Abstract
Cdc7 is a serine/threonine kinase conserved from yeasts to human and is known to play a key role in the regulation of initiation at each replication origin. Its catalytic function is activated via association with the activation subunit Dbf4/activator of S phase kinase (ASK). It is known that two conserved motifs of Dbf4/ASK are involved in binding to Cdc7, and both are required for maximum activation of Cdc7 kinase. Cdc7 kinases possess unique kinase insert sequences (kinase insert I–III) that are inserted at defined locations among the conserved kinase domains. However, precise mechanisms of Cdc7 kinase activation are largely unknown. We have identified two segments on Cdc7, DAM-1 (Dbf4/ASK interacting motif-1; amino acids 448–457 near the N terminus of kinase insert III) and DAM-2 (C-terminal 10-amino acid segment), that interact with motif-M and motif-C of ASK, respectively, and are essential for kinase activation by ASK. The C-terminal 143-amino acid polypeptide (432–574) containing DAM-1 and DAM-2 can interact with Dbf4/ASK. Characterization of the purified ASK-free Cdc7 and Cdc7-ASK complex shows that ATP binding of the Cdc7 catalytic subunit requires Dbf4/ASK. However, the “minimum” Cdc7, lacking the entire kinase insert II and half of kinase insert III, binds to ATP and shows autophosphorylation activity in the absence of ASK. However, ASK is still required for phosphorylation of exogenous substrates by the minimum Cdc7. These results indicate bipartite interaction between Cdc7 and Dbf4/ASK subunits facilitates ATP binding and substrate recognition by the Cdc7 kinase.
Keywords: Cell Cycle, DNA Replication, Phosphorylation Enzymes, Protein Kinases, Protein-Protein Interactions, Cdc7, Dbf4/ASK
Introduction
In eukaryotes, DNA replication begins with the assembly of a prereplicative complex (pre-RC)2 on the chromatin, which is generated on each potential origin through association of the origin recognition complex and MCM complexes during the G1 phase of the cell cycle. The MCM2–7 heterohexamer complex may function as the helicase that unwinds duplex DNA during S phase (1–7). Genetic and biochemical evidence has now emerged to support a model in which the initiation of DNA replication is regulated through two mutually exclusive chromatin states (1, 2). Although necessary, the assembly of pre-RCs during G1 is not sufficient to initiate DNA replication. During the G1-S transition, cells must enter the second state in which DNA replication can be initiated.
DNA replication initiation is strictly regulated by the cell cycle signals in the G1 phase. It can either be activated or suppressed by extracellular growth or differentiation signals, respectively (2). The G1 cell cycle signals are known to regulate Cdk-cyclin activity and ultimately activate E2F, leading to expression of various components of DNA replication machinery and activation of protein kinases (8). Among them, Cdk and Cdc7-Dbf4 are the two kinases activated by the G1 cell cycle signals and are known to play critical roles in activation of DNA replication origins (9–18).
In budding yeast, Cdc28 complexed with specific cyclin subunits controls the assembly and activation of pre-RCs (19). In addition, Cdc7 also plays an essential role as a second S phase-promoting kinase in promoting DNA replication (20). Like Cdks, whose activities require association with their activation subunit cyclins, Cdc7 kinase activity requires its own activation subunits, Dbf4/ASK (referred to ASK hereafter) or Drf1/ASKL1 (19–28), although there is little similarity in terms of the primary structure between Dbf4 and cyclins.
MCM is the key substrate of Cdc7 during DNA replication. Cdc7 is required throughout the S phase for activation of each replication origin (29, 30) presumably through phosphorylating MCM2, -4, and -6 subunits, which facilitates the loading of Cdc45 onto the pre-RC (31, 32). Hence, Cdc7 may play crucial roles in determining where and when DNA replication should be initiated in the eukaryotic genomes (2). Cdc7 has been implicated also in various other chromosome transactions, including meiotic recombination (33) and chromosome separation (34–36). Cdc7 may phosphorylate distinct targets in these processes. Thus, understanding the mechanism by which Cdc7 is activated by ASK and recognizes specific substrates would provide important information on how chromosome functions are regulated by Cdc7 kinase.
Dbf4-related subunits, conserved from yeasts to human, were shown to contain three conserved motifs, Dbf4-motif-N, -M, and -C (37). Both Dbf4-motif-M and -C are capable of binding to Cdc7 on their own, but each alone is not sufficient for full kinase activation (38, 39). The sequence between motif-M and motif-C is not essential, and its length can be varied without compromising the kinase activation, which leads to a bipartite binding model of ASK (38). In yeast, vegetative growth requires only motif-M (40), but the growth through meiosis requires both motif-M and motif-C (38). However, motif-N is also required for growth of mouse embryonic stem cells (41).
Cdc7 kinase consists of 11 kinase domains and two (or three) so-called “kinase insert” sequences unique to this family of kinases (20, 21, 42). The kinase domains are highly conserved through evolution, whereas the amino acid sequences of kinase insert II (amino acids, 275–368) and III (440–538) are more diverse (supplemental Fig. S1; kinase insert I of huCdc7 is very short). It has been reported that sequences within the Cdc7 kinase insert II interact with Importin and facilitate Cdc7 nuclear import (43). It was also reported that nuclear export signals are present in the C-terminal segments (44). However, how the two ASKs interact with Cdc7 and how this interaction leads to activation of Cdc7 kinase have been unclear.
The findings in this study show that a small segment within the Cdc7 kinase insert III and that near the C terminus of Cdc7, designated as DAM-1 and DAM-2, interact with motif-M and motif-C, respectively, and these bipartite interactions are essential for kinase activation. Furthermore, the 143-amino acid segment of Cdc7 containing these motifs can bind to ASK. We have purified free full-length Cdc7 and the Cdc7-ASK complex expressed in Escherichia coli and showed that Cdc7 in the complex, but not free Cdc7, bound to ATP. Deletion of kinase inserts led to the generation of a “minimum” Cdc7 that can bind to ATP and autophosphorylates in the absence of ASK. However, ASK is still required for efficient phosphorylation of exogenous substrates by the minimum Cdc7. These results lead us to conclude that ASK facilitates both ATP binding to the catalytic subunit and recognition of specific substrates.
EXPERIMENTAL PROCEDURES
Cells
HeLa and 293T cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum (Hana-Nesco Bio). muCdc7(−/−) ES cells were maintained in DMEM high glucose (Invitrogen, 11995-065) containing 20% FBS (Invitrogen, 16141-079), 1% nonessential amino acids (Invitrogen 11140-050), 2 mm l-glutamine (Invitrogen, 20530-081), 1% nucleotide mixture (Dainippon Sumitomo Pharma, R-ES-008D), 0.07% β-mercaptoethanol (Nacalai 214-38), and 103 units/ml ESGRO (Chemicon, ESG1107).
Antibodies
Phosphospecific antibodies recognizing phosphorylated S53 (A300-756A) and S41/42 (A300-788A) of MCM2 were from Bethyl Laboratories. The phosphospecific antibody, S6T7 (MCM4), was described before (31). Anti-ASK antibody was made against an ASK polypeptide (305–357 amino acids) in rabbit.
Plasmid Constructions
pME18S-HA vector was generated by inserting DNA sequences derived from the oligonucleotides (XhoI-HA-NotI-F 5′-TCG AGA TGT ACC CAT ACG ATG TTC CAG ATT ACG CTG C-3′ and XhoI-HA-NotI-R 5′-GGC CGC AGC GTA ATC TGG AAC ATC GTA TGG GTA CAT C-3′) encoding influenza hemagglutinin (HA) epitope tag between the XhoI and NotI sites of pME18S plasmid. The pME18S-HA-Cdc7 was constructed by cloning a cDNA encoding full-length human Cdc7 into the NotI and SpeI sites of pME18S-HA. A series of Cdc7 deletion mutants were generated by using Phusion® site-directed mutagenesis kit (New England Biolabs), as recommended by the supplier. Each construct was verified by nucleotide sequencing. The pCSII-EF-mKO2-HA-Cdc7 plasmid was generated by subcloning the HA-Cdc7 insert (XhoI-SpeI fragment) from pME18S-HA-Cdc7 plasmid into the CSII-EF-MCS vector at the XhoI-XbaI site. The pME18S-FLAG-ASK plasmid was generated as described previously (41).
Transfection
Either Lipofectamine 2000 (Invitrogen) or FuGENE HD (Roche Applied Science) was used for transfection into HeLa cells, and TransIT293 (Mirus) or polyethyleneimine (“MAX” reagent) solution (56) was used for transfection into 293T cells. Cells were harvested at 30 h after transfection, and protein expression was confirmed by Western blot analysis.
Ade-Cre Infection and Transient Transfection in muCdc7(−/−)tg ES Cells
Asynchronously growing muCdc7(−/−)tg ES cells (45) were infected by Ade-Cre (100 multiplicity of infection) and incubated for 24 h at 37 °C. Then pCSII-EF-mKO2-HA-Cdc7-derived plasmids expressing various forms of huCdc7 were transiently transfected into the cells by using FuGENE HD. Four days after transfection, growth recovery of transfected cells was examined under fluorescence microscopy.
Immunoprecipitation
Cells were resuspended in CSK buffer (10 mm PIPES-KOH, (pH 6.8), 100 mm potassium glutamate, 300 mm sucrose, 1 mm EGTA, 1 mm MgCl2, 1 mm dithiothreitol, 1 mm Na3VO4, 50 mm NaF, 0.1 mm ATP, complete protease inhibitor mixture (Roche Applied Science), and 0.1% Triton X-100). After incubation for 10 min at 4 °C, the suspension was centrifuged at 13,000 × g in a bench-top centrifuge for 10 min at 4 °C, and the supernatant was recovered. The anti-HA monoclonal antibody (clone 16B12, Covance) or the anti-FLAG M2 antibody (Sigma) was conjugated with Dynabeads protein G (Dynal) at room temperature for 40 min with rotation. The extract was incubated with antibody-conjugated beads at 4 °C for 1 h. The beads, washed four times with cold CSK buffer, were resuspended in SDS sample buffer for SDS-PAGE/Western blot analysis or washed once with 1× kinase buffer (40 mm Hepes-KOH (pH 7.6), 0.5 mm EDTA, 0.5 mm EGTA, 1 mm β-glycerophosphate, 1 mm NaF, and 2 mm dithiothreitol) and used in the in vitro kinase assay.
Expression and Purification of Cdc7T1, Cdc7T5, Cdc7T1-ASK, and Cdc7T5-ASK
For expression of Cdc7T5-ASK, the “minimum” Cdc7 sequence harboring amino acid residues 27–221, 371–486, and 537–574 was subcloned into the BamHI/SalI sites of the first multicloning site of modified pETDuet-1 (Novagen) with a His6 tag at the N terminus. The minimal ASKs, including residues 171–350, were subcloned into NdeI/XhoI sites of the second multicloning site. For expression of Cdc7T5 alone, the PCR product for Cdc7T5 was digested with BamHI/XhoI and inserted into pET21b. The recombinant plasmids were transformed in Escherichia coli BL21 (DE3) strain (Novagen). The expression of Cdc7T5 or Cdc7T5-ASK was induced with 0.5 mm isopropyl 1-thio-β-d-galactopyranoside at A600 = 0.45–0.7. Following incubation at 17 °C for 12 h, cells were harvested by centrifugation at 5,000 rpm for 15 min. The pellets were resuspended in lysis buffer (50 mm Tris-HCl (pH 7.5), 200 mm NaCl, 5 mm β-mercaptoethanol, 5% glycerol, and 1% Triton X-100). The cells were lysed by sonication and centrifuged at 12,000 rpm for 45 min. The supernatant was loaded onto a HisTrap HP column (Amersham Biosciences) pre-equilibrated with lysis buffer. The column was washed with 10 column volumes of 50 mm Tris-HCl (pH 7.5), 200 mm NaCl, 5 mm β-mercaptoethanol, 5% glycerol, and 20 mm imidazole, and Cdc7 T5 proteins were eluted with the same buffer containing 500 mm imidazole. The proteins were treated with tobacco etch virus protease for 12 h at 4 °C to cleave the His6 tag and further purified by the heparin HP column (Amersham Biosciences). The heparin fractions were reloaded onto HisTrap HP column (Amersham Biosciences), and flow-through fractions were collected. The proteins were finally polished through a Superdex 200 column (Amersham Biosciences) equilibrated with 2 column volumes of 20 mm Tris-HCl (pH 7.5), 200 mm NaCl, 1 mm DTT, and 5% glycerol. Fractions containing the Cdc7 T5 alone were collected and concentrated to 12 mg/ml using 10,000 nominal molecular weight limit (NMWL) device (Centricon). These fractions were further purified by the Mono S column, and activity was confirmed to comigrate with the T5 polypeptide (data not shown). Because there was no difference in activity between the Centricon-concentrated fraction and Mono S-purified fraction, we used the former fraction for our assays.
The full-length Cdc7 cDNA tagged with His6-tobacco etch virus protease site at the N terminus was subcloned at NdeI-XhoI sites of pETDuet, generating pETDuet-Cdc7 expressing His-tagged Cdc7T1. DNA fragment containing the minimal ASK was subcloned at NcoI-NotI sites of pETDuet-Cdc7 to express His-tagged Cdc7T1-ASK. Cdc7T1 and T1-ASK were expressed in BL21-CodonPlus(DE3)-RP strain and were purified by using Qiagen nickel-nitrilotriacetic acid-agarose in buffer A containing 40 mm Hepes-KOH (pH 7.6), 100 mm potassium glutamate, 1 mm EDTA, 10% glycerol, 1 mm DTT, and complete protease inhibitor mixture (Roche Applied Science). After the column was washed with buffer A containing 10 mm imidazole, protein was eluted with buffer A containing 50 mm imidazole. The peak fractions were further purified by a Mono Q column using SMART system. The protein was eluted with 0–1 m KCl gradient, and eluted fractions were dialyzed against buffer A.
In Vitro Kinase Assays of Cdc7
Standard in vitro kinase assay for Cdc7 (46) was conducted in a 25-μl reaction containing 40 mm Hepes-KOH (pH 7.6), 0.5 mm EDTA, 0.5 mm EGTA, 1 mm glycerophosphate, 1 mm NaF, 2 mm dithiothreitol, 2–8 mm MgOAc, 0.1 mm ATP, and 5–10 μCi of [γ-32P]ATP. Mouse MCM2-4-6-7 complex (0.1–0.5 μg) was used as substrate. Reactions were incubated at 30 °C for 60 min prior to loading onto 7.5% SDS-PAGE (59:1). The proteins were visualized with either silver or Coomassie Brilliant Blue staining. Gels were dried and subjected to autoradiography. GST-Cdc7-ASK complex, used as a control, was purified from insect cells, as described before (46).
In Vivo Photocross-linking Assays
A series of Cdc7 mutants carrying a termination codon TAG at various amino acid residues were generated by using Phusion® site-directed mutagenesis kit (New England Biolabs), as recommended by the supplier. Each of the Cdc7 mutants (HA-tagged) and full-length human ASK coding frame were inserted at two separate cloning sites in the pETDuet vector (Novagen) for co-expression. p-Benzoyl-l-phenylalanine (Bpa, Bachem) was used as photocross-link amino acid. BL21 (DE3), transformed with pSup-BpaRS-6TRN (47) and pETDuet-Cdc7Bpa mutant-ASK, was grown, and the two proteins were co-induced by addition of 1 mm isopropyl 1-thio-β-d-galactopyranoside for 20 h at 20 °C in the presence of 1 mm Bpa. The whole cell lysates were analyzed by SDS-PAGE/Western blotting. The successful cross-linking of the two proteins gives rise to a polypeptide with higher molecular mass, the identity of which can be verified by co-reactivity with anti-HA and anti-ASK antibodies.
In Vitro UV Photocross-linking Assays (48)
Proteins were mixed in 20 mm Tris-HCl (pH 7.5), 5 mm DTT, 10 mm MgCl2, 125 nm ATP, 9% glycerol with 10 μCi of [γ-32P]ATP (in 20 μl). The reaction mixtures placed on a parafilm (on ice) were UV-irradiated using a hand UV lamp for the times indicated. Reaction was terminated by adding 0.8 μl of 100 mm ATP and SDS sample buffer. The reaction mixtures were run on SDS-PAGE, stained with Coomassie Brilliant Blue, dried, and autoradiographed.
RESULTS
Requirement of the C-terminal Tail and a Part of Kinase Insert III of Cdc7 for Interaction with ASK and ASK-mediated Kinase Activation
We previously reported that the activation subunits of human and yeast interact with Cdc7 through their conserved segments, Dbf4-motif-M and Dbf4-motif-C (37–39). However, it is not known which segment of Cdc7 interacts with the Dbf4-related activation subunit. To determine the human Cdc7 segments involved in interaction with ASK, Cdc7 and its deletion derivatives were expressed in the CSII-EF-mKO2 vector plasmid, generating huCdc7-derived polypeptides tagged with mKO2 (monomeric Kusabira Orange 2) and HA (Fig. 1). These plasmids were transiently co-transfected into 293T cells or HeLa cells together with FLAG-tagged ASK plasmid. Extracts were prepared, and immunoprecipitation with anti-FLAG antibody was conducted. In addition to the wild-type Cdc7, several mutant Cdc7 polypeptides (Δ201–303(d3), Δ304–336(d4), Δ478–497(d8), and Δ497–525(d9)) were coimmunoprecipitated (supplemental Fig. S2 and data not shown; Table 1). All the mutants containing a deletion spanning the kinase domain (Δ1–100(d1), Δ101–200(d2), Δ337–438(d5), Δ525–544(d10), and Δ545–564(d11)) could not bind to ASK under the conditions employed, potentially due to the loss of conformational integrity. Two kinase insert III mutants (Δ438–456(d6) and Δ458–477(d7)) and a C-terminal tail mutant (Δ565–574(d12)) only inefficiently co-precipitated with ASK (d6) or did not co-precipitate with ASK (d7 and d12). These deletions are located outside the kinase domains and are less likely to induce a large conformational change. These results indicate that a portion of the kinase insert III and the C-terminal tail sequences are specifically required for binding to ASK. Some of the Cdc7 mutants that do not bind to ASK (Δ1–100(d1), Δ101–200(d2), Δ337–438(d5), Δ438–456(d6), Δ458–477(d7), and Δ525–544(d10)) were less stable than those binding to ASK. The ASK protein level also decreased when co-expressed with some of these mutants, suggesting that the complex formation may stabilize both proteins.
FIGURE 1.
Mapping of the segments of Cdc7 required for interaction with ASK. A, summary of various deletion derivatives of human Cdc7 cloned on monomeric Kusabira Orange2-HA fusion vector or on pME18S-HA. Black boxes represent the deleted segments in each mutant. Gray boxes indicate alanine substitution mutants generated (10A for amino acids (a.a.) 448–457 and 7A for 566–572). The numbers on both sides of the bars indicate the amino acids at the ends of each deleted fragment. A series of Cdc7 deletion derivatives was cloned into CSII-EF-MCS lentiviral vector carrying both HA- and mKO2 (monomeric Kusabira Orange 2)-fluorescent tags or the pME18S vector carrying HA tag. B, plasmid DNAs expressing various deletion derivatives of Cdc7, as indicated, were transfected into 293T cells alone or in combination with pME18S-FLAG-ASK. The immunoprecipitates prepared with anti-FLAG antibody were analyzed by Western blotting using anti-HA antibody (upper panel) to detect co-immunoprecipitated mKO2-HA-Cdc7 derivatives and anti-FLAG antibody (lower panel) to detect immunoprecipitated FLAG-ASK. IP, immunoprecipitate; Input, the starting extract used (10% of the immunoprecipitate). In most cases, mKO2-HA-Cdc7 bands appear as doublets due to proteolytic degradation. In some cases, ASK bands migrate as a series of mobility-shifted bands due to the autophosphorylation induced by the associated Cdc7. C, 293T cells were transfected by pEF-mKO2-HA-Cdc7 wild-type or deletion derivatives with or without pME18S-FLAG-ASK, as indicated, and extracts were prepared. The immunoprecipitates prepared with anti-HA antibody (lanes 1–7) were used for kinase assays by using MCM2-4-6-7 complex as a substrate. The reaction mixtures were run on SDS-PAGE (7.5%, 59:1) and the silver-stained pattern (lower panel) and autoradiogram (upper panel) are shown. The lower graph shows the quantification of the 32P incorporation into the MCM2 bands. D and E, wild-type Cdc7 and its deletion derivatives were co-transfected with GFP-ASK motif-M or motif-C polypeptide (39) into 293T cells. The immunoprecipitates prepared with anti-HA antibody (IP) were analyzed by Western blotting using anti-GFP antibody (upper panel) to detect co-immunoprecipitated GFP-ASK polypeptides and anti-HA antibody (lower panel) to detect immunoprecipitated HA-Cdc7. Input, the starting extract used (10% of the immunoprecipitate). The asterisk indicates degradation products of GFP-Motif-M and GFP-Motif-C polypeptides.
TABLE 1.
Summary of deletion derivatives of human Cdc7 kinase
KI indicates kinase insert. In the 2nd column (“Location of deletion”), the location of the deletion is shown in parentheses. Where there is no parentheses, the deletions are present in the kinase conserved domains.
| Name of the Cdc7 deletion | Location of deletion | ASK binding | Kinase activity |
|
|---|---|---|---|---|
| Mobility shift of ASK | In vitro phosphorylation of MCM | |||
| D1 | 1–100 | − | − | − |
| D2 | 101–200 | − | − | − |
| D3 | 201–303(KI-II) | + | + | + |
| D4 | 304–336(KI-II) | + | + | + |
| D5 | 337–438 | − | − | − |
| D6 | 438–456(KI-III) | +/− | − | − |
| D7 | 458–477(KI-III) | − | − | − |
| D8 | 478–497(KI-III) | + | + | + |
| D9 | 497–525(KI-III) | + | + | + |
| D10 | 525–544 | − | − | − |
| D11 | 545–564 | − | − | − |
| D12 | 565–574(C-ter) | − | − | − |
| Full length | − | + | + | + |
We next examined the kinase activities of Cdc7 mutant proteins. The active Cdc7-ASK complex autophosphorylates both subunits, resulting in the mobility shift of ASK subunits on SDS-PAGE primarily due to phosphorylation of the C-terminal serine and threonine-rich tail segment (39, 53). This phosphorylation is likely to occur in an intramolecular manner, because dilution of Cdc7-ASK did not affect the level of phosphorylation (supplemental Fig. S3). We used this mobility shift as an indicator for the kinase activity. FLAG-ASK and mKO2-HA-Cdc7 mutant proteins were transiently co-expressed in HeLa cells, and Triton-soluble extracts were analyzed by Western blotting. The Cdc7 mutants (Δ201–303(d3), Δ304–336(d4), Δ478–497(d8), and Δ497–525(d9)) induced phosphorylation of ASK, whereas the Cdc7 mutants (Δ1–100(d1), Δ101–200(d2), Δ337–438(d5), Δ438–456(d6), Δ458–477(d7), Δ525–544(d10), Δ545–564(d11), and Δ565–574(d12)) did not induce mobility shift of ASK, suggesting that they are not active kinases or deficient in autophosphorylation of the ASK C terminus. The latter eight mutants did not efficiently interact with ASK, consistent with the fact that complex formation is essential for Cdc7 kinase activation.
To measure Cdc7 kinase activity more precisely, we examined the kinase activity of the immunoprecipitated Cdc7-ASK from HeLa cells transiently co-expressing FLAG-ASK and mKO2-HA-Cdc7 mutant on the MCM2-4-6-7 complex in vitro. The results indicate that the same set of the mutants (Δ201–303(d3), Δ304–336(d4), Δ478–497(d8), and Δ497–525(d9)) supports the phosphorylation of MCM2 (Table 1). These results are basically consistent with those of the autophosphorylation assays, indicating that the N-terminal half of the kinase insert III and the very C-terminal segment (Δ565–574) are required for binding to ASK and activation of the kinase.
Identification of Two ASK Interacting Motifs, DAM-1 and DAM-2
To further specify a segment critical for ASK binding and kinase activity, mutants bearing smaller deletions at the N terminus of Cdc7, C terminus of kinase insert II, kinase domains VIII–X, and the N terminus of kinase insert III were generated (Fig. 1 and Table 2) and characterized. FLAG-ASK and these new mKO2-HA-Cdc7 mutants were transiently co-expressed in 293T cells, and ASK autophosphorylation was analyzed. Δ1–57(d13), Δ463–467(d23), and Δ468–472(d24) caused mobility shift of ASK. In comparison, mobility shift was reduced in Δ438–442(d18), and deletions between 443 and 462 (d19–22) as well as those between 337 and 438 (d14–17) did not cause significant mobility shift (Fig. 1B and data not shown), suggesting these segments are important for efficient kinase activity or phosphorylation of the C-terminal tail of ASK. The loss of kinase activity would be expected for d15–17, because these lack a part of the kinase domains. Co-immunoprecipitation assays showed that d13–19 and d22–24 bound to ASK (Fig. 1B).
TABLE 2.
Summary of deletion or mutation derivatives of human Cdc7 kinase
KI indicates kinase insert. In the second column (“Location of deletion (or mutation)”), the location of the deletion/mutation is shown in parentheses. Where there is no parentheses, the deletions are present in the kinase conserved domains.
| Name of the Cdc7 deletion | Location of deletion (or mutation) | ASK binding | Kinase activity |
|
|---|---|---|---|---|
| Mobility shift of ASK | In vitro phosphorylation of MCM | |||
| D13 | 1–57 | + | + | + |
| D14 | 337–361(KI-II) | + | − | +/− |
| D15 | 362–386 | + | − | − |
| D16 | 387–412 | + | − | − |
| D17 | 413–438 | + | − | − |
| D18 | 438–442(KI-III) | + | +/− | + |
| D19 | 443–447(KI-III) | + | − | + |
| D20 | 448–452(KI-III) | +/− | − | +/− |
| D21 | 453–457(KI-III) | − | − | − |
| D22 | 458–462(KI-III) | + | − | − |
| D23 | 463–467(KI-III) | + | + | + |
| D24 | 468–472(KI-III) | + | + | + |
| 10A(DAM-1) | 448–457(KI-III) alanine-substituted | +/− | +/− | − |
| 7A(DAM-2) | 566–572 alanine-substituted | w | − | − |
| Full length | − | + | + | + |
We have conducted kinase assays with these mutants. The results of kinase assays are largely consistent with those of mobility shift of ASK. d12 and d21 showed almost no kinase activity, whereas d20 exhibited significantly reduced activity (Fig. 1C). d18 and d19 were active despite reduced mobility shift of ASK (data not shown). d13 was also active. The results confirm that interaction with ASK is essential for efficient kinase activity.
d14 (Δ337–361; lacking a C-terminal segment of kinase insert II) and d22 (Δ458–462) interacted with ASK but did not cause mobility shift of ASK (Fig. 1B) and showed only a low level of kinase activity (Fig. 1C and data not shown). These segments may be required specifically for activation of kinase.
Because Dbf4-motif-M and -C are known to interact with Cdc7, we examined the interaction of Δ448–452(d20), Δ453–457(d21), and Δ565–574(d12) with GFP-fused ASK motif-M or motif-C polypeptides (Fig. 1, D and E) (30). As reported before, the wild-type Cdc7 interacted with both motif-M and motif-C polypeptides (Fig. 1, D and E, lanes 2 and 4). In contrast, Δ448–452(d20) and Δ453–457(d21) were shown to interact with motif-C but not with motif-M, whereas Δ565–574(d12) interacted with motif-M but not with motif-C (Fig. 1, D and E). This result indicates that the C-terminal tail and kinase insert III motif of Cdc7 may be critical for interaction with motif-C and motif-M, respectively, of ASK (see Fig. 8) and provides a molecular model for bipartite interaction between Cdc7 and ASK. We named the two critical segments, amino acid residues 448–457 and 566–572, DAM-1 (Dbf4/ASK interacting motif) and DAM-2, respectively.
FIGURE 8.
Dissection of human Cdc7 kinase, two segments, DAM-1 and DAM-2, required for interaction with ASK. A, schematic representation of human Cdc7 polypeptide showing two essential motifs (DAM-1 and DAM-2 indicated by the pink boxes) required for interaction with ASK. Double-arrowed lines indicate that DAM-1 and DAM-2 interact with motif-M and motif-C, respectively, of ASK. The segment shown by the purple box is not required for interaction with ASK but may be required for full kinase activity in the context of full-length polypeptide. The numbers on both sides of each box indicate the amino acids (a.a.) at the ends of each segment. B, schematic drawing of a proposed mode of interaction between Cdc7 and ASK. M and C indicate motif-M and -C, respectively, of ASK. It is proposed that ATP may not have access to the ATP-binding pocket of free full-length Cdc7 polypeptide possibly due to blockage caused by the kinase insert sequences (left, suggested by the fact that minimum Cdc7 lacking KI-II and half of KI-III showed ASK-independent kinase activity, but is not explicitly depicted in the figure). ASK may bind to the larger lobe of Cdc7 kinase, through bipartite interactions (motif-M versus DAM-1 and motif-C versus DAM-2), and this binding may induce conformational change on Cdc7 and permit the binding of ATP to the ATP pocket of the kinase (right). The binding also facilitates the recognition of specific target sites on the substrate. The location of ASK on Cdc7 in this model may provide basis for further structural and biochemical studies on the mechanisms of kinase activation and substrate recognition. KI-II, kinase insert II; KI-III, kinase insert III; C-ter, C-terminal tail segment.
Physical Interaction between ASK and Cdc7 Kinase Insert III
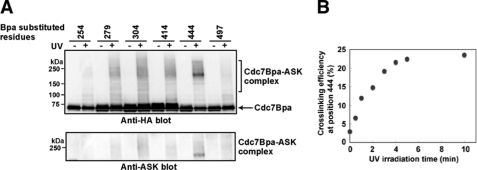
To evaluate the possibility that kinase insert III or C-terminal tail directly interacts with ASK, Bpa photocross-link assays were conducted. This system permits the expression of proteins containing the unnatural amino acid, Bpa, at specific sites designated by amber (TAG) nonsense codons introduced by mutagenesis (47). A TAG-containing Cdc7 and wild-type full-length ASK were co-expressed in E. coli in the presence of a multicopy plasmid carrying genes for an amber suppressor tRNA and BpaRS, a Bpa-tRNA synthetase. Bpa would be introduced at the TAG positions on Cdc7. Upon excitation at 365 nm, a wavelength that avoids protein damage, Bpa preferentially reacts with otherwise inactive carbon-hydrogen bonds (C–H). Furthermore, in contrast to other photocross-linking groups, Bpa does not photodissociate, and its photoexcited triplet state readily relaxes in the absence of a suitable C–H bond with which to react. Amber (TAG) substitutions were inserted at 22 different sites on Cdc7 (supplemental Fig. S4). After induction of expression, the cells were UV (365 nm)-irradiated, and extracts were then prepared. The successful cross-link would generate the Cdc7-ASK complex as a fused polypeptide on SDS-PAGE, which would be detected by both anti-HA (for Cdc7) and anti-ASK blot. The strongest signal was detected with Cdc7 (Bpa444), in which Gly-444 was substituted by Bpa (Fig. 2A). The efficiency of cross-link of Cdc7 (Bpa444)-ASK complex increased with UV irradiation time (Fig. 2B). The result suggests that the region near Gly-444 is geometrically close to ASK, suggesting that the Cdc7 kinase insert III directly interacts with ASK. Some cross-link signals were detected at amino acids 279, 304, and 414 as well as at 467, 483, and 513 (Fig. 2A and data not shown), but they were weaker compared with Gly-444 and gave rise to broader shifted bands. Thus, direct contact at these sites, if any, may need to be confirmed by other methods. At other sites, signals were not detected by one of the antibodies or only weak broad signals were detected by anti-HA antibody only (data not shown). It should also be noted that some of the Bpa mutations may result in disrupted protein structure of Cdc7. Thus, the absence of specific interaction in this assay may need to be evaluated carefully.
FIGURE 2.
Identification of the residues on Cdc7 directly interacting with ASK. A, UV photocross-linking between selected Cdc7-Bpa and ASK. Protein expression was induced in the E. coli BL21(DE3) strain carrying pSup-BpaRS-6TRN (47) and Bpa-substituted pETDuet-Cdc7-ASK by addition of 0.1 mm isopropyl 1-thio-β-d-galactopyranoside for 20 h at 20 °C in the presence of 1 mm Bpa. The harvested cells were exposed to 365 nm light for 5 min on ice. Then the cells were sonicated and loaded on a 5–20% gradient SDS-PAGE (ATTO). Cdc7 and ASK were detected with anti-HA and anti-ASK antibodies, respectively. Cdc7 and ASK are 574 (migrating at 65–70 kDa on SDS-PAGE) and 674 amino acids (migrating at 75–80 kDa) long, respectively, and the cross-linked protein is expected to be 140–150 kDa. However, it is expected to run at a larger size, because both subunits are autophosphorylated. B, efficiency of cross-link of Cdc7 (Bpa444)-ASK complex in response to different duration of UV irradiation. Cdc7 (Bpa444) and ASK were co-induced in BL21 (DE3) as described (A) and were UV-irradiated for 0–10 min on ice-cold dishes. The efficiency of the cross-link was estimated by calculating the ratio of the intensity of the shifted band to that of the sum of the noncross-linked Cdc7 band and shifted band. The efficiency increased with the time of UV irradiation and reached a plateau in 5 min.
Point Mutations in Each of the Two Segments Results in Loss of Interaction with ASK
DAM-1 and DAM-2 were further analyzed by point mutations. All the 10 or 7 amino acids, respectively, of DAM-1 or DAM-2 were substituted with alanine. The resulting DAM-1(10A) or DAM-2(7A) mutant was co-expressed with FLAG-ASK in 293T cells. The hyperphosphorylation of ASK was diminished with the DAM-1 mutant and was almost completely lost with the DAM-2 mutant (Fig. 3A), suggesting the mutations compromise the kinase activity of Cdc7. Interaction with ASK was reduced with the DAM-1 mutant and was completely lost with the DAM-2 mutant (Fig. 3B). The results indicate that these residues are specifically required for interaction with ASK. Consistent with this, these Cdc7 mutants were not active as kinase even in the presence of ASK (Fig. 3C).
FIGURE 3.
Two motifs, DAM-1 and DAM-2, of Cdc7 are required for interaction with ASK and kinase activation. pME18S-HA-Cdc7WT, pME18S-HA-Cdc7(448A457; 10A), or pME18S-HA-Cdc7(566A572; 7A) was transfected into 293T cells with or without pME18S-FLAG-ASK, as indicated. A, whole cell extracts were prepared and analyzed on SDS-PAGE (7.5% (59:1)) by Western blotting. Upper panel, anti-HA antibody; lower panel, anti-FLAG antibody. B, immunoprecipitates prepared with anti-FLAG antibody were analyzed by Western blotting using anti-HA antibody to detect co-immunoprecipitated HA-Cdc7 proteins. Upper panel, input (10% of the immunoprecipitate (IP)); lower panel, anti-FLAG antibody immunoprecipitate. C, immunoprecipitates prepared with anti-HA antibody were used for kinase assays on MCM2-4-6-7. The samples were analyzed on 7.5% SDS-PAGE (bisacrylamide:acrylamide, 99:1). Upper panel, autoradiogram; middle panel, silver staining; lower panel, immunoblot with anti-HA antibody to detect Cdc7 protein.
We then examined whether the small segment of Cdc7 containing DAM-1 and DAM-2 can interact with ASK. We expressed the 143-amino acid segment derived from the C-terminal section of Cdc7 (432–574) with the FLAG or Myc tag and examined the interaction between Myc- or GFP-tagged ASK, respectively, after co-expressing the two proteins in 293T cells. We noticed that the Cdc7-C fragment was not expressed efficiently by itself. However, its expression increased when ASK was co-expressed (Fig. 4, compare lanes 1 and 2). We detected the FLAG-ASK or GFP-ASK polypeptide in the Myc-Cdc7C or FLAG-Cdc7C immunoprecipitate, respectively (Fig. 4), although the efficiency was low. We suspect that the Cdc7C polypeptide can interact with ASK, and this interaction stabilizes the former polypeptide. This result strongly indicates that ASK interacts with the C-terminal segment of Cdc7 containing DAM-1 and DAM-2.
FIGURE 4.
Interaction of an isolated Cdc7 C-terminal polypeptide with ASK. A plasmid expressing FLAG- or Myc-tagged Cdc7 C-terminal polypeptide (432–574) was transfected into 293T cells together with Myc- or GFP-tagged ASK plasmid, respectively, as indicated in the figure. The immunoprecipitates (IP) prepared with anti-Myc antibody (lanes 1 and 2) or with anti-FLAG antibody (lanes 3 and 4) were analyzed by Western blotting using antibodies shown on the left side of each panel. Input, the starting extract used (10% of the immunoprecipitate). Note that only a very low level of myc-Cdc7C can be detected when transfected alone (lane 1) due to apparent instability of the polypeptide, which is stabilized when co-expressed with ASK (lane 2). For extracts used in lanes 3 and 4, MG132 (10 μm) was added for 6 h before the harvest to prevent protein degradation. FLAG-ASK alone expressed in 293T cells is not immunoprecipitated by anti-Myc antibody (data not shown). The asterisk indicates a nonspecifically reacting band.
Characterization of Free Cdc7 and Cdc7-ASK Complex
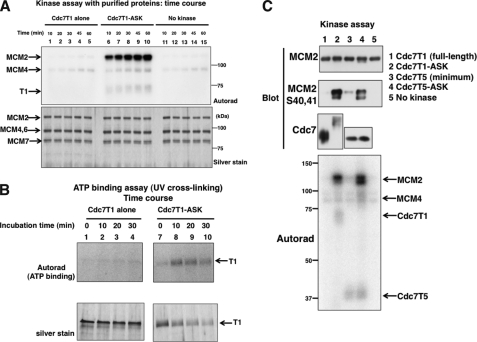
To investigate the mechanism of Cdc7 kinase activation by ASK, we attempted to purify the Cdc7-ASK complex and free Cdc7 polypeptide. The full-length Cdc7 construct (T1) was expressed alone or in combination with the minimum ASK (amino acids 171–350) containing motif-M and motif-C in E. coli. The Cdc7 was tagged with His6, and the protein was purified by a nickel column, followed by Mono Q ion chromatography. Although both free Cdc7 and Cdc7-ASK complex were eluted from Mono Q in a similar manner, only the Cdc7-ASK complex showed vigorous kinase activity on MCM2-4-6-7 (supplemental Figs. S5 and S6). We did not detect any phosphorylation over the background level with the free Cdc7 protein (Fig. 5A), indicating that Cdc7 kinase activity completely depends on the presence of ASK.
FIGURE 5.
Characterization of purified Cdc7 kinase (T1, full-length) protein with or without associated ASK subunit. A, purified full-length Cdc7T1 or full-length Cdc7T1-minimum ASK complex (fraction 11 from the Mono Q column fractionation; see supplemental Fig. S5) was used in kinase assays with MCM2-4-6-7 as a substrate. Incubation was at 30 °C for the time indicated. Lanes 1–5, T1 alone (25 ng); lanes 6–10, T1-ASK(25 ng); lanes 11–15, no kinase added. The proteins were analyzed on 8% SDS-PAGE (bisacrylamide:acrylamide, 29:1). B, ATP binding (UV cross-linking) assay of T1 and T1-ASK. ATP photocross-linking assays were conducted for the indicated time, as described under “Experimental Procedures.” Lanes 1–4, Cdc7T1 (50 ng); lanes 5–8, Cdc7T1-ASK (50 ng). Upper panel, autoradiogram; lower panel, silver staining of the same gel. C, MCM2-4-6-7 was phosphorylated in vitro by Cdc7 (T1 or T5; 25 ng each), as indicated in the figure, in the presence of [γ-32P]ATP. T5 is the minimum Cdc7 polypeptide. See Fig. 6A for details on Cdc7 T5. The products were run on 8% (29:1) SDS-PAGE. The bottom panel is the autoradiogram of the gel, and the phosphorylated proteins are indicated. In other panels, the proteins were detected by the antibodies indicated to the left of each panel.
We then examined the ATP binding by UV-photocross-linking assays. With the identical amount of the Cdc7 polypeptide, we saw the significant ATP binding only with the Cdc7-ASK complex (Fig. 5B), suggesting that ASK stimulates ATP binding of the Cdc7 catalytic subunit possibly by altering the conformation of the latter subunit.
MCM2 and MCM4 are major phosphorylation targets of Cdc7 kinase both in vivo and in vitro. We examined the phosphorylation of specific residues on them by using phospho-specific antibodies. The antibody recognizing the phosphorylation of MCM2, MCM2 S40S41 (49), was used. As expected, the phosphorylation of these residues was observed with the Cdc7T1-ASK complex but not with Cdc7T1 alone (Fig. 5C, lanes 1 and 2).
ASK-independent Kinase Activity and ATP Binding of the Minimum Cdc7
On the basis of the above results, we have constructed the smallest version of Cdc7 kinase containing the amino acid residues 27–221, 371–486, and 537–574 (designated as T5; Fig. 6A). The T5 construct was expressed alone or in combination with the minimum ASK in E. coli. The Cdc7 was tagged with His6, and the protein was purified by nickel column, followed by tobacco etch virus protease-mediated cleavage of the tag (supplemental Fig. S6). We compared the kinase activity of free Cdc7T5 and Cdc7T5-ASK complex. As shown in Fig. 6B, the titration of the kinase indicates that the free T5, at the highest concentration attempted, supported the phosphorylation of MCM at the level one-twentieth of that achieved by the T5-ASK complex. However, the level of Cdc7 autophosphorylation was not affected by the presence of ASK (T5 polypeptide shown by a arrows in Figs. 5C and 6B). The results indicate that the minimum Cdc7 retains basal phosphorylation activity on its own, in contrast to no detectable activity in the full-length Cdc7 alone (Fig. 5, A and C).
FIGURE 6.
Characterization of purified minimum Cdc7 kinase (T5) protein with or without associated ASK subunit. A, schematic drawing showing the segments of Cdc7 present in the minimum Cdc7 polypeptide (Cdc7T5; shown on top of the Cdc7 bar). Cdc7T5 was expressed alone or together with minimum ASK in E. coli, and Cdc7T5 and Cdc7T5-ASK were purified as described under “Experimental Procedures.” B, titration of Cdc7T5 and Cdc7T5-minimum ASK in the in vitro kinase assays with MCM2-4-6-7 as a substrate. Kinase added were as follows: lane 2, 1 ng; lane 3, 5 ng; lanes 4 and 9, 10 ng; lanes 5 and 10, 50 ng; lanes 6 and 11, 100 ng; lanes 7 and 12, 200 ng. Lane 13, 50 ng of GST-Cdc7-ASK (from insect cells); lanes 1 and 14, no kinase added. The asterisk indicates the mobility shifted band induced by Cdc7-mediated phosphorylation. C, ATP binding (UV photocross-linking) assay of T5 and T5-ASK. UV photocross-linking assays were conducted for the indicated time, as described under “Experimental Procedures.” Lanes 1–6, Cdc7T5 (0.5 μg); lanes 7–12, Cdc7T5-ASK (0.5 μg). Upper panel, autoradiogram; lower panel, Coomassie staining of the same gel. D, MCM2-4-6-7 was phosphorylated in vitro by Cdc7 (T5 or T5-ASK) in the presence of [γ-32P]ATP. Lanes 1–5 contain 25 ng of Cdc2-cyclinB1 protein. Amount of Cdc7 added was as follows: T5, 50 ng (lanes 2 and 7) and 200 ng (lanes 3 and 8); T5-ASK, 25 ng (lanes 4 and 9) and 100 ng (lanes 5 and 10). Upper panel, autoradiogram; upper middle panel, blot with anti-MCM2 phosphorylated S53 antibody, lower middle panel, blot with anti-MCM4 phosphorylated S6T7 antibody; lower panel, silver staining of the gel showing the T5 polypeptide.
We then examined the ATP binding of T5 and T5-ASK by UV-photocross-linking assays. The result indicates that there is no effect of the presence of ASK on the level of ATP binding (Fig. 6C), indicating that the minimum Cdc7 is able to bind to ATP on its own, consistent with its autophosphorylation activity.
However, much more efficient phosphorylation of the MCM subunits by the T5-ASK complex compared with the T5 alone suggests that the presence of ASK may affect the affinity of the kinase to exogenous substrate proteins. Therefore, we examined the phosphorylation by using phospho-specific antibodies. Kinase assays were conducted in the absence and presence of a low concentration of Cdc2-cyclinB1 protein, which was shown to significantly stimulate Cdc7-mediated phosphorylation of MCM proteins (46). Indeed, a more intense shifted band was observed in the presence of Cdc2-cyclinB1 (compare lanes 5 and 10 of Fig. 6D). Two different concentrations of T5 and T5-ASK were examined. 25 ng of T5-ASK and 200 ng of T5 gave similar levels of overall phosphorylation (Fig. 6D). Under this condition, anti-MCM2 S53 antibody detected the nonshifted form as well as the fast-migrating shifted bands that correspond to those observed in the cells during S phase on the chromatin (49). The intensities of the shifted forms were much stronger with 25 ng of T5-ASK than those with 200 ng of T5 (compare lanes 8 and 9 of Fig. 6D). The S53 signal with 100 ng of T5-ASK was nearly 10-fold stronger over that observed with 200 ng of T5, whereas total incorporation differed only by 3.5-fold between 100 ng of T5-ASK and 200 ng of T5 (data not shown). The presence of ASK also stimulated phosphorylation of S40 and S41 of MCM2 by T5 (Fig. 5C, lanes 3 and 4). In contrast, phosphorylation at S6T7 (of MCM4) depends on Cdc2-cyclinB1 and is observed at similar levels between T5 and T5-ASK (lanes 2–5 of Fig. 6D). These results indicate that the presence of ASK differentially affects Cdc7T5-mediated phosphorylation of different residues on the MCM substrate. Thus, the minimum Cdc7 is intrinsically active, but its affinity to specific substrate (or residues) is still mostly dependent on the presence of ASK.
In Vivo Functions of Mutant Cdc7
It has been well established in yeasts and Xenopus egg extracts that Cdc7 kinase activity is essential for cell viability. Because human Cdc7 kinase depends on ASK for expression of its kinase activity and ASK is indispensable for proliferation of mouse embryonic stem cells (41), it would be reasonable to speculate that the Cdc7 mutant defective in ASK binding and kinase activity would be nonfunctional in supporting the growth. However, it is not known whether cell cycle oscillation of the protein level, nuclear accumulation, or chromatin binding of Cdc7 kinase is required for maintenance of viability in mammalian cells. Therefore, we used the muCdc7 conditional knock-out ES cells (muCdc7(−/−)tg) in which Cdc7 genes can be conditionally inactivated (45) to evaluate the functions of each mutant. The removal of the transgene by infection of Ade-Cre leads to cessation of DNA synthesis, growth arrest, and eventual cell death of muCdc7(−/−)tg cells (supplemental Fig. S7) (45).
The ability of mutant Cdc7 to rescue the growth defect in this system was examined (Fig. 7). Asynchronous muCdc7(−/−)tg ES cells were infected by Ade-Cre and then a plasmid expressing a mKO2-HA-Cdc7 derivative was transiently transfected. Four days after transfection, cellular morphology and expression of mutant proteins were analyzed by fluorescence microscopy. The cells expressing wild-type and mutant Cdc7 (Δ201–303(d3), Δ304–336(d4), Δ478–497(d8), and Δ497–525(d9)) exhibited the presence of a viable cell population, whereas others showed cellular states similar to the control cells. The results show that the same set of the mutants supporting the ASK and MCM2 phosphorylation can at least partially complement the Cdc7 defect in vivo. Δ201–303(d3) and Δ304–336(d4) are partially defective in nuclear accumulation,3 but obviously this deficiency does not critically affect the essential role of Cdc7 in supporting viability under the experimental conditions employed.
FIGURE 7.
Complementation assays of mutant Cdc7 proteins using conditional muCdc7(−/−) ES cells. Asynchronously growing muCdc7(−/−)tg ES cells (45) were infected by Ade-Cre and incubated for 24 h. Then a plasmid expressing a mKO2-HA-Cdc7 derivative was transiently transfected into the cells. At 4 days after transfection, the cells were examined under a fluorescence microscope. Red, mKO2 signals; green, GFP signals (GFP is induced after successful Cre-mediated cleavage (45)) DIC, differential interference contrast.
DISCUSSION
Identification of Two ASK-binding Motifs, DAM-1 and DAM-2, on Cdc7
In this study, we examined the segments of human Cdc7 kinase required for interaction with ASK and kinase activation (Fig. 1). Among the Cdc7 truncated mutants generated, the ability to form a complex with ASK largely correlated with that to induce hyperphosphorylation of ASK and to support phosphorylation of MCM in vitro. We have identified two segments, DAM-1 and DAM-2, that are essential for ASK binding and full kinase activity. Fine mapping and mutational analyses showed that 10 amino acids in the kinase insert III (448–457; DAM-1) and 7 amino acids in the C-terminal tail segment (566–572; DAM-2) are required for interaction with ASK.
In yeasts, Cdc7 or Hsk1 kinase carries a C-terminal tail sequence that was reported to be required for interaction with the Dbf4 or Dfp1/Him1 activation subunit, respectively (23, 50), suggesting a conserved role of the C-terminal tail for interaction with the kinase. In fact, the alignment of Cdc7 of human, Xenopus, Schizosaccharomyces pombe, and Saccharomyces cerevisiae shows that the above 7 amino acids (HPFFKDM) are conserved in the Cdc7 family members (supplemental Fig. S1). We predicted secondary structures of kinase inserts II and III of human Cdc7 and also compared the sequences with those from Xenopus, S. pombe, and S. cerevisiae. Kinase insert II has little helical structures. In contrast, kinase insert III has many α-helices especially in its N-terminal half containing a putative ASK-binding segment. This feature of kinase insert III is conserved in mouse, Xenopus, S. pombe, and S. cerevisiae.
Prediction on the Mode of Interaction between Cdc7 and ASK
We further predict the mode of Cdc7-ASK interaction on the basis of the three-dimensional structure of kinases. Cdc7 is most closely related to casein kinase in terms of primary structure (20% identity and 57% similarity in the kinase conserved domains). On the basis of the known three-dimensional structure of the rat casein kinase 2 α subunit (Protein Data Bank code 2R7I), we predicted the spatial positions of the C-terminal tail and the N-terminal segment of the kinase insert III in Cdc7 (supplemental Fig. S8). We noted that the kinase insert III is located at the distant edge of the large lobe. The C-terminal tail is probably located closer to the cleft, but on a similar surface, and both of them are required for interaction with ASK. Indeed, the C-terminal 143 amino acids can interact with ASK albeit with low affinity (Figs. 4 and 8), suggesting that this can form a domain capable of interacting with ASK. The kinase insert III (DAM-1) is required for interaction with motif-M, whereas the C-terminal tail (DAM-2) is essential for interaction with motif-C. Thus, it is likely that ASK binds to Cdc7 at this surface of the molecule through the two Cdc7-binding modules (Fig. 8). The results presented in this study suggest that Dbf4-motif-M and Dbf4-motif-C (two Cdc7-binding modules on ASK) may directly interact with DAM-1 and DAM-2, respectively (Fig. 8). In contrast, the kinase insert II is located close to catalytic bases. Deletion of this segment does not affect the Cdc7-ASK interaction, but ASK autophosphorylation and kinase activity are reduced. This may suggest that the sequences present N-terminal to the kinase domain VIII, which are partially conserved across species (supplemental Fig. S1 and Fig. 8A), may affect the accessibility of the catalytic centers to the C-terminal tail of the associated ASK as well as to other exogenous substrates. It is also possible that partial deletion of the kinase insert II may have caused its conformational change or that of the kinase conserved domains adjacent to it, reducing the catalytic activity of the mutant protein.
These results are in contrast to the segments of Cdk required for cyclin binding. Most of the interacting surfaces were mapped to the N-terminal segment, including the conserved PSTAIRE sequence, of Cdk (51, 52). They are located primarily on the small lobe near its interface with the cleft (51). Thus, the mechanism for activation of Cdc7 by ASK may be distinct from that of Cdk by cyclins.
ASK interacts with the C-terminal 143 amino acids, albeit weakly. The C-terminal fragment bound to ASK lacking its long C-terminal segment more efficiently than to the full-length ASK.4 This suggests the possibility that the C-terminal segment of ASK inhibits the binding of Cdc7 DAM-1 and DAM-2 to ASK. This is consistent with the report that the C-terminal segment of ASK may be inhibitory for the kinase activity of Cdc7-ASK (53).
ASK-independent Cdc7 Kinase Activation in the Minimum Cdc7
The minimum Cdc7 lacking kinase insert II and a part of kinase insert III binds ATP on its own and undergoes autophosphorylation in the absence of ASK. This indicates that kinase insert sequences somehow exert negative effect on the access of ATP to the ATP pocket, and ASK binding relieves this inhibition. Constitutively active minimum Cdc7 may be a useful tool to probe the functions of Cdc7, because it will enable us to elevate the Cdc7 kinase activity in a manner independent of the cell cycle.
Summary and Perspectives
Detailed delineation of functional domains on Cdc7 could shed new lights on the mechanisms of Cdc7 kinase activation by the activation subunit, ASK. Through analyses of systematic deletion derivatives of Cdc7 kinase, the following conclusions were drawn.
1) Interaction with ASK requires DAM-1 and DAM-2, the 10 amino acids (448–457) within the kinase insert III, and the C-terminal 7 amino acids HPFFKDM, respectively. 2) DAM-1 and DAM-2 interact with Dbf4-motif-M and Dbf4-motif-C of ASK, respectively, and this interaction is essential for activity of the full-length Cdc7. 3) Binding of ASK to Cdc7 stimulates binding of ATP to the latter protein. 4) The minimum Cdc7 lacking most of kinase insert II and a part of kinase insert III is partially active on its own and binds to ATP in the absence of ASK, but it is inefficient in phosphorylating exogenous substrate proteins. 5) ASK facilitates the phosphorylation of specific target sites on exogenous substrates by Cdc7.
These results lead us to propose that ASK may activate the Cdc7 kinase by bipartite interactions at the edge of the large lobe of Cdc7 kinase. This interaction somehow leads to conformational change of Cdc7 that permits ATP binding and facilitates recognition of specific phosphorylation target sites on the exogenous substrates (Fig. 8). This initial phosphorylation will generate the acidic environment, resulting in increased affinity of the kinase catalytic sites to other target serine residues, because Cdc7 is known to be an acidophilic kinase (46, 54). This positive feedback would ultimately result in vigorous phosphorylation of the target protein by the Cdc7-ASK complex.
Delineation of the key interacting motifs on Cdc7 as well as on ASK would facilitate further studies on the structural basis of kinase activation and substrate recognition by Cdc7-ASK complex. The identification of specific interaction surfaces may permit design of chemicals or peptides that may compete with the binding and inhibit Cdc7 kinase activity. This would constitute a promising candidate for novel cancer agents, because Cdc7 has emerged as a potential target of cancer therapy (55). Alternatively, it may also be possible to activate the Cdc7 kinase by manipulating the two interacting surfaces with chemicals or small polypeptides, which may provide a useful tool for manipulating cell cycle progression or other chromosome transactions in which Cdc7 is involved.
Supplementary Material
Acknowledgments
We thank the members of our laboratory and Yoko Funahashi of SBI Biotech for helpful discussion. We also thank Ken-ichi Arai for support of this research. We thank Peter Schultz for the gift of Sup-BpaRS-6TRN plasmid for in vivo photocross-linking assays and Suguru Okuda for instructions on the procedure.
This work was supported in part by grants-in-aid for basic scientific research (A) and a grant-in-aid for scientific research on priority area “Chromosome Cycle” from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, by Takeda Science Foundation, and by Astellas Foundation for Research on Metabolic Disorders (to H. M.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S8.
R. Kitamura, R. Fukatsu, N. Kakusho, G.-t. Toh, and H. Masai, unpublished data.
R. Fukatsu, N. Kakusho, and H. Masai, unpublished data.
- pre-RC
- prereplicative complex, MCM, minichromosome maintenance, ASK, activator of S phase kinase
- Bpa
- p-benzoyl-l-phenylalanine
- Cdk
- cyclin-dependent kinase.
REFERENCES
- 1. Bell S. P., Dutta A. (2002) Annu. Rev. Biochem. 71, 333–374 [DOI] [PubMed] [Google Scholar]
- 2. Masai H., Matsumoto S., You Z., Yoshizawa-Sugata N., Oda M. (2010) Annu. Rev. Biochem. 79, 89–130 [DOI] [PubMed] [Google Scholar]
- 3. Ishimi Y. (1997) J. Biol. Chem. 272, 24508–24513 [DOI] [PubMed] [Google Scholar]
- 4. Lei M., Tye B. K. (2001) J. Cell Sci. 114, 1447–1454 [DOI] [PubMed] [Google Scholar]
- 5. Aparicio O. M., Weinstein D. M., Bell S. P. (1997) Cell 91, 59–69 [DOI] [PubMed] [Google Scholar]
- 6. Remus D., Beuron F., Tolun G., Griffith J. D., Morris E. P., Diffley J. F. (2009) Cell 13, 719–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Evrin C., Clarke P., Zech J., Lurz R., Sun J., Uhle S., Li H., Stillman B., Speck C. A. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 20240–20245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nevins J. R. (1998) Cell Growth Differ. 9, 585–593 [PubMed] [Google Scholar]
- 9. Stillman B. (1996) Science 274, 1659–1664 [DOI] [PubMed] [Google Scholar]
- 10. Sclafani R. A. (2000) J. Cell Sci. 113, 2111–2117 [DOI] [PubMed] [Google Scholar]
- 11. Jares P., Blow J. J. (2000) Genes Dev. 14, 1528–1540 [PMC free article] [PubMed] [Google Scholar]
- 12. Masai H., Miyake T., Arai K. (1995) EMBO J. 14, 3094–3104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sato N., Arai K., Masai H. (1997) EMBO J. 16, 4340–4351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang W., Hunter T. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 14320–14325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coverley D., Laman H., Laskey R. A. (2002) Nat. Cell Biol. 4, 523–528 [DOI] [PubMed] [Google Scholar]
- 16. Tanaka S., Umemori T., Hirai K., Muramatsu S., Kamimura Y., Araki H. (2007) Nature 445, 328–332 [DOI] [PubMed] [Google Scholar]
- 17. Zegerman P., Diffley J. F. (2007) Nature 445, 281–285 [DOI] [PubMed] [Google Scholar]
- 18. Walter J. C. (2000) J. Biol. Chem. 275, 39773–39778 [DOI] [PubMed] [Google Scholar]
- 19. Arias E. E., Walter J. C. (2007) Genes Dev. 21, 497–518 [DOI] [PubMed] [Google Scholar]
- 20. Masai H., Arai K. (2002) J. Cell. Physiol. 190, 287–296 [DOI] [PubMed] [Google Scholar]
- 21. Sclafani R. A., Jackson A. L. (1994) Mol. Microbiol. 11, 805–810 [DOI] [PubMed] [Google Scholar]
- 22. Brown G. W., Kelly T. J. (1998) J. Biol. Chem. 273, 22083–22090 [DOI] [PubMed] [Google Scholar]
- 23. Takeda T., Ogino K., Matsui E., Cho M. K., Kumagai H., Miyake T., Arai K., Masai H. (1999) Mol. Cell. Biol. 19, 5535–5547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kumagai H., Sato N., Yamada M., Mahony D., Seghezzi W., Lees E., Arai K., Masai H. (1999) Mol. Cell. Biol. 19, 5083–5095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jiang W., McDonald D., Hope T. J., Hunter T. (1999) EMBO J. 18, 5703–5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Montagnoli A., Bosotti R., Villa F., Rialland M., Brotherton D., Mercurio C., Berthelsen J., Santocanale C. (2002) EMBO J. 21, 3171–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoshizawa-Sugata N., Ishii A., Taniyama C., Matsui E., Arai K., Masai H. (2005) J. Biol. Chem. 280, 13062–13070 [DOI] [PubMed] [Google Scholar]
- 28. Takahashi T. S., Walter J. C. (2005) Genes Dev. 19, 2295–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bousset K., Diffley J. F. (1998) Genes Dev. 12, 480–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Donaldson A. D., Fangman W. L., Brewer B. J. (1998) Genes Dev. 12, 491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Masai H., Taniyama C., Ogino K., Matsui E., Kakusho N., Matsumoto S., Kim J. M., Ishii A., Tanaka T., Kobayashi T., Tamai K., Ohtani K., Arai K. (2006) J. Biol. Chem. 281, 39249–39261 [DOI] [PubMed] [Google Scholar]
- 32. Sheu Y. J., Stillman B. (2006) Mol. Cell 24, 101–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ogino K., Hirota K., Matsumoto S., Takeda T., Ohta K., Arai K., Masai H. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 8131–8136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matos J., Lipp J. J., Bogdanova A., Guillot S., Okaz E., Junqueira M., Shevchenko A., Zachariae W. (2008) Cell 135, 662–678 [DOI] [PubMed] [Google Scholar]
- 35. Bailis J. M., Bernard P., Antonelli R., Allshire R. C., Forsburg S. L. (2003) Nat. Cell Biol. 5, 1111–1116 [DOI] [PubMed] [Google Scholar]
- 36. Takeda T., Ogino K., Tatebayashi K., Ikeda H., Arai Ki., Masai H. (2001) Mol. Biol. Cell 12, 1257–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Masai H., Arai K. (2000) Biochem. Biophys. Res. Commun. 275, 228–232 [DOI] [PubMed] [Google Scholar]
- 38. Ogino K., Takeda T., Matsui E., Iiyama H., Taniyama C., Arai K., Masai H. (2001) J. Biol. Chem. 276, 31376–31387 [DOI] [PubMed] [Google Scholar]
- 39. Sato N., Sato M., Nakayama M., Saitoh R., Arai K., Masai H. (2003) Genes Cells 8, 451–463 [DOI] [PubMed] [Google Scholar]
- 40. Fung A. D., Ou J., Bueler S., Brown G. W. (2002) Mol. Cell. Biol. 22, 4477–4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yamashita N., Kim J. M., Koiwai O., Arai K., Masai H. (2005) Genes Cells 10, 551–563 [DOI] [PubMed] [Google Scholar]
- 42. Masai H., Sato N., Takeda T., Arai K. (1999) Front. Biosci. 4, D834–D840 [DOI] [PubMed] [Google Scholar]
- 43. Kim B. J., Lee H. (2006) J. Biol. Chem. 281, 12041–12049 [DOI] [PubMed] [Google Scholar]
- 44. Kim B. J., Kim S. Y., Lee H. (2007) J. Biol. Chem. 282, 30029–30038 [DOI] [PubMed] [Google Scholar]
- 45. Kim J. M., Nakao K., Nakamura K., Saito I., Katsuki M., Arai K., Masai H. (2002) EMBO J. 21, 2168–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Masai H., Matsui E., You Z., Ishimi Y., Tamai K., Arai K. (2000) J. Biol. Chem. 275, 29042–29052 [DOI] [PubMed] [Google Scholar]
- 47. Ryu Y., Schultz P. G. (2006) Nat. Methods 3, 263–265 [DOI] [PubMed] [Google Scholar]
- 48. You Z., Komamura Y., Ishimi Y. (1999) Mol. Cell. Biol. 19, 8003–8015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Montagnoli A., Valsasina B., Brotherton D., Troiani S., Rainoldi S., Tenca P., Molinari A., Santocanale C. (2006) J. Biol. Chem. 281, 10281–10290 [DOI] [PubMed] [Google Scholar]
- 50. Ohtoshi A., Miyake T., Arai K., Masai H. (1997) Mol. Gen. Genet. 254, 562–570 [DOI] [PubMed] [Google Scholar]
- 51. Marcote M. J., Knighton D. R., Basi G., Sowadski J. M., Brambilla P., Draetta G., Taylor S. S. (1993) Mol. Cell. Biol. 13, 5122–5131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Coleman K. G., Wautlet B. S., Morrissey D., Mulheron J., Sedman S. A., Brinkley P., Price S., Webster K. R. (1997) J. Biol. Chem. 272, 18869–18874 [DOI] [PubMed] [Google Scholar]
- 53. Hughes S., Jenkins V., Dar M. J., Engelman A., Cherepanov P. (2010) J. Biol. Chem. 285, 541–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lee J. K., Seo Y. S., Hurwitz J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 2334–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sawa M., Masai H. (2009) Drug Des. Dev. Ther. 2, 255–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Uno S., Masai H. (2011) Genes Cells, in press [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.