Abstract
The fly morphogen Hedgehog (Hh) and its mammalian orthologs, Sonic, Indian, and Desert hedgehog, are secreted signaling molecules that mediate tissue patterning during embryogenesis and function in tissue homeostasis and regeneration in the adult. The function of all Hh family members is regulated at the levels of morphogen multimerization on the surface of producing cells, multimer release, multimer diffusion to target cells, and signal reception. These mechanisms are all known to depend on interactions of positively charged Hh amino acids (the Cardin-Weintraub (CW) motif) with negatively charged heparan sulfate (HS) glycosaminoglycan chains. However, a precise mechanistic understanding of these interactions is still lacking. In this work, we characterized ionic HS interactions of multimeric Sonic hedgehog (called ShhNp) as well as mutant forms lacking one or more CW residues. We found that deletion of all five CW residues as well as site-directed mutagenesis of CW residues Lys33, Arg35, and Lys39 (mouse nomenclature) abolished HS binding. In contrast, CW residues Arg34 and Lys38 did not contribute to HS binding. Analysis and validation of Shh crystal lattice contacts provided an explanation for this finding. We demonstrate that CW residues Arg34 and Lys38 make contact with an acidic groove on the adjacent molecule in the multimer, suggesting a new function of these residues in ShhNp multimerization rather than HS binding. Therefore, the recombinant monomeric morphogen (called ShhN) differs in CW-dependent HS binding and biological activity from physiologically relevant ShhNp multimers, providing new explanations for functional differences observed between ShhN and ShhNp.
Keywords: Carbohydrate-binding Protein, Extracellular Matrix Proteins, Glycosaminoglycan, Heparan Sulfate, Heparin-binding Protein, Protein Motifs, Protein-Protein Interactions, Protein Multimers
Introduction
The proteins of the Hh family are powerful morphogens that control growth and patterning of developing embryos. Current models for Hh activity suggest that the morphogen disperses from a localized source and forms a gradient that patterns fields of responsive cells expressing the Hh receptor Patched (Ptc). Genetic evidence suggests that this process critically depends on heparan sulfate proteoglycan (HSPG)2 expression. Upon secretion to the cell surface, Drosophila Hh forms nanoscale oligomers on the cell surface that co-localize with HSPGs (1). HS binds to the Cardin-Weintraub (CW) motif found on all known Hhs and regulates their function in flies (2, 3) and mice (4). In Drosophila (5) as well as in mammalian cell culture (6, 7), Hhs are always released from producing cells in multimeric form, as demonstrated by gel filtration analysis of the soluble morphogen. Release of the multimeric morphogen (the processed Hh N-terminal signaling domain, designated HhNp) from the cell surface depends on the expression of Dispatched (8) and A disintegrin and metalloprotease (ADAM) family members that mediate ectodomain shedding from transfected Bosc23 cells (9). HS is involved in the formation of the HhNp extracellular gradient, which, in the fly, depends on the expression of the Drosophila Exostosin (Ext) family of proteins, encoded by the genes tout velu (ttv), brother of tout velu, and sister of tout velu and the glycosylphosphatidylinositol-linked HSPGs Dally and Dally-like, corresponding to vertebrate glypicans (2, 3, 10). HS expression and Dally-like/glypican expression are also essential for signal reception and modulation on Ptc-expressing receiving cells (10–14) and participate in HhNp-Ihog interaction (15). However, the essential role of direct morphogen-HSPG interactions in embryonic patterning was recently challenged (16). In that report, transgenic mice made deficient in two ShhNp CW amino acid residues implicated in HS binding (17) lacked an Shh-related phenotype, suggesting that direct morphogen-HS interactions were not essential for normal development. However, in that report as well as in others (16–18), CW-dependent HS interactions were characterized using a recombinant, non-physiological monomeric morphogen termed ShhN, whereas embryogenesis depends entirely on the activity of morphogen multimers. Potential variations between ShhN monomer-HS and ShhNp multimer-HS interactions, however, were not investigated.
HS is produced by most cell types in vertebrates and invertebrates. HS biosynthesis (as well as synthesis of heparin, a highly sulfated form of HS produced in connective tissue type mast cells) occurs in the Golgi compartment on proteoglycan core proteins (19). Exts synthesize the (GlcA1,4GlcNAc1,4)n carbohydrate backbone, which is subsequently modified by N-deacetylase/sulfotransferases, O-sulfotransferases, and a GlcA-C5 epimerase. This process results in the generation of specific sulfated protein binding sites (summarized in Ref. 20).
In this work, we compared HS interactions of physiologically relevant multimeric, wild-type, and CW mutant ShhNp with HS interactions of monomeric CW mutant and wild-type forms of ShhN. To this end, we conducted FPLC affinity chromatography employing coupled mouse embryo-derived HS and the various soluble forms of ShhNp and ShhN. We describe that HS elution profiles of multimeric CW mutants differ strikingly from those of the corresponding monomers, indicating that different CW amino acids participate in HS binding of the monomeric and multimeric morphogen. Indeed, we show that two positively charged CW residues that contribute to HS binding of the monomer interact with negatively charged pockets on adjacent molecules in the multimer. These findings shed new light on functional implications of ShhNp multimerization and provide mechanistic and structural insight into biologically relevant ShhNp-HS interactions.
EXPERIMENTAL PROCEDURES
Cloning and Expression of Recombinant Shh
Shh constructs were generated from murine cDNA (NM 009170) by PCR. Secreted, lipidated ShhNp (nucleotides 1–1314, corresponding to amino acids 1–438) was generated in Bosc23 cells (a human 293T derivative, an embryonic kidney cell line routinely used for the expression of lipidated ShhNp (6)), and secreted, unlipidated ShhN (nucleotides 1–594, corresponding to amino acids 1–198 of murine Shh) was generated in Bosc23 cells and in Escherichia coli. PCR products were ligated into pGEM (Promega), sequenced, and subsequently released and religated into pcDNA3.1 (Invitrogen) for the expression of secreted, lipidated 19-kDa ShhNp in Bosc23 cells; into pcDNA3.1/myc-HisC (Invitrogen) for the expression of secreted, C-terminally hexahistidine tagged 28-kDa ShhNHis6 (the large size due to the presence of a c-Myc and intervening cloning sequence) in Bosc23 cells; into pGEX4T-1 (Amersham Biosciences) for expression of N-terminally glutathione S-transferase-tagged 50-kDa GST-ShhN in E. coli; and into pWIZ-SEAP (Gene Therapy Systems) for the expression of secreted, N-terminally alkaline phosphatase-tagged 90-kDa secreted alkaline phosphatase (sAP)-ShhN in Bosc23 cells. Primer sequences for cloning and site-directed mutagenesis and an overview of the various proteins expressed are presented in supplemental Fig. 1. The biological activity of all recombinant proteins was confirmed by Shh-dependent alkaline phosphatase induction in C3H10T1/2 cells and firefly luciferase induction in Light 2 cells using the methods described below.
Cell Culture
Bosc23 cells were cultured in DMEM (PAA Laboratories) supplemented with 10% fetal calf serum (FCS) and 100 μg/ml penicillin/streptomycin. Cells were transfected with plasmids encoding secreted ShhN, ShhNp, or sAP-ShhN using PolyFect (Qiagen). Cells were grown for 36 h, washed with PBS, and incubated in DMEM or serum-free DMEM for various time periods. The serum-free supernatant was then TCA-precipitated, or proteins were enriched by heparin-agarose pull-down.
Protein Purification and Analysis
Proteins were resolved by 15% reducing SDS-PAGE and immunoblotted. Polyclonal anti-ShhN (goat IgG; R&D Systems) and polyclonal anti-CW (Cell Signaling Solutions) were used for primary detection. Visualization was performed after incubation with peroxidase-conjugated donkey anti-goat IgG (detecting anti-ShhN) and goat anti-rabbit IgG (detecting anti-CW) followed by chemiluminescent detection (Pierce). Quantification of Western blotted protein was conducted by ImageJ.
Gel filtration analysis was performed by FPLC (Äkta Protein Purifier (Amersham Biosciences)) using a Superdex200 10/300 GL column (Amersham Biosciences) equilibrated with PBS at 4 °C. Eluted fractions were TCA-precipitated, resolved by 15% SDS-PAGE, and immunoblotted. To determine heparin binding properties of ShhN and ShhNp, the supernatant of transfected Bosc23 cells was subjected to heparin affinity chromatography (Äkta Protein Purifier) using heparin-Sepharose columns (Amersham Biosciences) at 4 °C. Proteins were applied to the columns in the absence of salt, and bound material was eluted with a linear NaCl gradient from 0 to 1.5 m in 0.1 m sodium acetate buffer (pH 6.0). Fractions were precipitated, and eluted proteins were detected immunohistochemically. Shh and ShhNp binding to embryonic tissue HS coupled to NHS-activated Sepharose columns was conducted according to the same protocol. Cleared supernatants of transfected cells, containing the various morphogens, were subjected to automated Äkta affinity purification using a linear salt gradient from 0 to 1 m NaCl in 0.1 m sodium acetate buffer (pH 6.0) for protein elution. sAP-tagged morphogens eluted from HS columns were mixed with 0.1 m glycine buffer, pH 10.4, and directly visualized upon the addition of p-nitrophenolphosphate (Sigma) (Roche Applied Science). sAP quantification was conducted at 405 nm in a spectrophotometer.
Preparation and Analysis of Tissue HS
Embryos were digested overnight with 2 mg/ml Pronase in 320 mm NaCl, 100 mm sodium acetate (pH 5.5) at 40 °C, diluted 1:3 in water, and applied to 2.5 ml of DEAE-Sephacel columns. Glycosaminoglycans were applied to PD-10 (Sephadex G25) columns (Amersham Biosciences). Glycosaminoglycans were lyophilized, Chondroitinase ABC-digested overnight, purified on DEAE as described above, applied to PD-10 columns, and again lyophilized. Via the peptides attached to the HS chains, samples were coupled to NHS-activated Sepharose columns according to the manufacturer's protocol (Amersham Biosciences). For disaccharide analysis, 10-μg glycosaminoglycan samples were digested using heparin lyases I, II, and III (1.5 milliunits of each in 100-μl reactions) (IBEX, Montreal, Canada) at 37 °C for 1 h, and the resulting disaccharides were separated from undigested material using a 3-kDa spin column (Centricon, Bedford, MA). Compositional disaccharide analysis of cell-derived HS or HS derived from embryos was then carried out by liquid chromatography/mass spectrometry (LC/MS) (21). HS samples were also quantified by this method and/or by carbazole reaction.
For immunohistochemical detection, control slides were heparinase I–III-digested (IBEX) (50 mm Hepes, 100 mm NaCl, 1 mm CaCl2, 5 μg of BSA/ml, pH 7.0) overnight at 37 °C to prove specificity. sAP-ShhN and mutant forms generated by site-directed mutagenesis were cloned into pWIZ (Gene Therapy Systems, San Diego, CA), expressed in Bosc23 cells, and secreted into the medium. Heparin affinity purification was employed to test for HS binding of the fusion protein, showing elution at 0.9 m salt and thus strong HS interaction. sAP-ShhN-containing medium was adjusted to 0.6 m NaCl to increase binding specificity and was applied to 4% paraformaldehyde-fixed C3H10T1/2 osteoblast precursor cells and frozen embryo sections overnight, followed by three washing steps with PBS. sAP-ShhN bound to HS was directly visualized upon the addition of NBT-BCIP (Roche Applied Science).
Shh Reporter Assays
Assays for Hh pathway activation in Shh-LIGHT2 cells, a clonal NIH 3T3 cell line stably incorporating Gli-dependent firefly luciferase and constitutive Renilla luciferase reporters, were conducted as described (22). Differentiation of C3H10T1/2 osteoblast precursor cells was also used to determine Shh and ShhNp biological activity. 40 h post-transfection, media of ShhNp and mock-transfected BOSC23 cells were harvested. Proteins were first immunoblotted to check for protein release before being used in the subsequent assays. Conditioned media were sterile filtered, mixed with DMEM containing 2× FCS and antibiotics, and applied to C3H10T1/2 cells in 15-mm plates. To some samples, 2.5 μm cyclopamine, a specific inhibitor of Shh signaling, was added. Cells were lysed 5 days after induction (20 mm Hepes, 150 mm NaCl, 0.5% Triton X-100, pH 7.4), and alkaline phosphatase (AP) activity was measured at 405 nm after the addition of 120 mm p-nitrophenolphosphate (Sigma) in 0.1 m glycine buffer, pH 10.4. Background differentiation in the absence of exogenous Shh was also quantified. Assays were performed in triplicate.
Molecular Modeling
The crystal structure of human Shh (Protein Data Bank entry 3M1N) (23) was displayed and modified employing PyMOL. Crystal contacts were calculated employing CryCo (available on the World Wide Web).
RESULTS
ShhN and ShhNp Binding to Heparin Is Largely CW-independent
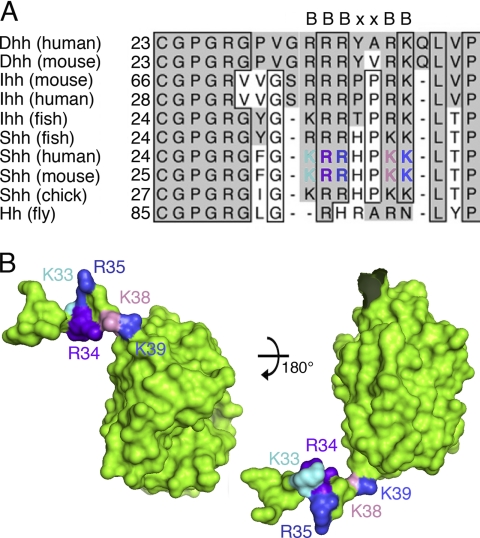
Sequence alignment of Shh N-terminal peptides with various corresponding N termini of vertebrate and invertebrate Hh family members shows that the CW sequence (amino acids 33–39 in the mouse Shh nomenclature; BBBXXBB, where B represents basic amino acid residues) (17) is highly conserved and follows a block of conserved residues required for N-terminal acylation (25) (Fig. 1A). Morphogen-HSPG ionic interactions are mediated by binding of positively charged amino acid residues lysine and arginine to sulfated, negatively charged motifs expressed on the HS chains. The three-dimensional structure of human ShhN (Protein Data Bank entry 3M1N) revealed that the N-terminal peptide extended 30 Å away from the globular domain of the protein (23, 26) and that CW residues Arg33 and Lys37 (human nomenclature; corresponding to residues Arg34 and Lys38 in the mouse) were located on one side of the extended peptide, whereas Lys32, Arg34, and Lys38 (corresponding to Lys33, Arg35, and Lys39 in the mouse) were located on the other side (Fig. 1B). We thus hypothesized that HS binding of the morphogen would be unlikely to depend equally on all five CW residues. Supporting this idea, N-terminal ShhNp peptides were demonstrated to contribute to morphogen multimerization as well (27). To us, this suggested that monomeric ShhN and multimeric ShhNp may bind to HS differently because some CW residues may contribute to ShhNp protein-protein interactions in the multimer rather than bind to HS. To test this possibility, we generated various monomeric and multimeric mutant forms of murine Shh (supplemental Fig. 1): ShhNΔCW/ShhNpΔCW lacking all CW residues (Lys33–Lys39), ShhN5xA/ShhNp5xA via site-directed mutagenesis of all five basic CW residues into alanine residues, ShhNR34A/K38A/ShhNpR34A/K38A upon site-directed mutagenesis of residues Arg34 and Lys38 into alanines (16), and ShhNK33E/R34L/ShhNpK33E/R34L upon site-directed mutagenesis of residues Lys33 and Arg34 into glutamic acid and leucine, respectively.
FIGURE 1.
The heparin-binding Cardin-Weintraub sequence is highly conserved and located at the Shh N terminus. A, comparison of the amino acid sequences of mammalian N-terminal Hh peptides and Drosophila Hh reveals the presence of two highly conserved motifs: a block of residues required for N-terminal acylation (25), ranging from 25 to 30 (mouse Shh), and the highly conserved CW sequence (where B represents a basic residue) (17). Boxes represent absolutely conserved amino acid residues in at least eight gene products, gray columns indicate the presence of conserved amino acids. B, structure of the CW sequence as revealed by the modeled murine ShhN crystal structure (template: Protein Data Bank entry 3M1N). The position of CW sequences located within the N-terminal extended peptides is indicated. Note that residues Arg34 and Lys38 are located on one side of the N-terminal peptide, and residues Lys33, Arg35, and Lys39 are located on the opposite side. Color coding of basic CW residues is as in A.
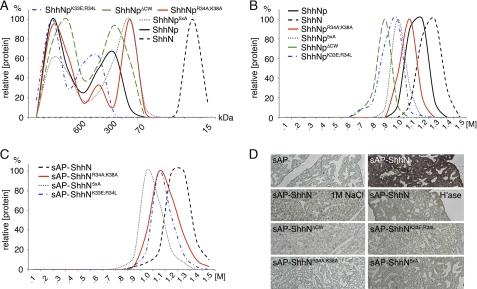
First, to confirm that CW mutagenesis did not impair the ability of the mutant proteins to multimerize, we analyzed recombinant murine full-length Shh cDNA, resulting in ShhNp, and the monomer control ShhN by gel filtration (Fig. 2A). Gel filtration analysis of media following 2 days of protein expression showed that ShhN was exclusively released in monomeric form into solution, as expected. In contrast, ShhNp formed multimers exceeding 600 kDa and a second fraction around 300 kDa, consistent with previous reports (28). Despite small differences in the relative abundance of lower and higher molecular weight fractions, all ShhNp mutants multimerized effectively. We thus concluded that all ShhNp mutant forms assemble properly on the cell surface and are exclusively released in multimeric form (29).
FIGURE 2.
Properties of CW mutant and wild-type Shh proteins. A, gel filtration analysis of supernatant derived from full-length ShhNp-expressing Bosc23 cells reveals the presence of “large” (>600 kDa) and “small” (70–600 kDa) multimers. In contrast, ShhN was exclusively monomeric. Site-directed mutagenesis of the Cardin-Weintraub sequence did not abolish ShhNp multimerization but affected the generation of large and small clusters to varying degrees. Representative results from one of three independently conducted experiments are shown. Molecular sizes of molecular weight standards are indicated. B, protein-heparin interactions of wild-type and CW mutant ShhNp as determined by heparin affinity chromatography. All multimeric CW mutant morphogens bind strongly to heparin. Recombinant, soluble ShhNp eluted at high salt concentrations (1–1.3 m NaCl), indicating strong ShhNp-heparin interactions. ShhN, however, showed even higher heparin affinity, eluting at 1.1–1.4 m NaCl. Reduced binding (>0.6 m NaCl) was observed for all mutant forms. C, heparin affinity chromatography of sAP-tagged ShhN monomers. Equal amounts of the wild-type and mutant morphogens were applied to the column, as assessed by sAP activity determined in the medium. Notably, all morphogens eluted at comparable salt concentrations from heparin. The heparin binding capacity was also comparable; integration of elution curves revealed 6.53, 5.12, 5.57, and 7.1 arbitrary units for sAP-ShhN, sAP-ShhNK33E/R34L, sAP-ShhNR34A/K38A, and sAP-ShhN5xA, respectively. D, ligand binding assay. Paraformaldehyde-fixed embryonic lung sections were treated with equal amounts of sAP alone, sAP-ShhN, and sAP-ShhN in the presence of 1 m NaCl, or tissues were pretreated with heparinase I–III to remove cell surface HS. Bound morphogens were detected via sAP activity upon incubation with NBT-BCIP. sAP-ShhN bound to HS in tissues, but CW mutants did not.
How does CW mutagenesis influence morphogen binding to heparin? To answer this question, we conducted heparin affinity chromatography of recombinant, mutant, and wild-type ShhNp as well as control ShhN secreted into serum-free medium. As shown in Fig. 2B, we found that all mutant and wild-type morphogens bound to heparin (eluting at 0.9–1.4 m NaCl). Mutant morphogens lacking the entire CW motif (ShhNpΔCW and ShhNp5xA) or containing a negatively charged residue (ShhNpK33E/R34L) eluted at lower salt concentrations (0.6–1.2 m NaCl); in contrast, ShhNpR34A/K38A binding to heparin was only slightly reduced if compared with the wild-type multimer (eluting at 0.9–1.3 m NaCl). The observed shifts in CW mutant elution profiles indicate that CW residues contribute to heparin binding; however, ionic interactions between heparin and all CW mutant morphogens were still strong, suggesting the presence of a separate heparin binding site. Moreover, although we initially expected stronger heparin binding of ShhNp due to the presence of an estimated 20–50 CW motifs within one multimer, we observed that monomeric ShhN eluted at higher salt concentrations compared with multivalent ShhNp (Fig. 2B). This suggests different heparin binding modes of the ShhN monomer compared with the multimeric morphogen.
In order to identify functional residues within the ShhN CW motif, sAP-tagged monomeric ShhN and corresponding CW mutants were expressed in Bosc23 cells, and the medium was subjected to heparin affinity chromatography as described above. We found that all monomeric mutant morphogens also bound strongly to heparin, eluting at 0.9–1.3 m NaCl (Fig. 2C). ShhN showed the highest heparin interactions (eluting at 1–1.5 m NaCl), but ShhN5xA lacking the entire functional CW motif also eluted at 0.9–1.2 m NaCl. This again suggests the presence of a separate Shh heparin binding site. However, heparin represents an artificial substrate, because it is exclusively produced in connective tissue type mast cells and is not known to interact with Shh in vivo.
ShhN Binding to Embryonic HS Is CW-dependent
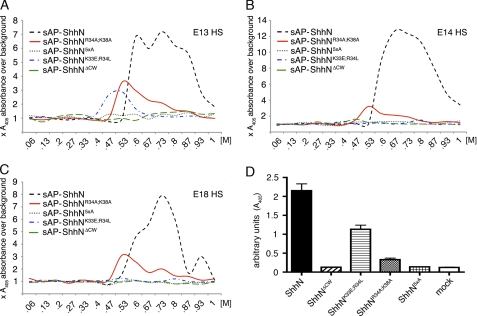
Thus, we next tested Shh binding to physiologically relevant forms of HS, such as mouse embryonic HS. To this end, embryonic day 14 (E14) embryo sections were incubated with equal amounts of recombinant mutant and wild-type ShhN linked to an sAP tag. Sections were washed, and sAP activity on slides was determined as a readout for the ability of the morphogens to bind HS (Fig. 2D) (16). Slide incubation with sAP-ShhN yielded a strong signal; in contrast, incubation with sAP alone, HS digestion with heparinase I–III prior to sAP-ShhN incubation, or sAP-ShhN incubation in the presence of 1 m NaCl did not yield detectable sAP activity. This demonstrated specific sAP-ShhN binding to HS. Notably, none of the CW mutant monomeric morphogens bound to HS. This demonstrated that, in contrast to heparin binding, HS binding of ShhN is absolutely dependent on the CW motif, consistent with a previous report (16). Moreover, because morphogens lacking the entire CW motif or just specific residues all failed to bind HS, our data suggest that HS binding depends on total positive charge of the CW motif. This assumption is backed by a report describing high affinity binding of Drosophila HhN to heparin, as determined by surface plasmon resonance, but undetectable binding to HS (18), consistent with the presence of only three basic CW residues (HhN, GRHRARN; murine ShhN, KRRHPKK (17)).
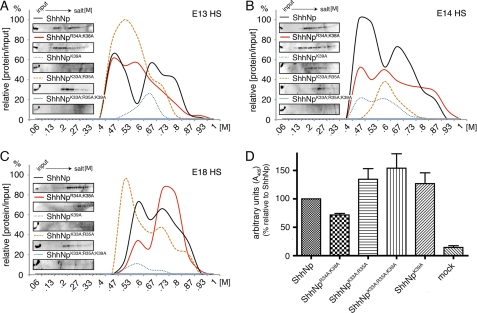
We next tested the interaction of mutant and wild-type sAP-ShhN with various forms of HS by FPLC affinity chromatography. To this end, HS was isolated from E13, E14, and E18 C57/Bl6 mouse embryos and coupled to HiTrap columns. We first established that sAP-Fgf8 and sAP-VEGF elution profiles from all three columns were comparable (supplemental Fig. 2), confirming efficient HS coupling to all three columns. We also confirmed that E18 HS elution profiles of ShhN and N-terminally sAP-tagged sAP-ShhN were comparable (supplemental Fig. 2). Next, equal amounts of wild-type and mutant sAP-ShhN (upon normalization of sAP activity in the input) were applied to each column. sAP activity in the flow-through was also monitored to confirm saturation of ShhN binding sites prior to elution. We detected multiple sAP-ShhN elution peaks from E13, E14, and E18 HS columns, suggesting weak and strong ionic ShhN interactions with HS. Consistent with lower overall HS sulfation if compared with heparin, ShhN eluted from HS columns at salt concentrations ranging from 0.53 to almost 1 m NaCl (Fig. 3, A–C). Weak and strong ionic ShhN interactions with different HS samples were variable, consistent with the observed variation in HS sulfation during development (7) (Table 1). We hypothesized that one possible explanation for the observed strong ionic interactions (0.9–1 m NaCl) would be the presence of “heparin-like,” highly sulfated domains on HS; if correct, we expected that CW mutant forms would also elute from HS columns at ∼0.9–1 m salt concentration because of their observed strong interaction with heparin. However, we found elution of only little sAP-ShhNR34A/K38A and sAP-ShhNK33E/R34L at low salt concentrations and no binding of sAP-ShhN5xA and sAP-ShhNΔCW to embryonic HS (Fig. 3, A–C) despite comparable sAP-activity in the input and flow-through. This finding shows that the separate heparin binding site does not contribute to the observed strong HS interactions of ShhN.
FIGURE 3.
CW mutant ShhN monomers do not bind to HS. Wild-type and mutant forms of sAP-ShhN were expressed in Bosc23 cells. The supernatants were filtered, and equal sAP activity was applied to columns coupled with E13, E14, and E18 embryonic HS. sAP activity in the flow-through confirmed saturation of Shh binding sites. Bound proteins were then eluted with a linear (0–1 m NaCl) gradient. sAP activity in the eluate was measured, and data were plotted as increased sAP activity over sAP base-line activity prior to elution. A, sAP-ShhN eluted from E13 HS at low (0.6 m) and high (>0.73 m) salt concentrations. In contrast, sAP-ShhN5xA and sAP-ShhNΔCW showed no detectable binding; little sAP-ShhNK33E/R34L and sAP-ShhNR34A/K38A binding to HS was detected (eluting at 0.5 and 0.53 m NaCl, respectively). B, sAP-ShhN bound to E14 HS. sAP-ShhN5xA, sAP-ShhNΔCW, and sAP-ShhNK33E/R34L showed no detectable binding, and little sAP-ShhNR34A/K38A binding to HS was detected (0.53 m NaCl). C, sAP-ShhN elution from E18 HS differed from both previous profiles. The relative amount of sAP-ShhN strong binding sites was increased over weaker binding sites, and a fraction of the wild-type monomer eluted at 0.93 m salt. No sAP-ShhN5xA, sAP-ShhNΔCW, and sAP-ShhNK33E/R34L activity was detected in the eluates, and sAP-ShhNR34A/K38 bound only weakly to HS (eluting at 0.53 m NaCl). D, reduced biological activity of ShhN CW mutants. C3H10T1/2 osteoblast precursor cells were incubated with equal amounts of untagged, wild-type, and CW mutant ShhN present in conditioned media, and the relative amount of Shh-induced AP activity was determined as a readout for C3H10T1/2 differentiation and hence for biological activity of the morphogens. Medium obtained from mock-transfected Bosc23 cells was used as a control (mock). Differences in biological activity were always significant (ShhN5xA, ShhNΔCW, and ShhNK33E/R34L: all p < 0.001, n = 3; ShhNK33E/R34L: p = 0.008, n = 3). Error estimates are standard deviations of the mean.
TABLE 1.
HS sulfation during development
HS was isolated from E13, E14, and E18 embryos, and samples were digested with heparin lyases. The resulting disaccharides were analyzed by quantitative LC/MS. All HS preparations showed somewhat comparable overall sulfation, E18-derived HS, however, showed reduced relative amounts of mono- and disulfated disaccharides and increased relative amounts of the trisulfated disaccharide D2S6. Values denote the mean percentage of total disaccharide. Disaccharides are abbreviated as follows. D0A0, ΔUA1,4GlcNAc; D0S0, ΔUA1,4GlcNS; D0A6, ΔUA1,4GlcNAc-6S; D0S6, ΔUA1,4GlcNS-6S; D2S0, ΔUA2S1,4GlcNS; D2S6, ΔUA2S1,4GlcNS-6S.
| E13 HS | E14 HS | E18 HS | |
|---|---|---|---|
| % | % | % | |
| D0A0 | 50.3 | 49.15 | 48 |
| D0S0 | 21 | 21.3 | 17 |
| D0A6 | 6 | 5.7 | 6.3 |
| D0S6 | 5.14 | 5.45 | 5.3 |
| D2S0 | 8.7 | 11.3 | 5.7 |
| D2S6 (trisulfated) | 5.12 | 6.6 | 16.5 |
| D2S6 (monosulfated) | 27 | 27 | 23.3 |
| D2S6 (disulfated) | 13.84 | 16.75 | 11 |
| SO3−/disaccharide | 0.7 | 0.8 | 0.95 |
We tested next whether HS binding of the monomeric morphogen was required for Shh-dependent differentiation of C3H10T1/2 osteoblast precursor cells into AP-producing osteoblasts (30). AP-catalyzed paranitrophenylphosphate conversion into paranitrophenol measured at 405 nm served as a biological readout (Fig. 3D). We first established that media obtained from untransfected cells did not induce differentiation (mock) and that (untagged) ShhN effectively induced C3H10T1/2 differentiation. Comparable amounts of ShhNK33E/R34L and ShhNR34A/K38A, as determined by immunoblotting, showed ∼50 and ∼90% reduced biological activity, respectively; ShhN5xA and ShhNΔCW were inactive. We thus conclude that a fully functional CW motif is essential for ShhN biological activity in this assay.
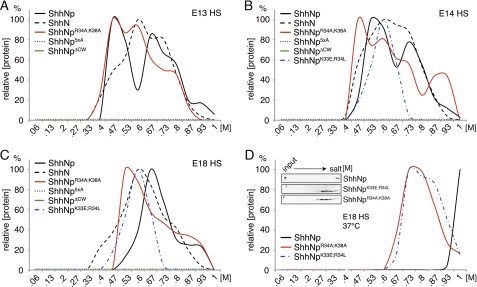
CW Residues Contribute Differently to HS Binding of ShhNp and ShhN
ShhNp multimers must have evolved because of specific advantages over the monomeric form. These advantages may include the possibility of allosteric control, higher local concentration of binding sites and larger binding surfaces, and the generation of novel epitopes at the subunit interfaces. Those features may all strongly affect HS binding of the multimer. Using the same HS-coupled FPLC columns, we thus determined binding of multimeric ShhNp and CW mutant forms to different preparations of HS. In this experimental setting, eluted ShhNp was detected by immunoblot analysis and quantified using ImageJ. The strongest signal was then set to 100%, and signals of other fractions were expressed as relative intensities compared with the peak value. As shown in Fig. 4, A and B, ShhNp eluted from E13 HS and E14 HS under high (0.73 m and up) and low (0.47 m) salt conditions, indicating strong and weak HS ionic interactions. This is consistent with the expected structural diversity of the HS mixture extracted from the various embryonic tissues. Ionic interactions of ShhNp with E18 HS were mostly of intermediate strength (around 0.67 m salt) (Fig. 4C). We confirmed these findings at 37 °C (Fig. 4D and inset); here, E18 HS binding of multimeric ShhNp was pronounced, suggesting that ShhNp-HS interactions are temperature-dependent. As expected, ShhNp5xA and ShhNpΔCW did not bind to any HS under the conditions employed. However, in contrast to the monomeric morphogens, substantial E13, E14, and E18 HS binding of ShhNpR34A/K38A and ShhNpK33E/R34L was always observed (Figs. 4, A–C). ShhNpR34A/K38A interactions with E13 HS were comparable with those of ShhNp (proteins eluting at 0.47 m and 0.73 m NaCl); in addition, ShhNpK33E/R34L elution at intermediate salt conditions was also observed (0.6 m NaCl). A fraction of ShhNpR34A/K38A eluted from E14 HS at even higher salt concentrations than the wild-type form; in contrast, binding to E18 HS was somewhat reduced. From these results, we concluded that CW residues Arg34 and Lys38 contribute to HS binding of monomeric ShhN but not to HS binding of the multimeric morphogen, ShhNp.
FIGURE 4.
CW mutant ShhNp multimers differ in their HS binding capacities and HS binding strength. Wild-type and mutant ShhNp were expressed in Bosc23 cells. The supernatant was filtered and applied to the same E13, E14, and E18 embryonic HS-coupled columns, and bound proteins were eluted with salt (0–1 m). ShhNp detected in the eluate was immunoblotted, and signals were quantified by ImageJ. The strongest signal was set to 100%. A, ShhNp and ShhNpR34A/K38A both bound E13 HS. In contrast, ShhNp5xA and ShhNpΔCW showed no detectable binding. ShhN served as a control. B, ShhNp5xA and ShhNpΔCW showed no detectable binding to E14 HS; however, ShhNpK33E/R34L and ShhNpR34A/K38A bound to E14 HS. ShhNp eluted at 0.63 and 0.73 m salt, and ShhNpR34A/K38A showed elution peaks around 0.47, 0.6, 0.73, and 0.93 m NaCl. In contrast, ShhNpK33E/R34L displayed a single elution peak at 0.6 m NaCl. C, ShhNp binding to E18 HS differed from both previous profiles. The relative amount of strong binding sites was increased over weak binding sites, and a fraction of the wild-type multimer, like the monomeric form, eluted at 0.93 m NaCl. ShhNp5xA and ShhNpΔCW elution was not detected, and most ShhNpR34A/K38A bound only weakly to HS (0.53 m NaCl). ShhNpK33E/R34L again displayed only one (low affinity) peak eluting at 0.6 m NaCl. D, at physiological temperature, ShhNp showed increased HS interaction (eluting at 0.8–1 m NaCl), indicating cooperative and temperature-dependent binding to E18 HS. Notably, both ShhNpK33E/R34L and ShhNpR34A/K38A also eluted at higher salt concentrations. Inset, immunoblotted input and eluates, demonstrating high capacity ShhNp binding to E18 HS. In contrast, ShhNp5xA and ShhNpΔCW binding to E18 HS were not observed.
In contrast to Fgf8 and VEGF that elute from the various columns at comparable salt concentrations (supplemental Fig. 2), ShhNp elution profiles from E18 HS differed from E13 HS and E14 HS profiles, suggesting that the source of HS determines ShhNp binding. One explanation for the pronounced shift of weak ionic interactions (0.47–0.53 m salt) to medium interactions (0.67 m salt) in E18 HS, in addition to substantial strong interactions (eluting at 0.93 m salt), would be the E18-specific expression of a defined ShhNp-specific oligosaccharide binding motif; another possibility would be increased overall E18 HS sulfation. E18 HS disaccharide analysis revealed strongly increased levels of trisulfated disaccharide ΔUA2S1,4GlcNS-6S (16.5% relative abundance in E18 HS versus 5.12% and 6.6% in E13 and E14 HS, respectively) despite only moderately increased overall sulfation (0.95 mol of sulfate/disaccharide in E18 HS versus 0.7 and 0.8 mol in E13 and E14 HS, respectively) (Table 1). The moderate increase in E18 HS overall sulfation was due to a decrease in relative amounts of monosulfated and disulfated disaccharides. Together, these results indicate that ShhNp may preferably bind to specifically sulfated HS domains. Moreover, our data suggest the presence of low, medium, and high strength HS binding sites for ShhNp. Last, comparison of ShhN elution profiles (black dashed line) and ShhNp profiles (solid black line) demonstrates different HS binding of multimeric ShhNp and the artificial yet widely employed monomeric morphogen.
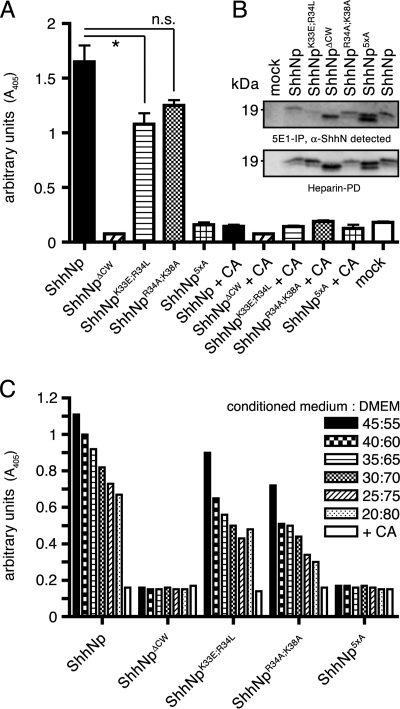
Retained Biological Activity of Selected ShhNp CW Mutants
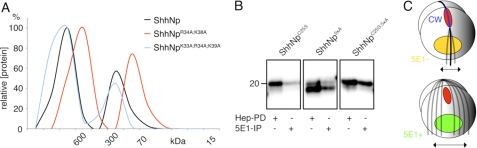
Based on their HS binding profiles, we anticipated largely unaltered biological activity of multimeric CW mutants ShhNpR34A/K38A and ShhNpK33E/R34L and biological inactivity of CW mutants ShhNp5xA and ShhNpΔCW. To test this idea, we again quantified Shh-dependent differentiation of C3H10T1/2 osteoblast precursor cells (30) (Fig. 5A). Media obtained from untransfected cells did not induce differentiation (mock), and ShhNp effectively induced C3H10T1/2 differentiation (1.650 ± 0.15 arbitrary units, n = 3). The teratogen cyclopamine (31) completely blocked biological activity of the ShhNp-conditioned media (0.145 ± 0.015, p ≤ 0.001, n = 2), demonstrating specificity of the assay. As expected, comparable ShhNpK33E/R34L protein amounts, as determined by immunoblotting, induced C3H10T1/2 differentiation into osteoblasts (1.08 ± 0.1, n = 2), as did ShhNpR34A/K38A (1.250 ± 0.05, n = 2). For all three morphogens, ShhNp, ShhNpK33E/R34L, and ShhNpR34A/K38A, biological activity was always significantly increased if compared with the mock negative control (p = 0.0051, p = 0.006, and p = 0.0023, respectively). However, compared with the wild-type morphogen, ShhNpK33E/R34L and ShhNpR34A/K38A activities were only insignificantly reduced (p = 0.04 and p = 0.12, respectively). As expected, ShhNp5xA and ShhNpΔCW did not induce C3H10T1/2 differentiation. We confirmed that their biological inactivity was not due to misfolding of the mutated morphogens. To this end, we compared total protein levels determined by heparin-Sepharose pull-down with immunoprecipitated ShhNp, employing the conformation-specific anti-Shh antibody 5E1 (32). As shown in Fig. 5B, 5E1 immunoprecipitates of the various mutant morphogens corresponded well with heparin-Sepharose pull-downs from the same supernatants, demonstrating proper folding of all forms. Finally, serial dilutions of all morphogens demonstrated concentration-dependent morphogen activities of multimeric ShhNp, ShhNpK33E/R34L and ShhNpR34A/K38A (Fig. 5C).
FIGURE 5.
Variable biological activities of ShhNp CW mutants. A, C3H10T1/2 osteoblast precursor cells were incubated with wild-type and CW mutant ShhNp conditioned media, and the relative amount of Shh-induced AP activity was determined as a readout for biological activity. Medium obtained from mock-transfected Bosc23 cells was used as a control (mock). ShhNp-induced AP activity was entirely blocked by the teratogen cyclopamine (CA) (2.5 μm, p ≤ 0.001, n = 2), a specific inhibitor of Shh signaling. Consistent with the previously observed loss of biological activity of monomeric CW-deficient morphogens, ShhNp5xA and ShhNpΔCW were also inactive in this assay. In contrast, both ShhNpK33E/R34L and ShhNpR34A/K38A retained significant biological activities in this assay if compared with the mock control (p < 0.001, n = 3), consistent with preserved HS binding of the mutant multimeric morphogens. *, p = 0.044, n = 3. n.s., not significant (p = 0.13, n = 3). One representative result of three independent experiments is shown. B, mutant and wild-type morphogens were subjected to immunoprecipitation using the conformation-dependent antibody 5E1 coupled to protein A-Sepharose, and the immune precipitates were subjected to SDS-PAGE and immunoblotting. For normalization of different expression levels, heparin-Sepharose pull-downs of the same expressions served as controls. 5E1 reactivity was detected toward all morphogens, demonstrating that loss of ShhNp5xA and ShhNpΔCW biological activities was not due to misfolding of the mutated morphogens. C, dose-dependent induction of C3H10T1/2 osteoblast precursor cell differentiation. C3H10T1/2 cells were incubated with equivalent amounts of wild-type or CW mutant ShhNp, and AP activity was measured at different morphogen concentrations as a readout for Shh-induced C3H10T1/2 differentiation. ShhNp5xA and ShhNpΔCW remained inactive at all concentrations used, and ShhNp, ShhNpK33E/R34L, and ShhNpR34A/K38A induced C3H10T1/2 cell differentiation in a dose-dependent manner. Cyclopamine was added as a control (2.5 μm).
Dual Functions of CW Residues in Multimeric and Monomeric Shh
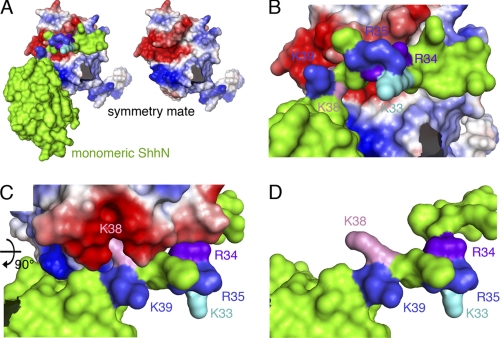
Our results show that deletion of the entire functional CW motif always resulted in the complete loss of HS binding and biological activity of monomeric and multimeric morphogens. In contrast, site-directed mutagenesis of CW residues that mediate HS binding of the monomeric morphogen, such as Arg34 and Lys38 (16), did not abolish HS binding and biological activity of the physiologically relevant, multimeric morphogen. Analysis of the human Shh crystal structure (Protein Data Bank entry 3M1N (23)) provided an explanation for this finding. We observed crystal lattice interactions between N-terminal extensions of ShhN substructures with hydrophobic pockets located on adjacent, symmetry-related Shh molecules (Fig. 6). Here, the basic N-terminal CW motif of one substructure located in close vicinity to a highly negatively charged area consisting of residues Asp88, Glu89, Glu90, Glu126, Asp129, Glu130, Asp131, Glu136, Glu137, and Asp146 on the surface of the symmetry-related substructure (33). Importantly, human Shh CW residues Arg33 and Lys37 (corresponding to residues Arg34 and Lys38 in the mouse) interact with this negatively charged area. CryCo Web analysis also predicted direct contact of residue Arg33 (Arg34 in murine Shh) with the adjacent molecule in the cluster. Residues Lys32, Arg34, and Lys38 (corresponding to Lys33, Arg35, and Lys39 in murine Shh), however, face the opposite, solvent-exposed side of the CW motif. Together, these observations suggested that only murine CW residues Lys33, Arg35, and Lys39 represent potential HS-binding amino acids.
FIGURE 6.
Intermolecular interactions observed in the human ShhN crystal structure (Protein Data Bank entry 3M1N) suggest CW interaction with adjacent molecules in the secreted morphogen cluster. Analysis of intermolecular electrostatic interactions as revealed by the human ShhN crystal structure suggests protein-protein contacts involving extended N-terminal peptides of symmetry-related molecules in the crystal. One subunit is colored green, the interacting molecule is colored according to electrostatic potential (red, negatively charged; blue, positively charged). A, the N-terminal CW motif interacts with a stretch of negatively charged residues present on the adjacent morphogen in the cluster (right). B and C, detailed view of the interaction site. CW residues Lys33, Arg35, and Lys39 (mouse nomenclature) are surface exposed, whereas residues Arg34 and Lys38 make contact with the negatively charged patch on the adjacent molecule. D, same orientation as C, with the interacting molecule removed for better visualization of residues Arg34 and Lys38.
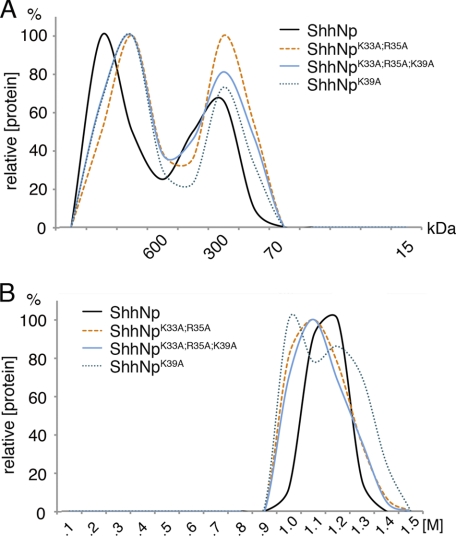
To test this idea, we deleted Lys33, Arg35, and/or Lys39 by site-directed mutagenesis. Gel filtration chromatography of solubilized mutant proteins demonstrated that morphogen multimerization was unaffected, as expected (Fig. 7A), and heparin affinity chromatography confirmed preserved binding of all forms to heparin (Fig. 7B). Subsequent HS affinity chromatography revealed that binding of ShhNpK33A/R35A/K39A to various forms of HS was strongly impaired; in comparison, HS binding of ShhNpR34A/K38A was affected less (Fig. 8, A–C). These results demonstrate that 3M1N crystal contacts accurately predict protein-protein contacts in soluble multimers, supporting a previous study (29). Because HS binding of ShhNpK39A to E13 and E18 HS was strongly affected, but ShhNpK33A/R35A binding was affected much less, we further conclude that CW residue Lys39 is most critically involved in the binding of ShhNp to E13 HS and E18 HS.
FIGURE 7.
CW mutagenesis does not interfere with ShhNp multimerization or heparin binding. Additional CW mutant morphogens were generated and analyzed by gel filtration and heparin affinity chromatography. A, gel filtration analysis of mutant forms lacking Lys39 (ShhNpK39A), Lys33 and Arg35 (ShhNpK33A/R35A), and all three residues (ShhNpK33A/R35A/K39A) revealed that morphogen multimerization was unaffected. Representative results from one of three independently conducted experiments are shown. Molecular weight standards are indicated. B, heparin affinity chromatography. All multimeric CW mutant morphogens eluted at high salt concentrations (1–1.3 m NaCl).
FIGURE 8.
CW residues 33, 35, and 39 selectively interact with HS. A–C, ionic interactions of CW mutant forms ShhNpK33A/R35A/K39A, ShhNpK33A/R35A, and ShhNpK39A with embryonic HS as determined by HS affinity chromatography. Bound proteins were eluted in a 0–1 m NaCl gradient; signal intensity is expressed relative to the signal intensity of the input set to 100%. ShhNpK33A/R35A/K39A did not bind to any HS column, but ShhNpK39A and ShhNpK33A/R35A variably bound to immobilized HS. ShhNpR34A/K38A always bound to all HS preparations. This indicates that residues Lys33, Arg35, and Lys39 contribute to HS binding, Lys39 being the most critical residue. Insets, representative immunoblotted fractions following HS affinity chromatography. D, C3H10T1/2 osteoblast precursor cell differentiation induced by mutant morphogens. C3H10T1/2 cells were stimulated with equivalent amounts of ShhNp, ShhNpR34A/K38A, ShhNpK33A/R35A/K39A, ShhNpK33A/R35A, and ShhNpK39A, and AP activity was quantified. ShhNp AP values were set to 100% in each experiment, and relative values for each mutant form were calculated. The observed increase in biological activity compared with ShhNp was always insignificant (ShhNpK33A/R35A/K39A: 154 ± 25%, p = 0,055, n = 7; ShhNpK33A/R35A: 134 ± 19%, p = 0.0891, n = 7; ShhNpK39A: 127 ± 19%, p = 0.18, n = 7). The observed decrease in ShhNpR34A/K38A biological activity was significant (72 ± 3%, p = 0,0001, n = 7).
Based on these findings, we hypothesized that biological activity of ShhNpK33A/R35A/K39A, ShhNpK33A/R35A, and ShhNpK39A should be reduced, as observed for ShhNpΔCW and ShhNp5xA. However, to our surprise, we found a consistent but insignificant increase in biological activity of ShhNpK33A/R35A/K39A, ShhNpK33A/R35A, and ShhNpK39A if tested in C3H10T1/2 differentiation (Fig. 8D) and a significant increase in ShhNpK33A/R35A/K39A- and ShhNpK33A/R35A-induced firefly luciferase induction in Light2 cells (supplemental Fig. 3). In agreement with previous results (Fig. 5A) (16), ShhNpR34A/K38A-dependent C3H10T1/2 differentiation and firefly luciferase induction in Light2 cells were somewhat reduced (Fig. 8D and supplemental Fig. 3). We thus conclude that CW residues Lys33, Arg35, and Lys39 modulate ShhNp biological activity in two cell-based bioassays for Shh activity; however, they are not required for ShhNp activity in these experimental settings.
What is the role of CW residues Arg34 and Lys38? In this work, we demonstrate that site-directed mutagenesis of these CW residues abolished HS binding of the monomeric morphogen but affected HS binding of the mutant multimer to a lesser degree. 3M1N crystal lattice contacts shown in Fig. 6 and CrystalCo predictions suggested that these residues may instead contribute to ShhNp multimerization (29). To test this possibility, gel filtration chromatography of ShhNpK33A/R35A/K39A, ShhNpR34A/K38A, and ShhNp were conducted. As shown in Fig. 9A, the relative size of ShhNpR34A/K38A multimers was indeed reduced if compared with those composed of ShhNpK33A/R35A/K39A and ShhNp, and the relative amount of lower molecular weight multimers was increased. We conclude from this result that CW residues Arg34 and Lys38 contribute to ShhNp multimerization or stabilize the multimeric morphogen in solution. Furthermore, crystal contact analysis and CrystalCo calculations predicted Arg34 to be instrumental for N-terminal peptide positioning on the surface of the adjacent subunit in the cluster because of the lack of any upstream ionic interactions, hydrogen bridges or hydrophobic interactions with the adjacent protein. This suggested Arg34-dependent positioning of the otherwise flexible ShhNp N terminus on the surface of the adjacent molecule in the cluster.
FIGURE 9.
CW residues 34 and 38 interact with adjacent molecules in the secreted morphogen cluster. A, gel filtration analysis of ShhNp, ShhNpR34A/K38A, and ShhNpK33A/R35A/K39A. ShhNpR34A/K38A multimer sizes were reduced, and the relative amounts of smaller clusters were increased, consistent with the suggested contribution of the two residues to protein-protein interactions. B, mutant and wild-type morphogens were subjected to immunoprecipitation using 5E1 coupled to protein A-Sepharose, and the immune precipitates were subjected to SDS-PAGE and immunoblotting. For normalization of different expression levels, heparin-Sepharose pull-downs of the same expressions served as controls. Soluble ShhNp5xA represents an N-terminally truncated morphogen (29), thereby exposing an epitope recognized by the 5E1 antibody (positive control). In contrast, ShhNpC25S is always released in non-truncated form, preventing binding of the 5E1 antibody to its (blocked) epitope (negative control). 5E1 binding is restored in a double mutant, untruncated form, suggesting increased spatial flexibility of the N-terminal peptide in the CW mutant morphogen. Note that ShhNpC25S shows a size comparable with that of ShhNpC25S/5xA. This indicates that both morphogens were released in N-terminally unprocessed form. C, model of CW-dependent spatial fixation of N-terminal peptides in the cluster. Functional inactivation of the CW motif (bottom) increases the spatial degree of freedom of the N-terminal peptide, resulting in increased accessibility of the 5E1 epitope.
To test this idea, we employed the conformation-dependent, monoclonal antibody 5E1 that specifically binds to the ShhNp zinc-coordinating surface epitope that also represents the binding site for the Shh receptor, Ptc (34). This epitope overlaps with the unprocessed N-terminal peptide of an adjacent molecule in the cluster, preventing 5E1 binding of the full-length ShhNp multimer. Upon N-terminal ShhNp processing and removal of the peptide during release (9, 29), however, 5E1 binding is restored. N-terminal processing depends on the presence of a conserved N-terminal cysteine residue serving as an acylation target (Fig. 1); site-directed mutagenesis of cysteine to serine (ShhNpC25S) prevents acylation and subsequent processing (29). For these reasons, 5E1 binding to soluble morphogen clusters indicates N-terminal truncation during release; in contrast, acylation-deficient, untruncated ShhNpC25S is not 5E1-immunoprecipitated (29). As expected, we detected strong 5E1 reactivity of N-terminally truncated ShhNp5xA and blockade of 5E1 binding by retained N-terminal peptides in clusters of unprocessed, full-length ShhNpC25S (Fig. 9B). This result supports the idea that the N terminus of one unprocessed morphogen blocks the 5E1-binding site of an adjacent molecule in the cluster (Fig. 9C, top). We next generated double mutant ShhNpC25S/5xA secreted in unprocessed form, as determined by immunoblotting. However, we observed strong 5E1 reactivity of the full-length morphogen, indicating 5E1 binding to the zinc coordination epitope despite the presence of N-terminal peptides in the cluster. We explain this observation by increased spatial flexibility of the N-terminal peptide in the absence of CW “anchoring” residues (Fig. 9C, bottom) and suggest a new role of CW residues in the positioning of ShhNp N-terminal peptides to the zinc coordination sites of adjacent molecules in the cluster.
DISCUSSION
HSPGs have been implicated in Hh release, spreading, and reception, and Shh binds to HS via its CW motif. In this work, we conducted the first biochemical characterization of HS interactions with the multimeric, physiologically relevant morphogen and a systematic analysis of the relative contribution of CW residues to HS binding. We demonstrate that monomeric and multimeric morphogens differ in their capacity to bind HS. Also, CW mutagenesis variably impairs HS binding capabilities of mutant morphogens. All mutant proteins employed in this study were effectively secreted from producing cells, interacted with heparin independent of CW function, and bound the conformation-dependent antibody 5E1, indicating correct folding and Ptc binding capability.
We found that CW residues Arg34 and Lys38 are not involved in HS binding but instead contribute to morphogen multimerization and positioning of the ShhNp N-terminal peptide, as predicted by the 3M1N crystal structure (23). This finding supports the predictive value of 3M1N crystal lattice interactions (29) and aids in interpretation of the observed phenotype of mice made deficient in residues Arg34 and Lys38 (16). In that work, it was demonstrated that HS binding of monomeric ShhNR34A/K38A was negligible, yet mice made transgenic for this mutation developed normally, except for a specific proliferation phenotype. From these observations, it was concluded that mouse development does not depend on direct Shh-HS interactions. However, monomeric ShhN does not contribute to mouse development, and as shown in our work, HS interactions of multimeric ShhNpR34A/K38A are only moderately affected. We thus suggest that mild phenotypes observed in ShhNpR34A/K38A transgenic mice (16) can be explained by only moderately impaired ShhNpR34A/K38A-HS interactions in vivo. The observed change in cell proliferation may result from the release of relatively smaller and less active morphogen clusters (6), consistent with our results (supplemental Fig. 4). Smaller ShhNpR34A/K38A multimers may further explain the reduced ShhNpR34A/K38A biological activity detected in C3H10T1/2 cells and Light 2 cells.
In contrast to ShhNpR34A/K38A, we found that HS binding of ShhNpK33A/R35A/K39A was strongly impaired. However, the functional role of CW residues Lys33, Arg35, and Lys39 in cells that receive the Hh signal is less clear. The observed increase in ShhNpK33A/R35A/K39A, ShhNpK33A/R35A, and ShhNpK39A biological activity in C3H10T1/2 and Light 2 cells may be explained by wild-type ShhNp binding to HS present in the serum, in turn competing with Ptc-binding of wild-type but not CW mutant morphogens on receiving cells. This, however, would imply that ShhNp signaling in C3H10T1/2 and Light 2 cells is not strictly dependent on HS expressed on these cells. Consistent with this assumption, disaccharide analysis of C3H10T1/2 cell-derived HS revealed very low relative amounts of trisulfated disaccharide D2S6 and disulfated forms D0S6 and D2S0, resulting in only 0.46 mol of sulfate/disaccharide (supplemental Fig. 5). Unfortunately, insufficient HS amounts isolated from this cell line prevented the generation of HS columns and further direct testing by affinity chromatography. However, three lines of evidence suggest that morphogen binding to C3H10T1/2 HS may be weak, consistent with its low degree of sulfation. First, in comparison with E13 HS, increased E18 HS-ShhNp ionic interactions (Fig. 4) correspond to increased expression of trisulfated disaccharides in E18 HS (Table 1), indicating ShhNp interaction with sulfated HS domains. This is consistent with impaired ShhN binding to low sulfated HS (18) and HS derived from mice made deficient in Ndst1 function (4). HS derived from Ndst1-deficient mouse embryos showed an almost 5-fold relative reduction in the abundance of disulfated D2S0, a 3-fold reduction in monosulfated D0S0, and about 2-fold reduction in trisulfated D2S6. Third, binding of monomeric sAP-ShhN to C3H10T1/2 cells was found to be weak (supplemental Fig. 6; compare with Fig. 2D). Therefore, the low degree of C3H10T1/2 HS sulfation, together with full biological activity of ShhNpK33A/R35A/K39A, challenges the general assumption that Shh signaling on receiving cells is HS-dependent (10, 11, 13, 35, 36). In these cells, HSPG core proteins may instead regulate ShhNp biological functions (13).
Instead, morphogen-HS interactions may be more essential in other parts of the Shh pathway. For example, HhNp multimerization in the fly depends on cell surface HSPG expression (1), ShhNp release from producing cells depends on HS-modulated shedding (9), and N-terminal morphogen processing at specific N-terminal sites is essential for biological activation of the solubilized multimer (29). Together, these findings indicate that regulated processing at specific, defined N-terminal peptide positions during release is required for the generation of biologically active morphogens (29) and that this process may be HS-regulated. If correct, biological inactivity of the CW deletion mutant ShhNpΔCW may thus be caused by the reduced length and aberrant positioning of the N terminus rather than by the lack of HS binding, as discussed above. We confirmed this idea by generating ShhNpN-5 (37), a mutant morphogen lacking the first 5 N-terminal amino acids. This modification resulted in complete loss of biological activity despite the presence of the CW motif (supplemental Fig. 7). Like ShhNpN-5 and ShhNpΔCW, ShhNp5xA was always secreted in N-terminally truncated form (Fig. 5B). Thus, based on the latter observation, we believe that the CW motif may be required to prevent nonspecific processing during shedding. Because processing at nonspecific, functionally “non-permissive” sites occurs only in the situation in which the spatial flexibility of the N-terminal peptide is increased and HS binding to the CW motif is impaired (in ShhNp5xA, not ShhNpR34A/K38A or ShhNpK33A/R35A/K39A), we suggest that HS binding protects the spatially disturbed N terminus from nonspecific processing.
Last, variable HS affinity of ShhN and ShhNp as described in our work may help explain conflicting results obtained by others when in vivo spreading of monomeric ShhN and multimeric ShhNp were compared. In vertebrates, ShhN showed reduced signaling capabilities and diffusion in comparison with the multimeric wild-type form (6, 38–40), but the opposite situation was found in the limb bud (24) and in the Drosophila wing imaginal disc (41, 42). Thus, based on those reports, it was unclear whether multimerization restricts or promotes morphogen spreading, also because observed differences had been linked to morphogen lipidation rather than multimerization or differential HS binding of monomeric and multimeric morphogens (24, 42, 43). In our work, we demonstrate variable ionic interactions between HS and ShhN or ShhNp and demonstrate that ionic interactions depend on HS sulfation. Thus, we postulate that, depending on the tissue and organism under investigation, ShhNp and ShhN bind to HSPGs differently, in turn affecting extracellular morphogen distribution. Extended ShhNp diffusion may be explained by relatively weaker ShhNp-HSPG interactions if compared with ShhN, as shown in this work, and an extended ShhN gradient by relatively stronger ShhNp-HSPG interactions possibly predominating in other systems or tissues (Fig. 4, compare A and C). If correct, this interpretation illustrates the essential roles that Shh multimerization and CW-dependent ShhNp-HSPG interactions play in morphogen gradient formation.
Supplementary Material
Acknowledgments
This work builds on information contained in Protein Data Bank entry 3M1N. The authors of this entry and the generous support of Biogen-IDEC (Cambridge, MA) providing the file in advance of Protein Data Bank deposition are therefore gratefully acknowledged. We also thank David J. Robbins (Dartmouth College, Hanover, NH) for Bosc23 cells and kind help in obtaining the human ShhN crystal coordinates, Jeff Esko (University of California San Diego) for pgs-D677 cells, and Andrew McMahon (Harvard College, Cambridge, MA) for Shh cDNA. The 5E1 antibody developed by T. M. Jessell was obtained from the Developmental Studies Hybridoma Bank maintained by the University of Iowa (Iowa City, IA). The excellent technical and organizational assistance of Sabine Kupich is gratefully acknowledged.
This work was supported by Deutsche Forschungsgemeinschaft (German Research Council) Grants SFB 492-B15 and GRK1549/1.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–7.
- HSPG
- heparan sulfate proteoglycan
- HS
- heparan sulfate
- CW
- Cardin-Weintraub
- AP
- alkaline phosphatase
- sAP
- secreted AP
- En
- embryonic day n.
REFERENCES
- 1. Vyas N., Goswami D., Manonmani A., Sharma P., Ranganath H. A., VijayRaghavan K., Shashidhara L. S., Sowdhamini R., Mayor S. (2008) Cell 133, 1214–1227 [DOI] [PubMed] [Google Scholar]
- 2. Bellaiche Y., The I., Perrimon N. (1998) Nature 394, 85–88 [DOI] [PubMed] [Google Scholar]
- 3. Desbordes S. C., Sanson B. (2003) Development 130, 6245–6255 [DOI] [PubMed] [Google Scholar]
- 4. Grobe K., Inatani M., Pallerla S. R., Castagnola J., Yamaguchi Y., Esko J. D. (2005) Development 132, 3777–3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Panáková D., Sprong H., Marois E., Thiele C., Eaton S. (2005) Nature 435, 58–65 [DOI] [PubMed] [Google Scholar]
- 6. Zeng X., Goetz J. A., Suber L. M., Scott W. J., Jr., Schreiner C. M., Robbins D. J. (2001) Nature 411, 716–720 [DOI] [PubMed] [Google Scholar]
- 7. Dierker T., Dreier R., Migone M., Hamer S., Grobe K. (2009) J. Biol. Chem. 284, 32562–32571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ma Y., Erkner A., Gong R., Yao S., Taipale J., Basler K., Beachy P. A. (2002) Cell 111, 63–75 [DOI] [PubMed] [Google Scholar]
- 9. Dierker T., Dreier R., Petersen A., Bordych C., Grobe K. (2009) J. Biol. Chem. 284, 8013–8022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gallet A., Staccini-Lavenant L., Thérond P. P. (2008) Dev. Cell 14, 712–725 [DOI] [PubMed] [Google Scholar]
- 11. Lum L., Yao S., Mozer B., Rovescalli A., Von Kessler D., Nirenberg M., Beachy P. A. (2003) Science 299, 2039–2045 [DOI] [PubMed] [Google Scholar]
- 12. Pallerla S. R., Pan Y., Zhang X., Esko J. D., Grobe K. (2007) Dev. Dyn. 236, 556–563 [DOI] [PubMed] [Google Scholar]
- 13. Capurro M. I., Xu P., Shi W., Li F., Jia A., Filmus J. (2008) Dev. Cell 14, 700–711 [DOI] [PubMed] [Google Scholar]
- 14. Li F., Shi W., Capurro M., Filmus J. (2011) J. Cell Biol. 192, 691–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McLellan J. S., Yao S., Zheng X., Geisbrecht B. V., Ghirlando R., Beachy P. A., Leahy D. J. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 17208–17213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chan J. A., Balasubramanian S., Witt R. M., Nazemi K. J., Choi Y., Pazyra-Murphy M. F., Walsh C. O., Thompson M., Segal R. A. (2009) Nat. Neurosci. 12, 409–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rubin J. B., Choi Y., Segal R. A. (2002) Development 129, 2223–2232 [DOI] [PubMed] [Google Scholar]
- 18. Zhang F., McLellan J. S., Ayala A. M., Leahy D. J., Linhardt R. J. (2007) Biochemistry 46, 3933–3941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Esko J. D., Selleck S. B. (2002) Annu. Rev. Biochem. 71, 435–471 [DOI] [PubMed] [Google Scholar]
- 20. Gandhi N. S., Mancera R. L. (2008) Chem. Biol. Drug. Des. 72, 455–482 [DOI] [PubMed] [Google Scholar]
- 21. Lawrence R., Olson S. K., Steele R. E., Wang L., Warrior R., Cummings R. D., Esko J. D. (2008) J. Biol. Chem. 283, 33674–33684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taipale J., Chen J. K., Cooper M. K., Wang B., Mann R. K., Milenkovic L., Scott M. P., Beachy P. A. (2000) Nature 406, 1005–1009 [DOI] [PubMed] [Google Scholar]
- 23. Pepinsky R. B., Rayhorn P., Day E. S., Dergay A., Williams K. P., Galdes A., Taylor F. R., Boriack-Sjodin P. A., Garber E. A. (2000) J. Biol. Chem. 275, 10995–11001 [DOI] [PubMed] [Google Scholar]
- 24. Li Y., Zhang H., Litingtung Y., Chiang C. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 6548–6553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Buglino J. A., Resh M. D. (2008) J. Biol. Chem. 283, 22076–22088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goetz J. A., Suber L. M., Zeng X., Robbins D. J. (2002) BioEssays 24, 157–165 [DOI] [PubMed] [Google Scholar]
- 27. Goetz J. A., Singh S., Suber L. M., Kull F. J., Robbins D. J. (2006) J. Biol. Chem. 281, 4087–4093 [DOI] [PubMed] [Google Scholar]
- 28. Feng J., White B., Tyurina O. V., Guner B., Larson T., Lee H. Y., Karlstrom R. O., Kohtz J. D. (2004) Development 131, 4357–4370 [DOI] [PubMed] [Google Scholar]
- 29. Ohlig S., Farshi P., Pickhinke U., van den Boom J., Höing S., Jakushev S., Hoffmann D., Dreier D., Schöler H., Dierker T., Bordych C., Grobe K. (2011) Dev. Cell, doi: 10.1016/j.devcel.2011.05.010 [DOI] [PubMed] [Google Scholar]
- 30. Nakamura T., Aikawa T., Iwamoto-Enomoto M., Iwamoto M., Higuchi Y., Pacifici M., Kinto N., Yamaguchi A., Noji S., Kurisu K., Matsuya T., Maurizio P. (1997) Biochem. Biophys. Res. Commun. 237, 465–469 [DOI] [PubMed] [Google Scholar]
- 31. Cooper M. K., Porter J. A., Young K. E., Beachy P. A. (1998) Science 280, 1603–1607 [DOI] [PubMed] [Google Scholar]
- 32. Ericson J., Morton S., Kawakami A., Roelink H., Jessell T. M. (1996) Cell 87, 661–673 [DOI] [PubMed] [Google Scholar]
- 33. Hall T. M., Porter J. A., Beachy P. A., Leahy D. J. (1995) Nature 378, 212–216 [DOI] [PubMed] [Google Scholar]
- 34. Maun H. R., Wen X., Lingel A., de Sauvage F. J., Lazarus R. A., Scales S. J., Hymowitz S. G. (2010) J. Biol. Chem. 285, 26570–26580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lin X. (2004) Development 131, 6009–6021 [DOI] [PubMed] [Google Scholar]
- 36. Yan D., Lin X. (2009) Cold Spring Harb. Perspect. Biol. 1, a002493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Williams K. P., Rayhorn P., Chi-Rosso G., Garber E. A., Strauch K. L., Horan G. S., Reilly J. O., Baker D. P., Taylor F. R., Koteliansky V., Pepinsky R. B. (1999) J. Cell Sci. 112, 4405–4414 [DOI] [PubMed] [Google Scholar]
- 38. Porter J. A., Ekker S. C., Park W. J., von Kessler D. P., Young K. E., Chen C. H., Ma Y., Woods A. S., Cotter R. J., Koonin E. V., Beachy P. A. (1996) Cell 86, 21–34 [DOI] [PubMed] [Google Scholar]
- 39. Lewis P. M., Dunn M. P., McMahon J. A., Logan M., Martin J. F., St-Jacques B., McMahon A. P. (2001) Cell 105, 599–612 [DOI] [PubMed] [Google Scholar]
- 40. Tian H., Jeong J., Harfe B. D., Tabin C. J., McMahon A. P. (2005) Development 132, 133–142 [DOI] [PubMed] [Google Scholar]
- 41. Burke R., Nellen D., Bellotto M., Hafen E., Senti K. A., Dickson B. J., Basler K. (1999) Cell 99, 803–815 [DOI] [PubMed] [Google Scholar]
- 42. Callejo A., Torroja C., Quijada L., Guerrero I. (2006) Development 133, 471–483 [DOI] [PubMed] [Google Scholar]
- 43. Huang X., Litingtung Y., Chiang C. (2007) Development 134, 2095–2105 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.