Abstract
The histone H3 variant CENP-A is the most favored candidate for an epigenetic mark that specifies the centromere. In fission yeast, adjacent heterochromatin can direct CENP-ACnp1 chromatin establishment, but the underlying features governing where CENP-ACnp1 chromatin assembles are unknown. We show that, in addition to centromeric regions, a low level of CENP-ACnp1 associates with gene promoters where histone H3 is depleted by the activity of the Hrp1Chd1 chromatin-remodeling factor. Moreover, we demonstrate that noncoding RNAs are transcribed by RNA polymerase II (RNAPII) from CENP-ACnp1 chromatin at centromeres. These analyses reveal a similarity between centromeres and a subset of RNAPII genes and suggest a role for remodeling at RNAPII promoters within centromeres that influences the replacement of histone H3 with CENP-ACnp1.
Keywords: Centromeres, Chromatin Remodeling, Nucleosome, Transcription, Yeast, CENP-A, Noncoding Transcripts
Introduction
Centromeres are the specific chromosomal loci at which kinetochore assembly occurs. Extensive investigation of centromeric DNA from a wide variety of eukaryotic cells indicates that the primary sequence is not conserved. Despite this, all functional centromeres, including neocentromeres formed at ectopic chromosomal loci, share a unique chromatin composition in which the evolutionarily conserved histone H3 variant CENP-A replaces canonical histone H3. At many centromeres, this CENP-A kinetochore chromatin is formed on repetitive arrays such as α-satellite on human chromosomes. However, the fact that this CENP-A chromatin is flanked by similar repeats assembled in heterochromatin (1–3) makes it difficult to distinguish repeats coated in heterochromatin from those assembled in CENP-A chromatin. In the fission yeast Schizosaccharomyces pombe, the heterochromatic outer repeats are distinct from the central domain over which CENP-ACnp1 and the kinetochore assemble (1). Although heterochromatin is required for the de novo assembly of CENP-ACnp1 chromatin on central domain DNA, it is dispensable for the subsequent maintenance of CENP-ACnp1 chromatin (4). Thus, kinetochore-associated DNAs may possess unidentified features that are not apparent from the primary sequences that make them favorable substrates for CENP-ACnp1 deposition and kinetochore assembly. Previous analyses have suggested that the acetylated state of histones may influence CENP-A assembly (5, 6). Moreover, transcripts homologous to centromere-associated DNAs have been detected in various organisms, and retrotransposon RNAs are implicated in centromere chromatin structure (7–10). In fission yeast, it has been shown that a GATA-like transcription factor is required for efficient CENP-ACnp1 deposition (11). Also in fission yeast, the ATP-dependent remodeling factor Hrp1 (orthologous to Saccharomyces cerevisiae Chd1 (chromo-helicase DNA-binding protein 1)) affects CENP-ACnp1 deposition (12). Chd1 is involved in transcriptional elongation by RNA polymerase II (RNAPII)4 (13) and has been shown to facilitate replication-independent histone H3 exchange (14). An attractive hypothesis is that transcription underlies chromatin remodeling in the centromeric DNAs, which in turn promotes CENP-ACnp1 deposition. Here, we investigated this possibility.
EXPERIMENTAL PROCEDURES
Standard procedures were used for growth and genetic manipulation. The details of PCR primers are listed in supplemental Table 1. The S. pombe strains used in this study are listed in supplemental Table 2. The procedures used are described under supplemental “Methods.”
ChIP
Cells were fixed with 1% paraformaldehyde and lysed by bead beating. Chromatin was solubilized by shearing with a Bioruptor sonicator and immunoprecipitated using 10 μl of anti-CENP-ACnp1 antiserum with protein G-agarose beads. ChIPs were then analyzed by quantitative PCR.
ChIP-Chip
DNA was immunoprecipitated and hybridized to Affymetrix GeneChip® S. pombe tiling 1.0FR arrays as described previously (15). 10 μl of anti-CENP-ACnp1 and 1.5 μg of anti-H3 (ab1791, ABCAM) antibodies were added to 100 μl of chromatin extract. Hrp1Chd1 binding data and histone H3 density maps are from a previous study (16).
Raw data from Affymetrix (.CEL format) were analyzed with Affymetrix TAS (tiling analysis) version 1.1 software and visualized with Affymetrix IGB (integrated genome browser). The data were normalized using quantile normalization plus scaling and run with a bandwidth of 20. p values were calculated using hypergeometric probability distribution in R version 2.12.0.
RESULTS
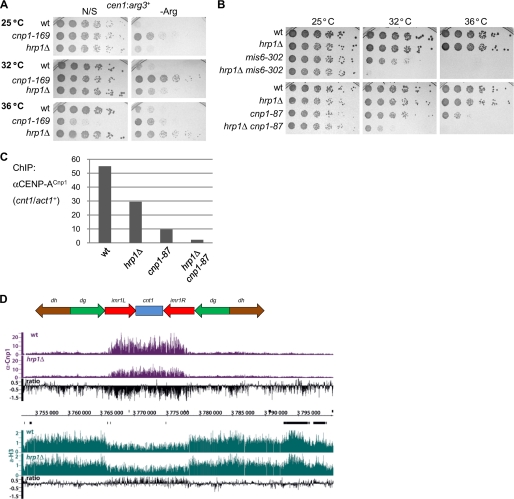
Hrp1Chd1 has previously been implicated in CENP-ACnp1 deposition but is not essential for cell viability (12). Expression of RNAPII-transcribed marker genes such as arg3+ is repressed when placed within the central domain, and silencing of cen1:arg3+ is highly sensitive to defective CENP-ACnp1 deposition (17); however, this silencing is only partially impaired in hrp1Δ cells (Fig. 1A). This suggests that redundant mechanisms operate to ensure CENP-ACnp1 deposition in the absence of Hrp1Chd1. In agreement with this and previous analyses (12), when hrp1Δ was combined with the mis6-302 temperature-sensitive mutation (defective in CENP-ACnp1 deposition), it reduced the restrictive temperature of mis6-302. Furthermore, hrp1Δ also reduced the restrictive temperature of cnp1-87, a weak temperature-sensitive allele (Fig. 1B) (18). Consistent with this, the levels of CENP-ACnp1 associated with the central domain were further reduced in hrp1Δ cnp1-87 double mutants compared with either single mutant (Fig. 1C). We conclude that Hrp1 facilitates the assembly of CENP-ACnp1 chromatin, and it becomes essential when Mis6 or CENP-ACnp1 function is impaired.
FIGURE 1.
Chromatin-remodeling factor Hrp1Chd1 contributes to CENP-ACnp1 chromatin formation. A, silencing of cen1:arg3+ in WT, cnp1-169, and hrp1Δ cells. The growth assay was performed on non-selective (N/S) or arginine-depleted (−Arg) plates at 25, 32, and 36 °C. B, viability of cells bearing hrp1Δ combined with mis6-302 (upper panels) or with cnp1-87 (lower panels) compared with wild-type and single mutants grown at 25, 32, or 36 °C. C, CENP-ACnp1 ChIP analyses in WT, hrp1Δ, cnp1-87, and hrp1Δ cnp1-87 cells grown at 36 °C. The enrichment of the cnt1 product was compared with input DNA relative to the act1+ control by quantitative PCR. D, genome browser view of cen1 showing ChIP-chip binding profiles for CENP-ACnp1 (purple) and H3 (green) in WT and hrp1Δ cells (as indicated) at 30 °C. The relative ratios of CENP-ACnp1 and H3 (hrp1Δ/WT) are indicated (black). Data on the y axis are presented in log 2 scale, and the x axis shows genome positions in base pairs.
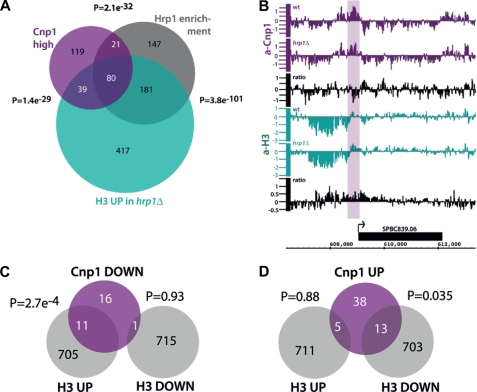
Genome-wide analyses of CENP-ACnp1 and histone H3 localization by ChIP on tiling arrays with anti-CENP-ACnp1 and anti-H3 antibodies confirmed that Hrp1Chd1 is required to maintain normal levels of CENP-ACnp1 and that, in hrp1Δ cells, H3 levels increase across the central domain (Fig. 1D and supplemental Fig. S1). Previously, we have shown that Hrp1Chd1 acts at a subset of gene promoters to disassemble histone H3-containing nucleosomes close to the transcription start sites (16). Further examination of the genome-wide analyses revealed that low but detectable levels of CENP-ACnp1 associate with a significant proportion of promoters in wild-type cells at which Hrp1Chd1 acts to disassemble H3-containing nucleosomes (Fig. 2A). CENP-ACnp1 association is significantly reduced at some, but not all, of these promoters in hrp1Δ cells, and in agreement with this, we saw an increase in H3 (p = 2.7e−4, hypergeometric probability) (Fig. 2, B and C). Some promoters show an increase in CENP-ACnp1 association and a decrease in H3 in hrp1Δ cells; however, this is less significant (Fig. 2D). These analyses imply that Hrp1Chd1 directly participates in a remodeling process that evicts H3 and allows CENP-ACnp1 deposition at the promoters of some genes. However, additional factors, such as Scm3, must also contribute to the replacement of H3 with CENP-ACnp1 and/or CENP-ACnp1 maintenance at centromeres (19, 20). This bears resemblance to the transcription-coupled replacement of H3.1 with H3.3 in metazoa (21) and suggests that remodeling at some promoters of RNAPII genes is intimately associated with the destabilization of H3-containing nucleosomes to encourage the assembly of CENP-ACnp1 chromatin. Nucleosome replacement in S. cerevisiae is more prominent at promoters than within coding regions (22), and the genome-wide effects of Hrp1Chd1 on H3 eviction in fission yeast are more pronounced at promoters compared with coding regions and 3′-intergenic regions (16), accounting for the preferential association of CENP-ACnp1 with the promoters of genes at which Hrp1Chd1 functions.
FIGURE 2.
Genome-wide CENP-ACnp1 localization correlates with Hrp1Chd1 occupancy and H3 density changes in hrp1Δ cells. A, Venn diagram illustrating the overlap of CENP-ACnp1 enrichment with Hrp1Chd1 occupancy and H3 density increase in hrp1Δ cells (16). A cutoff of 1.5 was used for CENP-ACnp1 enrichment. The p values indicate the probability of overlap generated by a hypergeometric probability test using R. B, genome browser view of gene SPBC839.06 with reduced CENP-ACnp1 (purple) and increased H3 (green) levels at its promoter in hrp1Δ cells. The arrow indicates the direction of transcription. The relative ratios of CENP-ACnp1 and H3 are indicated (black). Data on the y axis are presented in log 2 scale, and the x axis shows genome positions in base pairs. C, Venn diagram showing the overlap between promoters where CENP-ACnp1 is reduced, promoters where H3 is increased, and promoters where H3 is reduced in hrp1Δ cells. A cutoff of 1.2 was used for CENP-ACnp1 reduction. D, Venn diagram showing the overlap between promoters where CENP-ACnp1 is increased, promoters where H3 is increased, and promoters where H3 is reduced in hrp1Δ cells. A cutoff of 1.2 was used for the CENP-ACnp1 increase.
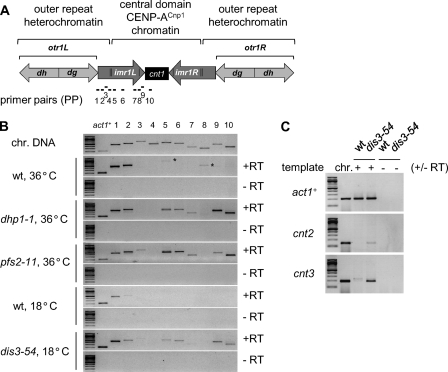
The close correlation between CENP-ACnp1 association and Hrp1Chd1 function at gene promoters as well as at centromeres raises the possibility that centromeric DNA may contain promoters for hitherto unidentified transcription elements. It is well established that transcripts are produced from the heterochromatic outer repeats of fission yeast centromeres and processed by the RNAi pathway (23, 24). To determine whether the central kinetochore domain is also transcribed, RT-PCR was performed with primer pairs (PP1–PP10) complementary to centromere 1 (cen1) (Fig. 3A). PP1–PP3 detect part of the outer repeat heterochromatic transcript. PP4 flanks the tRNAAla/tRNAGlu genes, whereas PP5–PP10 lie within the central subkinetochore domain. In wild-type cells, heterochromatic transcripts were detected in a region immediately adjacent to the outer repeats (PP1 and PP2), but transcripts were not apparent in the central domain (Fig. 3B). Outer repeat transcripts are known to accumulate in mutants defective in RNAi or heterochromatin integrity (23, 24). Several studies in S. cerevisiae indicate that aberrant or cryptic transcripts are degraded by 5′–3′- and/or 3′–5′-exoribonucleases; 3′-end processing can contribute to RNA stability (25). Indeed, in fission yeast, outer repeat heterochromatin transcript levels are also regulated by the exosome (26–28). We therefore tested whether conditional mutations in Pfs2 (pfs2-11 temperature-sensitive; polyadenylation factor I subunit 2) (29), Dhp1 (dhp1-1 temperature-sensitive; 5′–3′-exoribonuclease orthologous to Xrn2/Rat1) (30), or Dis3 (dis3-54 cold-sensitive; 3′–5′-exoribonuclease component of the exosome) (27) allow accumulation of RNA homologous to the central domain. Transcripts from the central domain were clearly detected with PP5, PP6, PP7, PP9, and PP10 in dhp1-1, pfs2-11, and dis3-54 cells, but not in wild-type cells, under restrictive conditions (36 or 18 °C) (Fig. 3B). We conclude that a large proportion of the central domain is transcribed but that the resulting transcripts are normally undetectable due to their rapid turnover. RT-PCR also detected transcripts homologous to the central domains of cen2 and cen3 in dis3-54 cells (Fig. 3C). Thus, transcription of subkinetochore chromatin is a general property of the three centromeres, and these transcripts from under kinetochores (TUKs) are normally degraded by the exosome.
FIGURE 3.
Accumulation of central domain transcripts in RNA-processing mutants. A, schematic of fission yeast cen1, indicating the central core (cnt), innermost repeat (imr), and outer repeats (otr/dg-dh). Regions amplified by primer pairs (PP1–PP10) used in RT-PCR are indicated below (black bars). B, RT-PCR analysis of transcripts from cen1 and the act1+ control. Wild-type, dhp1-1, and pfs2-11 cells were grown at 25 °C and then shifted to 36 °C for 6 h before RNA extraction. Wild-type and dis3-54 cells were grown at 36 °C and shifted to 18 °C for 9 h before RNA extraction. −RT, negative control performed without reverse transcriptase; *, unspecific products. PCR with a chromosomal DNA (chr. DNA) template was included as a positive control. C, RT-PCR analysis of transcripts from cnt2, cnt3, and the act1+ control. PP-cnt2 and PP-cnt3 are specific for the central domain of cen2 and cen3, respectively.
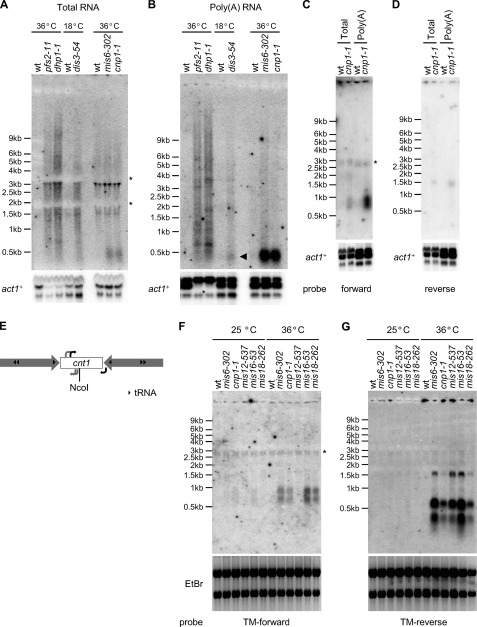
Northern analyses with an RNA probe specific for the cen1 central domain confirmed the presence of TUKs; no obvious signal was observed in wild-type cells, but a smear of transcripts was detected in RNA-processing mutants (pfs2-11, dhp1-1, and dis3-54) (Fig. 4A and supplemental Fig. S2). We also examined the temperature-sensitive mutants mis6-302 and cnp1-1, which have reduced levels of CENP-ACnp1 chromatin over the central domain (31, 32). Surprisingly, transcripts with discrete sizes (∼0.5 kb) were identified in both mutants (Fig. 4A); these discrete transcripts (discrete TUKs) were highly enriched in poly(A)-selected RNA (Fig. 4B). A lower level of these specific transcripts was also enriched in the poly(A) fraction from dis3-54 (Fig. 4B, arrowhead). Dis3 is the key catalytic subunit of the exosome required to degrade aberrant transcripts, whereas Pfs2 and Dhp1 are required for normal 3′-end formation/termination (27, 29, 30). pfs2-11 and dhp1-1 may generate transcripts with extended 3′-ends due to transcriptional read-through into downstream regions, resulting in the observed heterogeneous smear of transcripts (Fig. 4, A and B). The specific increase in discrete TUKs observed in mis6-302 and cnp1-1 cells could be interpreted to mean that intact CENP-ACnp1 chromatin prevents expression of these transcripts; however, our analyses suggest that TUKs are constitutively produced and turned over by the exosome in wild-type cells. The chromatin context in mis6-302 and cnp1-1 cells is dramatically altered from CENP-ACnp1 to H3 chromatin; CENP-ACnp1 chromatin may interfere with events required for the formation of specific transcripts, such as accurate 3′-termination, ensuring their turnover. Using RNA probes complementary to either reverse or forward strands of the cen1 central core (cc1/tm1) (supplemental Fig. S2), we detected other discrete transcripts in distinct central domain regions in poly(A) RNA from cnp1-1 cells (Fig. 4, C and D). To identify the 5′-ends of these central domain noncoding transcripts, we employed 5′-rapid amplification of cDNA ends (RACE)/PCR on 5′-capped poly(A) RNA extracted from wild-type and cnp1-1 cells. This demonstrated that these novel/unusual transcripts are produced from within the central domain and do not arise by read-through from outer repeat transcripts (Fig. 4E and supplemental Fig. S3). The high sensitivity of 5′-RACE/PCR allowed the detection of transcripts in wild-type cells with some primers (Fig. 4E, black arrows, and supplemental Fig. S3). The transcription start sites for these transcripts were identical in wild-type and cnp1-1 cells, suggesting that they are indeed produced from wild-type centromeres, albeit at a lower level. The fact that these transcripts are 5′-capped and polyadenylated indicates that TUKs are produced by RNAPII. Consistent with alterations in central domain chromatin affecting transcription (lower CENP-ACnp1 and higher H3 deposition) (Fig. 1D), increased levels of the central domain transcript were also detectable in hrp1Δ cells (supplemental Fig. S4).
FIGURE 4.
Northern and 5′-RACE/PCR analyses of central domain transcripts. A, Northern analysis of total RNAs in RNA-processing and kinetochore mutants. An RNA probe complementary to cnt1 was used. Cells grown at the permissive temperature (25 °C for WT, pfs2-11, dhp1-1, mis6-302, and cnp1-1 cells and 36 °C for WT and dis3-54 cells) were shifted to the restrictive temperature (6 h at 36 °C for WT, pfs2-11, dhp1-1, mis6-302, and cnp1-1 cells and 9 h at 18 °C for WT and dis3-54 cells) before RNA extraction. act1+ was used as a loading control. *, rRNA interference with hybridization. For additional EtBr images, see supplemental Fig. S5A. B, the same cnt1 probe used on poly(A) RNA. For additional EtBr images, see supplemental Fig. S5B. C and D, Northern analysis of total or poly(A) RNAs from WT and cnp1-1 cells with an RNA probe complementary to the reverse strand (forward probe; C) or the forward strand (reverse probe; D) of the cc1/tm1 sequence, which is shared by cnt1 and cnt3. *, nonspecific band. For additional EtBr images, see supplemental Fig. S5 (C and D). E, schematic representation of transcription start sites determined by 5′-RACE/PCR in WT and cnp1-1 cells. Black arrows, transcription start sites identified in WT and cnp1-1 cells; gray arrows, transcription start sites identified only in cnp1-1 cells. F and G, Northern blots showing transcripts complementary to the TM-forward (F) or TM-reverse (G) probes in WT, mis6-302, cnp1-1, mis12-537, mis16-53, and mis18-262 cells grown at permissive (25 °C) and restrictive (36 °C) temperatures. *, nonspecific band. EtBr staining confirmed equal loading.
On the basis of the 5′-RACE analyses above, we designed improved probes for Northern analyses to detect the forward and reverse strands of the cen1 central core (cc1/tm1) (supplemental Fig. S2) in additional mutants known to affect kinetochore integrity (mis12-537, mis16-53, and mis18-262 in addition to mis6-302 and cnp1-1) (6). At 36 °C, transcripts were detected in all mutants apart from mis12-537 with this TM-forward5 probe (Fig. 4F). Some transcripts were even detectable in mis6-302, cnp1-1, and mis16-53 at 25 °C, the permissive temperature. In all mutants, including mis12-537, this TM-reverse probe allowed detection of other transcripts (Fig. 4G) in addition to those observed with the original probe (Fig. 4D). Together, these analyses indicate that cryptic transcription is prevalent in the central kinetochore domain of fission yeast centromeres and revealed only in cells defective in RNA turnover or formation of subkinetochore chromatin. In support of this, low levels of H3K4 methylation, a modification associated with active transcription, have been shown to be enriched in the small amount of histone H3 that remains within subkinetochore chromatin in wild-type cells (33).
DISCUSSION
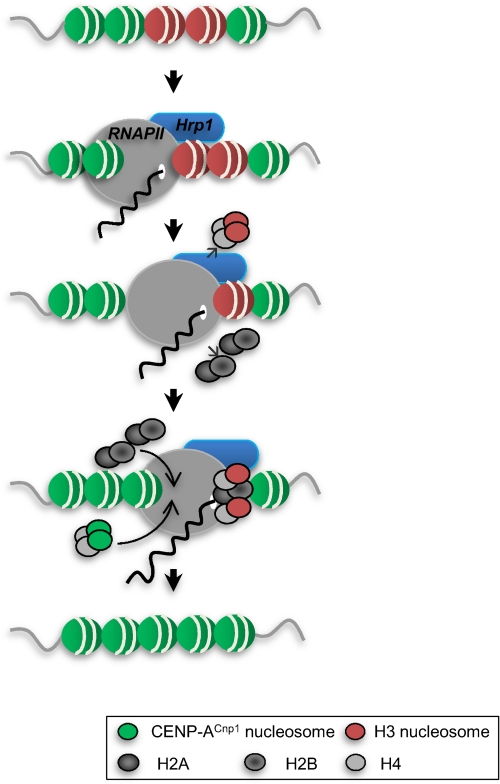
Centromere-associated DNA has been shown to be transcribed in plants and vertebrates (7, 10). 30 years ago, EM studies demonstrated the presence of RNase-sensitive material at the base of kinetochore microtubules in newt lung cells (34). Since then, centromeric transcripts have been found to associate with kinetochore proteins (9, 10). In this study, we demonstrated an analogy between the central domain-associated CENP-ACnp1 chromatin of centromeres and genes whose promoters are associated with Hrp1Chd1 in fission yeast. The fact that Hrp1Chd1 promotes eviction of histone H3 at a set of promoters suggests that similar remodeling processes may occur at RNAPII promoters within centromeres and may contribute to exchange of canonical H3-containing nucleosomes for CENP-ACnp1-containing nucleosomes (summarized in Fig. 5).
FIGURE 5.
Proposed model for the role of Hrp1 in the exchange of histone H3 with CENP-ACnp1 during RNAPII transcription. Transcription from the promoters within centromeric chromatin acts to promote the replacement of histone H3 with CENP-ACnp1 through the associated remodeling factor Hrp1Chd1. Similarly, Hrp1Chd1 acts at some euchromatic promoters to replace H3 with CENP-ACnp1. The resulting centromeric transcripts are degraded by the exosome.
It is possible that transcription within centromeres occurs merely as a consequence of having RNAPII promoters whose presence or activation is critical to act as a seed for remodeling events that promote CENP-ACnp1 deposition. The resulting TUK transcripts are degraded by the exosome and thus may represent just nonfunctional by-products of transcription. However, we cannot rule out the possibility that these unstable transcripts might also have a dedicated function in guiding some components of the CENP-ACnp1 deposition machineries and/or kinetochore complex to their cognate DNA sequences (10). Alternatively, the production of TUKs might influence chromatin modification within centromeric chromatin by processes analogous to those associated with cryptic or antisense noncoding RNA production in S. cerevisiae (35, 36). However, we found that the accumulation of TUKs in pfs2-11, dhp1-1, or dis3-54 cells had no obvious effect on CENP-ACnp1 chromatin formation.6 It is also conceivable that these transcripts are processed into a specific class of small RNAs that have a role in CENP-ACnp1 chromatin formation and/or kinetochore assembly analogous to how siRNAs derived from outer repeat transcripts induce heterochromatin formation. However, with our detection methods, we did not find evidence for the presence of small RNAs corresponding to the central domain of centromeres.5
Intriguingly, it has recently been reported that S. cerevisiae CENP-ACse4 also tends to associate with a number of RNAPII promoters where RNAPII binding is high (37); however, it is not known if these promoters share any structural or mechanistically related features with S. cerevisiae centromeres that contribute to CENP-ACse4 deposition. Interestingly, the S. cerevisiae centromeric protein Cbf1, which binds centromere DNA element I, functions as a transcription factor at the MET16 promoter (38). In this regard, it is possible that RNAPII transcription might also occur within or close to centromeres of S. cerevisiae. In fact, cryptic transcription was detected close to CEN3 in S. cerevisiae exosome mutants (39); it is not known if this is a general feature of all S. cerevisiae centromeres. Recently, the human chromatin-remodeling factor FACT (facilitates chromatin transcription), whose function is implicated in transcription, was found to associate with affinity-purified CENP-A chromatin (40, 41) Moreover, depletion of FACT was found to impair incorporation of newly synthesized CENP-A in chicken cells (42). CHD1 was also found at centromeres in chicken cells and is required for centromeric localization of CENP-A in human cells (42). These observations, together with the analyses presented here, implicate RNAPII transcription and the associated remodeling activities in the replacement of histone H3 with CENP-A.
Supplementary Material
Acknowledgments
We thank A. Pidoux, A. Buscaino, L. Harrington, and J. Houseley for comments on the manuscript. We are grateful to the following colleagues for advice, strains; and reagents: K. Takahashi and M. Yanagida.
This work was supported by a European Molecular Biology Organization long-term fellowship (to E. S. C.), Wellcome Trust Programme Grant (065061/Z (to R. C. A.), the Swedish Research Council (VR; to K. E.), the Görans Gustafsson Foundation for Research in Natural Sciences and Medicine (to K. E.), and the Cancer Society (CF; to K. E.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Methods,” Figs. S1–S5, and Tables 1 and 2.
TM represents a 3.3-kb element shared between the central domains of cen2 and cen3.
E. S. Choi and R. C. Allshire, unpublished data.
- RNAPII
- RNA polymerase II
- TUK
- transcript from under kinetochores
- RACE
- rapid amplification of cDNA ends.
REFERENCES
- 1. Allshire R. C., Karpen G. H. (2008) Nat. Rev. Genet. 9, 923–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cleveland D. W., Mao Y., Sullivan K. F. (2003) Cell 112, 407–421 [DOI] [PubMed] [Google Scholar]
- 3. Schueler M. G., Sullivan B. A. (2006) Annu. Rev. Genomics Hum. Genet. 7, 301–313 [DOI] [PubMed] [Google Scholar]
- 4. Folco H. D., Pidoux A. L., Urano T., Allshire R. C. (2008) Science 319, 94–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fujita Y., Hayashi T., Kiyomitsu T., Toyoda Y., Kokubu A., Obuse C., Yanagida M. (2007) Dev. Cell 12, 17–30 [DOI] [PubMed] [Google Scholar]
- 6. Hayashi T., Fujita Y., Iwasaki O., Adachi Y., Takahashi K., Yanagida M. (2004) Cell 118, 715–729 [DOI] [PubMed] [Google Scholar]
- 7. Carone D. M., Longo M. S., Ferreri G. C., Hall L., Harris M., Shook N., Bulazel K. V., Carone B. R., Obergfell C., O'Neill M. J., O'Neill R. J. (2009) Chromosoma 118, 113–125 [DOI] [PubMed] [Google Scholar]
- 8. Chueh A. C., Northrop E. L., Brettingham-Moore K. H., Choo K. H., Wong L. H. (2009) PLoS Genet. 5, e1000354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Topp C. N., Zhong C. X., Dawe R. K. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 15986–15991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wong L. H., Brettingham-Moore K. H., Chan L., Quach J. M., Anderson M. A., Northrop E. L., Hannan R., Saffery R., Shaw M. L., Williams E., Choo K. H. (2007) Genome Res. 17, 1146–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen E. S., Saitoh S., Yanagida M., Takahashi K. (2003) Mol. Cell 11, 175–187 [DOI] [PubMed] [Google Scholar]
- 12. Walfridsson J., Bjerling P., Thalen M., Yoo E. J., Park S. D., Ekwall K. (2005) Nucleic Acids Res. 33, 2868–2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simic R., Lindstrom D. L., Tran H. G., Roinick K. L., Costa P. J., Johnson A. D., Hartzog G. A., Arndt K. M. (2003) EMBO J. 22, 1846–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Konev A. Y., Tribus M., Park S. Y., Podhraski V., Lim C. Y., Emelyanov A. V., Vershilova E., Pirrotta V., Kadonaga J. T., Lusser A., Fyodorov D. V. (2007) Science 317, 1087–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Durand-Dubief M., Ekwall K. (2009) Methods Mol. Biol. 529, 279–295 [DOI] [PubMed] [Google Scholar]
- 16. Walfridsson J., Khorosjutina O., Matikainen P., Gustafsson C. M., Ekwall K. (2007) EMBO J. 26, 2868–2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pidoux A. L., Richardson W., Allshire R. C. (2003) J. Cell Biol. 161, 295–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Castillo A. G., Mellone B. G., Partridge J. F., Richardson W., Hamilton G. L., Allshire R. C., Pidoux A. L. (2007) PLoS Genet. 3, e121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pidoux A. L., Choi E. S., Abbott J. K., Liu X., Kagansky A., Castillo A. G., Hamilton G. L., Richardson W., Rappsilber J., He X., Allshire R. C. (2009) Mol. Cell 33, 299–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Williams J. S., Hayashi T., Yanagida M., Russell P. (2009) Mol. Cell 33, 287–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Henikoff S. (2008) Nat. Rev. Genet. 9, 15–26 [DOI] [PubMed] [Google Scholar]
- 22. Dion M. F., Kaplan T., Kim M., Buratowski S., Friedman N., Rando O. J. (2007) Science 315, 1405–1408 [DOI] [PubMed] [Google Scholar]
- 23. Cam H. P., Chen E. S., Grewal S. I. (2009) Cell 136, 610–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kloc A., Martienssen R. (2008) Trends Genet. 24, 511–517 [DOI] [PubMed] [Google Scholar]
- 25. Houseley J., Tollervey D. (2009) Cell 136, 763–776 [DOI] [PubMed] [Google Scholar]
- 26. Bühler M., Haas W., Gygi S. P., Moazed D. (2007) Cell 129, 707–721 [DOI] [PubMed] [Google Scholar]
- 27. Murakami H., Goto D. B., Toda T., Chen E. S., Grewal S. I., Martienssen R. A., Yanagida M. (2007) PLoS One 2, e317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang S. W., Stevenson A. L., Kearsey S. E., Watt S., Bähler J. (2008) Mol. Cell. Biol. 28, 656–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang S. W., Asakawa K., Win T. Z., Toda T., Norbury C. J. (2005) Mol. Cell. Biol. 25, 2288–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shobuike T., Tatebayashi K., Tani T., Sugano S., Ikeda H. (2001) Nucleic Acids Res. 29, 1326–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saitoh S., Takahashi K., Yanagida M. (1997) Cell 90, 131–143 [DOI] [PubMed] [Google Scholar]
- 32. Takahashi K., Chen E. S., Yanagida M. (2000) Science 288, 2215–2219 [DOI] [PubMed] [Google Scholar]
- 33. Cam H. P., Sugiyama T., Chen E. S., Chen X., FitzGerald P. C., Grewal S. I. (2005) Nat. Genet. 37, 809–819 [DOI] [PubMed] [Google Scholar]
- 34. Rieder C. L. (1979) J. Cell Biol. 80, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Camblong J., Iglesias N., Fickentscher C., Dieppois G., Stutz F. (2007) Cell 131, 1534–1545 [DOI] [PubMed] [Google Scholar]
- 36. Houseley J., Rubbi L., Grunstein M., Tollervey D., Vogelauer M. (2008) Mol. Cell 32, 685–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lefrançois P., Euskirchen G. M., Auerbach R. K., Rozowsky J., Gibson T., Yellman C. M., Gerstein M., Snyder M. (2009) BMC Genomics 10, 37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hemmerich P., Stoyan T., Wieland G., Koch M., Lechner J., Diekmann S. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 12583–12588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Houseley J., Kotovic K., El Hage A., Tollervey D. (2007) EMBO J. 26, 4996–5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Foltz D. R., Jansen L. E., Black B. E., Bailey A. O., Yates J. R., 3rd, Cleveland D. W. (2006) Nat. Cell Biol. 8, 458–469 [DOI] [PubMed] [Google Scholar]
- 41. Izuta H., Ikeno M., Suzuki N., Tomonaga T., Nozaki N., Obuse C., Kisu Y., Goshima N., Nomura F., Nomura N., Yoda K. (2006) Genes Cells 11, 673–684 [DOI] [PubMed] [Google Scholar]
- 42. Okada M., Okawa K., Isobe T., Fukagawa T. (2009) Mol. Biol. Cell 20, 3986–3995 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.