Abstract
The SNF1/AMP-activated protein kinases are central energy regulators in eukaryotes. SNF1 of Saccharomyces cerevisiae is inhibited during growth on high levels of glucose and is activated in response to glucose depletion and other stresses. Activation entails phosphorylation of Thr210 on the activation loop of the catalytic subunit Snf1 by Snf1-activating kinases. We have used mutational analysis to identify Snf1 residues that are important for regulation. Alteration of Tyr106 in the αC helix or Leu198 adjacent to the Asp-Phe-Gly motif on the activation loop relieved glucose inhibition of phosphorylation, resulting in phosphorylation of Thr210 during growth on high levels of glucose. Substitution of Arg for Gly53, at the N terminus of the kinase domain, increased activation on both high and low glucose. Alteration of the ubiquitin-associated domain revealed a modest autoinhibitory effect. Previous studies identified alterations of the Gal83 (β) and Snf4 (γ) subunits that relieve glucose inhibition, and we have here identified a distinct set of Gal83 residues that are required. Together, these results indicate that alterations at dispersed sites within each subunit of SNF1 cause phosphorylation of the kinase during growth on high levels of glucose. These findings suggest that the conformation of the SNF1 complex is crucial to maintenance of the inactive state during growth on high glucose and that the default state for SNF1 is one in which Thr210 is phosphorylated and the kinase is active.
Keywords: AMP-activated Kinase (AMPK), Glucose, Mutant, Protein Kinases, Protein Phosphatase, Signal Transduction, Yeast, Yeast Genetics
Introduction
The SNF1/AMP-activated protein kinase (AMPK)3 family is conserved from yeasts to humans. In mammals, AMPK regulates metabolism and energy status at both cellular and organismal levels (1, 2). SNF1 of the budding yeast Saccharomyces cerevisiae is the founding member of this family (3). SNF1 is inhibited during growth of cells in nutrient-rich conditions and is activated in response to nutrient limitation, notably glucose depletion, and other environmental stresses; the kinase regulates the transcription of a large set of genes and the activity of metabolic enzymes (4). Activation of SNF1 requires phosphorylation of the conserved Thr210 on the activation loop of the catalytic subunit Snf1 (5, 6) by any one of three activating kinases: Sak1, Tos3, or Elm1 (7–9). Reg1-Glc7 protein phosphatase 1 has roles in dephosphorylation of Thr210 and inhibition of SNF1 catalytic activity (6, 10, 11), and evidence suggests that access of Reg1-Glc7 to phosphorylated Thr210 is restricted under conditions of glucose limitation (12); however, Reg1-Glc7 is not solely responsible for dephosphorylation of Thr210, and Sit4, a type 2A-like protein phosphatase, also contributes (13).
SNF1/AMPK is a heterotrimer comprising the catalytic α subunit and regulatory β and γ subunits. For mammalian AMPK, AMP activation is mediated by the binding of AMP to the γ subunit (14, 15), which allosterically activates the kinase, inhibits dephosphorylation of the activation-loop Thr of the α subunit, and promotes phosphorylation by upstream kinases (16–19). For S. cerevisiae SNF1, the γ subunit Snf4 is required for catalytic function (20), but AMP does not allosterically activate SNF1 or protect SNF1 from dephosphorylation in vitro (17, 21, 22); however, alteration of several Snf4 residues analogous to those involved in AMP binding and regulation of AMPK modestly relieved glucose inhibition of SNF1 in vivo (23). In addition, alteration of residues at the interface between Snf4 and the β subunit (Asn177 and Arg169) caused substantial phosphorylation of Thr210 during growth on high glucose, as did alteration of Cys136 to Tyr, which is predicted to disrupt the structure of Snf4 (23).
The β subunits also play regulatory roles. SNF1 includes one of three alternate β subunits, Gal83, Sip1, or Sip2, which control the subcellular localization of the kinase and its interactions with substrates (24–26). Gal83 is the major isoform when cells are grown on glucose, whereas Sip2 is highly expressed during growth on a nonfermentable carbon source (26). A glycogen-binding domain (GBD), or carbohydrate-binding module, is conserved in Gal83 and Sip2 and to a much lesser extent in Sip1 (27). Deletion of the Gal83 GBD and substitutions of single residues within the GBD that interact with Snf4 resulted in Thr210 phosphorylation and SNF1 catalytic activity during growth on high glucose (23); several lines of evidence indicate that the effects of the GBD on SNF1 do not involve the binding of glycogen (13, 23).
The Snf1 catalytic subunit contains sequences C-terminal to the kinase domain that interact physically with Snf4 and the β subunit (28–30) (see Fig. 1A). Although the function of full-length Snf1 requires Snf4, truncation of Snf1 after the kinase domain at residue 309 or deletion of residues 381–414 alleviated the dependence on Snf4 in growth and other indirect assays, suggesting that Snf4 counteracts autoinhibition by C-terminal Snf1 sequences (20, 28, 31). The structure of the SNF1 heterotrimer core showed extensive interaction between Snf1 residues 460–498 (called the regulatory sequence) and Snf4 (30), but mutational analysis provided no evidence that this interaction serves a regulatory function (32). A putative ubiquitin-associated (UBA) domain, which contains the conserved MGY/F motif and hydrophobic residues but does not appear to bind ubiquitin (33, 34), lies distal to the kinase domain in Snf1 (residues 347–398) and AMPK-related kinases (33, 35–37). The UBA domain in the AMPK-related MARK protein kinases enhances phosphorylation and activation by LKB1 (33) but has a moderate inhibitory effect on activation of MARK1/2 by MARKK (38). The mammalian AMPK α subunit has a UBA-like domain lacking the MGY/F motif, which inhibits the catalytic activity of recombinant proteins and facilitates Thr dephosphorylation in vitro (39–41).
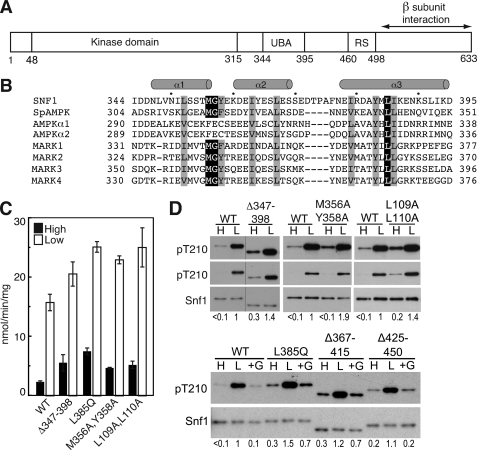
FIGURE 1.
Effects of the Snf1 UBA domain on Thr210 phosphorylation. A, schematic representation of Snf1 catalytic subunit. UBA, UBA domain. RS, regulatory sequence that interacts with Snf4 in the crystal structure (30). B, sequence alignment of UBA and UBA-like domains of the catalytic subunits of SNF1, S. pombe (Sp) AMPK, and human AMPKs and MARKs. Residues are indicated in single-letter code. Conserved hydrophobic residues are highlighted. Secondary structure designation is based on Chen et al. (39). C and D, wild-type (WT) and mutant Snf1 proteins were expressed in snf1Δ cells. Cells were collected after growth in 2% (high, H) glucose (filled bars) or after a shift to 0.05% (low, L) glucose for 10 min (open bars), as described under “Experimental Procedures.” In some cases, after incubation in 0.05% glucose, an aliquot of cells was incubated in SC plus 2% glucose (+G) for 10 min and collected. C, assays of SNF1 catalytic activity. D, immunoblot analysis to detect phosphorylated Thr210 (pT210) and Snf1 protein. Two exposures for detection of pT210 are shown in the upper panels. The bar indicates that lanes were from the same blot, but not adjacent. Immunoblot analysis was carried out on three independent transformants expressing each mutant protein; representative samples are shown. Values indicate the relative intensity of the bands corresponding to phosphorylated Snf1-Thr210 and total Snf1 protein, normalized to a value of 1 for WT in low glucose. For the upper panels, values were determined using the shorter exposures; for Snf1M356A,Y358A on high glucose, the longer exposure gave a value that was 4-fold greater than that for WT.
To assess the contribution of sequences within the Snf1 subunit to the regulation of SNF1 in response to glucose availability, we introduced deletions and substitutions that altered the UBA domain and other features, based on sequence conservation and structural information. We expressed each mutant Snf1 subunit in snf1Δ cells and assessed regulation of the resulting mutant SNF1 protein kinase by monitoring phosphorylation of Thr210 and catalytic activity. In addition, examination of the structure of the SNF1 heterotrimer core (30) led us to identify other residues of the Gal83 GBD that affect regulation of Thr210 phosphorylation. Our findings indicate that alterations at many sites within the SNF1 heterotrimer allow phosphorylation of Thr210 and activation of SNF1 during growth of cells on high levels of glucose, suggesting that the default state for SNF1 is one in which Thr210 is phosphorylated and the kinase is active.
EXPERIMENTAL PROCEDURES
Plasmids and Strains
Snf1, Snf4, and functional Gal83-green fluorescent protein fusion were expressed from their native promoters on centromeric plasmids pCE108 (20), pOV75 (23), and pRT12 (26), respectively. Mutations were introduced into plasmids using the QuikChange site-directed mutagenesis kit (Agilent) and confirmed by sequencing. Proteins were expressed in S. cerevisiae strains derived from W303–1A (MATa ade2 trp1 his3 can1 ura3 leu2): MCY4908 (W303–1A snf1Δ10) (42), MCY5713 (W303–1A snf1Δ10 snf4Δ::kanMX), MCY4099 (W303–1A gal83Δ::TRP1 sip1Δ::kanMX sip2Δ::kanMX) (27), MCY5733 (W303–1A gal83Δ::TRP1 sip1Δ::kanMX sip2Δ::kanMX snf4Δ::hphMX), and MCY5751 (W303–1A snf1Δ10 snf4Δ::hphMX gal83Δ::TRP1 sip1Δ::kanMX sip2Δ::kanMX).
Assay for Catalytic Activity
Cultures were grown in selective synthetic complete (SC) medium containing 2% glucose. After reaching exponential phase (A600 of 0.7–0.8), cells were collected by rapid filtration and either frozen in liquid nitrogen or resuspended in SC plus 0.05% glucose for 10 min and then harvested by filtration and frozen. For some experiments, after incubation in 0.05% glucose, an aliquot of cells was incubated in SC plus 2% glucose for 10 min and collected. SNF1 was partially purified from two to four independent cultures as described (43) except with an additional washing step with 0.1 m NaCl (3 ml) before the final elution from DEAE-Sepharose (GE Healthcare). Catalytic activity of SNF1 was assayed by measuring phosphorylation of SAMS peptide (HMRSAMSGLHLVKRR) (44) as described (21, 43), except ATP was at 1 mm, with different protein concentrations to confirm linearity. Kinase activity is expressed as nanomoles of phosphate incorporated into the peptide/min/mg of protein (44). Values are averages for 6–12 assays from 2–4 independent experiments.
Immunoblot Analysis
Partially purified protein samples, prepared as above, were separated on 8% SDS-PAGE, transferred to a PVDF membrane, and probed with anti-Thr(P)-172-AMPK (Cell Signaling) to detect phosphorylated Thr210 or anti-polyhistidine (Sigma) to detect Snf1, which contains a stretch of His residues. ECL Plus (GE Healthcare) was used for visualization. The intensity of the bands was quantified using ImageJ software from the National Institutes of Health (45).
RESULTS
Mutation of the UBA Domain Increases Activation of SNF1
To assess the role of the UBA domain in regulating SNF1, we expressed mutant Snf1 proteins from the native promoter on a centromeric plasmid in snf1Δ cells. Cultures were grown to exponential phase in medium containing 2% (high) glucose and were harvested by rapid filtration to maintain the phosphorylation state of Snf1. An aliquot was resuspended in medium containing 0.05% (low) glucose for 10 min and then harvested. We partially purified SNF1 and assayed Thr210 phosphorylation and kinase activity.
First, we deleted codons 347–398, encoding the UBA domain (Fig. 1, A and B). SNF1 protein kinase containing the Snf1Δ347–398 catalytic subunit showed modestly increased Thr210 phosphorylation and kinase activity, relative to wild-type SNF1, when cells were grown on high levels of glucose, and catalytic activity increased further when cultures were depleted for glucose (Fig. 1, C and D). We next introduced mutations to alter key residues. For the UBA-like domain of AMPK, alteration of the invariant Leu328 to Gln (L328Q) (Fig. 1B) increased the catalytic activity of a bacterially expressed AMPK fragment (41). The analogous Snf1L385Q mutant kinase was more highly phosphorylated on Thr210 and more active than wild-type kinase during growth on high glucose (Fig. 1, C and D). Substitution of the Met and Tyr residues of the MGY/F motif (Fig. 1B), which is predicted to disrupt the structure of the UBA domain in AMPK-related kinases (33, 34), resulted in increased activation of Snf1M356A,Y358A on high glucose (Fig. 1, C and D).
In a structure of the Schizosaccharomyces pombe kinase domain-autoinhibitory domain (KD-AID) (39), Leu88 in the kinase domain contributes to hydrophobic interactions with the UBA domain (called AID) involving the invariant Leu341 (equivalent to Snf1 Leu385). Leu88 is conserved in S. cerevisiae Snf1 (Leu109), as is the neighboring Leu89 (Leu110) at the end of the αC helix, and the double mutation L109A,L110A conferred a phenotype resembling that caused by alteration of Leu385 (Fig. 1, C and D). We note that the UBA domain is in a different position relative to the kinase domain in the MARK structures (38, 39) than in the S. pombe structure.
In each of the above cases, the effect was modest, but the mutations yielded a consistent pattern indicating that the UBA domain contributes to inhibition of Thr210 phosphorylation of SNF1. Deletion of sequences extending beyond or located immediately C-terminal to the UBA domain (Δ367–415, Δ345–441, and Δ425–450) did not identify any substantial regulatory role for this region (Fig. 1D and data not shown); these deletions shorten the linker to the Snf1 segment that is bound to Snf4.
Substitutions in the Snf1 Kinase Domain Cause Thr210 Phosphorylation during Growth on High Glucose
Crystal structures of the Snf1 kinase domain differ with respect to the position of the αC helix (46–48), which is important in the activation of various protein kinases; for example, in cyclin-dependent kinase, the binding of cyclin causes movement of the αC helix toward the kinase domain, which results in activation (49). To investigate the role of the αC helix in Snf1 function, we introduced Ala substitutions (Fig. 2, A and B). Most of the substitutions reduced SNF1 activity but did not affect regulation of Thr210 phosphorylation. Unexpectedly, substitution of Tyr106 relieved glucose inhibition, causing substantially increased Thr210 phosphorylation and activity during growth on high glucose. Tyr is not conserved at this position; the corresponding residue in human AMPKα isoforms is Asn. Snf1Y106A provided Snf1 function in growth assays and also conferred resistance to the glucose analog 2-deoxyglucose, which prevents growth of wild-type cells on sucrose (Fig. 2H). Mutations that relieve glucose inhibition of the SNF1 pathway, such as reg1, confer resistance (50), and addition of 2-deoxyglucose to glucose-limited cells inhibits SNF1 (51).
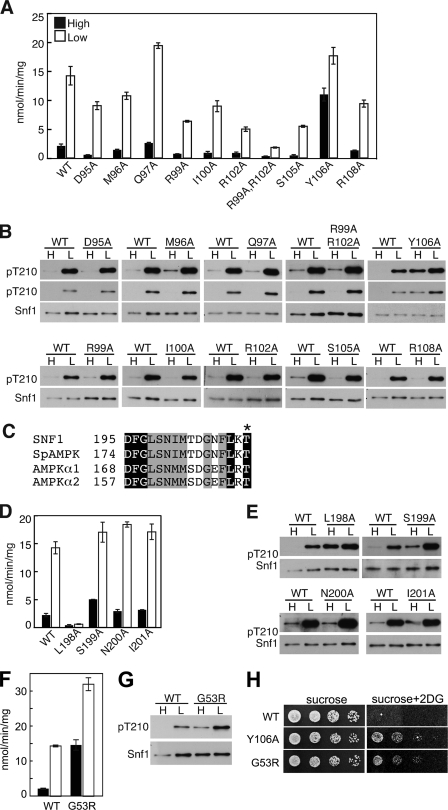
FIGURE 2.
Residues in the Snf1 kinase domain affect SNF1 activity and phosphorylation. A, B, and D–G, WT and mutant Snf1 proteins were expressed in snf1Δ cells. Assays of catalytic activity and immunoblot analysis were as in Fig. 1. Immunoblot analysis of three transformants expressing each mutant protein gave similar results. C, sequence alignment of the DFG motif and following residues for yeast and human members of the SNF1/AMPK family. Conserved residues are highlighted. Asterisk, phosphorylated Thr. H, cells were grown overnight in selective SC plus 2% glucose and spotted with serial 5-fold dilutions on selective SC solid medium containing 2% sucrose or 2% sucrose plus 200 μg/ml 2-deoxy-D-glucose (2DG). Plates were incubated at 30 °C for 2 or 3 days, respectively, and photographed. Rows were from the same plates.
Structures of the Snf1 kinase domain also differ with respect to the position of the invariant Asp195-Phe196-Gly197 (DFG) motif (46–48). We introduced Ala substitutions for Leu198, which is conserved as a hydrophobic residue in the SNF1/AMPK family (Fig. 2C), and the next three residues. The L198A substitution virtually abolished catalytic activity (Fig. 2D), but, unexpectedly, Snf1L198A was strongly phosphorylated on Thr210 in cells grown on high levels of glucose (Fig. 2E). Alteration of residues immediately following Leu198 did not cause comparable effects, although S199A resulted in some activation on high glucose. In one structure (47), Leu198 is located near Tyr106. Consistent with the lack of catalytic activity in vitro, Snf1L198A did not provide Snf1 function in vivo, as judged by growth on sucrose or glycerol plus ethanol.
Gly53 is located at the N terminus of the conserved kinase domain. The mutation SNF1-G53R was repeatedly isolated as a suppressor of growth defects of the snf4Δ mutant and was shown to partially restore SUC2 expression (5, 20). Assays of immunoprecipitated Snf1G53R for phosphorylation of coprecipitated proteins showed elevated activity, relative to Snf1, in both snf4Δ and wild-type SNF4 strains; however, this elevated activity was still markedly Snf4-dependent (5). Additional studies using a transcriptional activation assay confirmed that Snf1G53R has elevated catalytic activity in vivo (52).
At the time of the previous studies, procedures for maintaining the phosphorylation state of Snf1 Thr210 during preparation of cell extracts had not been developed, so we have revisited the properties of Snf1G53R. We found that Snf1G53R exhibits increased Thr210 phosphorylation and catalytic activity, relative to wild-type Snf1, in cells grown on high glucose, and is further activated in response to glucose depletion (Fig. 2, F and G). Cells expressing Snf1G53R exhibited resistance to 2-deoxyglucose (Fig. 2H).
Previous studies reported that Snf1G53R conferred glucose-regulated SUC2 expression and transcriptional activation (5, 20, 52). We note that the subcellular localization of SNF1 is regulated, and its enrichment in the nucleus, where key targets are localized, is inhibited during growth on high glucose (43, 51).
Negatively Charged Residues and a Conserved Sequence C-terminal to the Kinase Domain Are Not Required for Regulation of SNF1
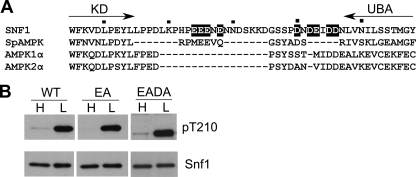
The region between the Snf1 kinase domain and the UBA domain is enriched for acidic residues (Fig. 3A), similar to the negatively charged common docking domains of MAPKs, which interact with other proteins, including kinases and phosphatases (53). To determine whether this region affects phosphorylation of Thr210, we substituted Ala for four of these acidic residues, Glu residues 324, 325, 326, and 328. The resulting quadruple mutant protein, designated Snf1EA, showed normal regulation of Thr210 phosphorylation (Fig. 3B). Additional mutation of Asp residues 340, 342, 345, and 346 and Glu343, to yield Snf1EADA, with nine Ala substitutions, did not alter Thr210 phosphorylation (Fig. 3B). Thus, the negatively charged residues in this region are not required for interactions with Snf1-activating kinases or protein phosphatases.
FIGURE 3.
C-terminal acidic residues of Snf1 are not required for regulated Thr210 phosphorylation. A, alignment of sequences lying between the kinase domain (KD) and UBA domain of yeast and human SNF1/AMPKs (residues 305–358 of Snf1). Residues that were changed to Ala in Snf1EA and Snf1EADA are highlighted. B, immunoblot analysis as in Fig. 1. Snf1 proteins were expressed in snf1Δ cells. The sequence encoding Snf1EADA was confirmed for codons 1–500.
The C terminus of Snf1 includes the sequence KKSKTRWHFGIRSRSYPLDV (residues 499 through 518; distal to the regulatory sequence segment), which is completely conserved among divergent yeast species, including Candida albicans, Debaryomyces hansenii, Kluyveromyces lactis, and Pichia stiptis (32). The position of the kinase domain relative to the SNF1 heterotrimer core (30) (see Fig. 5) is not known, but Snf1 residues 1–392 interacted with residues 392–518, and not with residues 392–513, in two-hybrid assays (28). To explore the functional importance of this region, we introduced various mutations. Snf1S511A,S513A,Y514A,P515A,L516A,D517A and Snf1S443A,S501A,T503A,S513A exhibited glucose-regulated SNF1 catalytic activity (1.1 ± 0.15 and 3.6 ± 0.59 nmol/min/mg on high glucose and 18 ± 1.8 and 20 ± 1.5 nmol/min/mg on low glucose, respectively). Alteration of basic residues in this conserved segment similarly had no effect, but we noted that these basic residues are positioned on a face of the core heterotrimer structure that has multiple positively charged residues; we therefore substituted Ala for thirteen basic residues on the Snf1 C terminus, Gal83, and Snf4 (Snf1K492A,K499A,K500A,K502A,R504A,R510A,R512A; Gal83K391A,H392A; and Snf4K51A,K52A,K246A,R263A) and expressed all three mutant proteins together in snf1Δ snf4Δ gal83Δ sip1Δ sip2Δ cells. The resulting multiply substituted SNF1 complex showed glucose-regulated SNF1 activity, 1.1 ± 0.04 and 8.0 ± 0.3 nmol/min/mg on high and low glucose, respectively; values were low when all subunits were expressed from plasmids (9.0 ± 0.07 nmol/min/mg for wild-type SNF1 on low glucose).
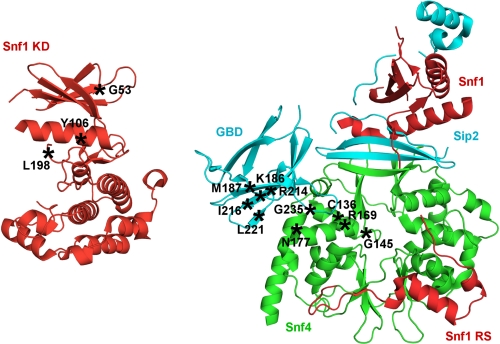
FIGURE 5.
Alterations that activate SNF1 during growth on high levels of glucose. Schematic representations are shown of the structures of the Snf1 kinase domain (PDB accession code 3HYH) (46) and the SNF1 core heterotrimer with Snf1 C-terminal sequence, including the regulatory sequence (residues 460–498) (red), Snf4 (green), and Sip2 C-terminal sequence, including the GBD (cyan) (PDB accession code 2QLV) (30). The β subunit residues are numbered as in Gal83. The positions of residues that, when altered, cause phosphorylation of Thr210 and activation of SNF1 during growth on high glucose are marked (asterisks). Representations of the structures were produced with PyMOL (DeLano, W. L. (2002) The PyMOL Molecular Graphics System, DeLano Scientific LLC, San Carlos, CA).
Substitutions in the Gal83 GBD Activate SNF1 during Growth on Glucose
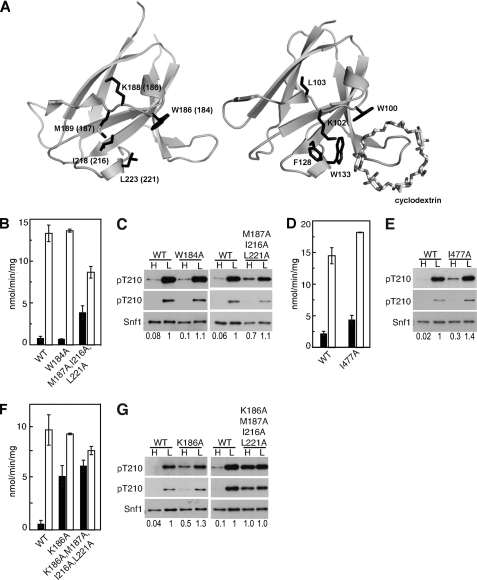
In the structure of the SNF1 heterotrimer core (30), Snf1 Ile477 interacts with hydrophobic residues Met189, Ile218, and Leu223 of the Sip2 GBD from another heterotrimer in the crystal. Substitution of the corresponding hydrophobic residues in Gal83M187A,I216A,L221A (Fig. 4A) caused substantial activation of SNF1 in gal83Δ sip1Δ sip2Δ cells on high glucose, with only a 2-fold increase in activity in response to glucose depletion (Fig. 4, B and C; note that Snf1 protein levels were reduced in the mutant samples). The substitution I477A caused lesser effects (Fig. 4, D and E), in accord with other evidence that the interaction in the structure is an artifact of crystal packing (32).
FIGURE 4.
Residues in the Gal83 GBD are required to maintain SNF1 in the dephosphorylated state in high glucose. A, schematic representation of the structures of Sip2 GBD (PDB accession code 2QLV) (30) with corresponding Gal83 residue numbers in parentheses, and mammalian GBD bound to β-cyclodextrin (PDB accession code 1Z0M) (54). For residues mentioned in the text, the corresponding side chains are shown for each structure. The structure figures were produced with PyMOL (DeLano, W. L. (2002) The PyMOL Molecular Graphics System, DeLano Scientific LLC, San Carlos, CA). B–G, assays of catalytic activity and immunoblot analysis as in Fig. 1. B, C, F, and G, Gal83 proteins were expressed in gal83Δ sip1Δ sip2Δ cells. D and E, Snf1 proteins were expressed in snf1Δ cells. Immunoblot analysis of three transformants gave similar results. Values indicate relative intensity of the bands corresponding to phosphorylated Snf1-Thr210 and total Snf1 protein on the longer exposures.
More important, these findings identified a set of GBD residues that affect glucose regulation and are not involved in interactions with Snf4. The Lys186 residue next to Met187 was also of interest, because the orientation of the corresponding side chain differs in the structures of Sip2 GBD and mammalian AMPK β1 GBD (30, 54) (Fig. 4A). Expression of Gal83K186A or Gal83K186A,M187A,I216A,L221A in gal83Δ sip1Δ sip2Δ cells caused substantial activation of SNF1 on high glucose (Fig. 4, F and G). Alteration of the highly conserved residue Trp184 in Gal83W184A did not alter regulation of Thr210 phosphorylation or SNF1 activity (Fig. 4, B and C); the analogous residue in mammalian β1 GBD, Trp100, is essential for the binding of glycogen to the GBD (54, 55). This finding is concordant with other evidence that glycogen binding to the GBD is not involved in regulation of SNF1 (13, 23).
DISCUSSION
In this study, we showed that residues in different regions of the Snf1 catalytic subunit are required to maintain inhibition of SNF1 during growth of cells on high levels of glucose (Fig. 5). Substitution of Ala for Tyr106 in the αC helix of the kinase domain resulted in substantial phosphorylation of Snf1 Thr210 and catalytic activity during growth on high glucose. Substitution of Ala for Leu198 adjacent to the DFG motif in the activation loop similarly relieved glucose inhibition of Thr210 phosphorylation, although alteration of this conserved residue abolished catalytic activity. Substitution of Arg for Gly53 at the N terminus of the kinase domain increased phosphorylation and activation of SNF1 on high glucose, with yet further activation occurring in response to glucose depletion. Finally, deletion of the UBA domain, or substitution of conserved residues, led to a modest increase in Thr210 phosphorylation and SNF1 activity on both high and low levels of glucose, consistent with a role in autoinhibition.
We also identified another set of residues of the Gal83 GBD that affect glucose inhibition of SNF1. Previously, we showed that the Gal83 GBD mutations W184A,R214Q and G235R, which affect interactions between residues at the interface of the GBD and Snf4 in the crystal structure of the SNF1 heterotrimer core (30), resulted in Thr210 phosphorylation and SNF1 catalytic activity during growth on high glucose, as did deletion of the entire Gal83 GBD (23). R214Q disrupts interaction with Snf4 Asn177, mutation of which confers a similar phenotype (23, 30). Here, we identified additional substitutions in the Gal83 GBD that have similar effects but alter residues that are not involved in interactions with Snf4 in the structure: K186A and M187A,I216A,L221A. These residues may participate in other interactions within the SNF1 complex that are not evident from the structure of the heterotrimer core, which lacks the kinase domain.
Residues in distinct regions of Snf4 contribute to inhibition of SNF1 in glucose-grown cells (23). In addition to Asn177 and Arg169, which interact with the β subunit, residues of Snf4 that are not located near the β subunit in the crystal structure affected glucose inhibition. Notably, substitution of Asp for Gly145 (analogous to His383 in the nucleotide-binding pocket of AMPK γ2) and Tyr for Cys136, which is predicted to disrupt the structure of Snf4, caused activation of SNF1 on high glucose.
Thus, alterations at dispersed sites within the SNF1 heterotrimer, in all three subunits, caused phosphorylation of Thr210 and activation of SNF1 during growth of cells on high levels of glucose. Maintenance of the dephosphorylated, inactive state in glucose-grown cells appears to require a specific conformation of the kinase complex that can be perturbed by a variety of alterations. It remains to be determined whether some of these alterations interfere with recognition of a glucose signal that acts directly on SNF1 to induce a particular conformation. The conformation assumed by wild-type SNF1 during growth on high glucose could either prevent phosphorylation or promote dephosphorylation, and either recognition of SNF1 or access to Thr210 by kinases or phosphatases could be affected. It has been reported that, under conditions of glucose limitation, Reg1-Glc7 phosphatase is active but its access to Thr210 is blocked (12); however, recent studies of the reg1Δ mutant (13) suggest a need to revisit the evidence that Reg1-Glc7 is active in low glucose. For AMPK, the binding of AMP inhibits dephosphorylation of the activation-loop Thr in vitro (17, 18) but also promotes phosphorylation by upstream kinases (19). Regardless of whether the regulated event during growth on high glucose is phosphorylation or dephosphorylation, our findings suggest that the conformation of the SNF1 complex is crucial to maintenance of the inactive state and that the default state for SNF1 is one in which the kinase is phosphorylated and active. These findings suggest new strategies for targeting AMPK to activate the kinase.
Acknowledgments
We thank Amparo Ruiz for growth assays and L. Tong and G. Amodeo for helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grant GM34095 (to M. C.).
- AMPK
- AMP-activated protein kinase
- GBD
- glycogen-binding domain
- UBA
- ubiquitin-associated
- SC
- synthetic complete medium.
REFERENCES
- 1. Kahn B. B., Alquier T., Carling D., Hardie D. G. (2005) Cell Metab. 1, 15–25 [DOI] [PubMed] [Google Scholar]
- 2. Steinberg G. R., Kemp B. E. (2009) Physiol. Rev. 89, 1025–1078 [DOI] [PubMed] [Google Scholar]
- 3. Celenza J. L., Carlson M. (1986) Science 233, 1175–1180 [DOI] [PubMed] [Google Scholar]
- 4. Hedbacker K., Carlson M. (2008) Front. Biosci. 13, 2408–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Estruch F., Treitel M. A., Yang X., Carlson M. (1992) Genetics. 132, 639–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCartney R. R., Schmidt M. C. (2001) J. Biol. Chem. 276, 36460–36466 [DOI] [PubMed] [Google Scholar]
- 7. Hong S. P., Leiper F. C., Woods A., Carling D., Carlson M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 8839–8843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sutherland C. M., Hawley S. A., McCartney R. R., Leech A., Stark M. J., Schmidt M. C., Hardie D. G. (2003) Curr. Biol. 13, 1299–1305 [DOI] [PubMed] [Google Scholar]
- 9. Nath N., McCartney R. R., Schmidt M. C. (2003) Mol. Cell. Biol. 23, 3909–3917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hong S. P., Momcilovic M., Carlson M. (2005) J. Biol. Chem. 280, 21804–21809 [DOI] [PubMed] [Google Scholar]
- 11. Ludin K., Jiang R., Carlson M. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 6245–6250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rubenstein E. M., McCartney R. R., Zhang C., Shokat K. M., Shirra M. K., Arndt K. M., Schmidt M. C. (2008) J. Biol. Chem. 283, 222–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ruiz A., Xu X., Carlson M. (2011) Proc. Natl. Acad. Sci. U.S.A. 108, 6349–6354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scott J. W., Hawley S. A., Green K. A., Anis M., Stewart G., Scullion G. A., Norman D. G., Hardie D. G. (2004) J. Clin. Invest. 113, 274–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xiao B., Heath R., Saiu P., Leiper F. C., Leone P., Jing C., Walker P. A., Haire L., Eccleston J. F., Davis C. T., Martin S. R., Carling D., Gamblin S. J. (2007) Nature 449, 496–500 [DOI] [PubMed] [Google Scholar]
- 16. Davies S. P., Helps N. R., Cohen P. T., Hardie D. G. (1995) FEBS Lett. 377, 421–425 [DOI] [PubMed] [Google Scholar]
- 17. Sanders M. J., Grondin P. O., Hegarty B. D., Snowden M. A., Carling D. (2007) Biochem. J. 403, 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Suter M., Riek U., Tuerk R., Schlattner U., Wallimann T., Neumann D. (2006) J. Biol. Chem. 281, 32207–32216 [DOI] [PubMed] [Google Scholar]
- 19. Oakhill J. S., Chen Z. P., Scott J. W., Steel R., Castelli L. A., Ling N., Macaulay S. L., Kemp B. E. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 19237–19241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Celenza J. L., Carlson M. (1989) Mol. Cell. Biol. 9, 5034–5044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Woods A., Munday M. R., Scott J., Yang X., Carlson M., Carling D. (1994) J. Biol. Chem. 269, 19509–19515 [PubMed] [Google Scholar]
- 22. Mitchelhill K. I., Stapleton D., Gao G., House C., Michell B., Katsis F., Witters L. A., Kemp B. E. (1994) J. Biol. Chem. 269, 2361–2364 [PubMed] [Google Scholar]
- 23. Momcilovic M., Iram S. H., Liu Y., Carlson M. (2008) J. Biol. Chem. 283, 19521–19529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vincent O., Carlson M. (1999) EMBO J. 18, 6672–6681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schmidt M. C., McCartney R. R. (2000) EMBO J. 19, 4936–4943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vincent O., Townley R., Kuchin S., Carlson M. (2001) Genes Dev. 15, 1104–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wiatrowski H. A., van Denderen B. J., Berkey C. D., Kemp B. E., Stapleton D., Carlson M. (2004) Mol. Cell. Biol. 24, 352–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jiang R., Carlson M. (1996) Genes Dev. 10, 3105–3115 [DOI] [PubMed] [Google Scholar]
- 29. Jiang R., Carlson M. (1997) Mol. Cell. Biol. 17, 2099–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Amodeo G. A., Rudolph M. J., Tong L. (2007) Nature 449, 492–495 [DOI] [PubMed] [Google Scholar]
- 31. Leech A., Nath N., McCartney R. R., Schmidt M. C. (2003) Eukaryot. Cell 2, 265–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Amodeo G. A., Momcilovic M., Carlson M., Tong L. (2010) Biochem. Biophys. Res. Commun. 397, 197–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jaleel M., Villa F., Deak M., Toth R., Prescott A. R., Van Aalten D. M., Alessi D. R. (2006) Biochem. J. 394, 545–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Murphy J. M., Korzhnev D. M., Ceccarelli D. F., Briant D. J., Zarrine-Afsar A., Sicheri F., Kay L. E., Pawson T. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 14336–14341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hofmann K., Bucher P. (1996) Trends Biochem. Sci. 21, 172–173 [PubMed] [Google Scholar]
- 36. Hicke L., Schubert H. L., Hill C. P. (2005) Nat. Rev. Mol. Cell Biol. 6, 610–621 [DOI] [PubMed] [Google Scholar]
- 37. Raasi S., Varadan R., Fushman D., Pickart C. M. (2005) Nat. Struct. Mol. Biol. 12, 708–714 [DOI] [PubMed] [Google Scholar]
- 38. Marx A., Nugoor C., Müller J., Panneerselvam S., Timm T., Bilang M., Mylonas E., Svergun D. I., Mandelkow E. M., Mandelkow E. (2006) J. Biol. Chem. 281, 27586–27599 [DOI] [PubMed] [Google Scholar]
- 39. Chen L., Jiao Z. H., Zheng L. S., Zhang Y. Y., Xie S. T., Wang Z. X., Wu J. W. (2009) Nature 459, 1146–1149 [DOI] [PubMed] [Google Scholar]
- 40. Crute B. E., Seefeld K., Gamble J., Kemp B. E., Witters L. A. (1998) J. Biol. Chem. 273, 35347–35354 [DOI] [PubMed] [Google Scholar]
- 41. Pang T., Xiong B., Li J. Y., Qiu B. Y., Jin G. Z., Shen J. K., Li J. (2007) J. Biol. Chem. 282, 495–506 [DOI] [PubMed] [Google Scholar]
- 42. Hedbacker K., Townley R., Carlson M. (2004) Mol. Cell. Biol. 24, 1836–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hedbacker K., Hong S. P., Carlson M. (2004) Mol. Cell. Biol. 24, 8255–8263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Davies S. P., Carling D., Hardie D. G. (1989) Eur. J. Biochem. 186, 123–128 [DOI] [PubMed] [Google Scholar]
- 45. Abramoff M. D., Magelhaes P. J., Ram S. J. (2004) Biophotonics Int. 11, 36–42 [Google Scholar]
- 46. Rudolph M. J., Amodeo G. A., Bai Y., Tong L. (2005) Biochem. Biophys. Res. Commun. 337, 1224–1228 [DOI] [PubMed] [Google Scholar]
- 47. Nayak V., Zhao K., Wyce A., Schwartz M. F., Lo W. S., Berger S. L., Marmorstein R. (2006) Structure 14, 477–485 [DOI] [PubMed] [Google Scholar]
- 48. Rudolph M. J., Amodeo G. A., Tong L. (2010) Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 66, 999–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pavletich N. P. (1999) J. Mol. Biol. 287, 821–828 [DOI] [PubMed] [Google Scholar]
- 50. Neigeborn L., Carlson M. (1987) Genetics 115, 247–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hedbacker K., Carlson M. (2006) Eukaryot. Cell 5, 1950–1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kuchin S., Treich I., Carlson M. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 7916–7920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tanoue T., Adachi M., Moriguchi T., Nishida E. (2000) Nat. Cell. Biol. 2, 110–116 [DOI] [PubMed] [Google Scholar]
- 54. Polekhina G., Gupta A., van Denderen B. J., Feil S. C., Kemp B. E., Stapleton D., Parker M. W. (2005) Structure 13, 1453–1462 [DOI] [PubMed] [Google Scholar]
- 55. Polekhina G., Gupta A., Michell B. J., van Denderen B., Murthy S., Feil S. C., Jennings I. G., Campbell D. J., Witters L. A., Parker M. W., Kemp B. E., Stapleton D. (2003) Curr. Biol. 13, 867–871 [DOI] [PubMed] [Google Scholar]