Abstract
The protozoan parasite Leishmania is the causative agent of serious human infections worldwide. The parasites alternate between insect and vertebrate hosts and cause disease by invading macrophages, where they replicate. Parasites lacking the ferrous iron transporter LIT1 cannot grow intracellularly, indicating that a plasma membrane-associated mechanism for iron uptake is essential for the establishment of infections. Here, we identify and functionally characterize a second member of the Leishmania iron acquisition pathway, the ferric iron reductase LFR1. The LFR1 gene is up-regulated under iron deprivation and accounts for all the detectable ferric reductase activity exposed on the surface of Leishmania amazonensis. LFR1 null mutants grow normally as promastigote insect stages but are defective in differentiation into the vertebrate infective forms, metacyclic promastigotes and amastigotes. LFR1 overexpression partially restores the abnormal morphology of infective stages but markedly reduces parasite viability, precluding its ability to rescue LFR1 null replication in macrophages. However, LFR1 overexpression is not toxic for amastigotes lacking the ferrous iron transporter LIT1 and rescues their growth defect. In addition, the intracellular growth of both LFR1 and LIT1 null parasites is rescued in macrophages loaded with exogenous iron. This indicates that the Fe3+ reductase LFR1 functions upstream of LIT1 and suggests that LFR1 overexpression results in excessive Fe2+ production, which impairs parasite viability after intracellular transport by LIT1.
Keywords: Differentiation, Iron, Oxidation-Reduction, Protozoan, Reductase, Leishmania
Introduction
Human infections with the protozoan parasite Leishmania spp. represent a serious public health problem. Depending on the parasite species, the clinical symptoms can range from self-healing cutaneous lesions to severe visceralizing disease, which is fatal if left untreated. Control of disease transmission is very challenging, because the parasite is transmitted by several sand fly species, which are present throughout the world in both rural and urban environments (1). No vaccines are currently available, and there is a strong need for less toxic drugs for the treatment of human and animal infections. The identification of essential parasite nutrient uptake pathways is therefore of paramount importance for the development of novel therapeutic strategies. Iron is one of the four most abundant elements in the Earth's crust, but its uptake is a challenging problem for living cells (2). Iron is an essential co-factor for many enzymatic activities, and therefore it must be transported from the extracellular environment into the cytosol. However, the ferrous iron (Fe2+) that is soluble at neutral pH cannot be allowed to accumulate, because it generates highly toxic radicals in the presence of oxygen (3). For this reason, in aerobic environments iron is found mostly in the poorly soluble oxidized ferric form (Fe3+) and stored as a complex with iron-binding proteins such as transferrin, lactoferrin, or ferritin. Prior to transport across membranes, Fe3+ must be converted to Fe2+ by a ferric iron reductase (4, 5).
Until recently, very little was known about the mechanism by which Leishmania deals with the problem of iron acquisition. An important advance was the identification and characterization of LIT1, a Leishmania amazonensis plasma membrane Fe2+ transporter. LIT1 is expressed by amastigotes replicating within the low iron environment of macrophage phagolysosomes and is required for parasite replication and lesion formation in vivo (6–8). The process by which Leishmania gains access to Fe2+, the LIT1 substrate, was suggested by an earlier study that detected NADPH-dependent reductase activity in Leishmania chagasi (9). In this work, we identify LFR1, a Leishmania gene that encodes a membrane protein with ferric reductase activity that acts in concert with the ferrous iron transporter LIT1. The ferric reductase activity of LFR1 can be detected on the cell surface of several Leishmania species and is required for the differentiation of L. amazonensis into forms capable of initiating infections in the mammalian host.
EXPERIMENTAL PROCEDURES
Parasites
L. amazonensis (IFLA/BR/67/PH8), Leishmania major (clone VI, MHOM/IL/80/Friedlin), Leishmania infantum (Spain isolate), L. chagasi (Brazil isolate), and Leishmania donovani (India isolate, MHOM/IN/83/mongi-142) were provided by D. L. Sacks (Laboratory of Parasitic Diseases, NIAID, National Institutes of Health). L. amazonensis Δlit1 was previously described (7). Promastigotes were maintained in vitro at 26 °C in promastigote media as follows: M199 (Invitrogen), 40 mm HEPES, pH 7.4, 20% heat-inactivated FBS, 5% penicillin/streptomycin, 0.1% hemin (from a 25 mg/ml stock in 50% triethanolamine), 10 mm adenine, and 5 mm l-glutamine. To generate L. amazonensis axenic amastigotes, log phase promastigotes were inoculated at 1 × 106 parasites/ml into amastigote media (M199, 40 mm sodium succinate, pH 4.5, 20% heat-inactivated FBS, 5% penicillin/streptomycin, 0.1% hemin, 10 mm adenine, 5 mm l-glutamine, 0.25% glucose, and 0.5% trypticase) and incubated at 32 °C. L. amazonensis metacyclic forms were purified from 7-day-old promastigote stationary phase cultures using the 3A.1 mAb, which specifically agglutinates L. amazonensis procyclic promastigotes but not the metacyclic forms (10, 11). Parasites were washed twice with PBS, resuspended at 1.0 × 108 parasites/ml in 1.0 ml of PBS containing a 1:30 dilution of 3A.1 culture supernatant for 30 min, and centrifuged at 250 × g for 5 min. Nonagglutinated parasites in the supernatant were washed twice and counted.
Identification and Cloning of LFR1 in L. amazonensis
Protein BLAST homology searches of the L. major data base were used to identify LmjF30.1610, which shared 36% identity and 45% similarity to the Arabidopsis thaliana ferric reduction oxidase 1, FRO1. Primers AFPNA10 and AFPNA11 (supplemental Table S1) were designed based on the LmjF30.1610 DNA sequence to amplify the predicted open reading frame (ORF) of the putative ferric reductase gene from genomic DNA of L. amazonensis. Genomic DNA was isolated from 1 × 108 parasites in TELT buffer (50 mm Tris-HCl, pH 8.0, 62.5 mm EDTA, 2.5 m LiCl, and 4% (v/v) Triton X-100) as described previously (12). The resulting 3.5-kb product was cloned into the PCR4Blunt-TOPO vector (Invitrogen) to generate the plasmid pTOPO-LFR1, and the correct coding sequence was confirmed by sequencing. The resulting amino acid sequence was used to find the predicted FAD and NADH binding pockets by conserved domain analysis (NCBI) (13, 14). The topology of the protein was modeled with the SOSUI algorithm (15), and the resulting predicted transmembrane topology was used to locate the two transmembrane helices containing the four invariant histidines. The protein sequences for L. major LFR1 (LmLFR1, accession number XP_001684755), A. thaliana FRO1 (AtFRO1, accession number NP_171665), A. thaliana FRO2 (AtFRO2, accession number NP_171664), Pisum sativum FRO1 (PsFRO1, accession number AAK95654), Homo sapiens GP91-PHOX (GP91-phox, accession number P04839), and Saccharomyces cerevisiae FRE1 (ScFRE1, accession number NP_013315) were downloaded from the National Center for Biotechnology Information server and aligned with ClustalW (16). For LFR1 ectopic expression, the LFR1 ORF was cloned into the expression vector pXG-SAT (courtesy of S. Beverley, Washington University, St. Louis, MO) (17, 18). The Leishmania pXG vectors, developed by the Beverley laboratory, utilize the dihydrofolate reductase-thymidylate synthase 5′ and 3′ UTR regions to control transcription and reach ∼10–20 copies per cell resulting in overexpression of cloned genes (18). The LFR1 ORF was PCR-amplified with the primer set AFPNA13/AFPNA20, digested with BamHI enzyme, and cloned into a BamHI linearized pXG-SAT plasmid to generate pLFR1-SAT.
Gene Deletion Constructs
The gene deletion constructs LaKOLFR1Hyg and LaKOLFR1Neo, required for sequential deletion of both alleles of the LFR1 gene, were based on the Leishmania expression vectors pXG-hyg and pXG-neo (courtesy of S. Beverly, (17)). A 1-kb flanking sequence upstream of the L. amazonensis LFR1 ORF was amplified using a nested PRC strategy utilizing the outer primer set (AFPNA25/AFPNA36) followed by the nested primer set (AFPNA35/AFPNA37). A 600-bp flanking sequence downstream of the L. amazonensis LFR1 ORF was amplified using a partial nested PCR strategy utilizing the outer primer set (AFPNA43/AFPNA38) followed by a second PCR using a primer set with one nested primer (AFPNA28/AFPNA43). The 5′- and 3′-flanking sequences were cloned into the pCR4-Blunt TOPO vector (Invitrogen) and sequenced to confirm the identity of the fragments. The 5′- and 3′-flanking regions were amplified with the primer sets AFPNA45/AFPNA46, encoding NruI and EcoRV restriction sites, and AFPNA47/AFPNA48, encoding EcoRV and HindIII restriction sites, and subsequently cloned into pCR4-Blunt TOPO vector creating the plasmids p5′-topo and p3′-topo. The 5′-flanking region was excised from the p5′-topo plasmid with SpeI and EcoRV enzymes and cloned into the p3′-topo plasmid linearized with the same enzymes to create the p5′3′-topo plasmid. To generate the deletion constructs, the spanning regions containing the DHFR-Hyg or (Neo)-TS were PCR-amplified from the pXG-based vectors with primers KO1 and KO2 and ligated into p5′3′-topo plasmid, linearized by EcoRV digestion. Plasmid DNA from each gene-targeted construct was digested with NruI and HindIII to release the integrating fragments, and the linearized gene deletion constructs were gel-purified.
Transfection and Generation of Δlfr1 L. amazonensis
Transfections of L. amazonensis promastigotes were performed as outlined in Huynh et al. (7). For generation of the Δlfr1 knock-out strain, the region containing the LFR1 gene was replaced sequentially on both alleles by the hygromycin B phosphotransferase (Hyg) and neomycin phosphotransferase (Neo) genes, to confer resistance to the antibiotics hygromycin B and G418, respectively. 100 μg/ml hygromycin B and/or 50 μg/ml G418 were added to the medium used to expand the isolated colonies after each round of homologous gene targeting. Southern blots were performed to determine integration of the selectable markers at the LFR1 locus. The digoxigenin-labeled probes for the Hyg, Neo, and LFR1 genes were generated by PCR amplification with primer sets AFPNA57/AFPNA58, AFPNA62/AFPNA63, and AFPNA14/AFPNA17, respectively, according to the manufacturer's protocol (Roche Applied Science). Genomic DNA from candidate clones was isolated in TELT buffer as described previously (12) and digested with EcoRI/NotI (Hyg-probed bots), EcoRI/MfeI (Neo-probed blots), or MfeI/XhoI (LFR1-probed blots) enzymes overnight. The digests were run on a 1% agarose gel and transferred to nylon membranes. The blots were then blocked, hybridized, and probed with digoxigenin-labeled PCR probes according to the manufacturer's protocol (Roche Applied Science). The homozygous Δlfr1 strain was cultured in promastigote media supplemented with 100 μg/ml hygromycin B and 50 μg/ml G418. Complementation of Δlfr1 parasites with wild type LFR1 was achieved by transfection of promastigotes with the episomal vector pLFR1-SAT. Agar-grown clones resistant to 50 μg/ml nourseothricin were selected for further characterization.
Quantification of LFR1 Transcripts by Quantitative PCR
The effect of iron deprivation on the LFR1 transcript levels was ascertained by quantitative real time PCR analysis (qPCR). Log phase wild type L. amazonensis promastigotes were washed twice in PBS, resuspended at 1 × 106 parasites per ml in promastigote medium lacking hemin, and incubated for 15 h at 26 °C. A total of 1 × 108 parasites was collected, and total RNA was isolated from three independent samples using Qiagen RNeasy kit (Qiagen) according to the manufacturer's protocol. cDNA synthesis was carried out using 1 μg of total RNA and qScript cDNA SuperMix (Quanta Biosciences). To quantify the levels of specific mRNA transcripts in individual samples, 1 μl of the cDNA was amplified with gene-specific primers (LFR1 Fv/LFR1 Rv) and L. amazonensis ubiquitin hydrolase (UbiqH FD/UbiqH Rv) as a reference gene with PerfeCTa SYBR Green FastMix (Quanta Biosciences) following the manufacturer's protocol using a C1000 thermocycler with the CFX96 real time system (Bio-Rad). Three technical and three biological replicates of each reaction were performed; amplification efficiencies were validated, and expression for the LFR1 gene was normalized to the mRNAs encoding ubiquitin hydrolase, which is known to be expressed constitutively in Leishmania (19, 20).
Parasite Growth Assays
For procyclic promastigotes, a late log phase culture of L. amazonensis was used to inoculate parasites at 1 × 105 parasites/ml in promastigote medium and incubated at 26 °C. For axenic amastigotes, a log phase culture of L. amazonensis was used to inoculate parasites at 1 × 105 parasites/ml in amastigote medium and incubated at 32 °C. A sample of the cultures was taken every 24 h and incubated with 5 μm fluorescein diacetate for 5 min. Fluorescent parasites were visualized with a Nikon Eclipse E200 microscope with a 40× NA 0.75 objective (Nikon) and counted on a bright line hemocytometer (Hausser Scientific). The data were analyzed for statistical significance using an unpaired Student's t test (p < 0.05 was considered significant).
Iron Reductase Assays
The ability of parasites to reduce extracellular ferric iron to ferrous iron was measured with the cell-impermeable compound potassium hexacyanoferrate (K3Fe(CN)6) as described previously (9, 21). Briefly, log phase promastigotes or axenic amastigotes were washed twice in PBS, resuspended at 1 × 106 parasites per ml in promastigote or amastigote media lacking or supplemented with hemin, and incubated for 15 h at 26 °C for promastigotes and 32 °C for amastigotes. The parasites were harvested by centrifugation, washed twice in PBS, and either 1 × 108 or 3 × 107 parasites were resuspended in 1 ml of HBSS containing 1 mm K3Fe(CN)6. The reduction of the ferric to ferrous form of K3Fe(CN)6 was monitored by the change in absorbance at 420 nm. Samples (50 μl) were taken every hour, centrifuged at 10,000 × g for 5 min, and read on a SpectraMax 5e plate reader (Molecular Devices). A standard curve of K3Fe(CN)6 was used to convert A420 readings into millimolar K3Fe(CN)6. Data were plotted with Microsoft Excel (Microsoft) and line-fitted to determine rates of Fe3+ reduction.
Field Emission Scanning Electron Microscopy
Log phase promastigotes, 7-day stationary phase metacyclic, or axenic amastigotes were attached to poly-l-lysine-coated glass coverslips and fixed for 2 h in 2% glutaraldehyde, 2% paraformaldehyde, 0.1 m HEPES, pH 7.0, at room temperature. Samples were then post-fixed in 1% OsO4 in 0.1 m HEPES, pH 7.4, for 1 h and then exposed to 1% tannic acid (in water) for 1 h before dehydration in an ethanol series, critical point dried from CO2, and gold-sputtered (22). Images were acquired on a Hitachi S-4800 field emission scanning electron microscope operated at 5 kV. All reagents were obtained from Electron Microscopy Sciences.
Live Microscopy
To determine the percentage of live and dead parasites, samples were taken 48 h after transfer to amastigote media at 32 °C and incubated with 5 μm fluorescein diacetate, to detect living parasites (green fluorescence), and 150 μm propidium iodide, to detect dead parasites (red fluorescence), for 5 min at room temperature. Parasites were transferred to a bright line hemocytometer and counted on a Nikon Eclipse E200 microscope with a ×40 NA 0.75 objective (Nikon). To determine the percentage of motile versus nonmotile parasites, samples of axenic amastigotes were taken at 48 h post-induction and observed via phase contrast microscopy on a Nikon Eclipse E200 microscope with a ×40 NA 0.75 objective. Parasites were scored as motile if movement of the parasite or flagellar beating was observed. Parasites failing to meet the former criteria were scored as nonmotile. To determine parasite length, 48 h post-induction axenic amastigotes or 7-day stationary phase metacyclic promastigotes were imaged by differential interference contrast microscopy on a Nikon Eclipse Ti inverted microscope with a ×100 NA 1.4 objective (Nikon) equipped with a Hamamatsu C9100-50 camera. Acquired images were analyzed with the Volocity software suite (PerkinElmer Life Sciences).
Immunofluorescence Microscopy
48 h post-induction, axenic amastigotes or mid-log phase promastigotes were harvested, washed three times in PBS, and fixed with 4% paraformaldehyde. The fixed parasites were permeabilized with 0.05% Triton X-100, washed in PBS, and adhered to poly-l-lysine-coated coverslips. The samples were blocked with 5% horse serum in PBS for 1 h, and incubated with mouse monoclonal antibodies against the P4 antigen (23) (courtesy of D. McMahon-Pratt, Yale University, New Haven, CT) for 1 h at room temperature. After incubation, the coverslips were washed three times with PBS and incubated with Texas Red-conjugated goat anti-mouse antibodies (Invitrogen) for 1 h at room temperature. The coverslips were then washed three times with PBS, incubated with 36 μm DAPI, washed with PBS, and mounted in ProLong mounting media (Invitrogen) prior to imaging on a Leica TCS SP5 X Supercontinuum confocal microscope using a ×63 NA 1.2 objective. Image acquisition was performed with the Leica application suite software package. Image analysis was performed with the Volocity software suite.
Infection of Primary Bone Marrow Macrophages
Bone marrow-derived macrophages from BALB/c mice (NCIS) were prepared as described previously (24), seeded onto 24-well plates containing coverslips at a density of 7 × 104 cells per well, and incubated overnight in BMM2 media (RPMI 1640 medium supplemented with 10% FBS and 5% L cell supernatant (as a source of M-CSF)) at 37 °C and 5% CO2. Adherent macrophages were washed with fresh RPMI 1640 medium and infected with axenic amastigotes in 0.5 ml of RPMI 1640 medium supplemented with 5% FBS (multiplicity of infection = 1.0 or 1.5). After 1 h at 34 °C and 5% CO2, free parasites were removed by three washes with PBS and further incubated for various amounts of time in RPMI 1640 medium supplemented with 10% FBS and 5% L cell supernatant at 34 °C and 5% CO2.
For rescue experiments with cationic ferritin, macrophages were isolated and plated as outlined above. One hour before infection, BMM media were replaced with fresh BMM media supplemented with 10 μg/ml cationized ferritin from horse spleen (Sigma). Adherent macrophages were washed with fresh RPMI 1640 medium and infected with axenic amastigotes in 0.5 ml of RPMI 1640 medium supplemented with 5% FBS (multiplicity of infection = 1.0). After 1 h at 34 °C and 5% CO2, cultures were washed with PBS to remove extracellular parasites and further incubated for various amounts of time in BMM media supplemented with 10 μg/ml cationized ferritin.
Coverslips were fixed after 1 h (zero hour time point) and 24, 48, and 72 h of incubation with 2% paraformaldehyde, permeabilized with 0.02% Triton X-100, and stained with 35 μm DAPI. The number of intracellular parasites (identified through the characteristic kinetoplast DNA staining pattern with DAPI and localization within parasitophorous vacuoles by phase contrast) was quantified on a Nikon Eclipse E200 microscope with a ×100 NA 1.4 objective (Nikon) in a minimum of 400 macrophages per coverslip, in triplicate. The data were expressed as the total number of intracellular parasites per 100 macrophages. The data were analyzed for statistical significance using an unpaired Student's t test (p < 0.01 was considered significant). Images of the infected macrophages were taken with a Nikon DS-Fi1 camera and processed with the Volocity software suite.
In Vivo Virulence and Persistence Assays
Female BALB/c mice were injected in the right hind footpad with 1.0 × 106 mAb 3A.1-purified metacyclic promastigotes of L. amazonensis (10, 11), and lesion progression was followed by blinded weekly measurements with a caliper. The parasite tissue load of wild type and Δlfr1 L. amazonensis was determined after 9 or 11 weeks, respectively, using a limiting dilution assay (7, 25). Promastigotes derived from parasites isolated from footpad lesions were transferred to amastigote axenic media and subsequently used for infections in BMM. Statistical significance between means of the two groups was determined using a two-tailed Student's t test for independent samples.
RESULTS
Leishmania LFR1 Gene Encodes a Membrane Protein with Ferric Reductase Activity That Is Induced by Iron Deficiency
Homology searches of the L. major data base identified LmjF30.1610, a gene on chromosome 30 (GenBankTM accession number XP_001684755) with 36% identity and 45% similarity to FRO1, the ferric reduction oxidase gene from Arabidopsis thaliana. PCR amplification and cloning of the corresponding 3.5-kb LFR1 from genomic DNA of L. amazonensis (the species responsible for cutaneous leishmaniasis in the New World) revealed extensive identity with the L. major sequence (supplemental Fig. S1). LFR1 contains the PFAM ferric reductase-like transmembrane component (Ferric_reduct (PF01794)) found in other NADPH-dependent oxidoreductases (A. thaliana FRO1 and FRO2; S. cerevisiae FRE1 and FRE2; Schizosaccharomyces pombe FRP1; and H. sapiens GP91-phox), along with the ferric reductase NAD binding domain (NAD_binding_6 (PF08030)) and the FAD binding domain (FAD_binding_8 (PF08022)), which together promote transmembrane electron transfer (26). Specifically, LFR1 contains the highly conserved residues HPFT in the FAD-binding motif, the residues GPyG in the NAD-binding motif, and the four invariant transmembrane histidines that are found in A. thaliana FRO1 and FRO2, P. sativum FRO1, and S. cerevisiae FRE1 (supplemental Fig. S2) (27–29).
The LFR1 gene encodes a 1084-amino acid protein with a calculated molecular mass of 119 kDa. Hydropathy analysis predicts 11 transmembrane domains, including an apparent N-terminal signal peptide. The C-terminal region containing the FAD- and NADPH-binding sites is predicted to be in contact with the cytosol. Four conserved histidines likely to mediate heme binding (29, 30) are present in transmembrane domains 9 (residues His-458 and His-472) and 11 (residues His-542 and His-556) (Fig. 1A and supplemental Fig. S1).
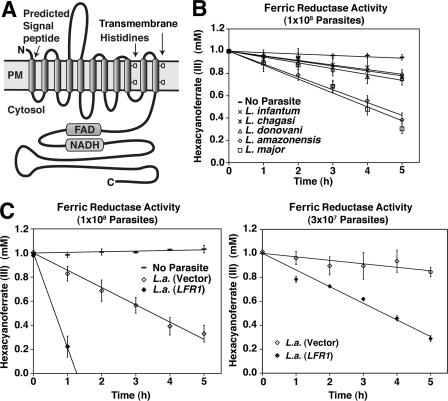
FIGURE 1.
Leishmania LFR1 gene encodes a membrane protein with ferric reductase activity. A, model of the predicted membrane topology of Leishmania ferric reductase 1 (LFR1) highlighting the transmembrane histidines, FAD, and NADH motifs present in ferric reductases. B, five isolates of Leishmania (L. infantum; L. chagasi; L. donovani; L. amazonensis; and L. major) were assayed for ferric reductase activity. Parasites were grown to mid-log phase, transferred to promastigote media lacking hemin for 15 h, washed, and resuspended in assay buffer at 1 × 108 parasites/ml, and ferric reductase activity was monitored spectrophotometrically. All five strains of Leishmania assayed showed ferric reductase activity, whereas no activity was detectable in assay buffer alone (——). C, L. amazonensis parasites were transfected with an LFR1 overexpression vector, pLFR1-SAT or vector alone, and 1 × 108 (left graph) or 3 × 107 (right graph) parasites/ml were assayed for ferric reductase activity. The data represent the mean ± S.D. of triplicate determinations and are representative of at least four independent experiments.
The sequence similarity with Arabidopsis FRO1 and the presence of functionally conserved motifs suggested that LFR1 encodes a plasma membrane ferric reductase. To directly test this hypothesis, we performed ferric reductase activity assays utilizing the membrane-impermeable compound, hexacyanoferrate, as a means to monitor the ferric reductase activity present on the surface of live promastigote forms of the parasites. Consistent with the high levels of sequence identity between LFR1 from L. amazonensis and the corresponding genes in L. major (causative agent of cutaneous leishmaniasis in the Old World) (supplemental Fig. S1), live promastigotes from five distinct Leishmania isolates were able to reduce extracellular ferric iron. Higher levels of reductase activity were observed associated with species that cause cutaneous lesions (L. major, 1.40 ± 0.29 fmol of hexacyanoferrate/h/cell; L. amazonensis, 1.27 ± 0.18 fmol of hexacyanoferrate/h/cell), when compared with species that cause visceral disease (L. donovani, 0.55 ± 0.16 fmol of hexacyanoferrate/h/cell; L. chagasi, 0.47 ± 0.09 fmol of hexacyanoferrate/h/cell; and L. infantum, 0.49 ± 0.03 fmol of hexacyanoferrate/h/cell) (Fig. 1B).
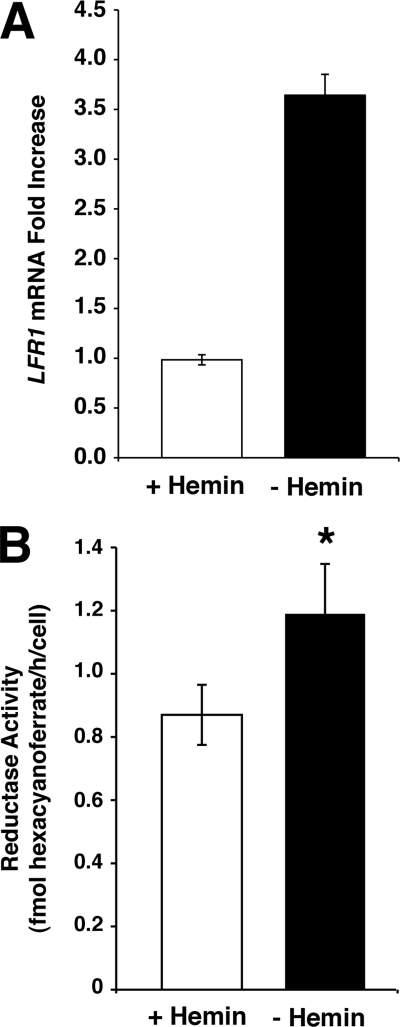
To demonstrate that LFR1 directly confers ferric reductase activity to Leishmania, L. amazonensis promastigotes were transfected with the LFR1 overexpressing vector, pLFR1-SAT, or vector alone. Parasites transfected with pLFR1-SAT showed a 3-fold increase in ferric reductase activity levels (from 1.37 ± 0.09 fmol of hexacyanoferrate/h/cell to 4.39 ± 0.44 fmol of hexacyanoferrate/h/cell) (Fig. 1C). These assays were performed with promastigotes incubated for 15 h in media lacking the major source of iron, hemin, because several previously described reductases are induced by iron deficiency (4, 5). As predicted, LFR1 transcript levels were >3-fold higher in L. amazonensis promastigotes incubated in iron-deficient media, when compared with parasites kept in the presence of hemin (Fig. 2A). In addition, the reductase activity of the parasites grown in iron-deficient media was increased by 27% (from 0.87 ± 0.10 fmol to 1.19 ± 0.16 fmol of hexacyanoferrate/h/cell) when compared with parasites grown in the presence of hemin (Fig. 2B).
FIGURE 2.
LFR1 transcript levels increase in response to iron deprivation. L. amazonensis promastigotes were grown to mid-log phase at 26 °C, washed, and transferred to complete promastigote medium containing hemin (white columns) or medium lacking hemin (black columns) for 15 h at 26 °C. A, total of 1 × 108 parasites were collected; total RNA was isolated, and transcript levels were quantified by quantitative PCR. The data represent the mean ± S.D. of triplicate determinations and are representative of three independent experiments. LRF1 transcript levels from parasites grown in media lacking hemin were increased 2.5-fold when compared with parasites grown in complete media. B, after 15 h of incubation in the presence or absence of hemin, the parasites were resuspended in assay buffer at 1 × 108 parasites/ml, and ferric reductase activity was monitored spectrophotometrically. Parasites grown without hemin had a significant increase in reductase activity, when compared with parasites grown in complete hemin-containing media (Student's t test, p < 0.01). The data represent the mean ± S.D. of triplicate determinations and are representative of three independent experiments.
LFR1 Is the Major Surface Ferric Reductase of L. amazonensis Promastigotes
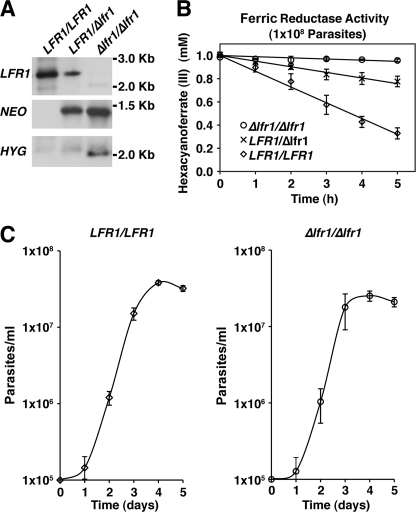
Two sequential rounds of transfection of L. amazonensis promastigotes with LFR1 gene deletion constructs followed by drug selection were used to generate heterozygote (LFR1/Δlfr1) and homozygote null (Δlfr1/Δlfr1) clones (Fig. 3A). No growth defects in complete promastigote culture media were observed between wild type (LFR1/LFR1) and LFR1 null (Δlfr1/Δlfr1) promastigotes (Fig. 3C), allowing expansion of the null strain. Ferric reductase activity was reduced in LFR1/Δlfr1 consistent with a single copy gene deletion and undetectable in null Δlfr1/Δlfr1 parasites. The ferric reductase activity, calculated from the linear slope fitted to the data, was as follows: LFR1/LFR1, 1.68 ± 0.17 fmol of hexacyanoferrate/h/cell; LFR1/Δlfr1, 0.49 ± 0.13 mm fmol of hexacyanoferrate/h/cell; and Δlfr1/Δlfr1, not detectable (Fig. 3B). This result demonstrates that LFR1 encodes the major ferric reductase protein exposed on the surface of Leishmania promastigotes. LFR1 is thus likely to be the major enzyme responsible for the conversion of Fe3+ into Fe2+ for intracellular transport by the Leishmania membrane transporter LIT1 (6, 7).
FIGURE 3.
LFR1 is responsible for the observed ferric reductase activity but is not required for normal promastigote growth. Generation and characterization of LFR1 null mutants. A, Southern blots of genomic DNA from wild type (LFR1/LFR1), heterozygous mutant (LFR1/Δlfr1), and homozygous null mutant (Δlfr1/Δlfr1) promastigotes digested with restriction enzymes and probed for Neo, Hyg, or LFR1 ORFs. The sequential replacement of the LFR1 gene is indicated by the LFR1-containing 2.5-kb fragment in lanes representing wild type and heterozygous parasites, a Neo-containing 1.5-kb fragment in lanes representing heterozygous and homozygous LFR1 null parasites, and a Hyg-containing 2-kb fragment in lanes representing homozygous LFR1 null parasites. B, ferric reductase activity of wild type (LFR1/LFR1, ◊), heterozygous (LFR1/Δlfr1, ×), or homozygous LFR1 null (Δlfr1/Δlfr1, ○) parasites. Promastigotes were grown to mid-log phase, transferred to promastigote media lacking hemin for 15 h, and washed, and 1 × 108 parasites/ml were assayed for ferric reductase activity. The data represent the mean ± S.D. of triplicate determinations and are representative of at least five independent experiments. C, growth curves of wild type (LFR1/LFR1) and LFR1 null (Δlfr1/Δlfr1) promastigotes in liquid culture. There were no significant differences in promastigote growth rates between the two strains.
LFR1-deficient L. amazonensis Promastigotes Are Defective in Differentiation into Infective Metacyclic and Amastigote Forms
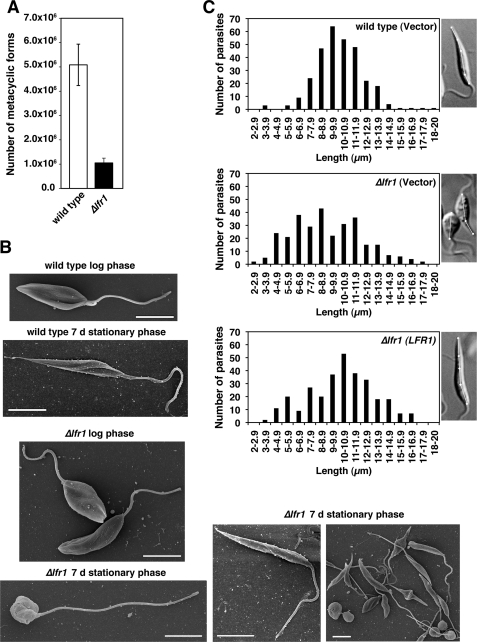
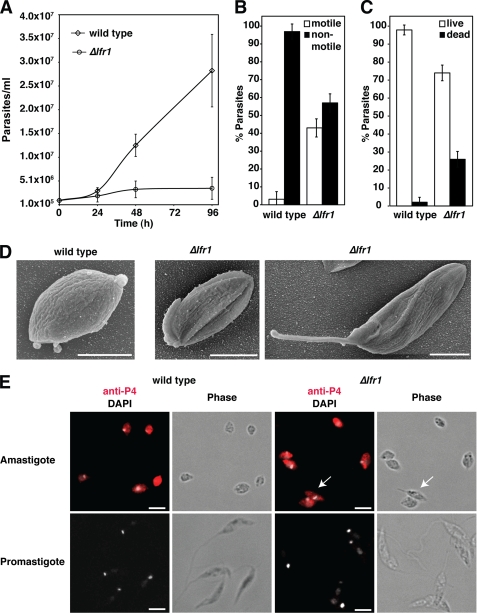
Wild type and LFR1 null (Δlfr1) L. amazonensis promastigotes were grown to stationary phase, a condition that induces differentiation to metacyclic promastigote forms capable of initiating infections in mammalian hosts (31). The expected number of metacyclic promastigotes was isolated from cultures of wild type L. amazonensis. In contrast, there was a 5-fold reduction in the number of metacyclic promastigotes (defined as not being agglutinated by the 3A.1 mAb) isolated from Δlfr1 L. amazonensis cultures (Fig. 4A).
FIGURE 4.
LFR1 null L. amazonensis is defective in differentiation into infective metacyclic forms. A, total of 1 × 108 parasites were used to isolate metacyclic forms from 7-day stationary phase promastigote cultures by preferential agglutination of promastigotes with the 3A.1 mAb. The data represent the mean number of metacyclic forms recovered ± S.D. of triplicate determinations and are representative of three independent experiments. B, parasites from mid-log or stationary phase cultures were adhered to polylysine-coated coverslips and processed for scanning electron microscopy. Images show representative morphological forms for each condition. Quantification of a total of 140 Δlfr1 parasites revealed three main morphological forms: long cylindrical (35%), long bulbous (31%), or short bulbous (34%). Scale bars, 5 μm. C, wild type and Δlfr1 parasites were transfected with empty pXG-SAT vector (wild type (vector) and Δlfr1 (vector)) or pLFR1-SAT (Δlfr1 (LFR1)) and grown to stationary phase. Parasites from each strain were imaged by differential interference contrast microscopy, and the longest cell body axis was measured as indicated with the white lines in the insets. Wild type length = 12.0 μm, Δlfr1 (vector) = 5.0 and 8.5 μm, and Δlfr1 (LFR1) = 13.5 μm. A total of 300 parasites was measured for each strain.
Scanning electron microscopy examination showed that wild type and Δlfr1 parasites present in mid-log phase had a normal morphology, typical of replicating promastigote forms. However, examination of stationary phase cultures revealed striking morphological alterations in the Δlfr1 strain. Although most wild type parasites from the stationary phase cultures had the elongated cylindrical morphology typical of this life cycle stage, Δlfr1 parasites showed a very heterogeneous morphology, ranging from long cylindrical forms resembling normal metacyclic forms to bulbous forms displaying long flagella (Fig. 4B). To quantify this phenotype, wild type parasites transfected with vector alone and Δlfr1 parasites transfected with pLFR1-SAT or vector alone were grown to stationary phase and subjected to microscopic imaging and long axis cell body measurements. Wild type parasites transfected with vector alone displayed elongated forms with the majority of the cell bodies ranging from 7 to 12 μm in length, whereas Δlfr1 parasites transfected with vector alone had a heterogeneous distribution of cell body length, ranging from 4 to 14 μm. The Δlfr1 promastigotes transfected with pLFR1-SAT displayed a partially restored morphology, as determined by long axis cell body measurements. However, although the number of elongated cylindrical forms typical of metacyclic promastigotes was increased, numerous shorter and bulbous forms were still present (Fig. 4C). Transfection of wild type and Δlfr1 promastigotes with the empty pXG-SAT vector did not alter the metacyclic form morphology observed in the absence of transfection (data not shown).
Next, we investigated whether LFR1 was required for the generation of amastigotes, the rounded nonmotile Leishmania life cycle stages that replicate within host macrophages. In L. amazonensis, promastigotes can be induced to differentiate into axenic amastigotes by shifting the parasites to culture medium that resembles the intracellular conditions of low pH (pH 4.5) and elevated temperature (32 °C) (32). When this procedure was performed in parallel with wild type and Δlfr1 promastigotes, wild type parasites differentiated normally and replicated as axenic amastigotes. In contrast, no detectable growth as amastigotes was observed with Δlfr1 parasites (Fig. 5A). After 48 h of incubation in amastigote medium, 95% of wild type parasites had assumed the rounded amastigote morphology and were no longer motile, whereas only 60% of Δlfr1 parasites were nonmotile (Fig. 5B). The viability of Δlfr1 parasites was also reduced under these conditions, as determined with a fluorescent live/dead assay (Fig. 5C). Scanning electron microscopy analysis of the morphology of parasites after 48 h in amastigote culture conditions showed that the recently differentiated wild type amastigotes had the expected rounded and compact cell shape, whereas Δlfr1 parasites showed a more heterogeneous morphology, with different degrees of incomplete shortening of the cell body and flagella (Fig. 5D). Despite the observed differences in morphological differentiation, both wild type and Δlfr1 L. amazonensis expressed the amastigote marker P4 (23) when promastigote cultures were shifted to amastigote growth medium (Fig. 5E). These findings suggest that in the absence of LFR1 the differentiation process into amastigotes is initiated but is not completed.
FIGURE 5.
LFR1 null L. amazonensis is defective in differentiation into axenic amastigotes. Mid-log phase wild type or LFR1 null promastigotes (Δlfr1) were washed, resuspended in pH 4.5 amastigote growth media at 1 × 105 parasites/ml, and cultured at 32 °C. A, growth curves of wild type (◊) and Δlfr1 parasites (○). The data represent the mean ± S.D. of triplicate determinations and are representative of four independent experiments. B–D, wild type and Δlfr1 L. amazonensis 48 h after switching to amastigote media. B, parasite motility was assayed by live phase microscopy. The percentages of motile (white columns) or nonmotile (black columns) parasites for wild type or Δlfr1 are shown. The data represent the mean ± S.D. of quadruplicate determinations. C, viability was assessed by parasite treatment with 5 μm fluorescein diacetate (green) and 150 μm propidium iodide (red) followed by live fluorescence imaging. Green cells were scored as “live” (white columns), and red cells were scored as “dead” (black columns). The data represent the mean ± S.D. of quadruplicate determinations. D, samples of wild type and Δlfr1 amastigote cultures were adhered to polylysine-coated coverslips and processed for scanning electron microscopy. Scale bars, 5 μm. E, samples were taken from wild type and Δlfr1 mid-log cultures or 48-h axenic amastigote cultures, fixed, permeabilized, and stained by immunofluorescence with antibodies against the amastigote-specific antigen P4 (red) and the nuclear stain DAPI (gray). The white arrows indicate an incompletely differentiated Δlfr1 parasite displaying P4 staining. Scale bars, 5 μm.
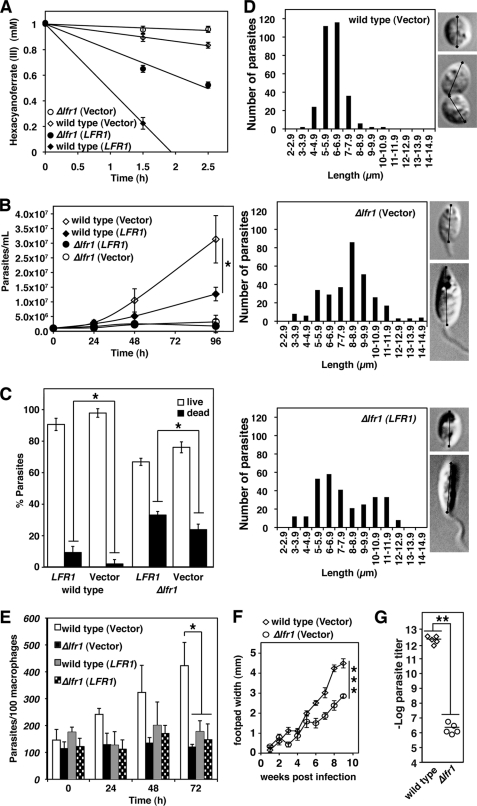
Transfection with pLFR1-SAT, followed by a shift to amastigote medium at 32 °C and pH 4.5, resulted in increased ferric reductase activity, from 0.71 ± 0.10 to 4.32 ± 0.60 fmol of hexacyanoferrate/h/cell in wild type and from undetectable levels to 1.98 ± 0.08 fmol of hexacyanoferrate/h/cell in Δlfr1 promastigotes (Fig. 6A). The increase in LFR1 expression did not rescue the inability of Δlfr1 to grow as axenic amastigotes (Fig. 6B). Notably, LFR1 overexpression markedly inhibited the growth of wild type axenic amastigotes, suggesting a toxic effect (Fig. 6B). Viability assays confirmed an increase in the number of dead parasites after LFR1 overexpression, in both wild type and Δlfr1 parasites (Fig. 6C). Imaging followed by cell body length measurements revealed that LFR1 expression in Δlfr1 parasites only partially restored the morphological shift from the elongated promastigote shape to the shortened round amastigotes (Fig. 6D). To determine the infectivity of these forms, parasites transfected with pXG-SAT alone or pLFR1-SAT were incubated for 48 h in amastigote medium, and infection of BMM was performed. In agreement with their inability to replicate as axenic amastigotes, Δlfr1 parasites did not replicate within macrophage phagolysosomes. In contrast, wild type parasites expressing the empty vector showed vigorous intracellular replication, confirming that transfection alone did not reduce parasite viability. The inhibition in intracellular growth was also not due to an attenuation caused by extended culturing, as Δlfr1 parasites freshly isolated from mice displayed the same phenotype (supplemental Fig. S3). A strong inhibition in the intracellular replication of both wild type and Δlfr1 parasites was observed when LFR1 was overexpressed, consistent with the toxic effect of high levels of LFR1 (Fig. 6E).
FIGURE 6.
LFR1 null L. amazonensis has a strong growth defect as axenic amastigotes and has reduced virulence. LFR1 overexpression partially rescues the defective differentiation defect of LFR1 null L. amazonensis but impairs viability and replication of wild type and LFR1 null parasites. Wild type and Δlfr1 promastigotes were transfected with the pXG-SAT vector alone (vector) or pLFR1-SAT (LFR1). Mid-log phase promastigotes were resuspended in amastigote growth media at 1 × 105 parasites/ml and cultured at 32 °C. A, ferric reductase activity of axenic amastigotes after 48 h in amastigote media. Wild type (⧫) and Δlfr1 (●) parasites transfected with pLFR-SAT1 have higher ferric reductase activity than wild type (◊) or Δlfr1 (○) transfected with vector alone. The data represent the mean ± S.D. of triplicate determinations. B, growth curves of wild type and Δlfr1 parasites transfected with vector alone (vector) or pLFR1-SAT (LFR1). The data represent the mean ± S.D. of quadruplicate determinations. The asterisk indicates significant differences between wild type (vector) and wild type (LFR1) (Student's t test, p < 0.02). C, parasite viability was determined 48 h after switching to amastigote media by incubation with 5 μm fluorescein diacetate (green) and 150 μm propidium iodide (red). Green fluorescent parasites were scored as live, and red fluorescent parasites were scored as dead. The data represent the mean ± S.D. of quadruplicate determinations. The asterisk indicates significant differences between wild type (vector) and wild type (LFR1) or between Δlfr1 (vector) and Δlfr1 (LFR1) (Student's t test, p < 0.02). D, wild type and Δlfr1 parasites were imaged by differential interference contrast microscopy, and the cell body axis was measured as indicated in the insets (n = 300). E, BMM were infected for 1 h with parasites cultured for 48 h in amastigote growth media (multiplicity of infection = 1.5) and fixed after the indicated periods for determining the number of intracellular parasites. The data represent the mean ± S.D. of the results of three independent experiments. The asterisk indicates significant differences when wild type (vector) was compared with Δlfr1 (vector), wild type (LFR1), or Δlfr1 (LFR1) (Student's t test, p < 0.01). F and G, BALB/c mice were inoculated in the left hind footpad with 1 × 106 wild type or Δlfr1 purified metacyclic forms. F, lesion development was measured by weekly caliper measurements. The data correspond to the mean ± S.D. of values from five mice. The asterisks indicate significant differences between wild type (vector) and Δlfr1 (vector) (Student's t test, p < 0.0002). G, parasite loads were determined by limiting dilution in footpads of mice inoculated with wild type or Δlfr1. The asterisks indicate significant differences between wild type (vector) and Δlfr1 (vector) (Student's t test, p < 0.003).
To determine whether the inability of Δlfr1 amastigotes to replicate within cultured BMM also occurred in vivo, wild type and Δlfr1 metacyclic promastigotes were inoculated into the footpads of BALB/c mice. As shown in Fig. 4A, despite the reduction in number, metacyclic parasites could be purified from the mixed morphology 7-day stationary phase cultures of Δlfr1. As expected, a progressive growth in the size of cutaneous lesions was observed in mice injected with wild type L. amazonensis metacyclic promastigotes. In contrast, lesion development was significantly delayed in mice inoculated with Δlfr1 metacyclic promastigotes (Fig. 6F). Because lesion development measurements based on footpad swelling reflect inflammation and not the absolute number of parasites in the tissue, quantification of parasite load in the mouse footpads was also performed using a limiting dilution assay (33). The average number of Δlfr1 parasites per footpad was reduced by a factor of 106 when compared with wild type parasites (Fig. 6G), demonstrating their impaired virulence.
LFR1 Overexpression Inhibits Growth of Wild Type L. amazonensis Amastigotes but Rescues Replication of Amastigotes Lacking the Fe2+ Transporter LIT1
The results discussed above indicate that LFR1 is required for the transformation of L. amazonensis promastigotes into amastigotes, and for their sustained axenic growth. Overexpression of LFR1 was found to have toxic effects on the parasites, precluding a rescue of the metacyclic and amastigote differentiation defects. This finding was not totally unexpected, because it is known that cells have to exert tight control over the levels of cellular Fe2+, to avoid the generation of toxic radicals in aerobic environments (3). To test the hypothesis that the toxicity caused by overexpression of LFR1 is a result of excessive production and uptake of Fe2+, we overexpressed LFR1 in Δlit1, an L. amazonensis null mutant lacking the Fe2+ iron transporter LIT1 (7).
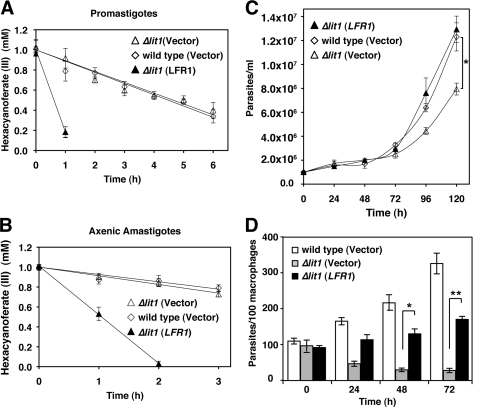
Reflecting the endogenous levels of LFR1, wild type and Δlit1 promastigotes transfected with pXG-SAT alone had similar levels of ferric reductase activity (wild type (vector) = 1.01 ± 0.09 fmol of hexacyanoferrate/h/cell; Δlit1 (vector) = 1.03 ± 0.09 fmol of hexacyanoferrate/h/cell), and Δlit1 promastigotes transfected with pLFR1-SAT showed the expected activity increase (Δlit1 (LFR1) = 4.66 ± 0.90 fmol of hexacyanoferrate/h/cell) (Fig. 7A). This pattern of expression was maintained when the transfected promastigotes were induced to differentiate into axenic amastigotes at pH 4.5 at 32 °C (wild type (vector) = 0.63 ± 0.15 fmol of hexacyanoferrate/h/cell; Δlit1 (vector) = 0.90 ± 0.14 fmol of hexacyanoferrate/h/cell; Δlit1 (LFR1) = 3.38 ± 0.05 fmol of hexacyanoferrate/h/cell) (Fig. 7B). When the rate of growth of these transfected parasites as axenic amastigotes was determined, we observed a reduction in Δlit1 replication when compared with wild type parasites, which is consistent with the data presented above and with the results of previous studies (7). Remarkably, overexpression of LFR1 in Δlit1 did not result in the toxic effects observed in wild type L. amazonensis (Fig. 6B), allowing vigorous amastigote replication and a full rescue of the Δlit1 growth defect (Fig. 7C).
FIGURE 7.
Overexpression of LFR1 in LIT1 null L. amazonensis is not toxic and rescues axenic and intracellular replication of amastigotes. Wild type and Δlit1 L. amazonensis promastigotes were transfected with vector alone or pLFR1-SAT. Mid-log phase promastigotes and axenically grown amastigotes were washed and transferred to promastigote or amastigote media lacking hemin, respectively. After 15 h at 26 °C (A, promastigotes) or 32 °C (B, amastigotes), parasites were washed and assayed for ferric reductase activity at 1 × 108 parasites/ml. The data represent the mean ± S.D. of triplicate determinations and are representative of at least four independent experiments. B, axenic amastigotes from wild type (◊) and Δlit1 (△) promastigotes transfected with vector alone had similar ferric reductase activity, and Δlit1 amastigotes transfected with pLFR1-SAT (▴) had increased ferric reductase activity. C, growth rates of wild type (◊) and Δlit1 (△) L. amazonensis axenic amastigotes transfected with pXG-SAT vector alone and Δlit1 transfected with pLFR1-SAT (▴). Overexpression of LFR1 gene is not deleterious to growth of Δlit1 parasites and complements the growth defect seen in Δlit1 transfected with vector alone. The data represent the mean ± S.D. of triplicate determinations. The asterisk indicates a significant difference between wild type (vector) and Δlit1 (vector) (Student's t test, p < 0.01). D, BMM were infected (multiplicity of infection = 1.0) for 1 h with axenic amastigotes (wild type and Δlit1 parasites transfected with vector alone or Δlit1 parasites transfected with pLFR1-SAT) and fixed after the indicated incubation periods for determining the number of intracellular parasites. The data represent the mean ± S.D. of the results of three independent experiments. The asterisks indicate significant differences between Δlit1 (vector) and Δlit1 (LFR1) (Student's t test 48 h, p < 0.01; 72 h, p < 0.001).
Previous studies revealed that Δlit1 amastigotes were unable to replicate in BMM (7). To test if overexpression of LFR1 rescued the growth of intracellular amastigotes, wild type and Δlit1 parasites transfected with pXG-SAT alone or pLFR1-SAT were used to infect BMM. As reported previously, the number of intracellular Δlit1 amastigotes decreased over time (7). In contrast, overexpression of LFR1 led to a significant increase in the numbers of intracellular amastigotes, when compared with Δlit1 parasites transfected with vector alone (Fig. 7D). Thus, the toxic effects of LFR1 overexpression on axenic or intracellular amastigotes require expression of the Fe2+ iron transporter LIT1.
LFR1-deficient Parasites Are Able to Replicate within Macrophages Loaded with an Exogenous Source of Iron
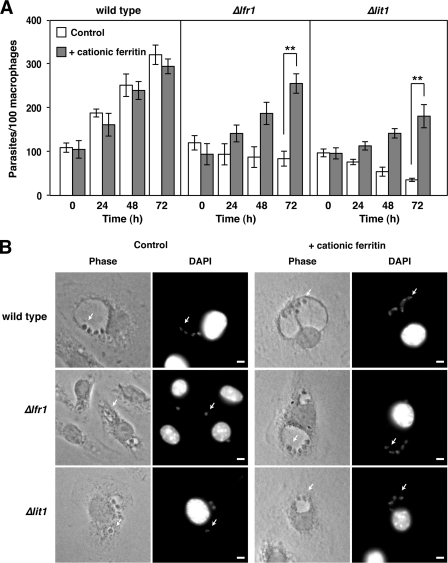
Cationic ferritin binds to negative charges on the plasma membrane, is endocytosed, concentrated in lysosomes, and degraded releasing Fe3+ for transport to the cytoplasm (34, 35). Because Δlfr1 parasites retain a functional LIT1 transporter that promotes ferrous iron acquisition by amastigotes within phagolysosomes (7), we hypothesized that loading the lysosomal compartment of macrophages with excess exogenous iron should be able to promote the replication in Δlfr1 amastigotes. To test this hypothesis, BMM were incubated in BMM medium alone or BMM medium supplemented with 10 μg/ml cationic ferritin for 1 h, followed by infection with wild type, Δlfr1, and Δlit1 parasites. As expected, Δlfr1 and Δlit1 parasites failed to replicate in BMM grown in regular BMM medium, whereas the number of wild type parasites increased progressively between 24 and 72 h after infection (Fig. 8, A and B). However, in BMM preincubated with cationic ferritin, wild type, Δlfr1, and Δlit1 parasites all displayed progressive intracellular growth and formed the typically enlarged parasitophorous vacuoles containing multiple amastigotes (Fig. 8, A and B).
FIGURE 8.
Endocytic pathway loading with cationic ferritin rescues the intracellular growth of LFR1 null and LIT1 null L. amazonensis. A, BMM were pretreated for 1 h with fresh BMM medium (control) or BMM medium supplemented with 10 μg/ml cationic ferritin. BMM were infected (multiplicity of infection = 1.0) for 1 h with wild type, Δlfr1, or Δlit1 axenic amastigotes. The BMM were washed, incubated in fresh BMM medium or BMM medium supplemented with 10 μg/ml cationic ferritin, and fixed after the indicated periods for determining the number of intracellular parasites. The data represent the mean ± S.D. of the results of three independent experiments. The asterisks indicate significant differences between the growth of Δlfr1 or Δlit1 parasites in BMM media versus BMM media supplemented with cationic ferritin (Student's t test, p < 0.0 01). B, phase contrast and DAPI-stained fluorescent images of BMM supplemented with or without cationic ferritin and infected with wild type, Δlfr1, or Δlit1 amastigotes at 48 h. The arrows indicate intracellular amastigotes. Scale bars, 5 μm.
DISCUSSION
Evidence has been accumulating in support of the notion that Leishmania spp. can obtain iron from a variety of sources, including heme, hemoglobin, transferrin, lactoferrin, or ferric nitrilotriacetate (36–40). However, unlike mammalian cells, these protozoan parasites do not appear to utilize a receptor-mediated mechanism for the uptake of Fe3+ chelated to carrier proteins such as transferrin (6, 9, 40). Rather, Leishmania directly translocates iron across their plasma membrane through LIT1, a ZIP family Fe2+ membrane transporter that is expressed on the plasma membrane of L. amazonensis under conditions of iron deprivation (7). LIT1-deficient parasites are unable to replicate within host macrophages and do not cause skin lesions in mice, indicating that this pathway of iron acquisition is essential for the ability of Leishmania to cause disease (7).
Ferrous iron transporters are expected to work in concert with ferric reductases, which are essential for the generation of the soluble Fe2+ substrate for membrane translocation (4). Indeed, earlier studies using membrane-impermeable substrates detected an NADPH-dependent ferric reductase activity associated with intact promastigote stages of L. chagasi, suggesting a plasma membrane association for this enzyme (9). In this study, we have identified LFR1, a gene induced under low iron availability that accounts for all the detectable ferric iron reductase activity associated with the surface of L. amazonensis promastigotes. The LFR1 gene and the surface-associated ferric reductase activity are present in several Leishmania species, including L. infantum, L. chagasi, and L. donovani, species that cause visceralizing disease (Fig. 1B). Interestingly, under the culture conditions utilized, the species causing visceralizing infections expressed lower levels of LFR1 activity than the species that induce cutaneous lesions. This observation suggests the existence of differences in iron acquisition and homeostasis pathways that might be associated with the different environments encountered by the parasites in vivo. The characterization of LFR1 null mutants performed in this study indicates that the ferric reductase LFR1 is essential for the ability of Leishmania to differentiate into infective forms and to establish infections.
LFR1 is predicted to have multiple transmembrane domains, a bis-heme-binding motif and a cytoplasmic loop that includes FAD- and NADH-binding sites (Fig. 1A and supplemental Fig. S2). These three motifs are a hallmark of the large family of NADPH oxidases, of which one of the best characterized members is GP91-phox, a protein essential for superoxide production by phagocytes (supplemental Fig. S2) (41). NADPH oxidase proteins transfer electrons from NADPH in the cytosol, across the lipid bilayer via the noncovalently bound FAD and histidine-coordinated heme to an acceptor molecule on the outer face of the membrane (42). In the case of the GP91-phox, the acceptor molecule is molecular oxygen, and for ferric reductases the electron acceptor is Fe3+, which is converted to Fe2+ (28, 43–45).
The Leishmania LFR1 protein shows close similarity to the ferric reductases of A. thaliana (FRO1 and FRO2) and P. sativum (FRO1) that are involved in root iron acquisition (28, 29, 45). LIT1, the only additional Leishmania gene identified to date with a role in iron acquisition, was also identified based on its close similarity to an Arabidopsis gene, the ZIP family ferrous iron transporter IRT1. Interesting questions regarding the phylogenetic origin of genes involved in iron acquisition and homeostasis in these ancient trypanosomatid protozoa are likely to emerge, as soon as additional genes involved in these pathways are identified.
Despite its severe defect in differentiation into infective life cycle stages, LFR1 null mutants grow normally as promastigotes, the insect forms that can be axenically cultivated in the presence of abundant sources of iron. Because LFR1 expression is up-regulated upon iron deprivation, it is conceivable that in iron-abundant environments Leishmania spp. utilizes low affinity iron transport systems, as described for S. cerevisiae (4). Alternatively, cultured promastigotes may be able to internalize iron in a form that is independent of Fe3+ to Fe2+ conversion by a plasma membrane ferric reductase. Leishmania parasites are heme auxotrophs, and promastigotes have been proposed to have plasma membrane receptors and/or transporters that mediate heme uptake (36, 38), raising the possibility that heme can serve as a source of iron for these parasites.
Under conditions that promote differentiation into metacyclic promastigotes or axenic amastigotes, the essential role of LFR1 in L. amazonensis becomes evident. Without the LFR1 reductase, even in the presence of abundant sources of iron, differentiation into infective stages is incomplete, and viability is reduced. Because overexpression of LFR1 inhibits parasite growth and reduces viability, we could not directly demonstrate with complementation assays that the differentiation defects of the null mutant are directly attributable to the absence of LFR1. However, taking advantage of the ability to load macrophage endosomal compartments with exogenous iron, we were able to rescue the intracellular growth of LFR1 null parasites. This result indicates that under excess iron availability, the need for reduction of Fe3+ to Fe2+ by LFR1 can be bypassed, possibly through the generation of Fe2+ by the lysosomal reductase cytochrome b561 (46). In addition, utilizing a null mutant lacking the LIT1 Fe2+ transporter (7), we showed that LFR1 overexpression rescues the growth defect of LIT1 null amastigotes, perhaps by providing additional substrate for alternative lower affinity Fe2+ transporters. This result provides strong evidence in support of the hypothesis that LFR1 functions upstream of LIT1 by generating the ferrous form of iron that can be transported across the parasite's membrane. Furthermore, our results strongly suggest that the toxicity of LFR1 overexpression is a direct consequence of increased Fe2+ production. Without LIT1 to transport Fe2+ intracellularly, LFR1 overexpression is no longer toxic, which allowed us to demonstrate that LFR1 can compensate for the growth defect of LIT1 null mutant amastigotes. Taken together, our results show that the Leishmania transmembrane protein LFR1 functions as a ferric iron reductase, converting extracellular chelated Fe3+ into the soluble Fe2+ form, which is then transported into the parasites by the ZIP family transporter LIT1.
Supplementary Material
Acknowledgments
We are grateful to Dr. D. L. Sacks (NIAID, National Institutes of Health) for providing the different Leishmania isolates and for helpful discussions and to Dr. J. Kaplan (University of Utah) for useful suggestions. We also thank Drs. L. A. Rodrigues and B. Kachar (NIDCD, National Institutes of Health) for providing technical help and access to the FE-scanning electron microscope and Dr. D. McMahon-Pratt (Yale University) for the anti-P4 antibodies.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 AI067979 (to N. W. A.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3 and Table 1.
The nucleotide sequence(s) reported in this paper has been submitted to the GenBankTM/EBI Data Bank with accession number(s) JF830585.
- BMM
- bone marrow-derived macrophage.
REFERENCES
- 1. Sacks D., Kamhawi S. (2001) Annu. Rev. Microbiol. 55, 453–483 [DOI] [PubMed] [Google Scholar]
- 2. Morris C. J., Earl J. R., Trenam C. W., Blake D. R. (1995) Int. J. Biochem. Cell Biol. 27, 109–122 [DOI] [PubMed] [Google Scholar]
- 3. Imlay J. A., Chin S. M., Linn S. (1988) Science 240, 640–642 [DOI] [PubMed] [Google Scholar]
- 4. Askwith C. C., de Silva D., Kaplan J. (1996) Mol. Microbiol. 20, 27–34 [DOI] [PubMed] [Google Scholar]
- 5. Wu H., Li L., Du J., Yuan Y., Cheng X., Ling H. Q. (2005) Plant Cell Physiol. 46, 1505–1514 [DOI] [PubMed] [Google Scholar]
- 6. Huynh C., Andrews N. W. (2008) Cell. Microbiol. 10, 293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huynh C., Sacks D. L., Andrews N. W. (2006) J. Exp. Med. 203, 2363–2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jacques I., Andrews N. W., Huynh C. (2010) Mol. Biochem. Parasitol. 170, 28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wilson M. E., Lewis T. S., Miller M. A., McCormick M. L., Britigan B. E. (2002) Exp. Parasitol. 100, 196–207 [DOI] [PubMed] [Google Scholar]
- 10. Courret N., Prina E., Mougneau E., Saraiva E. M., Sacks D. L., Glaichenhaus N., Antoine J. C. (1999) Eur. J. Immunol. 29, 762–773 [DOI] [PubMed] [Google Scholar]
- 11. Pinto-da-Silva L. H., Fampa P., Soares D. C., Oliveira S. M., Souto-Padron T., Saraiva E. M. (2005) Int. J. Parasitol. 35, 757–764 [DOI] [PubMed] [Google Scholar]
- 12. Medina-Acosta E., Cross G. A. (1993) Mol. Biochem. Parasitol. 59, 327–329 [DOI] [PubMed] [Google Scholar]
- 13. Marchler-Bauer A., Anderson J. B., Chitsaz F., Derbyshire M. K., DeWeese-Scott C., Fong J. H., Geer L. Y., Geer R. C., Gonzales N. R., Gwadz M., He S., Hurwitz D. I., Jackson J. D., Ke Z., Lanczycki C. J., Liebert C. A., Liu C., Lu F., Lu S., Marchler G. H., Mullokandov M., Song J. S., Tasneem A., Thanki N., Yamashita R. A., Zhang D., Zhang N., Bryant S. H. (2009) Nucleic Acids Res. 37, D205–D210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marchler-Bauer A., Bryant S. H. (2004) Nucleic Acids Res. 32, W327–W331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hirokawa T., Boon-Chieng S., Mitaku S. (1998) Bioinformatics 14, 378–379 [DOI] [PubMed] [Google Scholar]
- 16. Chenna R., Sugawara H., Koike T., Lopez R., Gibson T. J., Higgins D. G., Thompson J. D. (2003) Nucleic Acids Res. 31, 3497–3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ha D. S., Schwarz J. K., Turco S. J., Beverley S. M. (1996) Mol. Biochem. Parasitol. 77, 57–64 [DOI] [PubMed] [Google Scholar]
- 18. LeBowitz J. H., Coburn C. M., McMahon-Pratt D., Beverley S. M. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 9736–9740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Depledge D. P., Evans K. J., Ivens A. C., Aziz N., Maroof A., Kaye P. M., Smith D. F. (2009) PLoS Negl. Trop. Dis. 3, e476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rochette A., Raymond F., Ubeda J. M., Smith M., Messier N., Boisvert S., Rigault P., Corbeil J., Ouellette M., Papadopoulou B. (2008) BMC Genomics 9, 255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Olakanmi O., Stokes J. B., Pathan S., Britigan B. E. (1997) J. Biol. Chem. 272, 2599–2606 [DOI] [PubMed] [Google Scholar]
- 22. Goldberg M. W., Allen T. D. (1992) J. Cell Biol. 119, 1429–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kar S., Soong L., Colmenares M., Goldsmith-Pestana K., McMahon-Pratt D. (2000) J. Biol. Chem. 275, 37789–37797 [DOI] [PubMed] [Google Scholar]
- 24. Becker S. M., Delamarre L., Mellman I., Andrews N. W. (2009) Immunobiology 214, 495–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tabbara K. S., Peters N. C., Afrin F., Mendez S., Bertholet S., Belkaid Y., Sacks D. L. (2005) Infect. Immun. 73, 4714–4722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Finn R. D., Mistry J., Tate J., Coggill P., Heger A., Pollington J. E., Gavin O. L., Gunasekaran P., Ceric G., Forslund K., Holm L., Sonnhammer E. L., Eddy S. R., Bateman A. (2010) Nucleic Acids Res. 38, D211–D222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dancis A., Roman D. G., Anderson G. J., Hinnebusch A. G., Klausner R. D. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 3869–3873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Waters B. M., Blevins D. G., Eide D. J. (2002) Plant Physiol. 129, 85–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schagerlöf U., Wilson G., Hebert H., Al-Karadaghi S., Hägerhäll C. (2006) Plant Mol. Biol. 62, 215–221 [DOI] [PubMed] [Google Scholar]
- 30. Finegold A. A., Shatwell K. P., Segal A. W., Klausner R. D., Dancis A. (1996) J. Biol. Chem. 271, 31021–31024 [DOI] [PubMed] [Google Scholar]
- 31. Sacks D. L., Perkins P. V. (1984) Science 223, 1417–1419 [DOI] [PubMed] [Google Scholar]
- 32. Hodgkinson V. H., Soong L., Duboise S. M., McMahon-Pratt D. (1996) Exp. Parasitol. 83, 94–105 [DOI] [PubMed] [Google Scholar]
- 33. Titus R. G., Marchand M., Boon T., Louis J. A. (1985) Parasite Immunol. 7, 545–555 [DOI] [PubMed] [Google Scholar]
- 34. Anderson E., Batten B. E. (1983) Tissue Cell 15, 853–871 [DOI] [PubMed] [Google Scholar]
- 35. Radisky D. C., Kaplan J. (1998) Biochem. J. 336, 201–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chang C. S., Chang K. P. (1985) Mol. Biochem. Parasitol. 16, 267–276 [DOI] [PubMed] [Google Scholar]
- 37. Segovia M., Navarro A., Artero J. M. (1989) Ann. Trop. Med. Parasitol. 83, 357–360 [DOI] [PubMed] [Google Scholar]
- 38. Sengupta S., Tripathi J., Tandon R., Raje M., Roy R. P., Basu S. K., Mukhopadhyay A. (1999) J. Biol. Chem. 274, 2758–2765 [DOI] [PubMed] [Google Scholar]
- 39. Soteriadou K., Papavassiliou P., Voyiatzaki C., Boelaert J. (1995) J. Antimicrob. Chemother. 35, 23–29 [DOI] [PubMed] [Google Scholar]
- 40. Wilson M. E., Vorhies R. W., Andersen K. A., Britigan B. E. (1994) Infect. Immun. 62, 3262–3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sumimoto H. (2008) FEBS J. 275, 3249–3277 [DOI] [PubMed] [Google Scholar]
- 42. Cross A. R., Segal A. W. (2004) Biochim. Biophys. Acta 1657, 1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Anderson G. J., Lesuisse E., Dancis A., Roman D. G., Labbe P., Klausner R. D. (1992) J. Inorg. Biochem. 47, 249–255 [DOI] [PubMed] [Google Scholar]
- 44. Shatwell K. P., Dancis A., Cross A. R., Klausner R. D., Segal A. W. (1996) J. Biol. Chem. 271, 14240–14244 [DOI] [PubMed] [Google Scholar]
- 45. Vasconcelos M., Eckert H., Arahana V., Graef G., Grusak M. A., Clemente T. (2006) Planta 224, 1116–1128 [DOI] [PubMed] [Google Scholar]
- 46. Zhang D. L., Su D., Bérczi A., Vargas A., Asard H. (2006) Biochim. Biophys. Acta 1760, 1903–1913 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.