Abstract
Toll-like receptor 2 (TLR2) plays an essential role in innate immunity by the recognition of a large variety of pathogen-associated molecular patterns. It induces its recruitment to lipid rafts induces the formation of a membranous activation cluster necessary to enhance, amplify, and control downstream signaling. However, the exact composition of the TLR2-mediated molecular complex is unknown. We performed a proteomic analysis in lipopeptide-stimulated THP1 and found IMPDHII protein rapidly recruited to lipid raft. Whereas IMPDHII is essential for lymphocyte proliferation, its biologic function within innate immune signal pathways has not been established yet. We report here that IMPDHII plays an important role in the negative regulation of TLR2 signaling by modulating PI3K activity. Indeed, IMPDHII increases the phosphatase activity of SHP1, which participates to the inactivation of PI3K.
Keywords: Lipid Raft, Lipoprotein, NF-κB, PI 3-Kinase (PI3K), Toll-like Receptors (TLR), Tyrosine Protein Phosphatase/Tyrosine Phosphatase, IMPDH, Mycophenolic Acid
Introduction
Toll-like receptors (TLRs)4 play an essential role in innate immunity by recognizing invading microorganisms through conserved pathogen-associated molecular patterns. The binding of microbial ligands to TLRs elicit a sequence of molecular events leading to the activation of transcription factors such as nuclear factor κB (NF-κB) that control the organization of the proinflammatory response (1, 2). Among the 10 TLRs involved in human antimicrobial defense, TLR2 is characterized by its wide repertoire of recognized pathogens (3, 4) and its ability to heterodimerize with TLR1 or TLR6 to bind diacyl or triacyl lipopeptides, respectively (5, 6).
These preformed heterodimers are recruited to membrane microdomains or lipid rafts where they contribute to the constitution of a molecular cluster essential to downstream signal transduction (7, 8). In immune cells such as monocyte/macrophages, the TLR2 activation cluster includes the adaptor protein myeloid differentiation factor 88 (MyD88) upstream of a cascade of protein kinases that ultimately lead to the activation of MAP kinase pathways, phosphorylation of IκB, and nuclear translocation of NF-κB (1, 2). TLR2-mediated activation of NF-κB requires phosphorylation of its p65 subunit through a second pathway that involves Rho-GTPase Rac1, phosphoinositide 3-kinase (PI3K), and AKT (9–13).
Because prolonged or excessive activation may result in excessive inflammation, tissue injury septic shock, and multiple organ failure (14), TLR activation is fine-tuned through complex interactions with negative regulators. Signaling proteins that inhibit TLR signaling include IRAKM, ST2, SIGIRR, SOCS1, TANK, tumor suppressors CYLD and A20 (14–18). Of note, they all interact with components of the NF-κB canonical activation pathway. We characterized the proteome of lipid rafts following stimulation of TLR2 by lipopeptides to identify new proteins involved in TLR2 signaling. Following an Ingenuity (Ingenuity Systems, Redwood City, CA) analytic output of differentially regulated proteins focused on NF-κB signaling pathways, we found that inosine monophosphate dehydrogenase II (IMPDHII), a rate-limiting enzyme for GTP de novo synthesis, is a negative regulator of TLR2-mediated NF-κB activation and is recruited and phosphorylated in lipid rafts. We report that IMPDHII inhibits NF-κB transactivation via the activation of SHP1 phosphatase, which in turn negatively regulates PI3K activation. This study provides the first evidence for a negative role of IMPDHII in TLR2 signaling.
EXPERIMENTAL PROCEDURES
Reagents and Cell Culture
We used the human monocyte cell line THP1 stably transfected with human CD14 and human embryonic kidney (HEK) 293 cells stably transfected with TLR2 (HEK293-T2). HEK293-T2 cells were maintained in low glucose Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), HEPES (10 mm), l-glutamine (2 mm), penicillin (100 units/ml), and streptomycin (100 μm). THP-1-CD14 cells were cultured in RPMI 1640 supplemented with 10% heat-inactivated FBS, HEPES (10 mm), l-glutamine (2 mm), penicillin (100 units/ml), and streptomycin (100 μm).
Human peripherical blood mononuclear cells (PBMCs) were isolated from healthy volunteers using a discontinuous 33/66% Ficoll gradient (GE Healthcare). PBMCs were washed in RPMI medium and then incubated in RPMI 1640 medium containing and 10% FBS. Cells were incubated in low attachment plates (Corning) for stimulation studies with synthetic lipopeptides.
Pam3-Cys-KKKK (Pam3; EMC Microcollections) mimics the bacterial triacyl component after ligation to the heterodimer TLR-1/2. Pam2-Cys-FEPPPATTT (Pam2; EMC Microcollections) mimics bacterial diacyl lipoprotein after ligation to the heterodimer TLR-2/6. LPS (Sigma-Aldrich), agonist of TLR4, and flagellin (Salmonella typhimurium flagellin; InvivoGen, San Diego, CA), agonist of TLR5, were also used. Mycophenolic acid (MPA; Sigma-Aldrich) was used in vitro at a sufficient dose to induce inhibition of IMPDHII as described previously (19–21) and to induce inhibition of NF-κB activity with a minimum cell death (<5%). LY-294002 and guanosine were obtained from Sigma-Aldrich.
Protein G-agarose was from Sigma. Polyclonal anti-p85 was a kind gift from Dr. Tamborini, Cochin Institute. Polyclonal anti-IMPDHII and anti-SHP1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal antibodies to phospho-Akt (Ser-473), Akt, phospho-p65 (Tyr-536), p65, phospho-p38, phospho-Erk, phospho-Sap/Jnk, Erk, Sap-Jnk, IκB, P-SHIP-1, and SHIP-1 and monoclonal antibody p38 were obtained from Cell Signaling (Danvers, MA). Monoclonal antibody to FLAG was from Sigma. Monoclonal antibodies against amino acid 800–1139 of human p110 isoforms and antibodies against SHP1, TLR2, and Rac1 were from Santa Cruz Biotechnology. Anti-phosphotyrosine (4G10 clone) and anti-phosphoserine (4A4 clone) antibodies were obtained from Upstate Biotechnology Inc. (Charlottesville, VA).
Preparation of Lipid Raft Proteins and Two-dimensional Electrophoresis
Lipid rafts isolation is based on their resistance to Triton X-100 detergent (detergent-resistant membrane). THP1 cells (1 × 109) were lysed at 4 °C in 1.4 ml of MES buffer saline (MBS) (25 mm MES, 150 mm NaCl, and 1 mm EDTA, pH 6.5) containing 1% Triton X-100 (Sigma), 1 mm sodium vanadate, and protease inhibitor mixture (Sigma) and homogenized 10 times with a Dounce homogenizer. The homogenates were then mixed with an equal volume of 85% sucrose/MBS and transferred to Ultracentrifuge tubes. The samples were overlaid by 4 ml of 30% sucrose/MBS and 2 ml of 5% sucrose/MBS. The gradients were centrifuged at 250,000 × g at 4 °C for 16 h in a Beckman SW 41 rotor (Beckman L-80 Ultracentrifuge). 12 fractions (833 ml each) were collected from the top; fractions 2–4 were pooled as detergent-resistant membrane fractions. To concentrate lipid rafts, the pooled fractions were diluted four times in a HEPES buffer without NaCl (25 mm HEPES, 1 mm EDTA, and 1 mm PMSF, pH 6,5) and ultracentrifuged at 140,000 × g for 1 h at 4 °C in a SW 41 Beckman rotor. After centrifugation, the resulting pellets were homogenized for 1 h at 4 °C in 120 μl of UTCD buffer (8 m urea, 2 m thiourea, 4% CHAPS detergent, and 50 mm dithiothreitol) and then centrifuged. Supernatants were collected, and proteins were precipitated with a 2-D Clean-Up Kit (GE Healthcare). Pellets were solubilized in 100 μl of UTCD buffer without DTT, and the protein concentration was determined using a Bradford assay (Bio-Rad).
Four duplicates of each condition (namely, samples of lipid rafts from nonstimulated, Pam2-stimulated, and Pam3-stimulated THP1) were used for two-dimensional difference gel electrophoresis (DIGE) analysis. 50 μg of each sample were alternatively labeled with Cy3 and Cy5 CyDyesTM Fluor minimal dyes (GE Healthcare) according to the manufacturer's instructions. The internal standard, prepared by combining equal portions of each of the samples used for the study, was labeled with Cy2. Two-dimensional gel electrophoresis was performed with pH 4–7 IPG strips (24 cm, GE Healthcare) at 20 °C using IPGphor3 (GE Healthcare) for a total of 100 kV/h. After equilibration the IPG strips were placed onto homemade polyacrylamide gels (8–18%), and electrophoresis was done at 20 °C in the EttanDalt II system (GE Healthcare) at 4 watts/gel for 30 min then 18 watts/gel for ∼5 h (until the bromphenol blue dye front reached the bottom of the gel). We performed all electrophoresis procedures in the dark. The Cy2, Cy3, and Cy5 components in each gel were imaged individually using a Typhoon 9400 variable mode imager (GE Healthcare). The images were scanned using optimal excitation/emission wavelength for each DIGE Fluor (Cy2, 488/520 nm; Cy3, 532/580 nm; Cy5, 633/670 nm). Spot detection, relative quantification of spot intensity, and statistical evaluation were carried out with DeCyder 7.0 software (GE Healthcare). Protein spot fold changes ≥+1.4 or ≤−1.4 were set as thresholds. Spots with a Student's t test p value of less than 0.05 in a comparison of log-standardized abundance values of Pam2- or Pam3-stimulated lipid raft samples versus nonstimulated samples were retained for protein identification.
For two-dimensional electrophoresis gel multiplexed staining and Western immunoblotting, 80 μg of proteins for each condition were resolved by two-dimensional electrophoresis using pH 4–7 IPG strips (7 cm, GE Healthcare). The first dimension was preformed at 20 °C using the IPGphor system (GE Healthcare) for a total of 6 kV/h, and then equilibrated strips were inserted onto 11% polyacrylamide gels (Mini Protean III, Bio-Rad) in the second dimension. Two-dimensional gels were either stained or transferred onto nitrocellulose membrane. For multiplexed staining, two-dimensional electrophoresis gels were fixed in a mixture of 50% ethanol and 10% acetic acid three times for 30 min. Afterward, gels were washed three times for 10 min in ultra-pure water and stained for 1 h with Pro-Q Diamond as recommended by the manufacturer (Molecular Probes, Invitrogen). Phosphoproteins were visualized by gel scanning with a Typhoon 9400 Imager (GE Healthcare). Afterward, a SYPRO Ruby (Bio-Rad) total protein stain was performed. For Western analysis, nitrocellulose membranes were blocked for 1 h using blocking buffer (20 mm Tris, 150 mm NaCl, pH 7.5, 0.5% Tween 20, 1% BSA). Membranes were probed overnight at 4 °C with anti-IMPDHII antibodies, washed three times in washing buffer (20 mm Tris, 150 mm NaCl, pH 7.5, and 0.5% Tween 20), and probed with HRP-conjugated secondary antibodies. Immunopositive proteins were visualized using ECL+ (GE Healthcare) as directed by the manufacturer.
Identification by Mass Spectrometry (MS)
Spots of interest were excised in a Coomassie Blue-colored gel following differential analysis. Spots were first destained twice with a mixture of 100 mm ammonium bicarbonate (ABC) and 50% acetonitrile (ACN) for 45 min at 22 °C and then dried using 100% ACN for 15 min. Protein spots were then reduced with 25 mm ABC containing 10 mm DTT for 1 h at 60 °C and then alkylated by 55 mm iodoacetamide in 25 mm ABC for 30 min in the dark at 22 °C. Gel pieces were washed twice with 25 mm ABC; finally they were shrunk with 100% ACN for 15 min and dried using 100% ACN for 10 min. Bands were completely dehydrated after 1 h at 60 °C. Gel pieces were incubated with 13 μl of sequencing grade modified trypsin (Promega, Madison, WI; 12.5 μg/ml in 40 mm ABC, 10% ACN, pH 8.0) overnight at 40 °C. After digestion, peptides were washed with 30 μl of 25 mm ABC, shrunk with 100% ACN, and extracted twice with a mixture of 50% ACN, 5% formic acid. Extracts were dried using a vacuum centrifuge (Eppendorf). Peptides were then desalted using C18 ZipTips (Millipore) with two elutions, first with 50% ACN, 5% formic acid and then with 80% ACN, 5% formic acid. Pooled elutions were left to dry at ambient temperature. For MS and MS/MS analysis, peptides were redissolved in 4 μl of α-cyano-4-hydroxycinnamic acid (5 mg/ml in 50% ACN, 0.1% trifluoroacetic acid). 1 μl of each sample was spotted directly onto a MALDI plate (Applied Biosystems, Foster City, CA). Sample analysis was performed using a MALDI-TOF-TOF 4800 mass spectrometer (Applied Biosystems). Spectral acquisition and processing was performed using the 4000 Series Explorer software (Applied Biosystems) version 3.5.28193 in positive reflectron mode at fixed laser fluency with low mass gate and delayed extraction. External plate calibration was performed with four calibration points spotted onto the four corners of the plate using a mixture of five external standards (PepMix 1, LaserBio Labs, Sophia Antipolis, France). Peptide masses were acquired in steps of 50 spectra for the range of 900 to 4000 Da. MS spectra were summed from 500 laser shots from an Nd-YAG laser operating at 355 nm and 200 Hz. After filtering of tryptic, keratin, and matrix contaminant peaks, up to 15 parent ions were selected for subsequent MS/MS fragmentation according to mass range, signal intensity, signal to noise ratio, and absence of neighboring masses in the MS spectrum. MS/MS spectra were acquired in 1 kV positive mode, and 1000 shots were summed by increments of 50. Database searches were carried out using Mascot version 2.2 (Matrix Science, London, UK) via GPS Explorer software (Applied Biosystems), version 3.6, combining MS and MS/MS interrogations on human proteins from the SwissProt data bank. The search parameters were as follows: carbamidomethylation was used as a variable modification for cysteins and oxidation as a variable modification for methionines. Up to one missed tryptic cleavage was permitted, and a mass accuracy tolerance of 30 ppm for precursors and 0.3 Da for fragments was used for all tryptic mass searches. Positive identification was based on a Mascot score above the significance level (i.e. <5%). The reported proteins were always those with the highest number of peptide matches. Under our identification criteria, no result was found to match to multiple members of a protein family.
Two-dimensional Data Analysis
Protein lists of differentially expressed proteins in lipid rafts were analyzed using the Ingenuity Pathways Analysis application (IPA, Ingenuity Systems). Proteins that interacted with the NF-κB canonical pathway were selected for further functional analyses.
Transfection
HEK293-T2 cells were transfected with NF-κB-responsive luciferase reporter (5× NF-κB-Luc, 40 ng) and β-galactosidase (40 ng) plasmids (Promega) to measure luciferase activity. Luciferase assays were done using the Luciferase Assay System (Promega) according to the manufacturer's instructions. Luciferase activity was normalized to β-galactosidase to standardize transfection efficiency. Wild type IMPDHII and the negative dominant form of PI3K regulatory subunit p85 (DP85N) were subcloned in pCMV6 vector; the GFP-Akt-PH construct was a kind gift from Dr. G. Bismuth (22). cDNA for wild type SHP1 subcloned into the pJ3 vector was from Addgene (Cambridge, MA). All constructs were confirmed by DNA sequencing. These constructs were transiently transfected using Lipofectamine Plus (Invitrogen). IMPDHII and SHP1 siRNA SMARTpools ON-TARGETplus and the negative control siRNA Nontargeting Pool were obtained from Dharmacon (Logan, UT). Transient transfection of siRNA constructs was done using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol.
Western Blotting and Immunoprecipitation Assays
Cells (1.106) stimulated or not with Pam2 or Pam3 were washed three times in PBS and then solubilized in a buffer containing 25 mm Tris, pH 7.5, 150 mm NaCl, 1 mm EDTA, 5 mm MgCl2, 10% glycerol, 1 mm sodium vanadate, 1 mm DTT, protease inhibitors (Roche), and 1% Nonidet P-40. Protein extracts were solubilized for 5 min at 100 °C in Laemmli buffer (65 mm Tris, pH 6.8, 20% glycerol, 5% β-mercaptoethanol, 0.01% bromphenol blue, and 2% SDS). Proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane (Bio-Rad). Membranes were incubated in TBS-Tween (10 mm Tris, pH 7.5, 150 mm NaCl, and 0.1% Tween 20) and 5% low-fat milk for 2 h to saturate nonspecific sites and then in specific antibody overnight at 4 °C. The membranes were then washed with TBS-Tween and incubated with secondary antibody for 1 h at room temperature. In some experiments, mouse TrueBlot (1:1,000, Cliniscience Laboratories, Montrouge, France) was used as the secondary antibody for enhanced chemiluminescence to avoid immunodetection of immunoglobulins. Proteins were revealed with a chemiluminescent kit according to the manufacturer's instructions (Amersham Biosciences).
A Rac PBD (p21-binding domain) pulldown assay was realized as described (Benard, Bohl, Bokoch, 1999). Briefly, lysates (600 μg) from HEK293-T2 cells incubated with MPA and stimulated with Pam3 were incubated with recombinant GST-PBD beads. Rac-GTP was observed by immunoblot with anti-Rac antibodies.
Protein extracts obtained from THP1 and HEK293-T2 cells were quantified with a BCA colorimetry assay (Pierce) using a SMARTSpec 3000 spectrometer (Bio-Rad). Regarding immunoprecipitation assays, cell lysates (300–500 μg) were incubated at 4 °C with 3 μg of primary antibodies for 2 h and then incubated with protein G-agarose beads (Roche). Immunoprecipitates were washed three times with lysis buffer and then incubated with Laemmli buffer at 95 °C for 10 min.
Immunofluorescence Staining and Confocal Microscopy
293-TLR2 cells (12.103/ml) were plated in their culture medium on glass coverslips (BD Biocoat; Bedford, MA) and incubated at 37 °C for 1 day. Cells were transfected with an Akt-PH construct and then starved for 20–24 h before the experiment in basal culture medium. For IMPDHII inhibition experiments, 293-TLR2 cells were co-transfected with pCMV6-IMPDHII or preincubated for 120 min with MPA (10 μm) before the TLR2 agonist Pam3 was added. Cells were then washed in PBS, fixed with 4% paraformaldehyde, and washed with 0.1 m glycine.
THP1-CD14 cells (1 × 106) were seeded on 6-well dishes and starved for 20 h before stimulation with Pam3. Cells were then washed in PBS, fixed with 4% paraformaldehyde and then washed with 0.1 m glycine. Cells were then permeabilized with PBS, 1% BSA containing 0.05% saponin for 20 min and labeled with anti-TLR2 and anti-SHP1 antibodies for 1 h at room temperature. Cells were subsequently incubated with Alexa Fluor 488 and Alexa Fluor 594 anti-mouse and anti-rabbit secondary antibodies for 30 min. Cells were washed with PBS and mounted on slides. All images were obtained using a confocal Leica DMIRE2 microscope and analyzed using ImageJ 1.40 software.
Assay of Kinase and Phosphatase Activities
PI3K activity was assayed with a PI3K ELISA kit (Echelon Biosciences, Salt Lake City, UT) according to manufacturer's instructions. In brief, cell lysates were prepared, and PI3K was immunoprecipitated with antibody against the p85α subunit and incubated with phosphatidylinositol 4,5-diphosphate (PI(4,5)P2). The reaction products were incubated with a PI(3,4,5)P3 detector protein and then added to a PI(3,4,5)P3-coated microplate for competitive binding. A peroxidase-linked secondary detection reagent and colorimetric detection were used to detect PI(3,4,5)P3 detector protein binding to the plate. The colorimetric signal was inversely proportional to the amount of PI(3,4,5)P3 produced by PI3K activity.
SHP1 activity was assayed as described (23). THP1 cells (15 × 106) were starved for 20 h and incubated or not with MPA before stimulation with Pam3. Cells were lysed in a lysis buffer without any phosphatase inhibitors. Proteins (800 μg) were immunoprecipitated with 3 μg of anti-SHP1 mouse monoclonal antibody and incubated for 2 h with agarose beads. Immunoprecipitates were washed twice with lysis buffer and then twice with a phosphatase buffer (50 mm HEPES, pH 5.0, 6.25 mm EDTA, and 12.5 mm dithiothreitrol). The reaction was initiated by the addition of 25 mm para-nitrophenyl phosphate for 30 min at 30 °C. The reaction was stopped by the addition of 0.6 ml of 1 n NaOH, and sample absorbances were measured at 410 nm.
Measurement of Cytokine Production
The IMPDHII activity of THP1 and HEK293-T2 cells (106/ml) was inhibited by MPA or siRNA, and cells were stimulated by Pam3. Concentrations of TNFα in culture supernatants were determined by ELISA according to the manufacturer's protocol (R&D Systems, Minneapolis, MN).
Statistical Analysis
Continuous variables were represented as means ± S.D. Statistical comparisons were assessed using the Student's t test (for comparison between two groups) or one-way analysis of variance with Tukey's post hoc test (for multiple groups). p values < 0.05 indicated statistically significant differences.
RESULTS
IMPDHII Is Recruited to Lipid Rafts following TLR2 Stimulation and Subjected to Post-translational Modifications
Synthetic lipopeptides Pam2 and Pam3 initiate effective immune response after specific recognition by TLR2/6 and TLR2/1, respectively, by inducing the formation of a signaling cluster within lipid rafts (4, 7). To characterize the molecular composition of the signaling cluster following TLR2 stimulation, we used a proteomic approach to identify proteins recruited to or excluded from lipid rafts 5 min after stimulation of TLR2 with Pam3 or Pam2. Rafts were isolated from THP1 cells; the proteome of the lipid rafts was compared between TLR2-stimulated and control cells. Among the spots revealed by two-dimensional DIGE analysis as differentially expressed following Pam2 or Pam3 stimulation, IMPDHII was found theoretically to interact with the NF-κB canonical pathway in an Ingenuity analysis (data not shown). The levels of IMPDHII in lipid rafts of THP1 cells increased 1.7-fold after stimulation of TLR2/TLR1 or TLR2/TLR6 with Pam3 or Pam2 (Fig. 1A). To confirm the recruitment of IMPDHII to lipid rafts, we performed an immunoblot on THP1 lipid rafts obtained from the solubilization of low density fractions (2–3 and 4) in a sucrose gradient (Fig. 1B, upper panel). IMPDHII levels in the lipid rafts fraction increased significantly 5 min after stimulation of THP1 cells with Pam2 or Pam3 and remained high for up to 30 min (Fig. 1B, lower panel). Two-dimensional gel analysis facilitates the identification of post-translational modifications.
FIGURE 1.
IMPDHII is recruited to lipid rafts following TLR2 stimulation and is subjected to post-translational modifications. A, left panel, image of one gel selected from the 12 gels realized for two-dimensional DIGE analysis. This gel contains lipid rafts proteins prior to (Cy3, green) and following a 5-min stimulation with Pam3 (Cy5, red). Cy2 is an internal standard and corresponds to a 1:1 ratio of unlabeled proteins from both groups. Right panel, DeCyder relievo presentation of the identified IMPDHII spot volume in control, Pam2-, and Pam3-stimulated THP1 cells. Gels were analyzed by DeCyder software, and statistics were generated for up-regulated and down-regulated proteins such as IMPDHII. B, confirmation of two-dimensional DIGE findings by immunoblotting. THP1 cells were stimulated with Pam2 or Pam3 (100 ng/ml) and lysed with 1% Triton X-100. Lipid rafts were isolated by ultracentrifugation (fractions 2–4), and lipid raft proteins were subjected to SDS-PAGE. IMPDHII was revealed by immunoblot in lipid rafts. C, lipid rafts were isolated from THP1 cells stimulated with Pam3 (100 ng/ml) for 5 min and subjected to two-dimensional electrophoresis. Two-dimensional gels were then transferred onto nitrocellulose membranes and subjected to immunoblot with anti-IMPDHII antibodies. D and E, phosphorylation changes following stimulation of TLR2 by lipopeptides. D, lipid raft phosphoprotein profiles of nonstimulated and stimulated (Pam3, 5 min) THP1 were viewed on a two-dimensional gel using in-gel staining with ProQ Diamond. Total protein stain of lipid rafts was performed with SYPRO Ruby. Here is a “zoom” view of a two-dimensional gel, centered on one spot identified by MALDI-TOF as IMPDHII (circles). E, THP1 cells were stimulated with Pam3, and cell lysates were immunoprecipitated with 3 μg of IMPDHII monoclonal antibodies. Tyrosine-phosphorylated proteins were then visualized by immunoblot with anti-phosphotyrosine antibodies (4G10 clone). IB, immunoblot; NS, nonstimulated.
To determine whether IMPDHII was subjected to post-translational modifications within lipid rafts following TLR2 stimulation, we used a polyclonal anti-IMPDHII antibody to perform an immunoblot after two-dimensional separation of lipid raft proteins isolated from THP1 stimulated with Pam3. As shown in Fig. 1C, TLR2 stimulation induced a change in the isoelectrophoretic mobility of IMPDHII, suggesting that post-translational modifications of this protein are associated with its recruitment in lipid rafts. As phosphorylation results in changes to the isoelectric profile of proteins and because IMPDHII contains serine and tyrosine phosphorylation sites, we investigated whether IMPDHII is phosphorylated upon TLR2 stimulation. In-gel staining with a ProQ Diamond dye specific for phosphorylated proteins revealed spots that showed a significant increase in their phosphorylated forms following TLR2 stimulation with Pam2 and Pam3. Mass spectrometry analysis enabled us to identify IMPDHII as one the proteins that undergoes phosphorylation after TLR2 stimulation (Fig. 1D, upper panel). To determine the nature of these phosphorylations, we performed immunoprecipitation of protein extracts with an anti-IMPDHII antibody and submitted the immunoprecipitates to immunoblotting with anti-phosphotyrosine antibodies. As shown in Fig. 1E, stimulation of THP1 cells with TLR2 agonists induced tyrosine-phosphorylation of IMPDHII.
IMPDHII Inhibits NF-κB Activity following TLR2 Stimulation
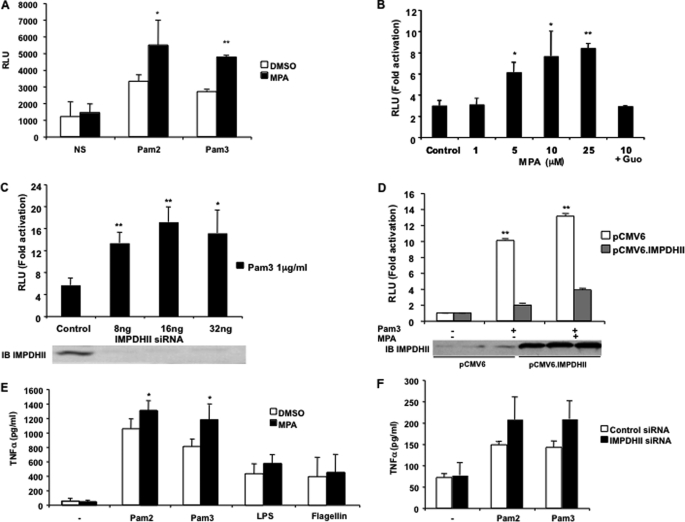
Stimulation of TLR2 initiates a cascade of signaling events that leads to activation of NF-κB. To investigate whether IMPDHII plays a role in the TLR2-dependent activation of innate immune responses, we used a gene reporter assay to measure NF-κB activity in HEK293-T2 stimulated with Pam2 or Pam3 in the presence or absence of MPA, the chemically noncompetitive inhibitor of IMPDHII. Stimulation with either Pam2 or Pam3 resulted in a 3–4-fold increase in NF-κB activity (Fig. 2A). Inhibition of IMPDHII with MPA further increased NF-κB activity in a significant and dose-dependent manner in the stimulated cells (Fig. 2, A and B), indicating that IMPDHII negatively regulates NF-κB activity under physiological conditions. The addition of guanosine in the cell media before stimulation reversed the potentiating effect of MPA on NF-κB activity. To confirm that IMPDHII negatively regulates NF-κB activity, we transfected HEK293-T2 with a siRNA targeting IMPDHII. As shown in Fig. 2C, extinction of IMPDHII significantly increased TLR2-mediated NF-κB activity. Conversely, overexpression of IMPDHII inhibited NF-κB activity following TLR2 stimulation and reversed the effects of MPA (Fig. 2D).
FIGURE 2.
IMPDH II inhibits NF-κB activation following TLR2 stimulation. A–D, in vitro NF-κB-driven luciferase activity in HEK293-T2 cultures after stimulation for 6 h with Pam2 (200 ng/ml) or Pam3 (1 μg/ml). HEK293-T2 cells were co-transfected with 5× κB-luciferase promoter gene plasmid (40 ng/ml) and β-galactosidase expression vector (40 ng/ml). Cells were stimulated with either Pam2 or Pam3 for 6 h following a 2-h incubation with 10 μm MPA (A) or various concentrations of MPA (B). NS, nonstimulated; Guo, guanosine (50 μm) added to the cell media 2 h before stimulation. HEK293-T2 cells were stimulated with Pam3 (1 μg/ml) following a 24-h transfection with various concentrations of siRNA targeting IMPDHII (C) or with pCMV6.IMPDHII expression vector (250 ng/ml) (D). IMPDHII expression was quantified by immunoblot (IB) using anti-IMPDHII antibody. Control conditions correspond to DMSO, non-interfering scramble siRNAs, or empty pCMV6 vector. NF-κB luciferase activity was rationalized upon β-galactosidase expression. Results are expressed as arbitrary units (RLU, relative luciferase units) corresponding to “-fold activation.” Data are the means ± S.D. representative of three independent experiments. E and F, TNFα secretion following TLR stimulation. THP1 cells were incubated with MPA (10 μm) and stimulated with TLR2, TLR4, or TLR5 agonists (E). TNFα concentrations were measured by ELISA in the supernatants of cells incubated with 10 μm MPA or DMSO following a 24-h stimulation with Pam2 (200 ng/ml), Pam3 (1 μg/ml), LPS (1 μg/ml), or flagellin (5 μg/ml). HEK293-T2 cells were transfected with IMPDHII siRNA or nontargeting scrambled siRNA (16 ng/ml) for 36 h and then were stimulated with Pam2 (200 ng/ml) or Pam3 (1 μg/ml) for 24h (F). TNFα concentrations were measured in cell supernatants. Control conditions correspond to DMSO or non-interfering scramble siRNAs. Data are the means ± S.D. representative of three independent experiments. Group comparisons were assessed using Student's t test or analysis of variance with post hoc test. *, p < 0.05; **, p < 0.01; control versus each treated group (HEK293-T2 treated with MPA, siRNA targeting IMPDHII, or pCMV6.IMPDHII expression vector).
NF-κB activation following stimulation of TLR2 leads to the synthesis of proinflammatory cytokines such as TNFα (2, 24). To determine whether inhibition IMPDHII modifies cytokine expression, we measured the amounts of TNFα excreted by THP1 or HEK293-T2 cells following Pam2 and Pam3 stimulation. THP1 cells incubated with MPA and HEK293-T2 transfected with 16 ng/ml siRNA specific for IMPDHII were compared with control cells. As shown under control conditions, the stimulation of THP1 and HEK293-T2 by Pam2 and Pam3 induced a significant increase in TNFα excretion (Fig. 2, E and F). Inhibition of IMPDHII by either MPA or IMPDHII-specific siRNA increased TNFα excretion in the media regardless of the agonist used. We also evaluated the effect of IMPDHII inhibition on TLR4- and TLR5-mediated release of TNFα. In THP1 cells stimulated with LPS or flagellin, the inhibition of IMPDHII by MPA did not increase the levels of TNFα in the culture media (Fig. 2E), suggesting that IMPDHII is a TLR2-specific negative regulator of NF-κB activity.
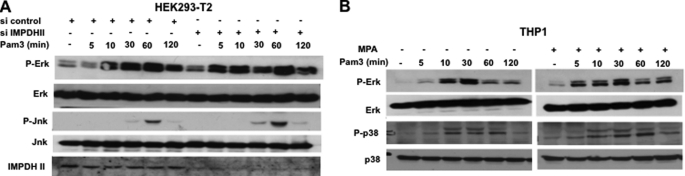
In addition to activation of NF-κB, stimulation of TLR2 initiates the signaling events that lead to the activation of MAP kinases (2). Investigating the role of IMPDHII in MAP kinase activation after stimulation of TLR2, we used immunoblotting to study the phosphorylation of Erk, Jnk, and p38 in HEK293-T2 and THP1 cells stimulated by Pam3 (Fig. 3A). Whereas stimulation of TLR2 induced phosphorylation of MAP kinases, IMPDHII inhibition by MPA or siRNA did not modify MAP kinase activation, suggesting that IMPDHII does not play any role in the TLR2-dependent activation of this pathway. Altogether, these data suggest that IMPDHII acts as a negative regulator of TLR2-stimulated NF-κB activity.
FIGURE 3.
IMPDHII does not interfere with MAP kinase activation pathways. A, HEK293-T2 were transfected with IMPDHII siRNA (16 ng/ml) for 36 h and stimulated with Pam3 (1 μg/ml). Cell lysates were subjected to SDS-PAGE, and phosphorylation of Erk and Jnk was quantified by Western blot. B, THP1 were pretreated with MPA (10 μm) for 2 h and stimulated with Pam3 at different time points. Phosphorylation of Erk and p38 were evaluated by Western blot with specific antibodies. Results are representative of two independent experiments.
IMPDHII Inhibits Phosphorylation of p65
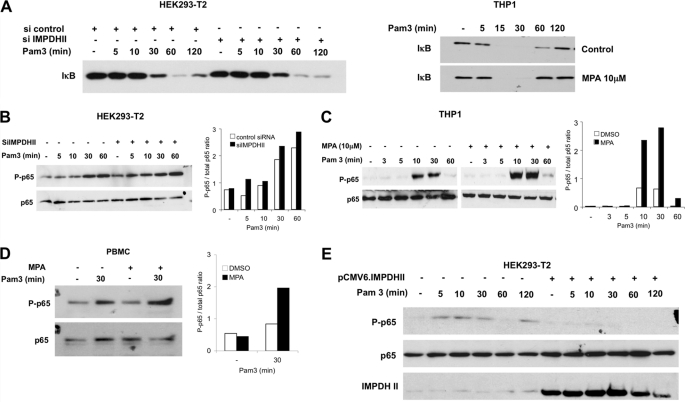
Stimulation of TLR2 activates a MyD88-dependent signaling pathway that results in the nuclear translocation of NF-κB subunits after phosphorylation degradation of IκB. To test whether IMPDHII interfere with this MyD88-dependent pathway, we analyzed the patterns of IκB degradation by immunoblot in HEK293-T2 and THP1 cells stimulated with Pam3. In both cell types, IκB decreased following Pam3 stimulation (Fig. 4A). Neither transfection of siRNA targeting IMPDHII nor exposure to MPA modified the decrease of IκB expression, suggesting that IMPDHII does not prevent IκB degradation. As TLR2 stimulation activates a Rac1/PI3K-dependent pathway that is crucial to transactivation of NF-κB (9), we assessed the effect of IMPDHII inhibition on this pathway. We used immunoblotting to study the phosphorylation of p65 on serine 536 in Pam3-stimulated HEK293-T2 cells transfected with siRNA interfering with IMPDHII or control scrambled siRNA. In HEK293-T2 cells, the engagement of TLR2 induced phosphorylation of p65 between 10 and 60 min (Fig. 4B). Inhibition of IMPDHII expression with specific siRNA further increased the ratio between the phosphorylated forms and total expression of p65 following TLR2 stimulation. Similar results were observed in HEK293-T2 cells stimulated with Pam2 (data not shown). Phosphorylation of p65 was also increased in THP1 cells (Fig. 4C) and human PBMC (Fig. 4D) incubated previously with MPA. To confirm that IMPDHII interferes with the phosphorylation of p65, we transfected HEK293-T2 cells with pCMV6-IMPDHII and assessed the phosphorylation of p65 following TLR2 stimulation. Overexpression of IMPDHII in HEK293-T2 cells led to a significant decrease of p65 phosphorylation, as nearly undetectable levels of phosphorylation were observed after Pam3 exposure (Fig. 4E), suggesting that IMPDHII inhibits NF-κB transactivation. Finally, inhibition of IMPDHII by MPA did not increase phosphorylation of p65 in THP1 cells stimulated with LPS (supplemental Fig. 3) or flagellin (data not shown), suggesting that the negative regulation mediated by IMPDHII is restricted to TLR2 signaling.
FIGURE 4.
IMPDHII inhibits NF-κB transactivation. A, quantification of IκB expression by Western blot. Left panel, HEK293-T2 cells were transfected with siRNA targeting IMPDHII (16 ng/ml) for 36 h. Cells were then stimulated with Pam3 (1 μg/ml), lysed, and subjected to SDS-PAGE. The kinetics of IκB degradation following stimulation was observed by Western blot with anti-IkB antibodies. Right panel, THP1 cells incubated or not with MPA (10 μm) for 2 h were stimulated with Pam3 and lysed, and IκB degradation was observed by Western blot. B–E, phosphorylation of the p65 subunit of NF-κB. IMPDHII activity of HEK293-T2 cells (B) and THP1 monocyte cells (C) was inhibited using siRNA targeting IMPDHII (16 ng/ml) and MPA (10 μm), respectively. Cells were stimulated with Pam3 (1 μg/ml), lysed, and subjected to SDS-PAGE. Controls correspond to non-interfering scramble siRNAs or DMSO. The phosphorylated form of p65 (P-p65) and the expression of p65 were visualized by Western blot with anti-phospho-p65 (Ser-536) and p65 antibodies. D, PBMCs were incubated with MPA or DMSO for 2 h and then stimulated with Pam3, lysed, and subjected to SDS-PAGE. The phosphorylated forms of p65 and total p65 were visualized by Western blot. E, HEK293-T2 cells were transfected with pCMV6.IMPDHII (250 ng/ml) or pCMV6 empty vector (250 ng/ml) and stimulated with Pam3 (1 μg/ml) 36 h after transfection. P-p65, p65, and IMPDHII expression were visualized by Western blot. Densitometry quantification of P-p65 was performed using ImageJ software and normalized with the densities of total p65 for each stimulated condition. Results are representative of three independent experiments.
IMPDHII Modulates PI3K Activation Upstream of Akt
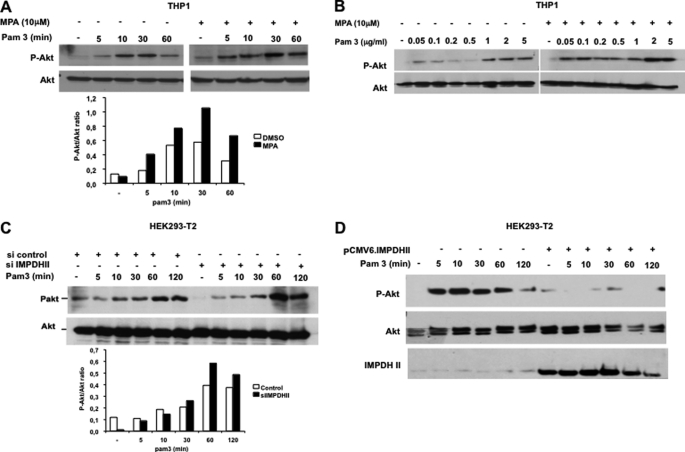
Transactivation of NF-κB following TLR2 stimulation depends upon Akt activation (9, 13). To test the effect of IMPDHII on TLR2-mediated Akt activation, we stimulated THP1 cells with Pam3 after incubation with the IMPDHII inhibitor MPA and studied the phosphorylation of Akt on serine 473 using immunoblotting. Whereas phosphorylation of Akt was observed 10 min after stimulation of THP1 cells under control conditions, phosphorylation occurred earlier and the intensity of Akt phosphorylation was stronger when IMPDHII was inhibited with 10 μm MPA (Fig. 5A). The dose dependence of Pam3-stimulated Akt observed in control cells was much stronger in MPA-treated cells (Fig. 5B). Similar results were obtained in Pam3-stimulated HEK293-T2 cells in the presence of siRNA targeting IMPDHII (Fig. 5C). Furthermore, overexpression of IMPDHII in HEK293-T2 cells inhibited TLR2-mediated phosphorylation of Akt (Fig. 5D).
FIGURE 5.
IMPDHII inhibits Akt activation following TLR2 stimulation. A, THP1 cells were incubated with MPA for 2 h and then stimulated with Pam3 (1 μg/ml). Cells were lysed and subjected to SDS-PAGE. Phosphorylation of Akt (P-Akt) on serine 473 was evaluated by Western blot. Control conditions correspond to cells incubated with DMSO. Densitometry quantification of P-Akt was performed using ImageJ software and normalized with the densities of total Akt for each stimulated condition. B, dose dependence of Pam3-stimulated Akt activation (30 min) in THP1 cells pretreated for 2 h with MPA or DMSO. The phosphorylated form of Akt and total expression of Akt was evaluated by Western blot. C, HEK293-T2 cells were transfected with siRNA targeting IMPDHII (16 ng/ml) and then stimulated with Pam3 (1 μg/ml) 36 h after transfection. Cell lysates were subjected to SDS-PAGE, and P-Akt and total Akt were visualized by Western blot. Control conditions correspond to cells transfected with scrambled siRNA. Densitometry quantification of P-Akt was performed using ImageJ software and normalized with the densities of total Akt for each stimulated condition. D, HEK293-T2 cells were transfected with either IMPDHII expression vector (pCMV6.IMPDHII, 250 ng/ml) or empty vector (pCMV6, 250 ng/ml) and stimulated with Pam3 (1 μg/ml). P-Akt amounts and IMPDHII expression were evaluated by Western blot. Results are representative of three independent experiments.
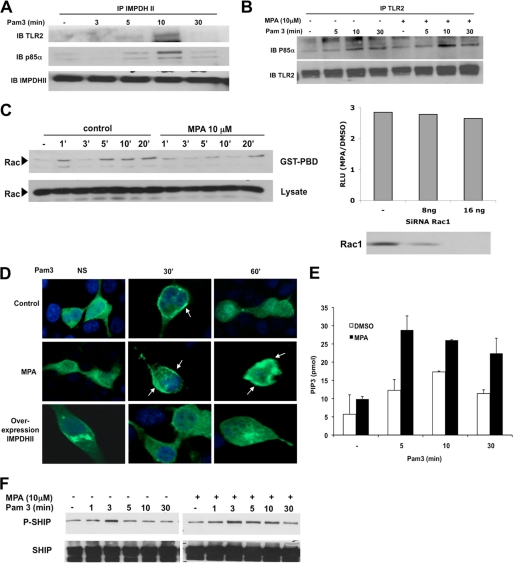
Following stimulation of TLR2, phosphorylation of Akt depends on the constitution of a molecular cluster involving TLR2, PI3K, and Rac. To determine whether IMPDHII is part of this activation cluster, we immunoprecipitated IMPDHII within HEK293-T2 whole cell lysates. Immunoblots revealed TLR2 and the p85α regulatory subunit of PI3K, confirming that IMPDHII was transiently recruited to the TLR2-dependent activation cluster after 10 min (Fig. 6A). We then tested whether inhibition of IMPDHII would alter the interaction between the p85α subunit of PI3K and TLR2. As shown in Fig. 6B, preincubation with MPA does not modify the interaction between the two molecules, suggesting that IMPDHII does not control the recruitment of PI3K to the activation cluster (Fig. 6B). We then tested whether Rac was activated after Pam3 stimulation using pulldown experiments. As expected, we observed an early activation of Rac1 after stimulation of TLR2. This activation decreased in HEK293-T2 cells incubated with MPA (Fig. 6C, left panel) or siIMPDHII (data not shown). The hyperactivation of NF-κB in MPA-treated cells persisted despite the absence of Rac1 (Fig. 6C, right panel).
FIGURE 6.
IMPDHII is recruited to the TLR2-dependant activation cluster and negatively regulates PI3K activity. A, HEK293-T2 cells were stimulated with Pam3 (1 μg/ml). Cell lysates were immunoprecipitated (IP) with 3 μg of IMPDHII antibodies, and TLR2, the p85α subunit of PI3K, or immunoprecipitated IMPDHII was visualized by Western blot (IB). B, HEK293-T2 cells were incubated with MPA (10 μm) for 2 h and stimulated with Pam3 (1 μg/ml). Cell lysates were immunoprecipitated with 3 μg of anti-TLR2 monoclonal antibody. The P85α subunit of PI3K and immunoprecipitated TLR2 were revealed by Western blot. C, left panel, time-dependent activation of Rac was quantified by the presence of Rac-GTP detected by Rac PBD pulldown in HEK293-T2 treated with MPA or DMSO (control cells) and stimulated with Pam3. Right panel, HEK293-T2 cells were co-transfected with siRNA targeting Rac1 or non-interfering scramble siRNA and with 5× κB-luciferase promoter gene plasmid (40 ng/ml) and β-galactosidase expression vector (40 ng/ml). Cells were then incubated or not with MPA for 2 h and stimulated with Pam3 for 6 h; NF-κB-driven luciferase activity was measured and rationalized upon β-galactosidase expression. Results are expressed as arbitrary units corresponding to the MPA/DMSO ratio of NF-κB activation. D, HEK293-T2 cells were transfected with a fusion protein combining GFP and the PH domain of Akt. Transfected cells were either co-transfected with IMPDHII expression vector or incubated with MPA (10 μm). Cells were then stimulated with Pam3 (1 μg/ml) and fixed with paraformaldehyde. Cell nuclei were stained with DAPI. Recruitment of the construct to the membrane was observed by confocal microscopy for each condition. NS, nonstimulated. E, THP1 cells were incubated with MPA (10 μm) or DMSO for 2 h and then stimulated with Pam3 (1 μg/ml). PI3K was then immunoprecipitated with antibody against the p85α subunit and incubated with PI(4,5)P2. The reaction products were incubated with a PI(3,4,5)P3 detector protein and then added to a PI(3,4,5)P3-coated microplate for competitive binding. A peroxidase-linked secondary detection reagent and colorimetric detection buffer were used to detect PI(3,4,5)P3 detector protein binding to the plate. The amount of PI(3,4,5)P3 produced by PI3K activity was inversely proportional to the colorimetric signal. Data are the means ± S.D. representative of three independent experiments. F, THP1 cells were incubated with MPA (10 μm) and stimulated with Pam3 (1 μg/ml). Immunoblot was assessed on lysates to evaluate the phosphorylation of SHIP-1 on Tyr-1020, reflecting its activation state. Results are representative of two independent experiments.
As phosphorylation of Akt requires activation of PI3K and the formation of phosphatidylinositol 3-phosphate (PIP3), we used confocal microscopy to investigate the role of IMPDHII on PI3K activity through the recruitment of Akt to membrane-bound PIP3. We transfected HEK293-T2 cells with a construct encoding a fusion protein between the PH domains of Akt and GFP and studied the translocation of Akt to the plasma membrane after stimulation with Pam3. In control cells, Pam3 induced a transient translocation of the fluorescent probe to the plasma membrane 30 min after stimulation (Fig. 6D). The inhibition of IMPDHII activity with MPA resulted in a sustained recruitment of Akt to PIP3, whereas overexpression of IMPDHII prevented the recruitment of Akt to the cell membrane, suggesting that IMPDHII negatively regulates PI3K activity (Fig. 6D). To confirm the effect of IMPDHII on PI3K activity, we used a PI3K activity assay to measure the formation of PIP3 in Pam3-stimulated THP1 cells in the absence or presence of MPA. Whereas stimulation with Pam3 induced TLR2-dependent PI3K activity after 5 min, the formation of PIP3 was significantly increased in MPA-treated cells at all time points (Fig. 6E). Similar results were observed in THP1 cells stimulated with Pam2 (Data not shown). As the levels of PIP3 also depend upon its conversion to phosphatidylinositol 2-phosphate (PIP2) by SHIP-1 (SH2-containing inositol 5-phosphatase 1) (12), we studied the phosphorylation profile of the tyrosine 1020 of SHIP-1 using immunoblotting to investigate the activity of SHIP-1 (12, 25, 26). Under control conditions, phosphorylation of SHIP-1 increased after 3 min and then gradually decreased to return to baseline after 30 min (Fig. 6F). The chemical inhibition of IMPDHII by MPA did not change the SHIP-1 phosphorylation profile, suggesting that IMPDHII does not regulate the conversion of PIP3 to PIP2. Finally, to confirm that IMPDHII regulates TLR2-mediated NF-κB activation through the PI3K pathway, we tested whether inhibition of IMPDHII still had an impact on NF-κB activity in PI3K inhibited cells. Inhibition of PI3K activity by the chemical inhibitor LY294002 or by overexpression of Dp85N, a negative form of the p85 subunit, reversed the effect of MPA on TLR2-mediated NF-κB activity (supplemental Fig. 1). Furthermore, the increased phosphorylation of Akt observed in MPA-treated cells was reversed by the addition of LY294002 in cell media.
IMPDHII Inhibits Tyrosine Phosphorylation of PI3K p85α Subunit via Phosphatase SHP1
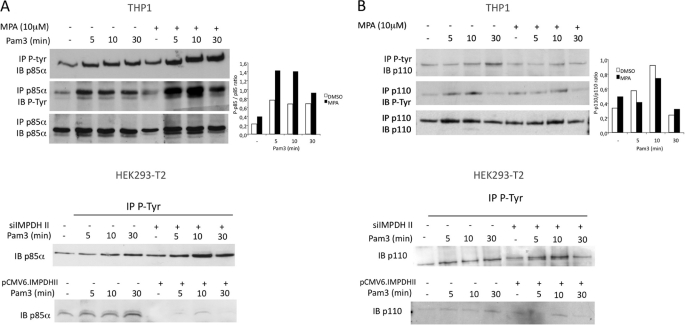
As several reports indicate that PI3K activity depends on its phosphorylation on tyrosine (27–29), we performed immunoprecipitation studies to test whether IMPDHII regulates PI3K activation through changes in the phosphorylation pattern of its subunits. In cell extracts of THP1 or HEK293-T2 cells stimulated with Pam3, we observed phosphorylation of p85α after 5 min. Phosphorylation was increased by IMPDHII inhibition in THP1 (Fig. 7A, left panel). Overexpression of IMPDHII in HEK293-T2 cells was associated with a significant decrease in tyrosine phosphorylation of p85α (Fig. 7A). In contrast, inhibition of IMPDHII did not significantly alter the phosphorylation pattern of the p110 catalytic subunit (Fig. 7B). These data suggest that IMPDHII inhibits PI3K activation through the regulation of p85α tyrosine phosphorylation.
FIGURE 7.
IMPDHII modulates tyrosine phosphorylation of p85α regulatory subunit of PI3K. A, upper panel, THP1 monocyte cells were incubated or not with MPA for 2 h and stimulated with Pam3. Lysates were immunoprecipitated (IP) with either 3 μg of anti-phosphotyrosine (P-Tyr, 4G10 clone) or anti-p85α antibodies, and precipitates were then immunoblotted with p85α or P-Tyr antibodies. Densitometry quantification of the phosphorylated form of p85α was performed using ImageJ software and normalized with the densities of precipitated p85α for each condition. Lower panel, HEK293-T2 cells were transfected either with siRNA targeting IMPDHII or with IMPDHII expression vector. Control conditions correspond to non-interfering scramble siRNAs and empty pCMV6 vector, respectively. Cells were then stimulated with Pam3, lysed, and immunoprecipitated with 3 μg of anti-phosphotyrosine antibodies (4G10 clone). The p85α subunit of PI3K was revealed by Western blot (IB). B, upper panel, THP1 lysates were immunoprecipitated with 3 μg of either P-tyrosine (4G10 clone) or p110 antibodies, and precipitates were then immunoblotted with anti-p110 or anti-4G10 antibodies. Densitometry quantification of the phosphorylated form of p110 was performed using ImageJ software and normalized with the densities of precipitated p110 for each stimulated condition. Lower panel, HEK293-T2 cells were transfected either with siRNA targeting IMPDHII or with IMPDHII expression vector. Control conditions correspond to non-interfering scramble siRNAs and empty pCMV6 vector, respectively. Cells were then stimulated with Pam3, lysed, and immunoprecipitated with 3 μg of anti-phosphotyrosine antibodies (4G10 clone). The p110 catalytic subunit of PI3K was revealed by Western blot. Results are representative of three independent experiments.
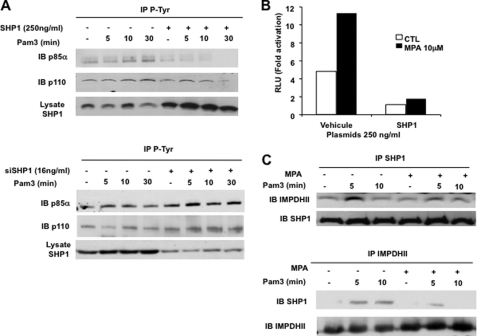
Several reports indicate that SHP1 regulates the phosphorylation of p85α (27, 29). We first confirmed that SHP1 regulates phosphorylation of p85α by comparing the patterns of p85α phosphorylation in HEK293-T2 cells transfected with wild type SHP1 or with empty vector. As shown in Fig. 8A (upper panel), overexpression of SHP1 significantly reduced tyrosine phosphorylation of p85α following TLR2 stimulation. In contrast, silencing SHP1 in HEK293-T2 cells increased the intensity of p85α, Akt, and p65 phosphorylation (Fig. 8A, lower panel, and supplemental Fig. 2). These data confirm that the phosphatase SHP1 is a negative regulator of PI3K-dependent activation of NF-κB downstream of TLR2. To test whether IMPDHII interferes with SHP1-dependent regulation of NF-κB activity, we measured NF-κB activity in HEK293-T2 cells transfected with pJ3.SHP1 and treated with DMSO or MPA. Whereas MPA significantly increased NF-κB activity in DMSO-treated cells, overexpression of SHP1 decreased NF-κB activity following stimulation with Pam3 (Fig. 8B). Moreover, the increase in NF-κB activity induced by pharmacologic inhibition of IMPDHII was totally abolished in HEK293-T2 transfected by pJ3.SHP1. These data suggest that IMPDHII interacts with the TLR2 signaling pathway upstream of or at the level of SHP1. We then tested whether IMPDHII interacted with SHP1. THP1 cells incubated with DMSO or MPA were stimulated with Pam3, and cell extracts were immunoprecipitated with anti-SHP1 or anti-IPMDHII. Immunoblotting with anti-IMDHII or anti-SHP1 antibodies revealed an interaction between SHP1 and IMPDHII between 5 and 10 min after TLR2 stimulation (Fig. 8C). Inhibition of IMPDHII by MPA did not significantly affect the physical interaction between IMDHII and SHP1. Similar results were obtained in HEK293-T2 cells (data not shown).
FIGURE 8.
IMPDHII interacts with SHP1, which negatively regulates TLR2-mediated phosphorylation of PI3K. A, HEK293-T2 cells were transfected with SHP1 expression vector (250 ng/ml) (upper panel) or siRNA targeting SHP1 (16 ng/ml) (lower panel) and stimulated with Pam3 (1 μg/ml). Cells were then stimulated with Pam3 and immunoprecipitated with anti-phosphotyrosine antibodies (4G10 clone) (IP). The P85α and p110 subunits of PI3K were visualized by Western blot (IB). B, HEK293-T2 cells were co-transfected with SHP1 expression vector (250 ng/ml), 5× κB-luciferase promoter gene plasmid (40 ng/ml), and β-galactosidase expression vector (40 ng/ml). Cells were then incubated or not with MPA for 2 h and stimulated with Pam3 for 6 h; NF-κB-driven luciferase activity was measured and rationalized upon β-galactosidase expression. Results are expressed as arbitrary units (RLU, relative luciferase units) corresponding to “-fold activation.” C, THP1 cells were exposed to MPA (10 μm) or DMSO for 2 h. Cells were then stimulated with Pam3 (1 μg/ml) and immunoprecipitated with 3 μg of anti-SHP1 or anti-IMPDHII antibodies. IMPDHII and SHP1 were detected by Western blot. Results are representative of three independent experiments.
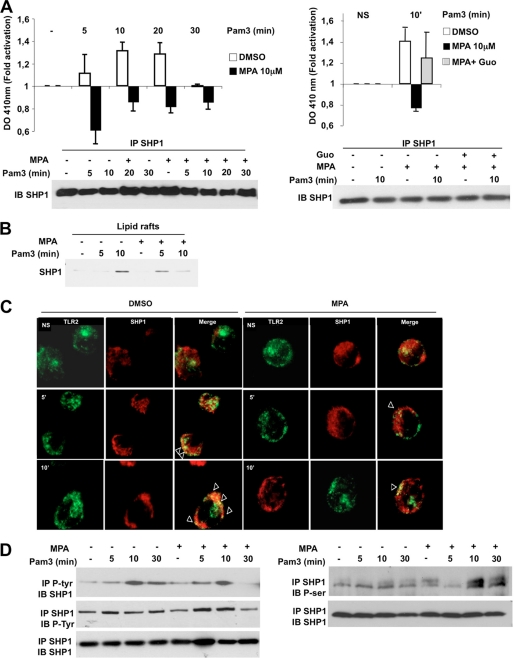
To further examine the interaction between IMPDHII and SHP1 upon engagement of TLR2, we examined the impact of IMPDHII inhibition on SHP1 activity. We immunoprecipitated SHP1 from Pam3-stimulated THP1 cells treated with MPA or MPA and guanosine. Then, after incubation of immunoprecipitates with a chromogenic substrate of SHP1, phosphatase activity was assessed quantitatively by measuring the absorbance (A410 nm), and activation was evaluated as the ratio between stimulated and unstimulated SHP1 activity. As shown in Fig. 9A, equal amounts of SHP1 were immunoprecipitated under each condition. Stimulation with Pam3 induced maximal activation of SHP1 after 10 min in control conditions. In contrast, inhibition of IMPDHII with MPA dramatically decreased SHP1 activity (Fig. 9A, left panel). The addition of guanosine in the cell media before stimulation reversed the inhibitory effect of MPA on SHP1 activity (Fig. 9A, right panel). Similar results were observed in THP1 cells stimulated with Pam2 (data not shown). These data suggest that IMPDHII contributes to the activation of SHP1 following TLR2 engagement. The activity of SHP1 depends upon the conformational changes induced by its recruitment to the ITIM domains of membranous interacting molecules. To check whether IMPDHII activates SHP1 through its recruitment within the TLR2 activation cluster, we performed immunoblotting of protein extracts from TLR2-stimulated THP1 cells treated with DMSO or MPA. As shown in Fig. 9B, SHP1 was recruited to lipid rafts following TLR2 stimulation independently of IMPDHII activity. We then tested whether IMDHII activity controls the interaction between SHP1 and TLR2 within its activation cluster. Whereas confocal microscopy studies revealed that SHP1 and TLR2 had a distinct localization in resting cells (Fig. 9C), stimulation with Pam3 induced colocalization of these two proteins in the cell membrane. Inhibition of IMPDHII by MPA did not prevent this interaction, suggesting that the activation of SHP1 by IMPDHII requires post-translational modifications in addition to its recruitment within the TLR2 activation cluster. Activity of SHP1 depends upon its phosphorylation status (30). Phosphorylation of tyrosine residues in the C-terminal tail of the phosphatase conveys its activation, whereas phosphorylation of serine 591 is associated with decreased activity of SHP1 (31, 32). We performed immunoprecipitation studies to investigate tyrosine and serine phosphorylation profiles after TLR2 stimulation (Fig. 9D). Whereas phosphorylation of tyrosine residues was not influenced by inhibition of IMPDHII activity, incubation of THP1 cells with MPA significantly increased phosphorylation of serine residues. These data are consistent with decreased SHP1 activity following inhibition of IMPDHII.
FIGURE 9.
IMPDHII regulates phosphatase activity of SHP1 downstream of TLR2 but does not affect its recruitment the TLR2 activation cluster. A, THP1 cells were incubated with DMSO, MPA (10 μm), or MPA with guanosine (Guo, 50 μm) before stimulation with Pam3. Cells were lysed in a lysis buffer without any phosphatase inhibitors. Proteins were immunoprecipitated with 3 μg of anti-SHP1 monoclonal antibody and incubated for 2 h with agarose beads. Immunoprecipitates were washed, and the reaction was initiated by the addition of 25 mm para-nitrophenyl phosphate for 30 min at 30 °C. The reaction was stopped by the addition of 1 n NaOH, and sample absorbances were measured at 410 nm. Results were expressed as “-fold activation.” An equal amount of precipitated proteins was controlled by Western blot (IB). Controls correspond to cells incubated with DMSO. Data are the means ± S.D. representative of three independent experiments. NS, nonstimulated. B, THP1 cells were incubated with MPA (10 μm), stimulated with Pam3 (1 μg/ml), and lysed with 1% Triton X-100. Lipid rafts were isolated by ultracentrifugation (fractions 2–4), and lipid raft proteins were subjected to SDS-PAGE. SHP1 was revealed by immunoblot in lipid rafts. C, THP1 cells were incubated with MPA, stimulated with Pam3 (1 mg/ml), and then fixed with 4% paraformaldehyde and washed with 0.1 m glycine. Cells were then permeabilized with PBS-1% BSA containing 0.05% saponin and incubated with anti-TLR2 and anti-SHP1 antibodies. Cells were subsequently labeled with Alexa Fluor 488- and Alexa Fluor 594-conjugated secondary antibodies combined. Cells were washed with PBS and mounted on slides. All images were obtained using a confocal Leica DMIRE2 microscope and analyzed using ImageJ 1.40 software. D, THP1 cells were incubated or not with MPA, stimulated with Pam3, and lysed. Cell lysates were immunoprecipitated (IP) with anti-phosphotyrosine (P-Tyr, 4G10 clone) or anti-SHP1 antibodies. Immunoprecipitates were then submitted to Western blot with anti-SHP1, anti-P-Tyr, and anti-P-Ser antibodies. The results are representative of three independent experiments.
DISCUSSION
Using a proteomics approach to find novel partners in TLR2-dependent signaling pathways, we have identified IMPDHII as an important negative regulator of NF-κB activation. The regulation of NF-κB activity downstream of TLRs is a very complex process that controls the balance between an appropriate immune response to combat infection or a dysregulated pathological inflammation that leads to tissue damage and organ failure. In recent years, positive or negative regulators of NF-κB activity have been identified at almost every step of the TLR-signaling pathways (14). Recent evidence indicates that lipid rafts play a pivotal role in the fine regulation of the innate immune response through various mechanisms including the recruitment or exclusion of signaling molecules (7, 33, 34). In contrast to a preconceived hypothesis, our proteomics strategy provided an unbiased differential analysis of proteins recruited in lipid rafts following TLR2 engagement, allowing for a systematic characterization of previously unidentified proteins. We report for the first time that IMPDHII is recruited to a TLR2-dependent membrane activation cluster within the lipid rafts after stimulation with lipopeptides. IMPDHII decreases NF-κB activation through increased SHP1 activity and subsequent dephosphorylation of the p85α subunit of PI3K, supporting the role of IMPDHII as a negative regulator of the PI3K/Akt-dependent pathway of NF-κB transactivation.
IMPDHII is a key enzyme of the de novo guanosine synthesis through the NAD-dependent conversion of inosine monophosphate (IMP) to xanthosine monophosphate (35, 36). In mammals, the two isoforms (type I and type II) of IMPDH are present as homotetramers (37). In contrast to IMPDHI, which display ubiquitous expression, IMPDHII expression is restricted to hematopoietic stem cells. IMPDHII is essential for B- and T-cell proliferation (38, 39) and is hence targeted by immunosuppressive therapies such as mycophenolic acid (19). The role of IMPDHII in innate immune cells has been much less studied. It has been suggested previously that IMPDHII contributes to the production of TNFα and nitric oxide following macrophage stimulation with LPS and interferon-γ through a guanosine-dependent mechanism (40). Paradoxically, noninfectious acute inflammatory syndromes with increased polymorphonuclear leukocyte activation have been reported in patients treated with mycophenolate mofetil, the pro-drug of MPA, following kidney transplantation or systemic diseases. Recent studies report that inhibition of IMPDHII by MPA in IL-1-β-stimulated endothelial cells potentiates the NF-κB-dependent production of intercellular adhesion molecule 1 (ICAM-1), suggesting that IMPDHII is involved in the negative regulation of signals mediated through the IL-1 receptor family. In this model, IMPDHII prevented degradation of IκB and subsequent nuclear translocation of the p50/p65 dimer (41). Lee et al. (42) have also demonstrated that inhibition of IMPDHII increases TLR7-dependent NF-κB activity in murine macrophages or human leukocytes. Consistently, we found that IMPDHII is a negative regulator of NF-κB activation and TNFα production downstream of TLR2. The effect of IMPDHII on NF-κB activity was specific to the TLR2 signaling pathway, as inhibition of IMPDHII did not significantly influence NF-κB activity downstream of the membrane-bound TLR4 and TLR5 receptors. The type of TLR2 heterodimers involved had no impact on the inhibitory effect of IMPDHII on NF-κB activity. Inhibition of IMPDHII activity had no effect on the activation profiles of Jnk, p38, and Erk. Altogether, these data suggest that IMPDHII has a fundamental inhibitory role in TLR2-dependent signal transduction to NF-κB.
Our results indicate that the inhibitory effect of IMPDHII is mediated through the negative regulation of PI3K activity. Several studies have indicated that in lymphocytes and neutrophils, PI3K activity is regulated by tyrosine phosphorylation of its p85α subunit (27). This phosphorylation state seems, however, tightly controlled by the tyrosine phosphatase SHP1, which has been found to decrease phosphorylation of p85α and inhibit human T-cell, neutrophil, and macrophage cell activation (27, 28, 43). Consistent with data demonstrating that SHP1 also modulates PI3K activity downstream of TLR2 in human pulmonary epithelial cells (44), we found that silencing SHP1 expression increased p85α phosphorylation and Akt-dependent transactivation of p65. Our data show that inhibition of IMPDHII increases phosphorylation of p85α through increased phosphorylation of SHP1 serine residues and decreased SHP1 activity. The mechanisms underlying the IMPDHII-mediated increased activation of SHP1 in our model are not completely understood. Several hypotheses can be discussed. First, IMPDHII could modulate the activation of SHP1 through as yet unidentified intermediate proteins, such as G-proteins, for which activation is fully dependent on the production of GTP (19, 45). Consistent with this hypothesis, the administration of GTP reversed the NF-κB potentiation observed after inhibition of IMPDHII. As expected, the activation of the Rho GTPase Rac1 was sensitive to MPA. However, silencing Rac1 did not prevent the MPA-induced increase in NF-κB activity following stimulation of TLR2. Altogether, these data infer that Rac1 is involved in TLR2 activation upstream of PI3K, as suggested by Arbibe et al. (9), and that IMPDHII negatively regulates NF-κB through PI3K independently and downstream of Rac1. Other candidates could include proteins containing immunoreceptor tyrosine-based activation or inhibition motifs (ITAM or ITIM). Indeed, SHP1 activation requires its interaction with phosphorylated tyrosines on these motifs (30, 46). Several receptor and non-receptor molecules such as CEACAM1 (44) and TLR-signaling proteins, including IRAK1, contain ITIM motifs (23). Moreover, some receptors, such as β-integrins, coupled with ITAM-containing adaptors interfere with the signaling of TLRs (46, 47). Interestingly, molecules such as β-integrin also interact with PKC (48), the kinase that phosphorylates SHP1 on serine residues (31). Whether IMPDHII regulates an ITIM- or ITAM-containing protein that is yet to be identified remains unknown.
In summary, using an unbiased proteomic differential analysis of the lipid rafts content following TLR2 stimulation, we identified IMPDHII as a novel negative regulator of NF-κB activation that contributes to the dephosphorylation of the p85α subunit of PI3K through increased SHP1 activity. The exact mechanism underlying the effect of IMPDHII on SHP1 activity remains to be elucidated. These findings highlight the importance of balanced signals coordinating the inflammatory response to infection and may help to find novel targets in the control of TLR2-mediated sepsis.
Supplementary Material
This work was supported by grants from INSERM and the Cochin Association for Research on Inflammation, Sepsis, and Molecular Advances (CARISMA).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
- TLR
- Toll-like receptor
- IMPDHII
- inosine monophosphate dehydrogenase type II
- PBMC
- peripherical blood mononuclear cell
- MAP
- mitogen-activated protein
- MPA
- mycophenolic acid
- MBS
- MES buffer saline
- IPG
- immobilized pH gradient
- DIGE
- differential in-gel electrophoresis
- ABC
- ammonium bicarbonate
- ACN
- acetonitrile
- PI
- phosphatidylinositol
- DMSO
- dimethyl sulfoxide
- ITIM
- immunoreceptor tyrosine-based inhibition motif
- ITAM
- immunoreceptor tyrosine-based activation motif.
REFERENCES
- 1. Akira S., Takeda K. (2004) Nat. Rev. Immunol. 4, 499–511 [DOI] [PubMed] [Google Scholar]
- 2. Kawai T., Akira S. (2010) Nat. Immunol. 11, 373–384 [DOI] [PubMed] [Google Scholar]
- 3. Farhat K., Riekenberg S., Heine H., Debarry J., Lang R., Mages J., Buwitt-Beckmann U., Röschmann K., Jung G., Wiesmüller K. H., Ulmer A. J. (2008) J. Leukoc. Biol. 83, 692–701 [DOI] [PubMed] [Google Scholar]
- 4. Triantafilou M., Gamper F. G., Haston R. M., Mouratis M. A., Morath S., Hartung T., Triantafilou K. (2006) J. Biol. Chem. 281, 31002–31011 [DOI] [PubMed] [Google Scholar]
- 5. Hajjar A. M., O'Mahony D. S., Ozinsky A., Underhill D. M., Aderem A., Klebanoff S. J., Wilson C. B. (2001) J. Immunol. 166, 15–19 [DOI] [PubMed] [Google Scholar]
- 6. Ozinsky A., Underhill D. M., Fontenot J. D., Hajjar A. M., Smith K. D., Wilson C. B., Schroeder L., Aderem A. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 13766–13771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Soong G., Reddy B., Sokol S., Adamo R., Prince A. (2004) J. Clin. Invest. 113, 1482–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Triantafilou M., Triantafilou K. (2002) Trends Immunol. 23, 301–304 [DOI] [PubMed] [Google Scholar]
- 9. Arbibe L., Mira J. P., Teusch N., Kline L., Guha M., Mackman N., Godowski P. J., Ulevitch R. J., Knaus U. G. (2000) Nat. Immunol. 1, 533–540 [DOI] [PubMed] [Google Scholar]
- 10. Liljeroos M., Vuolteenaho R., Morath S., Hartung T., Hallman M., Ojaniemi M. (2007) Cell. Signal. 19, 625–633 [DOI] [PubMed] [Google Scholar]
- 11. Santos-Sierra S., Deshmukh S. D., Kalnitski J., Küenzi P., Wymann M. P., Golenbock D. T., Henneke P. (2009) EMBO J. 28, 2018–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Strassheim D., Kim J. Y., Park J. S., Mitra S., Abraham E. (2005) J. Immunol. 174, 8064–8071 [DOI] [PubMed] [Google Scholar]
- 13. Strassheim D., Asehnoune K., Park J. S., Kim J. Y., He Q., Richter D., Kuhn K., Mitra S., Abraham E. (2004) J. Immunol. 172, 5727–5733 [DOI] [PubMed] [Google Scholar]
- 14. Liew F. Y., Xu D., Brint E. K., O'Neill L. A. (2005) Nat. Rev. Immunol. 5, 446–458 [DOI] [PubMed] [Google Scholar]
- 15. Shembade N., Ma A., Harhaj E. W. (2010) Science 327, 1135–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kawagoe T., Takeuchi O., Takabatake Y., Kato H., Isaka Y., Tsujimura T., Akira S. (2009) Nat. Immunol. 10, 965–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoshida H., Jono H., Kai H., Li J. D. (2005) J. Biol. Chem. 280, 41111–41121 [DOI] [PubMed] [Google Scholar]
- 18. Wald D., Qin J., Zhao Z., Qian Y., Naramura M., Tian L., Towne J., Sims J. E., Stark G. R., Li X. (2003) Nat. Immunol. 4, 920–927 [DOI] [PubMed] [Google Scholar]
- 19. Allison A. C., Eugui E. M. (2000) Immunopharmacology 47, 85–118 [DOI] [PubMed] [Google Scholar]
- 20. Franklin T. J., Morris W. P., Jacobs V. N., Culbert E. J., Heys C. A., Ward W. H., Cook P. N., Jung F., Plé P. (1999) Biochem. Pharmacol. 58, 867–876 [DOI] [PubMed] [Google Scholar]
- 21. Jain J., Almquist S. J., Ford P. J., Shlyakhter D., Wang Y., Nimmesgern E., Germann U. A. (2004) Biochem. Pharmacol. 67, 767–776 [DOI] [PubMed] [Google Scholar]
- 22. Harriague J., Bismuth G. (2002) Nat. Immunol. 3, 1090–1096 [DOI] [PubMed] [Google Scholar]
- 23. Abu-Dayyeh I., Shio M. T., Sato S., Akira S., Cousineau B., Olivier M. (2008) PLoS Negl. Trop. Dis. 2, e305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Russell J. A. (2006) N. Engl. J. Med. 355, 1699–1713 [DOI] [PubMed] [Google Scholar]
- 25. Chacko G. W., Tridandapani S., Damen J. E., Liu L., Krystal G., Coggeshall K. M. (1996) J. Immunol. 157, 2234–2238 [PubMed] [Google Scholar]
- 26. Sattler M., Verma S., Pride Y. B., Salgia R., Rohrschneider L. R., Griffin J. D. (2001) J. Biol. Chem. 276, 2451–2458 [DOI] [PubMed] [Google Scholar]
- 27. al-Shami A., Bourgoin S. G., Naccache P. H. (1997) Blood 89, 1035–1044 [PubMed] [Google Scholar]
- 28. Cuevas B. D., Lu Y., Mao M., Zhang J., LaPushin R., Siminovitch K., Mills G. B. (2001) J. Biol. Chem. 276, 27455–27461 [DOI] [PubMed] [Google Scholar]
- 29. Mazerolles F., Barbat C., Fischer A. (1997) Eur. J. Immunol. 27, 2457–2465 [DOI] [PubMed] [Google Scholar]
- 30. Poole A. W., Jones M. L. (2005) Cell. Signal. 17, 1323–1332 [DOI] [PubMed] [Google Scholar]
- 31. Brumell J. H., Chan C. K., Butler J., Borregaard N., Siminovitch K. A., Grinstein S., Downey G. P. (1997) J. Biol. Chem. 272, 875–882 [DOI] [PubMed] [Google Scholar]
- 32. Jones M. L., Craik J. D., Gibbins J. M., Poole A. W. (2004) J. Biol. Chem. 279, 40475–40483 [DOI] [PubMed] [Google Scholar]
- 33. Triantafilou M., Miyake K., Golenbock D. T., Triantafilou K. (2002) J. Cell Sci. 115, 2603–2611 [DOI] [PubMed] [Google Scholar]
- 34. Olsson S., Sundler R. (2006) Mol. Immunol. 43, 607–612 [DOI] [PubMed] [Google Scholar]
- 35. Collart F. R., Huberman E. (1988) J. Biol. Chem. 263, 15769–15772 [PubMed] [Google Scholar]
- 36. Colby T. D., Vanderveen K., Strickler M. D., Markham G. D., Goldstein B. M. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 3531–3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carr S. F., Papp E., Wu J. C., Natsumeda Y. (1993) J. Biol. Chem. 268, 27286–27290 [PubMed] [Google Scholar]
- 38. Jackson R. C., Weber G., Morris H. P. (1975) Nature 256, 331–333 [DOI] [PubMed] [Google Scholar]
- 39. Sintchak M. D., Nimmesgern E. (2000) Immunopharmacology 47, 163–184 [DOI] [PubMed] [Google Scholar]
- 40. Jonsson C. A., Carlsten H. (2002) Cell. Immunol. 216, 93–101 [DOI] [PubMed] [Google Scholar]
- 41. Weigel G., Bertalanffy P., Wolner E. (2002) Mol. Pharmacol. 62, 453–462 [DOI] [PubMed] [Google Scholar]
- 42. Lee J., Wu C. C., Lee K. J., Chuang T. H., Katakura K., Liu Y. T., Chan M., Tawatao R., Chung M., Shen C., Cottam H. B., Lai M. M., Raz E., Carson D. A. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 1828–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhou D., Collins C. A., Wu P., Brown E. J. (2010) J. Leukoc. Biol. 87, 845–855 [DOI] [PubMed] [Google Scholar]
- 44. Slevogt H., Zabel S., Opitz B., Hocke A., Eitel J., N′guessan P. D., Lucka L., Riesbeck K., Zimmermann W., Zweigner J., Temmesfeld-Wollbrueck B., Suttorp N., Singer B. B. (2008) Nat. Immunol. 9, 1270–1278 [DOI] [PubMed] [Google Scholar]
- 45. Mondin M., Moreau V., Genot E., Combe C., Ripoche J., Dubus I. (2007) Biochem. Pharmacol. 73, 1491–1498 [DOI] [PubMed] [Google Scholar]
- 46. Hamerman J. A., Ni M., Killebrew J. R., Chu C. L., Lowell C. A. (2009) Immunol. Rev. 232, 42–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Han C., Jin J., Xu S., Liu H., Li N., Cao X. (2010) Nat. Immunol. 11, 734–742 [DOI] [PubMed] [Google Scholar]
- 48. Abram C. L., Lowell C. A. (2009) Annu. Rev. Immunol. 27, 339–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.