defined domains for binding PDK1, AC5, RyR2, and PDE4D3, and below, showing the regions that were cloned as GST fusion proteins. B, GST fusion proteins corresponding to the larger fragments shown in A were tested for binding to purified PLCϵ. The top panel is a Western blot (IB) for PLCϵ after pulldown with the indicated GST fragments. The bottom panel is a Coomassie Blue-stained gel of the purified GST fusion proteins. C, GST fusion proteins corresponding to the smaller subfragments of the 586–1286 binding domain defined in B were tested for binding to purified PLCϵ. The top panel is a Western blot for PLCϵ after pulldown with the indicated GST fragments. The bottom panel is a Coomassie Blue-stained gel of the purified GST fusion proteins. D, the 615–915 fragment defined in C was mutated by site-directed mutagenesis (W793P), purified, and tested for binding to purified PLCϵ. The top panel is a Western blot for PLCϵ after pulldown with the indicated GST fragments. The bottom panel is a Coomassie Blue-stained gel of the purified GST fusion proteins. Each experiment was repeated at least 3 times with similar results.
Identification of PLCϵ Functional Domains Required for mAKAP Interactions
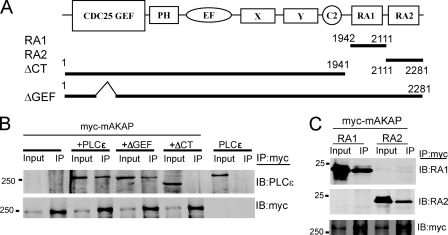
To identify specific domains in PLCϵ that mediate mAKAP binding, PLCϵ truncated at the C terminus (ΔCT) to delete the Ras association (RA) domains or PLCϵ with a GEF domain deletion (ΔGEF) was co-expressed with Myc-mAKAP in HEK293 cells (Fig. 6A). The ΔCT mutant was unable to interact with mAKAP in co-immunoprecipitation assays, whereas the interaction was unaffected by the ΔGEF mutation (Fig. 6B). Next, either the PLCϵ-RA1 or the PLCϵ-RA2 domains were expressed with Myc-mAKAP followed by Myc-mAKAP immunoprecipitation. Both PLCϵ-RA1 and PLCϵ-RA2 independently bound to co-expressed mAKAP (Fig. 6C). These data indicate that the RA domains are necessary and sufficient for binding of PLCϵ to mAKAP. Given that the RA domains are important for Rap and Ras interactions, we determined whether activated RapG12V or RasG12V altered PLCϵ-mAKAP binding in transfected cells. Expression of activated Rap or Ras did not affect PLCϵ-mAKAP co-immunoprecipitation (data not shown).
FIGURE 6.
The RA domains of PLCϵ bind to mAKAP. A, domain structure of PLCϵ showing the CDC25 guanine nucleotide exchange factor domain (CDC25 GEF), a putative pleckstrin homology domain (PH), the EF hand domain, the X and Y catalytic domain, the C2 lipid binding domain, and the RA homology domains. Only RA2 binds Ras. B, Myc-mAKAP alone or with the indicated PLCϵ constructs was cotransfected into HEK293 cells, and Myc-mAKAP was immunoprecipitated (IP) followed by immunoblotting (IB) with anti-PLCϵ antibody (top panel) or Myc antibody as a control (bottom panel). C, Myc-mAKAP was cotransfected with either PLCϵ-RA1 or PLCϵ-RA2 domains. Cells were lysed and immunoprecipitated with anti-Myc antibodies and immunoblotted with the antibodies indicated on the right. Each experiment was repeated at least 3 times with similar results.
Disruption of mAKAP-PLCϵ Binding by Expression of PLCϵ RA1 or mAKAP-SR1
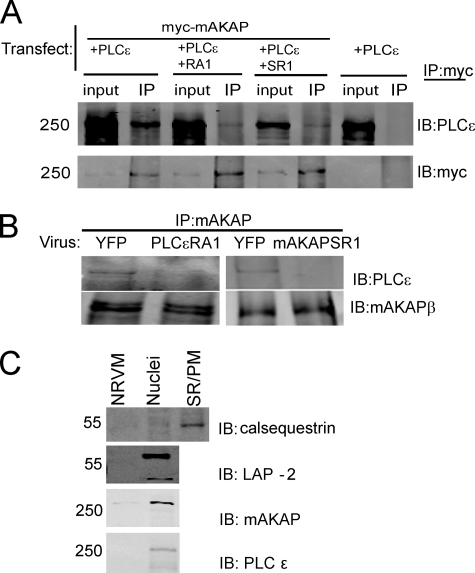
To determine whether expression of PLCϵ-RA1 or mAKAP-SR1 could compete for PLCϵ binding to mAKAP, mAKAP and PLCϵ were co-expressed in HEK293 cells, plus or minus co-expression of PLCϵ-RA1 or mAKAP-SR1 domains. Myc-mAKAP protein complexes were immunoprecipitated with Myc antibodies and immunoblotted for PLCϵ. PLCϵ co-immunoprecipitated with mAKAP, but expression of either PLCϵ-RA1 or mAKAP-SR1 prevented mAKAP-PLCϵ co-immunoprecipitation (Fig. 7A). To test whether PLCϵ-RA1 or mAKAP-SR1 can disrupt endogenous mAKAP-PLCϵ signaling complexes in NRVMs, NRVMs were infected with adenoviruses expressing either domain followed by immunoprecipitation of mAKAP and immunoblotting for PLCϵ. PLCϵ co-immunoprecipitated with mAKAP when YFP was expressed as a control (Fig. 7B), although the complex was difficult to detect. In general, endogenous PLCϵ is difficult to detect and has to be enriched by direct IP from most tissues to enable adequate detection by immunoblotting. Nevertheless, expression of either PLCϵ-RA1 or mAKAP-SR1 in NRVMs significantly reduced the association of PLCϵ with mAKAP immunoprecipitates (Fig. 7B), indicating that expression of either of these domains disrupts endogenous mAKAPβ-PLCϵ complexes in NRVMs.
FIGURE 7.
The PLCϵ-RA1 and mAKAP-SR1 domains compete for mAKAP-PLCϵ interactions. A, PLCϵ and Myc-mAKAP were cotransfected (Transfect.) into HEK393 cells in the presence or absence of PLCϵ-RA1 or mAKAP-SR1 domains. Myc-mAKAP was immunoprecipitated (IP) and Western blotted (IB) for PLCϵ (top panel) or Myc (bottom panel). Each experiment was repeated at least 3 times with similar results. B, NRVMs were infected with adenoviruses expressing YFP, PLCϵ-RA1, or mAKAP-SR1 for 48 h. Lysates were prepared (1 mg of protein, PLCϵ-RA1-infected cells, or 2 mg of protein, mAKAP-SR1-infected cells), and mAKAPβ was immunoprecipitated. Immunoprecipitates were immunoblotted for either PLCϵ or mAKAPβ. This experiment was repeated twice. C, NRVMs were fractionated to isolate nuclei and 10 μg of protein was analyzed by Western blotting. SR/PM, sarcoplasmic reticulum/plasma membrane.
To confirm the location of endogenous mAKAPβ in NRVMs, isolated myocytes were fixed and stained with mAKAP antibody (data not shown). As reported previously, there was a very strong perinuclear staining of mAKAP (25). Reliable detection of PLCϵ by immunocytochemistry was not possible, likely due to its low abundance coupled with nonspecific background staining with PLCϵ antibodies. Given the interaction with mAKAPβ and the predominant perinuclear localization of mAKAP, it is likely that PLCϵ interaction with mAKAPβ localizes some of the PLCϵ pool to the perinuclear region of the cell. To confirm the nuclear localization of PLCϵ, NRVMs were fractionated to isolate nuclei and immunoblotted for PLCϵ. PLCϵ was significantly enriched in the nuclear fraction when compared with whole cell NRVM lysate, consistent with association with nuclear-bound mAKAP. Nuclear enrichment was confirmed by measuring the enrichment of the nuclear protein LAP-2 and the mAKAPβ. Both proteins were greatly enriched relative to the whole cell NRVM lysates. To confirm that the nuclei were free of SR, the lysates were probed for calsequestrin, which was enriched in an SR fraction but was absent from the nuclear fraction.
mAKAPβ spectrin repeat domain 3, but not repeats 1 or 2, has been shown to mediate interactions with nesprin-1α, which localizes mAKAP to the perinuclear region (25) of cardiac myocytes. Nevertheless, because PLCϵ interacts with spectrin repeat domain 1, we tested whether expression of either PLCϵ-RA1 or mAKAP-SR1 disrupted perinuclear localization of mAKAPβ in NRVMs. Neither domain altered the localization of mAKAPβ as assessed by immunocytochemistry of NRVMs (supplemental Fig. 3), indicating that these domains effectively disrupt endogenous mAKAPβ-PLCϵ interactions without influencing mAKAPβ localization to the nuclear envelope. Thus, expression of PLCϵ-RA1 or mAKAP-SR1 likely disrupts PLCϵ localization at the nuclear envelope, while leaving the mAKAPβ scaffold itself undisturbed.
Importance of PLCϵ Scaffolding to mAKAP in Cardiomyocyte Function
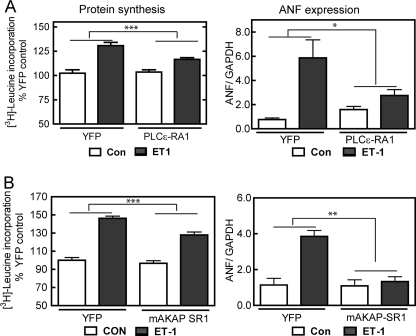
We have shown that PLCϵ is an important central mediator of hypertrophy and that it is scaffolded to mAKAPβ, which we have also shown to be important for cardiac myocyte hypertrophy (16). To establish the functional relevance of mAKAPβ-PLCϵ complexes, we tested whether mAKAPβ-PLCϵ binding was required for the hypertrophy of NRVMs. NRVMs were infected with an adenovirus expressing either PLCϵ-RA1 or mAKAP-SR1 to disrupt PLCϵ-mAKAP binding followed by treatment with 10 nm ET-1 for 48 h. Expression of either PLCϵ-RA1 or mAKAP-SR1 significantly blunted ET-1-dependent increases in [3H]leucine incorporation and ANF mRNA expression (Fig. 8, A and B). PLCϵ-RA1 is related to Ras binding domains but does not bind Ras or Rap (34). Nevertheless, to be sure that PLCϵ-RA1 was not inhibiting hypertrophy by blocking Ras or Rap signaling, we measured IGF-1-stimulated ERK phosphorylation, in the presence or absence of PLCϵ-RA1 expression. The IGF-1-stimulated increase in ERK1/2 phosphorylation in NRVMs was not affected by expression of PLCϵ-RA1 (data not shown), indicating that these proteins do not globally interfere with Ras signaling. That two independent approaches to disrupting mAKAPβ-PLCϵ complexes, using two different binding domains from two different proteins, both significantly inhibited ET-1-dependent hypertrophy strongly suggests that scaffolding of PLCϵ to mAKAPβ is required for hypertrophic responses mediated by ET-1. PLCϵ-RA1 expression in NRVMs also significantly inhibited [3H]leucine incorporation in response to Iso, NE, and IGF-1, indicating a general role for nuclear scaffolding of PLCϵ (supplemental Fig. 4).
FIGURE 8.
PLCϵ-RA1 or mAKAP-SR1 expression in neonatal cardiac myocytes inhibits development of cardiac hypertrophy in response to ET-1 treatment. Cells infected with adenovirus expressing either PLCϵ-RA1 or YFP (A) or mAKAP-SR1 or YFP (B) were treated with or without (CON) 10 nmol/liter ET-1 for 48 h followed by measurement of [3H]leucine incorporation or ANF mRNA. Data were normalized to YFP with no ET-1 treatment (CON). Data are the combined results from 3 separate experiments performed in triplicate for leucine incorporation or 2 separate experiments for ANF mRNA. Error bars indicate S.E. *, p < 0.05, **, p < 0.01, ***, p < 0.001.
DISCUSSION
Here we demonstrate that PLCϵ plays a central role in mediating hypertrophy downstream of multiple agonists and that subcellular scaffolding of PLCϵ to mAKAPβ, likely at the nuclear envelope, is critical for this process. Mechanistically, the upstream pathways that regulate PLCϵ for these hypertrophic responses remain to be defined in NRVMs. Possible mechanisms for activation include G12/13-dependent Rho pathways, cAMP-Epac pathways, and Ras pathways. ETA receptors can activate G12/13, IGF-1 receptors can activate Ras, and βAR can activate cAMP-Epac-Rap, all of which are potential PLCϵ regulators in NRVMs. α1-Adrenergic receptors signal primarily through Gq, which would not activate PLCϵ, but Gq activation can lead to activation of Rho and Ras pathways that could regulate PLCϵ activity. That PLCϵ can respond to such diverse signals ideally positions it to be a central integrator of multiple hormonal signals that impinge on the cardiac myocytes during physiologic and pathological stimulation.
Regardless of the mechanism for activation, the catalytic activity of PLCϵ is required for hypertrophy because expression of a catalytically inactive form of PLCϵ does not stimulate hypertrophy and blocks development of ET-1-dependent hypertrophy. PLC activity results in the production of two second messengers, DAG and IP3, and roles for both of these molecules have been suggested in hypertrophy. Nevertheless, the data show that PLCϵ is not responsible for the majority of global IP3 generation downstream of ETA or α1AR. An attractive hypothesis to explain this is that PLCϵ scaffolded to mAKAPβ at the nuclear envelope generates IP3 and DAG locally to regulate nuclear Ca2+ or PKC signals without significantly contributing to the pool of total IPs. In ventricular myocytes, IP3 receptors are localized primarily to the nuclear envelope and release a pool of nuclear calcium distinct from that involved in calcium-induced calcium release (35, 36). This nuclear calcium pool has been implicated in regulating hypertrophic gene expression through two pathways: (i) activation of nuclear CamKII leading to phosphorylation of histone deacetylase 5 (HDAC5) and derepression of myocyte enhancer factor (MEF)-dependent transcription; and (ii) activation of calcineurin-dependent dephosphorylation of nuclear factor of activated T-cells (NFAT) leading to increased nuclear factor of activated T-cell-dependent transcription. Additionally, PKC activation has been implicated in hypertrophy, and PKC-PKD-dependent phosphorylation of HDAC leads to export of HDAC5 from the nucleus (37). PLCϵ could be involved in the local generation of either IP3 or DAG at the nuclear envelope necessary for these processes.
The PLC isoform responsible for the majority of IP3 pro-duction downstream of Gq-coupled receptors has been suggested to be PLCβ because the major known target of Gαq is PLCβ (6, 38), although Gαq can also regulate Rho activation through direct stimulation of RhoGEFs (39). Data directly implicating PLCβ in cardiac hypertrophy have been lacking until recently. These new data show that PLCβ1b overexpression increases NRVM hypertrophy and that inhibition of PLCβ1b sarcolemmal membrane association can inhibit α1AR-dependent hypertrophy (9). Our published results in astrocytes (17) and fibroblasts (28) indicate that surprisingly, many Gq-coupled receptors generate significant accumulation of total IPs through PLCϵ, suggesting the possibility that some portion of hypertrophy driven through Gq-linked receptors could be downstream of PLCϵ activation. Nevertheless, the bulk of IP3 generation in NRVMs does not appear to involve PLCϵ and is likely PLCβ-driven, suggesting that PLCϵ-dependent local phosphatidylinositol 4,5-bisphosphate hydrolysis, distinct from the bulk IP pool, is required for hypertrophic responses. It is possible that hypertrophy in response to ET-1 and NE is both PLCβ1b-dependent and PLCϵ-dependent and that two distinct pools of IP3 and/or DAG are required for the hypertrophic response.
We previously reported that PLCϵ activity is important for CamKII-dependent phosphorylation of Ryr2 and regulation of cardiac Ca2+ cycling (41). Based on this observation, we predict that mAKAPβ scaffolded PLCϵ represents a pool of PLCϵ that is distinct from a separately scaffolded pool that regulates RyR2. mAKAPβ has been reported to bind to RyR2 (26), but this is at odds with the observation that the majority of mAKAPβ is found at the nuclear envelope, whereas the bulk of RyR2 protein is in the SR. Nevertheless, it remains possible that a small pool of mAKAPβ can scaffold PLCϵ to Ryr2. This hypothesis remains to be investigated.
Our data demonstrating a requirement for PLCϵ in hormone-regulated hypertrophic responses contrast with our previously reported in vivo observation that global PLCϵ−/− mice are more sensitive to the development of hypertrophy in response to chronic Iso treatment (18). A possible explanation for the difference in the cellular and in vivo data is that in the context of development of the PLCϵ−/− mice, compensatory pathways were up-regulated that sensitized mice to hypertrophy. In particular, we found that CamKII phosphorylation is basally increased in PLCϵ−/− mice (supplemental Fig. 5), which could lead to enhanced sensitivity to stress-induced hypertrophy because CamKII activation has been shown to be prohypertrophic (40). CamKII activation is not elevated in PLCϵ siRNA-treated myocytes (data not shown). It is not unusual for compensatory pathways to be up- or down-regulated in global knock-out mice where the gene product is lost from birth. This suggests that our previous conclusion from global PLCϵ−/− mice suggesting a protective role for PLCϵ was incorrect. Analysis of mice with cardiac myocyte-specific, acute PLCϵ knockdown would help to resolve this discrepancy.
Overall, these data demonstrate that PLCϵ plays a central role in mediating hypertrophy in response to multiple neurohumoral stimuli and that scaffolding to mAKAPβ is critical for this. The human heart is exposed to multiple hormonal inputs during the development of heart failure. PLCϵ represents a potentially interesting therapeutic target as a central integrator of these inputs, possibly allowing for more efficacious treatment of heart failure. Because PLCϵ plays an important role in the CNS, pancreas, and kidney as well as other tissues, targeting PLCϵ activity directly might not be desirable. Development of an approach that could specifically interfere with PLCϵ-mAKAPβ interactions could provide a more selective approach to targeting PLCϵ and its role in hypertrophy.
Supplementary Material
Acknowledgments
We thank the Dr. Shey-Shing Sheu laboratory for assistance with preparation of NRVMs and Dr. Tzong-Jen for sharing neonatal rats.
This work was supported, in whole or in part, by National Institutes of Health Grants GM R01 053536 (to A. V. S.) and HL075398 (to M. S. K.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–5.
- PLC
- phosphatidylinositol-specific phospholipase C
- AKAP
- A kinase anchoring protein
- mAKAPβ
- muscle-specific AKAP
- NRVM
- neonatal rat ventricular myocyte
- NE
- norepinephrine
- Iso
- isoproterenol
- ET-1
- endothelin-1
- Epac
- exchange protein activated by cAMP
- IP
- inositol phosphate
- IP3
- inositol trisphosphate
- DAG
- diacylglycerol
- CamK
- calcium calmodulin-dependent kinase
- RA
- Ras association domain
- SR
- sarcoplasmic reticulum
- SR1
- spectrin repeat domain 1
- AR
- adrenergic receptor
- GEF
- guanine nucleotide exchange factor
- ANF
- atrial natriuretic factor.
REFERENCES
- 1. Knowlton K. U., Michel M. C., Itani M., Shubeita H. E., Ishihara K., Brown J. H., Chien K. R. (1993) J. Biol. Chem. 268, 15374–15380 [PubMed] [Google Scholar]
- 2. Barki-Harrington L., Perrino C., Rockman H. A. (2004) Cardiovasc. Res. 63, 391–402 [DOI] [PubMed] [Google Scholar]
- 3. Shubeita H. E., McDonough P. M., Harris A. N., Knowlton K. U., Glembotski C. C., Brown J. H., Chien K. R. (1990) J. Biol. Chem. 265, 20555–20562 [PubMed] [Google Scholar]
- 4. Rhee S. G. (2001) Annu. Rev. Biochem. 70, 281–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adams J. W., Sakata Y., Davis M. G., Sah V. P., Wang Y., Liggett S. B., Chien K. R., Brown J. H., Dorn G. W., 2nd (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 10140–10145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smrcka A. V., Hepler J. R., Brown K. O., Sternweis P. C. (1991) Science 251, 804–807 [DOI] [PubMed] [Google Scholar]
- 7. Bers D. M. (2008) Annu. Rev. Physiol. 70, 23–49 [DOI] [PubMed] [Google Scholar]
- 8. Foncea R., Andersson M., Ketterman A., Blakesley V., Sapag-Hagar M., Sugden P. H., LeRoith D., Lavandero S. (1997) J. Biol. Chem. 272, 19115–19124 [DOI] [PubMed] [Google Scholar]
- 9. Filtz T. M., Grubb D. R., McLeod-Dryden T. J., Luo J., Woodcock E. A. (2009) FASEB J. 23, 3564–3570 [DOI] [PubMed] [Google Scholar]
- 10. Singer W. D., Brown H. A., Sternweis P. C. (1997) Ann. Rev. Biochem. 66, 475–509 [DOI] [PubMed] [Google Scholar]
- 11. Harden T. K., Sondek J. (2006) Annu. Rev. Pharmacol. Toxicol. 46, 355–379 [DOI] [PubMed] [Google Scholar]
- 12. Kelley G. G., Reks S. E., Ondrako J. M., Smrcka A. V. (2001) EMBO J. 20, 743–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kelley G. G., Reks S. E., Smrcka A. V. (2004) Biochem. J. 378, 129–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lopez I., Mak E. C., Ding J., Hamm H. E., Lomasney J. W. (2001) J. Biol. Chem. 276, 2758–2765 [DOI] [PubMed] [Google Scholar]
- 15. Song C., Hu C. D., Masago M., Kariyai K., Yamawaki-Kataoka Y., Shibatohge M., Wu D., Satoh T., Kataoka T. (2001) J. Biol. Chem. 276, 2752–2757 [DOI] [PubMed] [Google Scholar]
- 16. Wing M. R., Houston D., Kelley G. G., Der C. J., Siderovski D. P., Harden T. K. (2001) J. Biol. Chem. 276, 48257–48261 [DOI] [PubMed] [Google Scholar]
- 17. Citro S., Malik S., Oestreich E. A., Radeff-Huang J., Kelley G. G., Smrcka A. V., Brown J. H. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 15543–15548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang H., Oestreich E. A., Maekawa N., Bullard T. A., Vikstrom K. L., Dirksen R. T., Kelley G. G., Blaxall B. C., Smrcka A. V. (2005) Circ. Res. 97, 1305–1313 [DOI] [PubMed] [Google Scholar]
- 19. Simpson P., McGrath A., Savion S. (1982) Circ. Res. 51, 787–801 [DOI] [PubMed] [Google Scholar]
- 20. Scott J. D., Santana L. F. (2010) Circulation 121, 1264–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dodge-Kafka K. L., Langeberg L., Scott J. D. (2006) Circ. Res. 98, 993–1001 [DOI] [PubMed] [Google Scholar]
- 22. Dodge-Kafka K. L., Soughayer J., Pare G. C., Carlisle Michel J. J., Langeberg L. K., Kapiloff M. S., Scott J. D. (2005) Nature 437, 574–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kapiloff M. S., Piggott L. A., Sadana R., Li J., Heredia L. A., Henson E., Efendiev R., Dessauer C. W. (2009) J. Biol. Chem. 284, 23540–23546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pare G. C., Bauman A. L., McHenry M., Michel J. J., Dodge-Kafka K. L., Kapiloff M. S. (2005) J. Cell Sci. 118, 5637–5646 [DOI] [PubMed] [Google Scholar]
- 25. Pare G. C., Easlick J. L., Mislow J. M., McNally E. M., Kapiloff M. S. (2005) Exp. Cell Res. 303, 388–399 [DOI] [PubMed] [Google Scholar]
- 26. Marx S. O., Reiken S., Hisamatsu Y., Jayaraman T., Burkhoff D., Rosemblit N., Marks A. R. (2000) Cell 101, 365–376 [DOI] [PubMed] [Google Scholar]
- 27. Michel J. J., Townley I. K., Dodge-Kafka K. L., Zhang F., Kapiloff M. S., Scott J. D. (2005) Mol. Cell 20, 661–672 [DOI] [PubMed] [Google Scholar]
- 28. Kelley G. G., Kaproth-Joslin K. A., Reks S. E., Smrcka A. V., Wojcikiewicz R. J. H. (2006) J. Biol. Chem. 281, 2639–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Duale H., Kasparov S., Paton J. F., Teschemacher A. G. (2005) Exp. Physiol. 90, 71–78 [DOI] [PubMed] [Google Scholar]
- 30. Malik S., Ghosh M., Bonacci T. M., Tall G. G., Smrcka A. V. (2005) Mol. Pharmacol. 68, 129–136 [DOI] [PubMed] [Google Scholar]
- 31. Kapiloff M. S., Jackson N., Airhart N. (2001) J. Cell Sci. 114, 3167–3176 [DOI] [PubMed] [Google Scholar]
- 32. Wing M. R., Snyder J. T., Sondek J., Harden T. K. (2003) J. Biol. Chem. 278, 41253–41258 [DOI] [PubMed] [Google Scholar]
- 33. Simpson P. (1985) Circ. Res. 56, 884–894 [DOI] [PubMed] [Google Scholar]
- 34. Bunney T. D., Harris R., Gandarillas N. L., Josephs M. B., Roe S. M., Sorli S. C., Paterson H. F., Rodrigues-Lima F., Esposito D., Ponting C. P., Gierschik P., Pearl L. H., Driscoll P. C., Katan M. (2006) Mol. Cell 21, 495–507 [DOI] [PubMed] [Google Scholar]
- 35. Higazi D. R., Fearnley C. J., Drawnel F. M., Talasila A., Corps E. M., Ritter O., McDonald F., Mikoshiba K., Bootman M. D., Roderick H. L. (2009) Mol. Cell 33, 472–482 [DOI] [PubMed] [Google Scholar]
- 36. Wu X., Zhang T., Bossuyt J., Li X., McKinsey T. A., Dedman J. R., Olson E. N., Chen J., Brown J. H., Bers D. M. (2006) J. Clin. Invest. 116, 675–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vega R. B., Harrison B. C., Meadows E., Roberts C. R., Papst P. J., Olson E. N., McKinsey T. A. (2004) Mol. Cell. Biol. 24, 8374–8385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smrcka A. V., Sternweis P. C. (1993) J. Biol. Chem. 268, 9667–9674 [PubMed] [Google Scholar]
- 39. Aittaleb M., Boguth C. A., Tesmer J. J. G. (2010) Mol. Pharmacol. 77, 111–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang T., Brown J. H. (2004) Cardiovasc. Res. 63, 476–486 [DOI] [PubMed] [Google Scholar]
- 41. Oestreich E. A., Malik S., Goonasekera S. A., Blaxall B. C., Kelley G. G., Dirksen R. T., Smrcka A. V. (2009) J. Biol. Chem. 284, 1514–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.