Abstract
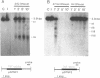
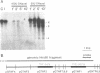
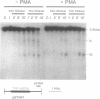
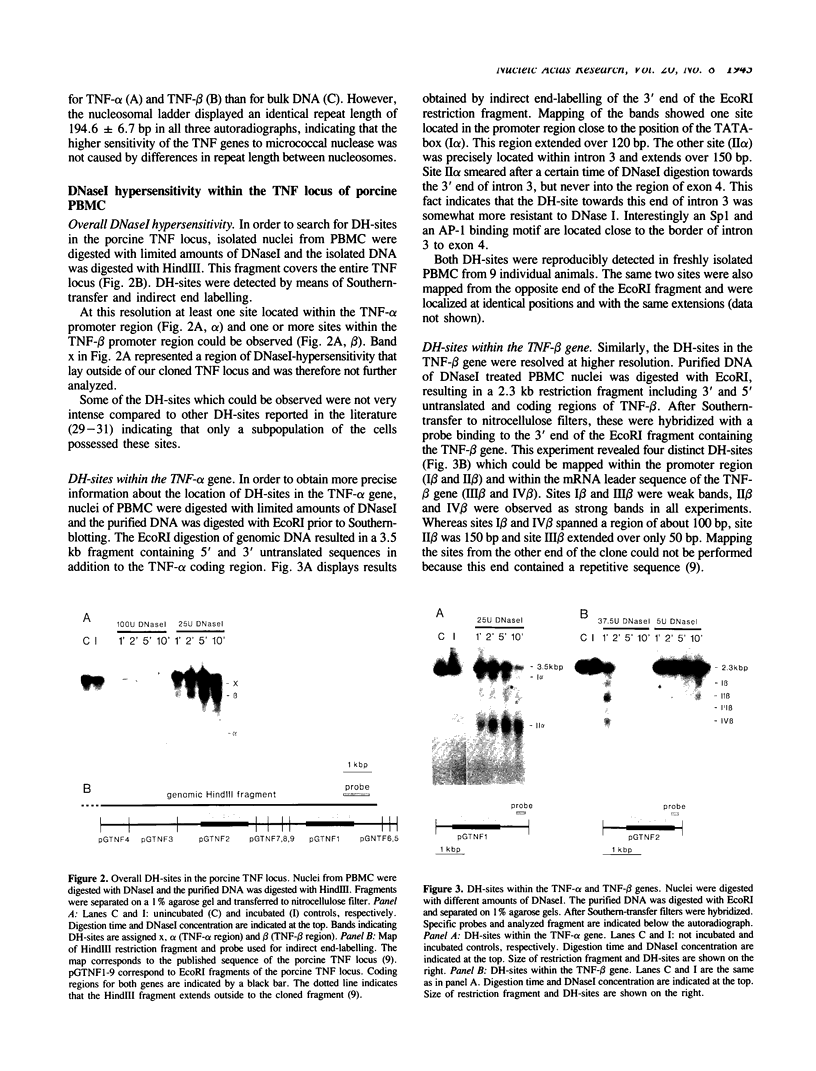
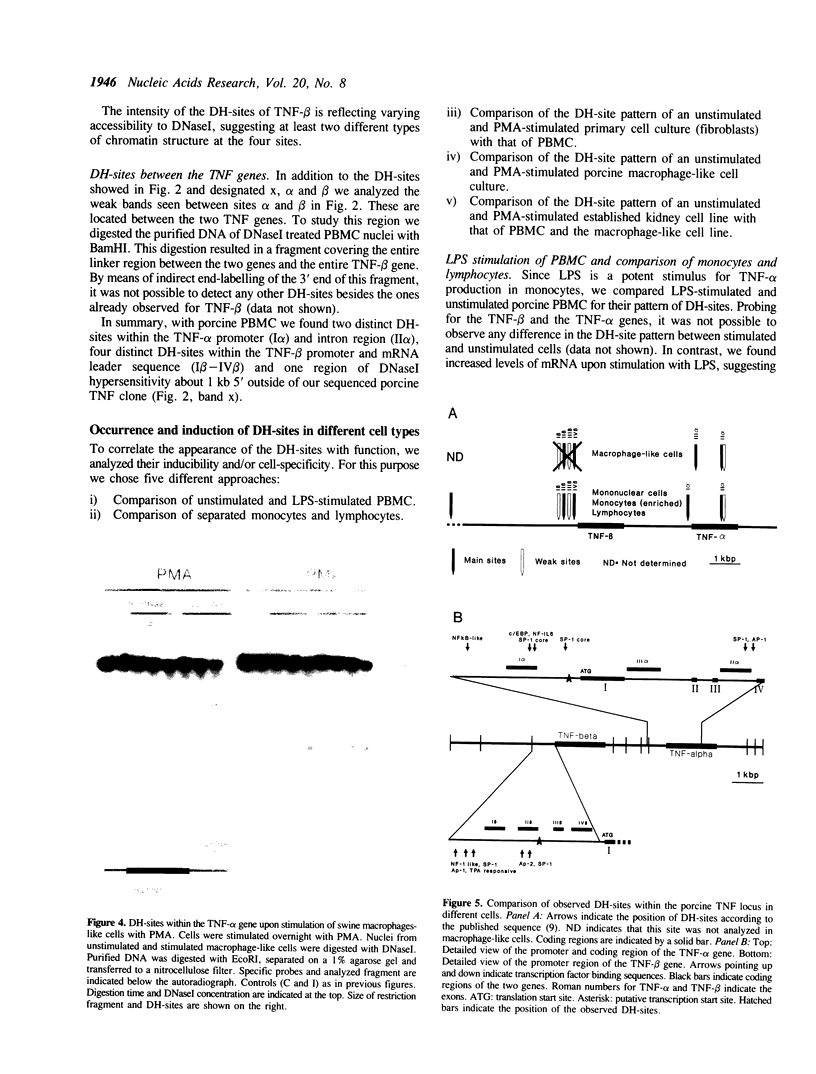
We have analyzed the chromatin structure of the porcine tumor necrosis factor gene locus (TNF-alpha and TNF-beta). Nuclei from porcine peripheral blood mononuclear cells were digested with different nucleases. As assessed with micrococcal nuclease, the two TNF genes displayed slightly faster digestion kinetics than bulk DNA. Studies with DNaseI revealed distinct DNaseI hypersensitive sites (DH-sites) within the porcine TNF locus. Four DH-sites could be observed in the promoter and mRNA leader regions of the TNF-beta gene. Two DH-sites could be observed for the TNF-alpha gene, one located in the promoter region close to the TATA-box and the other site in intron 3. This pattern of DH-sites was present independently of the activation state of the cells. Interestingly in a porcine macrophage-like cell line, we found that the TNF-alpha promoter DH-site disappeared and another DH-site appeared in the region of intron 1. Additionally, the DH-site of intron 3 could be enhanced by PMA-stimulation in these cells. TNF-beta sites were not detected in this cell line. However, DH-sites were totally absent in fibroblasts (freshly isolated from testicles) and in porcine kidney cells (PK15 cell line) both of which do not transcribe the TNF genes. Therefore, the pattern of DH-sites corresponds to the transcriptional activity of analyzed cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler B., Brown T. A CAT reporter construct allows ultrasensitive estimation of TNF synthesis, and suggests that the TNF gene has been silenced in non-macrophage cell lines. J Clin Invest. 1991 Apr;87(4):1336–1344. doi: 10.1172/JCI115137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- Bryan P. N., Olah J., Birnstiel M. L. Major changes in the 5' and 3' chromatin structure of sea urchin histone genes accompany their activation and inactivation in development. Cell. 1983 Jul;33(3):843–848. doi: 10.1016/0092-8674(83)90026-0. [DOI] [PubMed] [Google Scholar]
- Collart M. A., Baeuerle P., Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Mol Cell Biol. 1990 Apr;10(4):1498–1506. doi: 10.1128/mcb.10.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouet C., Shakhov A. N., Jongeneel C. V. Enhancers and transcription factors controlling the inducibility of the tumor necrosis factor-alpha promoter in primary macrophages. J Immunol. 1991 Sep 1;147(5):1694–1700. [PubMed] [Google Scholar]
- English B. K., Weaver W. M., Wilson C. B. Differential regulation of lymphotoxin and tumor necrosis factor genes in human T lymphocytes. J Biol Chem. 1991 Apr 15;266(11):7108–7113. [PubMed] [Google Scholar]
- Fashena S. J., Tang W. L., Sarr T., Ruddle N. H. The murine lymphotoxin gene promoter. Characterization and negative regulation. J Immunol. 1990 Jul 1;145(1):177–183. [PubMed] [Google Scholar]
- Fraser P., Hurst J., Collis P., Grosveld F. DNaseI hypersensitive sites 1, 2 and 3 of the human beta-globin dominant control region direct position-independent expression. Nucleic Acids Res. 1990 Jun 25;18(12):3503–3508. doi: 10.1093/nar/18.12.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritton H. P., Igo-Kemenes T., Nowock J., Strech-Jurk U., Theisen M., Sippel A. E. Alternative sets of DNase I-hypersensitive sites characterize the various functional states of the chicken lysozyme gene. Nature. 1984 Sep 13;311(5982):163–165. doi: 10.1038/311163a0. [DOI] [PubMed] [Google Scholar]
- Goldfeld A. E., Maniatis T. Coordinate viral induction of tumor necrosis factor alpha and interferon beta in human B cells and monocytes. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1490–1494. doi: 10.1073/pnas.86.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfeld A. E., Strominger J. L., Doyle C. Human tumor necrosis factor alpha gene regulation in phorbol ester stimulated T and B cell lines. J Exp Med. 1991 Jul 1;174(1):73–81. doi: 10.1084/jem.174.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Shirai T., Yamamoto S., Akira M., Kawahara S., Todd C. W., Wallace R. B. Molecular cloning of the gene encoding rabbit tumor necrosis factor. DNA. 1986 Apr;5(2):157–165. doi: 10.1089/dna.1986.5.157. [DOI] [PubMed] [Google Scholar]
- Jongeneel C. V., Shakhov A. N., Nedospasov S. A., Cerottini J. C. Molecular control of tissue-specific expression at the mouse TNF locus. Eur J Immunol. 1989 Mar;19(3):549–552. doi: 10.1002/eji.1830190321. [DOI] [PubMed] [Google Scholar]
- Jungi T. W. A rapid and sensitive method allowing photometric determination of erythrophagocytosis by mononuclear phagocytes. J Immunol Methods. 1985 Sep 3;82(1):141–153. doi: 10.1016/0022-1759(85)90233-9. [DOI] [PubMed] [Google Scholar]
- Kasid A., Director E. P., Stovroff M. C., Lotze M. T., Rosenberg S. A. Cytokine regulation of tumor necrosis factor-alpha and -beta (lymphotoxin)-messenger RNA expression in human peripheral blood mononuclear cells. Cancer Res. 1990 Aug 15;50(16):5072–5076. [PubMed] [Google Scholar]
- Kuhnert P., Wüthrich C., Peterhans E., Pauli U. The porcine tumor necrosis factor-encoding genes: sequence and comparative analysis. Gene. 1991 Jun 30;102(2):171–178. doi: 10.1016/0378-1119(91)90075-m. [DOI] [PubMed] [Google Scholar]
- Kunkel S. L., Remick D. G., Strieter R. M., Larrick J. W. Mechanisms that regulate the production and effects of tumor necrosis factor-alpha. Crit Rev Immunol. 1989;9(2):93–117. [PubMed] [Google Scholar]
- Li C. B., Gray P. W., Lin P. F., McGrath K. M., Ruddle F. H., Ruddle N. H. Cloning and expression of murine lymphotoxin cDNA. J Immunol. 1987 Jun 15;138(12):4496–4501. [PubMed] [Google Scholar]
- Lozano G., Levine A. J. Tissue-specific expression of p53 in transgenic mice is regulated by intron sequences. Mol Carcinog. 1991;4(1):3–9. doi: 10.1002/mc.2940040103. [DOI] [PubMed] [Google Scholar]
- Martinet Y., Yamauchi K., Crystal R. G. Differential expression of the tumor necrosis factor/cachectin gene by blood and lung mononuclear phagocytes. Am Rev Respir Dis. 1988 Sep;138(3):659–665. doi: 10.1164/ajrccm/138.3.659. [DOI] [PubMed] [Google Scholar]
- Müller U., Jongeneel C. V., Nedospasov S. A., Lindahl K. F., Steinmetz M. Tumour necrosis factor and lymphotoxin genes map close to H-2D in the mouse major histocompatibility complex. Nature. 1987 Jan 15;325(6101):265–267. doi: 10.1038/325265a0. [DOI] [PubMed] [Google Scholar]
- Nedwin G. E., Naylor S. L., Sakaguchi A. Y., Smith D., Jarrett-Nedwin J., Pennica D., Goeddel D. V., Gray P. W. Human lymphotoxin and tumor necrosis factor genes: structure, homology and chromosomal localization. Nucleic Acids Res. 1985 Sep 11;13(17):6361–6373. doi: 10.1093/nar/13.17.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli U., Beutler B., Peterhans E. Porcine tumor necrosis factor alpha: cloning with the polymerase chain reaction and determination of the nucleotide sequence. Gene. 1989 Sep 1;81(1):185–191. doi: 10.1016/0378-1119(89)90350-8. [DOI] [PubMed] [Google Scholar]
- Sariban E., Imamura K., Luebbers R., Kufe D. Transcriptional and posttranscriptional regulation of tumor necrosis factor gene expression in human monocytes. J Clin Invest. 1988 May;81(5):1506–1510. doi: 10.1172/JCI113482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenzato G. Tumour necrosis factor: a cytokine with multiple biological activities. Br J Cancer. 1990 Mar;61(3):354–361. doi: 10.1038/bjc.1990.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semon D., Kawashima E., Jongeneel C. V., Shakhov A. N., Nedospasov S. A. Nucleotide sequence of the murine TNF locus, including the TNF-alpha (tumor necrosis factor) and TNF-beta (lymphotoxin) genes. Nucleic Acids Res. 1987 Nov 11;15(21):9083–9084. doi: 10.1093/nar/15.21.9083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhov A. N., Collart M. A., Vassalli P., Nedospasov S. A., Jongeneel C. V. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J Exp Med. 1990 Jan 1;171(1):35–47. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino B. P., Ney P. A., Nienhuis A. W. Localization and characterization of the DNase I-hypersensitive site II (HS II) enhancer. A critical regulatory element within the beta-globin locus-activating region. Ann N Y Acad Sci. 1990;612:141–151. doi: 10.1111/j.1749-6632.1990.tb24300.x. [DOI] [PubMed] [Google Scholar]
- Taffet S. M., Singhel K. J., Overholtzer J. F., Shurtleff S. A. Regulation of tumor necrosis factor expression in a macrophage-like cell line by lipopolysaccharide and cyclic AMP. Cell Immunol. 1989 May;120(2):291–300. doi: 10.1016/0008-8749(89)90198-6. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Lee T. H. Tumor necrosis factor. New insights into the molecular mechanisms of its multiple actions. J Biol Chem. 1991 Apr 25;266(12):7313–7316. [PubMed] [Google Scholar]
- Voitenok N. N., Misuno N. I., Panyutich A. V., Kolesnikova T. S. Induction of tumor necrosis factor synthesis in human monocytes treated by transcriptional inhibitors. Immunol Lett. 1989 Jan 15;20(1):77–82. doi: 10.1016/0165-2478(89)90072-2. [DOI] [PubMed] [Google Scholar]
- Weil D., Brosset S., Dautry F. RNA processing is a limiting step for murine tumor necrosis factor beta expression in response to interleukin-2. Mol Cell Biol. 1990 Nov;10(11):5865–5875. doi: 10.1128/mcb.10.11.5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. The 5' ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature. 1980 Aug 28;286(5776):854–860. doi: 10.1038/286854a0. [DOI] [PubMed] [Google Scholar]