Abstract
Exposure of nonself surfaces such as those of biomaterials or transplanted cells and organs to host blood frequently triggers innate immune responses, thereby affecting both their functionality and tolerability. Activation of the alternative pathway of complement plays a decisive role in this unfavorable reaction. Whereas previous studies demonstrated that immobilization of physiological regulators of complement activation (RCA) can attenuate this foreign body-induced activation, simple and efficient approaches for coating artificial surfaces with intact RCA are still missing. The conjugation of small molecular entities that capture RCA with high affinity is an intriguing alternative, as this creates a surface with autoregulatory activity upon exposure to blood. We therefore screened two variable cysteine-constrained phage-displayed peptide libraries for factor H-binding peptides. We discovered three peptide classes that differed with respect to their main target binding areas. Peptides binding to the broad middle region of factor H (domains 5–18) were of particular interest, as they do not interfere with either regulatory or binding activities. One peptide in this group (5C6) was further characterized and showed high factor H-capturing activity while retaining its functional integrity. Most importantly, when 5C6 was coated to a model polystyrene surface and exposed to human lepirudin-anticoagulated plasma, the bound peptide captured factor H and substantially inhibited complement activation by the alternative pathway. Our study therefore provides a promising and novel approach to produce therapeutic materials with enhanced biocompatibility.
Therapeutic medicine increasingly relies on applications involving artificial or nonself surfaces, like the implantation or extracorporeal use of biomaterials (e.g., hemodialysis filters, medical devices, and drug delivery systems) or the transplantation of cell clusters (e.g., Langerhans islets) (1–3). Although considerable progress has been made in improving the biocompatibility of such nonself materials, their use in medical applications is still hampered by adverse reactions related to the activation of innate immunity and proinflammatory pathways: the level of inflammation and associated tissue damage can range from moderate to lethal for the patient (4, 5). By using soluble complement inhibitors [i.e., compstatin (6, 7)] or by coating surfaces with heparin, several groups showed that inhibition of complement activation largely attenuates biomaterial-induced activation and surface adhesion of cells (8–11). These observations clearly demonstrate that activation of the complement cascade on biomaterials or cell clusters, and the subsequent cross-talk with cytokine and coagulation pathways, marks a major cause of detrimental inflammatory responses (12). In particular, the anaphylatoxin C5a is known to potently attract immune cells and trigger their activation (13, 14), whereas surface-bound C3b largely contributes to cell adhesion (13). Very recently, the beneficial impact of complement inhibition in clinical biomaterial application was impressively demonstrated for the case of hemodialysis, where addition of compstatin to blood not only inhibited filter-induced complement response but also the subsequent activation of immune cells and the expression of pro-coagulative factors (15). Yet for many applications, a continuous administration of soluble inhibitors is not feasible, making modified surfaces with direct autoregulatory activity highly desired.
Recent data indicate that foreign surfaces rapidly adsorb abundant plasma proteins such as human serum albumin (HSA), IgG, and fibrinogen upon contact with blood or tissue, thereby forming an initial monolayer of proteins (16–18) on which complement activation occurs (18–20). Whereas the classical pathway (CP) of complement activation is likely to be involved in the initiation of the cascade (18, 21, 22), for example, via recognition of adsorbed IgG by C1q, the alternative pathway (AP) of complement activation appears to be the driving force behind the overall response. For one, adsorption of C3 to the protein layer may induce transformations that enable the formation of initial C3 convertases. More importantly, nascent C3b generated by either pathway binds to HSA and IgG (but not fibrinogen) in the protein layer, thereby leading to the assembly of the main AP convertase, C3bBb, and rapid amplification of complement response (Fig. 1A) (12, 18). The contribution of direct AP activation is further underscored by the observation that hemodialysis treatment of C4-deficient patients can induce complement activation, although at a slower rate compared with that of complement-sufficient individuals (21, 23). More importantly, Harboe et al. (24) have shown that the AP may contribute more than 80% of the C5a and terminal complement complex (C5b-9) that is produced during complement response. This observation suggests that the effector phase of complement, following recognition by the CP or the lectin pathway, is indeed dependent on AP-mediated amplification.
FIGURE 1.
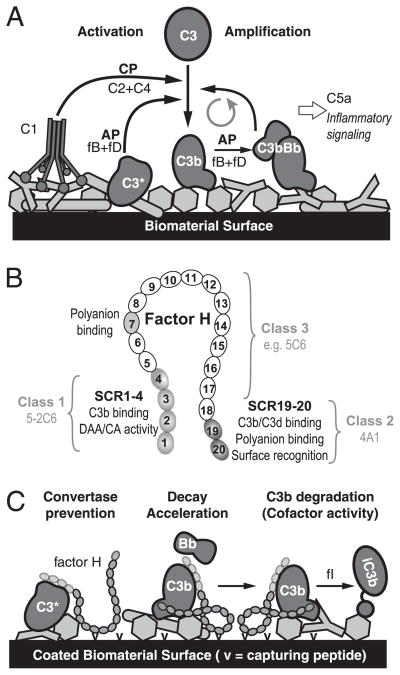
Schematic representation of biomaterial-induced complement activation and its prevention by factor-H binding peptides. A, Initiation of the complement cascade by adsorbed plasma proteins (light gray) via the CP or AP, and AP-driven amplification of the response that leads to attraction of immune cells and proinflammatory signaling. B, Domain organization of factor H with SCR domains involved in regulatory activity, C3b binding or polyanion recognition marked in gray. The major binding areas of the three peptide classes are indicated by brackets. C, Proposed mechanism of biomaterial surface protection by factor H-capturing peptides. Immobilized capturing entities (v) recruit factor H via binding to its nonregulatory domains, thereby enabling active regulation of convertase activity on the modified surface.
Thus, the AP plays a vital role in the complement-related incompatibility of nonself materials and is considered a suitable target for inhibition aimed at increasing the biocompatibility of these materials. Under homeostatic conditions, complement activation is tightly and precisely controlled by membrane-bound and soluble regulators of complement activation (RCA) (25, 26). Factor H, the second most abundant complement protein in plasma, is the primary regulator of the AP. It has an elongated structure consisting of 20 homologous short consensus repeats (SCR), each comprising ~60 amino acids held together by four conserved cysteine residues. Whereas the complement regulatory functions are concentrated to the N terminus (SCR 1–4) of factor H, two distinct regions (SCR 7, SCR 19–20) define the recognition of self surfaces via binding to polyanion patches (e.g., glycosaminoglycans) on host cells (Fig. 1B). Factor H regulates the AP by inhibiting the formation of the AP C3 convertase and accelerating its dissociation and by acting as cofactor for the degradation of C3b by factor I (Fig. 1C) (27–32). In the fluid phase, factor H was reported to have a bent or hairpin-like structure rather than a linear structure (33–36).
Surface coating with modulatory proteins or peptides is considered a promising approach for increasing biomaterial biocompatibility (37, 38). Theoretically, biomaterial surface-immobilized RCA proteins should confer a complement-regulatory capacity on the surface and increase its biocompatibility. Indeed, immobilization of the AP regulators factor H and decay accelerating factor (CD55) both attenuated biomaterial-induced complement in previous studies (39–41). However, although feasible to perform on a laboratory scale, the preparation and immobilization of the large RCA proteins is costly and likely associated with great loss of function. As a consequence, this approach would hardly be practical on a commercial scale. An alternative way to increase the blood compatibility of a surface is to conjugate molecules (e.g., Abs or peptides) with affinity for a plasma protein like factor H or C4b-binding protein (C4BP). The aim for such a procedure is that the structure on the surface should capture its ligand, ideally in an active conformation, when exposed to blood, recruiting soluble RCA to the artificial surface (Fig. 1C). Intriguingly, several human pathogens use recruitment of host regulators as part of their immune evasion strategy (25). A first attempt to use this approach for creating a complement-autoregulatory surface was made by Engberg et al. (42), who demonstrated that surface coating with C4BP-binding peptides from Streptococcus pyogenes inhibited complement activation via the CP on a model biomaterial surface. The aim of the current work was to discover factor H-binding peptides and create artificial or nonself surfaces that selectively inhibit activation and amplification of the AP.
Materials and Methods
Complement components and recombinant proteins
Human factor H was purified from normal human serum by 5–12% polyethylene glycol precipitation. The pellet was resuspended in 3 mM KH2PO4, 50 mM NaCl, pH 7.4, and the sample was injected onto a Source Q column (GE Healthcare). The protein was eluted using a step gradient with increasing salt concentrations. Fractions containing factor H were identified by direct ELISA using rabbit anti-human factor H polyclonal Ab (raised by standard procedures) followed by HRP-conjugated goat anti-rabbit IgG (Bio-Rad), pooled, dialyzed, and subsequently injected onto a Mono-S column (GE Healthcare). The eluted samples were identified by SDS-PAGE analysis, pooled, and dialyzed against PBS, pH 7.4. Human C3 was purified from normal human serum as previously described (43). C3b was generated by limited trypsin digestion on an activated thiol–Sepharose 4B column (GE Healthcare) and eluted with 20 mM L-cysteine (44). The eluted protein was treated with 100 mM iodoacetamide and further purified on a Mono-Q column (GE Healthcare). The expression and purification of factor H SCR 1–4 (referred to as “fH1–4” in this study) have been described (27), and fH19–20 was kindly provided by Dr. P. Barlow (University of Edinburgh). Factor I was a generous gift of Dr. S.A. Tsiftsoglou (University of Oxford).
Phage-displayed peptide libraries and biopanning of phage libraries
To discover factor H-binding peptides, we screened two variable cysteine-constrained phage-displayed libraries (ANL4, ANL5) that were constructed as previously described (45). Factor H-binding phage clones were isolated through three rounds of library screening. In the first round, microtiter wells (Nunc, Naperville, IL) were coated overnight with factor H (5 μg per well) or BSA (20 μg per well) in PBS at 4°C and saturated with 5% nonfat milk in PBS for 1 h at room temperature. After washing, the libraries were prescreened by incubating 2 × 1012 PFU of each library in BSA-coated wells for 1 h at room temperature; supernatants were then transferred to factor H-coated wells for binding at room temperature for 2 h. The wells were washed six times with PBST buffer (PBS with 0.1% Tween 20), and bound phage particles were eluted with 0.2 M glycine, pH 2.2, and immediately neutralized with 1 M Tris-HCl, pH 9.1. Recovered phage particles were amplified in Escherichia coli strain XL1Blue F′ TetR for the next round of screening; the screening procedure was repeated twice as described above. After the third round, the recovered phage particles were plated for identification by monoclonal phage ELISA.
Monoclonal phage ELISA
Microtiter wells were coated overnight with either factor H or BSA at 5 μg/ml in PBS at 4°C and saturated with 5% nonfat milk in PBST for 1 h. Completely separated individual phage plaques from the third round of screening were picked and amplified. Phage particles of each amplified individual phage plaque were added to both factor H- and BSA-coated wells in parallel and allowed to bind at room temperature for 1 h. After six washes with PBST, bound phages were detected with HRP-conjugated anti-M13 mAb (GE Healthcare), using ABTS (Roche) for color development. Clones that bound to factor H but not to BSA were considered positive; single-stranded DNA from positive clones was prepared using an M13 kit (Qiagen, Valencia, CA) and sequenced by the DNA sequencing facility of the University of Pennsylvania.
To localize the binding sites on factor H of the positive clones, we further tested each clone by monoclonal phage ELISA for binding to factor H and its N- and C-terminal fragments (i.e., fH1–4 and fH19–20). An unrelated phage clone (CK) from library ANL4, expressing the cyclic peptide ASASHCSFKLRVNC, was used as a control.
Peptide synthesis
Nα–Fmoc amino acids, PyBOP, and Rink amide MBHA resin (0.34 mmol/g) were obtained from Novabiochem (San Diego, CA). Diisopropyl-carbodiimide was purchased from AnaSpec (San Jose, CA). 1-Hydroxy-7-aza-benzotriazole was purchased from Advanced ChemTech (Louisville, KY). HSW syringes (10 ml; Torviq, Niles, MI) with frits on the bottom were used for all peptide syntheses. Dichloromethane (DCM) and N-methyl-pyrrolidinone were obtained from Fisher Scientific. All other chemical reagents for synthesis were purchased from Sigma-Aldrich (St. Louis, MO) and used without further purification.
The N-terminally Fmoc-protected linear peptides used in this study were synthesized on an Applied Biosystems 433A peptide synthesizer using FastMoc chemistry. Linear peptides were cyclized manually on resin in an HSW polypropylene syringe using thallium acetate in dimethylformamide/anisole (19:1, room temperature, 3 h). To couple biotin to the cyclized peptides, the C-terminal lysine side chain Mmt protecting group was selectively removed in 1% trifluoroacetic acid (TFA) (with 5% triisopropylsilane as scavenger). The coupling was then carried out in N-methyl-pyrrolidinone using PyBOP and 1-hydroxy-7-aza-benzotriazole until a negative Kaiser test result was observed. After removal of the Fmoc protecting group, the resin was washed with DCM (4 × 5 ml), then DCM/diethylether (1:1, 4 × 5 ml) and dried under a vacuum for 4 h. The peptides were cleaved from the resin with a mixture of 95% TFA, 2.5% water, and 2.5% triisopropylsilane for 3 h. After evaporation of the TFA under vacuum, the peptides were precipitated and washed three times with 5 ml each of cold diethylether. The liquid was separated from the solid by centrifugation and was decanted. The crude peptides were dried in air and dissolved in acetonitrile and 0.1% TFA in water (1:1) before purification by preparative reversed-phase HPLC to >95% purity.
Analytical HPLC was performed on a Waters Autopurification System using an XBridge BEH130 C18 column (Waters, Milford, MA). Preparative HPLC was performed on a Waters Autopurification System using a Waters XBridge BEH130 Prep C18 OBD column. Mass spectra were obtained online using a Waters MICROMASS ZQ 4000 mass spectrometer or separately on a Waters MALDI micro MX.
Peptide binding to factor H
Two approaches were used to evaluate the binding of synthetic peptides to factor H. First, we used peptide-phage competition assays, in which serially diluted synthetic peptide was added to its parent phage at the step of phage binding to coated factor H. The rest of the steps were performed as described above for monoclonal phage ELISA. Second, ELISA experiments were used to evaluate the binding of factor H to the immobilized peptide. Microtiter plates were coated with streptavidin (New England Biolabs) at 10 μg/ml in PBS at 4°C overnight and saturated with 2% BSA in PBS. After washing, either the biotinylated 5C6 peptide or a biotinylated control peptide [linear compstatin; IAVVQDWGHHRAT (46)] was added at 10 μg/ml and allowed to bind at room temperature for 30 min. The wells were washed again, and factor H serially diluted in PBS, starting with 2.5 μg/ml, was added. After 1 h of binding, the plates were washed, and bound factor H was detected with goat anti-human factor H polyclonal Ab (Quidel, San Diego, CA), followed by HRP-conjugated rabbit anti-goat IgG (Bio-Rad). In an alternative approach, human plasma that was anticoagulated with the thrombin inhibitor lepirudin (14) (final concentration 50 μg/ml; Refludan; Behring GmbH, Marburg, Germany), serially diluted in veronal-buffered saline (VBS), pH 7.4, containing 0.15 mM Ca2+ and 0.5 mM Mg2+, was used instead to assess the capture of factor H from plasma by the immobilized peptide.
Cofactor activity assays and hemolytic assays
To determine whether the binding of the 5C6 peptide interferes with the regulatory activity and cell-surface binding capacity of factor H, we performed factor H cofactor activity assays using purified C3b, factor H, and factor I, as well as hemolytic assays, both of which were performed in the presence of different concentrations of the 5C6 peptide. The hemolysis assay experiments were repeated at least three times using lepirudin plasma from different blood donors.
Cofactor activity assay
The factor H cofactor activity assay was performed as previously described (47). In brief, 1.14 μM C3b, 16 nM factor I, and 72 nM factor H were incubated with various amounts of the 5C6 peptide (0.4, 4, and 40 mM, respectively) in PBS at 37°C for 1 h in a final volume of 20 ml. The reaction was stopped by adding SDS-PAGE loading buffer, and the samples were analyzed by 8% SDS-PAGE under reducing conditions, followed by Coomassie blue staining.
Hemolytic assay
Hemolytic assays were performed to determine whether the peptide altered factor H cell-surface binding activity. Sheep erythrocytes (Cocalico Biological, Reamstown, PA) were washed with VBS containing 5 mM Mg2+/EGTA (VBS–Mg2+/EGTA) until the supernatant was clear. The assay was titrated by lysing erythrocytes in serial dilution with water in a final volume of 300 μl. The OD405 of the supernatants was measured, and the amount of the erythrocytes yielding an OD405 of ~1 was used in the assay. Serial dilutions of the 5C6 peptide or mAb MH10 (which binds fH19–20 and inhibits factor H cell surface binding) with the determined amount of sheep erythrocytes and lepirudin plasma diluted 1:10 in VBS–Mg2+/EGTA in a final volume of 300 μl were incubated at 37°C for 1 h in a shaking water bath. After centrifugation, the OD405 of the supernatants was measured, and the OD value was used to represent the cell lysis.
Interaction analysis of factor H to immobilized peptide 5C6
The binding of factor H to surface-bound 5C6 peptide was further characterized by surface plasmon resonance (SPR) on a ProteOn XPR36 instrument (Bio-Rad, Hercules, CA) using 10 mM sodium phosphate, 150 mM NaCl, 0.005% Tween 20 as running buffer. Streptavidin (50 μg/ml in 10 mM sodium acetate, pH 5.0) was immobilized on a GLC sensor chip at 30°C using amine coupling. The biotinylated 5C6 peptide and a biotinylated control peptide (linear compstatin; see earlier) were captured on separate streptavidin-coated channels to a density of ~200 resonance units. Purified factor H (Complement Technologies, Tyler, TX) or recombinant fragments fH1–4 and fH19–20 were injected at a concentration of 100 nM for 2 min at a flow rate of 25 μl/min with a dissociation phase of 6 min. A single injection of 2 M NaCl for 30 s was used to regenerate the surface between injections. Data were processed using ProteOn Manager software by subtracting the signals from interspots and a blank streptavidin channel. To perform a kinetic evaluation of the interaction, a 2-fold dilution series of factor H (0.24–500 nM) was injected using the same protocol and fitted to distinct kinetic models using ProteOn Manager. To exclude sensor chip matrix-specific surface binding, the same experiment was repeated on a Biacore 3000 instrument (GE Healthcare) using an SA chip under the same conditions as described above.
Inhibition of biomaterial-induced AP activation by immobilized peptide
Polystyrene microtiter wells, which served as a model biomaterial, were coated overnight with streptavidin (10 μg/ml in PBS) at 4°C. After washing, the biotinylated 5C6 peptide and the control peptide (10 μg/ml in PBS) were added to the wells and incubated at room temperature for 30 min. To form a defined plasma protein layer, the wells were washed and saturated with 2% HSA in PBS for 1 h. The wells were again washed, and serially diluted lepirudin plasma in VBS–Mg2+/EGTA was added and incubated at room temperature for 1 h to allow complement activation and amplification via the AP. In a parallel experiment intended to monitor the effect of a more natural plasma protein layer on complement inhibition by immobilized peptide 5C6, the washed peptide-coated wells were directly incubated with lepirudin plasma, and residual sites were blocked with 2% BSA in PBS after plasma incubation. In both experiments, bound fragments of activated C3 were detected with an HRP-conjugated goat anti-human C3 polyclonal Ab (Cappel, Aurora, OH). In parallel, captured factor H from serum was detected with goat anti-human factor H polyclonal Ab followed by HRP-conjugated rabbit anti-goat IgG in duplicate wells as described above. Undiluted lepirudin plasma was always included as a control having all complement pathways intact. The results show representatives of at least three independent experiments performed with plasma from different donors.
Results
Isolation and characterization of factor H-binding phage clones
Combinatorial peptide libraries are a rich source of structural diversity, and the screening of such libraries has proved to be an effective strategy for identifying peptide ligands to target proteins (6, 45). To isolate factor H-binding phage peptides, we screened two variable cysteine-constrained phage-displayed peptide libraries, ANL4 and ANL5, each containing ~2 × 1010 unique clones (45). A prescreening step was performed to eliminate potential nonspecific binding phages, for example, those that bind to plastic (48). After the third round of screening, we tested 96 individual clones from each library by monoclonal phage ELISA for their ability to bind factor H; 24 positive clones from each library were identified and sequenced.
All sequenced positive clones from library ANL4 were found to have identical sequence (class 1; clone 4A1; Fig. 1B, Table I) and bind to the C-terminal two SCR of factor H (i.e., fH19–20; Fig. 2A). From library ANL5, we identified six clones with unique sequences (Table I). One of those, clone 5-2C6, bound to the N-terminal regulatory region of factor H (i.e., fH1–4; class 2), whereas the remaining clones did not show significant binding to any of the terminal fragments (class 3); these clones therefore likely have a major binding site in the wide middle region of factor H between SCR5 and 18 (Fig. 2A). Neither 4A1 nor 5-2C6 was considered for subsequent experiments as they may potentially interfere with either the C3b binding or the regulatory activity of factor H. Therefore, we focused on the further characterization of the rest of the positive clones from ANL5. Sequence alignment showed a consensus sequence of xCxYSY/HWCxH among these clones (Table I), suggesting that their binding sites on factor H are overlapping or even identical.
Table I.
Phage display screening-derived factor H-binding peptides
| Classa | Phage Library | Phage Clone | Peptide Sequence | Binding Area on Factor Hb |
|---|---|---|---|---|
| 1 | ANL4 | 4A1 | ASSGMCFTKKTVLC | SCR19–20 |
| 2 | ANL5 | 5-2C6c | ASSYDVGYSHDCRF | SCR1–4 |
| 3 | ANL5 | 5C6 | ASSSRCTYDHWCSH | SCR5–18 |
| ANL5 | 5D1 | ASPSWCSYSHWCRH | SCR5–18 | |
| ANL5 | 5E10 | ASSFKCDYSHWCLH | SCR5–18 | |
| ANL5 | 5G11 | ASSNVCSYSYWCAH | SCR5–18 | |
| ANL5 | 5-2B2 | ASS--CMYSYWCTH | SCR5–18 | |
| Consensus sequenced | ASSxxCxYSHWCxH | — | ||
| Control peptide (LC)e | IAVVQDWGHHRAT | N/A | ||
Peptide class based on binding site region on factor H.
Determined by monoclonal phage ELISA. The SCR5–18 area was attributed in absence of stable binding to either the N or C terminus of factor H.
Peptide 5-2C6 is the only factor H-binding peptide that is not cysteine-constrained and therefore linear.
Based on sequence alignment of class 3 peptides and selection of most common residue; x designates variable preference.
Linear compstatin (LC; Cys-to-Ala double mutant).
N/A, no activity.
FIGURE 2.
Specificity characterization of factor H-binding phage clones. A, Major binding areas of positive clones on factor H were elucidated by monoclonal phage ELISA. Each clone was tested for binding to factor H, its N/C-terminal fragments fH1–4 and fH19–20, and C3b (negative control). CK, unrelated control phage from library ANL4. B, Binding of all class 3 clones (Table I) to a common area on SCR5–18 as confirmed by strong competition with the 5C6 peptide for binding to factor H. Data are representative of three separate experiments showing similar results.
Synthetic 5C6 peptide directly binds factor H with high affinity
Clone 5C6 was selected for further evaluation, and the corresponding peptide was prepared using solid-phase peptide synthesis. To confirm that the synthetic peptide binds factor H the same way as the parent phage does, we conducted a peptide-phage competition experiment in which the peptide, if active, would prevent the parent phages from binding to factor H. Indeed, the 5C6 peptide inhibited its parent phages from binding to factor H in a dose-dependent manner (Fig. 2B), indicating that the synthetic peptide bound to the same site on factor H as its parent phages. In addition, the 5C6 peptide also strongly inhibited the rest of the phages in this category from binding to factor H in a similar dose-dependent manner (Fig. 2B), thereby supporting the hypothesis that their binding sites on factor H are overlapping or identical (see earlier).
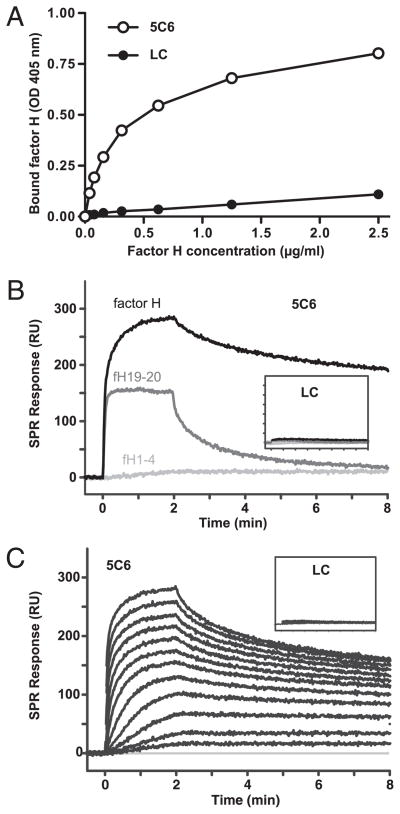
The direct binding between the peptide and factor H was initially evaluated in an ELISA experiment, in which the biotinylated 5C6 peptide and a biotinylated control peptide of similar size and charge (i.e., linear compstatin) were captured by coated streptavidin, and the binding of purified factor H was detected by polyclonal Abs. A strong binding signal for factor H was observed in wells with immobilized 5C6 peptide but not in wells bearing the control peptide (Fig. 3A), thereby confirming a direct capturing of factor H by the peptide.
FIGURE 3.
Binding of factor H to surfaces coated with synthetic 5C6 peptide as assessed by ELISA (A) and SPR (B, C). A, Polystyrene wells were coated with streptavidin, saturated with BSA, and incubated with biotinylated 5C6 peptide or negative control peptide; after incubation with serially diluted factor H, bound factor H was detected by a polyclonal Ab. Data shown are means ± SD (within the symbols) from a representative of three experiments. B and C, Biotinylated 5C6 peptide or control (LC, insets) was captured on streptavidin-coated SPR sensor chips, and factor H fragments at a fixed concentration (100 nM; B) or a dilution series of factor H (0.24–500 nM; C) was injected for 2 min with a dissociation phase of 6 min. The data are representative of two independent experiments on different SPR instruments showing comparable results. Additional evaluation of SPR data can be found in Supplemental Fig. 1. LC, linear compstatin; RU, resonance units.
The binding activity and specificity was further validated using SPR assays by simultaneously injecting factor H or its fragments over biotinylated 5C6 peptide and the control peptide, which were captured on a streptavidin-coated sensor chip. In agreement with the ELISA, the 5C6 peptide showed a high binding activity and stability for factor H, whereas no significant binding was observed on the control surface (Fig. 3B, inset). Although a significant SPR response could also be detected for the fH19–20 fragment, this interaction was not stable and the signal rapidly returned to baseline. Importantly, no binding of the regulatory fH1–4 fragment could be observed under the same conditions (Fig. 3B). Together, these results indicate that the middle region likely mediates the stable capturing of full-length factor H. Screening of a dilution series of factor H (0.24–500 nM) revealed concentration-dependent binding with high activity, yet the kinetic evaluation showed a significant deviation from a single-site model (Fig. 3C, Supplemental Fig. 1). Although the SPR results were closely comparable between two different SPR instruments featuring distinct surface chemistries (alginate versus carboxymethyl dextran matrix; data not shown), the observed binding pattern and affinities are nevertheless likely to be largely influenced by surface properties (peptide density, surface charges, etc.) and have to be regarded as apparent effects under the given conditions. Overall, however, the SPR results clearly confirmed a direct and specific binding of factor H to surface-immobilized peptide 5C6 as observed in ELISA.
The 5C6 peptide does not interfere with factor H activity
To inhibit the activation of complement, factor H recruited by the 5C6 peptide immobilized on a biomaterial surface must retain its regulatory activities. To confirm that this was the case, we first assayed the cofactor activity of factor H for the factor I-mediated degradation of C3b in the presence of increasing concentrations of the 5C6 peptide. As expected, 5C6 did not inhibit factor H cofactor activity, even at a 550-fold excess (40 μM) over factor H (72 nM) (Fig. 4A).
FIGURE 4.
Maintenance of the functional integrity of factor H in the presence of 5C6 peptide. A, The effects of the 5C6 peptide on the factor I-mediated cleavage of C3b (cofactor activity) was tested using a solution cleavage assay and analyzed by SDS-PAGE: lane 1, C3b; lanes 2–5, C3b, factor H, factor I, and increasing concentrations of 5C6 peptide (0–40 μM). B, Effect of 5C6 peptide on surface recognition by factor H as measured in a hemolytic assay in the presence of increasing concentrations of either the 5C6 peptide or an mAb against SCR19–20 of factor H (MH10). Data are means ± SD from a representative of three experiments.
Next, we performed hemolytic assays to determine whether the 5C6 peptide influenced the cell-surface binding of factor H. Sheep erythrocytes are known for their strong binding to human factor H and are often used to evaluate the effect of cell-surface binding on human factor H activity. No lysis of sheep erythrocytes mixed with 10-fold diluted lepirudin plasma was seen in the presence of the 5C6 peptide up to a concentration of 40 μM. (Fig. 4B). In contrast, the presence of mAb MH10 that recognizes the C terminus of factor H (49) induced quantitative cell lysis at a concentration of 0.2 μM, most likely due to inhibited factor H cell-surface binding (Fig. 4B). Taken together, these results indicate that the 5C6 peptide does not interfere with the cofactor activity or cell-surface binding properties of factor H.
Importantly, when expressed as fusion proteins with phage PIII domain, the peptides that bound to either the N terminus (i.e., 5-2C6) or C terminus of factor H (i.e., 4A1) affected the cofactor and hemolytic assays, respectively, whereas no such effect was observed for the 5C6 fusion protein (Supplemental Fig. 2). Whereas this suggests that class 1 and 2 peptides are less feasible for functional capturing of factor H, they may serve as interesting lead structures for developing important tools in complement research.
Inhibition of biomaterial-induced AP activity by the immobilized 5C6 peptide
To prevent biomaterial-induced complement activation, the surface-coated 5C6 peptide must be able to capture functional factor H from circulation even in presence of a plasma protein layer. To test this capability, we first assessed the complement-inhibitory capacity of the 5C6 peptide on a model biomaterial surface (polystyrene) carrying an HSA monolayer. After capture of the biotinylated peptide by coated streptavidin, the microtiter wells were saturated with 2% HSA. The major purpose of this experiment was to ascertain that the conjugated peptide would not be masked by the initial protein film, consisting mainly of HSA, which is rapidly adsorbed on a surface that is exposed to human blood.
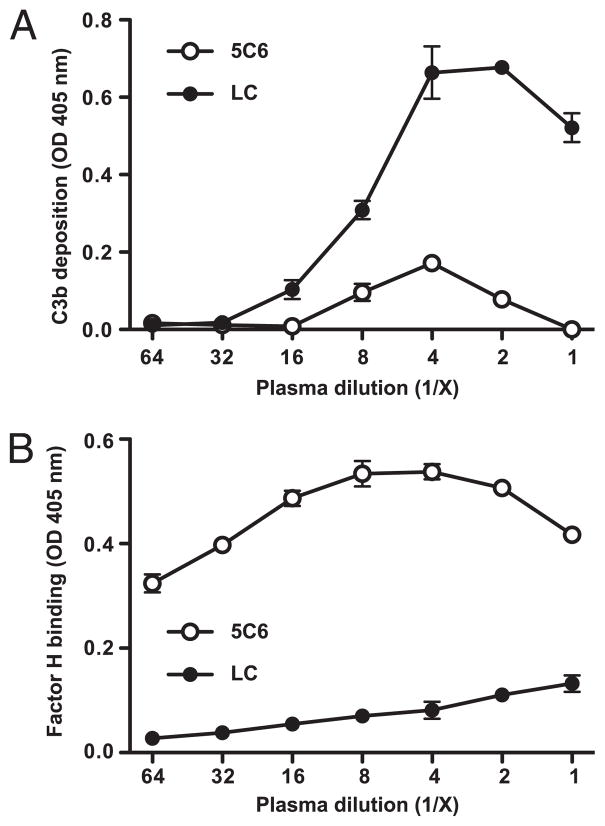
Anticoagulated plasma was serially diluted in VBS–Mg2+/EGTA and incubated in wells coated with either the 5C6 peptide or the control, followed by detection of deposited C3 activation fragments (i.e., C3b/iC3b) and captured factor H. Complement activation was substantially inhibited on the 5C6 peptide-immobilized surface (Fig. 5A), and there was pronounced factor H capture by immobilized 5C6 peptide compared with that of immobilized control peptide (Fig. 5B). These results demonstrated that the immobilized 5C6 peptide is capable of recruiting factor H from the plasma to the surface, resulting in a concomitant inhibition of complement activation by the AP.
FIGURE 5.
Inhibition of complement activation by immobilized 5C6 on a polystyrene surface carrying an HSA layer. Biotinylated 5C6 and the control peptide (linear compstatin, LC) were captured by coated streptavidin. After being saturated with HSA, wells were incubated with serially diluted lepirudin plasma to allow complement activation and factor H binding to occur. Complement activation was detected as surface-bound activated C3 (A), and bound factor H (B) was measured in duplicates per sample. Data are means ± SD from a representative of three experiments.
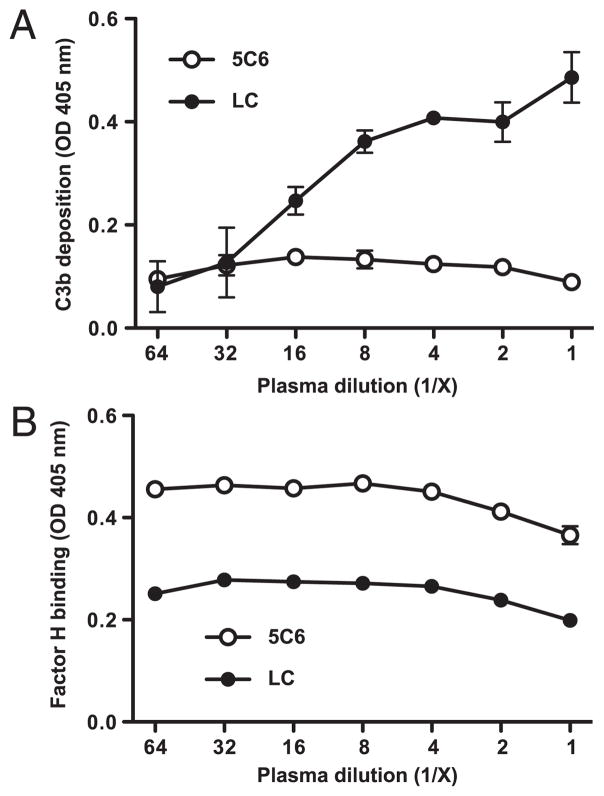
In an attempt to mimic the real application even closer, we exposed the peptide-coated polystyrene wells directly to plasma without HSA presaturation, thereby allowing formation of a spontaneous and more realistic protein layer. We then assayed for inhibition of complement activation by the 5C6 peptide as described earlier. Also under these conditions, the immobilized 5C6 peptide captured more factor H from plasma compared with that of the control peptide (Fig. 6B) and strongly inhibited complement activation on the surface (Fig. 6A). Importantly, there was no significant decline in factor H concentration in plasma that had been incubated on surfaces with immobilized 5C6 peptide (data not shown), thereby indicating that the surface capturing of factor H does not interfere with the regulatory capacity in circulation.
FIGURE 6.
Inhibition of complement activation by immobilized 5C6 peptide on a model polystyrene surface in absence of an initial plasma protein layer. Biotinylated 5C6 peptide and the negative control peptide (linear compstatin, LC) were captured by coated streptavidin. After washing, wells were directly incubated with serially diluted lepirudin plasma to allow complement activation and factor H binding to occur and then saturated with BSA. Complement activation was detected as surface-bound activated C3 (A), and bound factor H (B) was measured in duplicates per sample. Data are means ± SD from a representative of three experiments.
Discussion
In this study, we successfully used a nature-mimicking approach of preventing complement activation on blood-exposed materials by actively recruiting complement regulators to their surface by means of coating with small factor H-binding peptides. This strategy is especially important, as current research indicates that complement activation is a primary event and the main mediator of inflammatory response induced by bioincompatibility, thereby affecting both the function of therapeutic materials and the patient’s quality of life (4, 12). As a consequence, inhibition of complement activation on artificial or altered surfaces represents a targeted and highly promising approach for improving the biocompatibility and tolerability, and the AP should be selected as primary target due to the decisive role of this pathway in complement activation and amplification (24).
Under physiological conditions, human cells protect themselves by a panel of surface-bound regulators and by recruiting soluble RCA directly from circulation; the self-recognition capability of factor H and C4BP, mediated by binding to glycosaminoglycans and other polyanions, is essential in this process (26, 29, 50). Although microorganisms lack these self-recognition patterns, many human pathogens were shown to expose distinct RCA-binding proteins that “hijack” regulators as an integral part of their immune evasion portfolio (25, 51). One interesting example is Neisseria meningitidis, which escapes complement attack due to the presence of a surface protein (known as fHbp) that binds to SCR6 and thereby recruits factor H from host blood (51). In analogy to these natural self-protection strategies, immobilization or recruitment of RCA proteins to artificial biomaterial surfaces is expected to result in inhibition of complement activation, and factor H is an attractive candidate due to its strong AP-specific inhibitory activity. In previous studies, heparin coating was expected to recruit factor H from serum and inhibit biomaterial-induced complement activation, as an interaction between these two molecules has been well documented (28, 52). Indeed, a highly concentrated heparin coating inhibited biomaterial-induced complement activation (14, 53–55), yet this inhibition was found to be independent of factor H capturing by heparin (55). Furthermore, the use of heparin coating has to be critically examined because heparin exerts a broad binding specificity for plasma proteins (56, 57), induces activation of various cells (11, 58, 59), and may actually enhance complement activation at low concentrations in the fluid phase (14, 60). A more specific and complement-directed approach was applied by Andersson et al. (39, 41), who used two different chemical conjugation approaches to immobilize factor H on biomaterial surfaces and found that complement activation on the surface was efficiently inhibited. However, these approaches were deemed to be too complicated and costly for transfer to a commercial scale. Based on bacterial RCA-recruiting templates, Engberg et al. (42) immobilized streptococcal M protein-derived peptides that specifically bind human C4BP on polystyrene plates and found that complement activation via the CP was efficiently inhibited on coated surfaces under conditions in which the AP was not operative (42). Although representing an important proof of concept, inhibition of the CP alone may not be sufficient in the majority of biomaterial-induced complement activation where activation and amplification via the AP appears to be a driving force.
In this work, we wanted to exploit further this strategy to create a complement-autoregulatory surface in an efficient yet less costly manner. Therefore, our aim was to discover novel factor H-binding peptides that could be used to recruit autologous factor H to biomaterial surfaces, in analogy with the effect of M protein-derived peptides on C4BP (30), thereby inhibiting biomaterial-induced complement activation to a more substantial degree via the AP. We screened two variable cysteine-constrained phage-displayed peptide libraries and identified three classes of factor H-binding phage clones that differed with respect to their apparent binding sites on factor H. We believe that the 5C6 peptide, which primarily binds to the region between SCR5 to SCR18 of factor H, is the most promising candidate, as this peptide was able to capture factor H at high activity without interfering with its regulatory activity. Whereas SPR suggested a heterogeneous interaction pattern with some binding of fH19–20 to the 5C6 peptide-coated surface, the fast dissociation of this interaction renders it unlikely to be responsible for the observed stable capturing of full-length factor H. In this respect, the ELISA, competition, and SPR analysis all indicate a major binding site in the SCR5–18 region. It remains to be seen, however, whether the secondary interaction with the positively charged fH19–20 fragment is surface-specifically enhanced (e.g., on polyanionic surfaces as used in SPR) and whether it may even improve the capturing efficacy on certain materials. In any case, the soluble 5C6 peptide did not interfere with the surface recognition properties of factor H in the hemolytic assay, suggesting there is no overlap with the polyanion-binding sites.
We further assessed the degree of complement inhibition produced by the 5C6 peptide immobilized on a polystyrene-based model biomaterial surface using streptavidin–biotin chemistry under a variety of conditions. We found that immobilized 5C6 peptide captured factor H from plasma and substantially inhibited complement activation via the AP on polystyrene surfaces, with or without an HSA layer (Figs. 5, 6), suggesting that the captured factor H is bound in a functional conformation and that a direct correlation exists between the presence of factor H on the surface and the inhibition of complement activation. When we compared the amount of factor H captured by the 5C6 and control peptides on surfaces with or without an HSA layer (Figs. 5B, 6B), we noticed that relatively more factor H was detected on the control peptide-immobilized wells without HSA saturation. This result could be a reflection of direct binding or adsorption of factor H from plasma to the polystyrene surface to sites, which were inaccessible on the HSA-saturated surface. It should also be pointed out that a specific conjugation procedure was necessary to obtain a biological effect. Neither factor H binding nor inhibition of complement was seen when the 5C6 peptide was coated directly onto the polystyrene, suggesting that the peptide detached when the surface was exposed to plasma or that it had bound in a nonfunctional conformation. In the current preparation, however, both ELISA and SPR confirmed that the C-terminally biotinylated and surface-attached peptide largely retains its capturing activity, which is a critical requirement for the future development of coated therapeutic materials or cells. Because a biotin–streptavidin–based coating approach is not considered feasible both technically and economically, and because streptavidin itself may trigger immunogenicity, future development steps will aim at replacing the biotin group by tailored anchor and spacer moieties that will allow for a direct coating of specific biomaterials or cells.
In conclusion, we have discovered a novel factor H-binding peptide that binds to the region between SCR5 and SCR18 of this potent regulator. When immobilized onto a model biomaterial surface, this peptide efficiently recruited factor H, which led to a substantial inhibition of biomaterial-induced complement activation in undiluted lepirudin plasma. Although further refinements to increase the peptide’s affinity and optimize its immobilization are required, our discovery provides a promising approach for improving complement-related biocompatibility of materials or cells in therapeutic medicine at higher specificity and potentially lower cost compared with those of other strategies currently under consideration.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants EB003968, AI068730, and AI030040; Swedish Research Council Grants 60761701, 2009-4462, and 2009-4675; and by grants from the Natural Science Faculty, Linnæus University.
We thank Dr. Deborah McClellan for excellent editing of the manuscript, Antigoni Oulntreou and Dr. Paola Magotti for preliminary SPR analyses, and Dr. Paul N. Barlow (University of Edinburgh) and Dr. S.A. Tsiftsoglou (University of Oxford) for providing fH19–20 and factor I, respectively.
Abbreviations used in this article
- AP
alternative pathway
- C4BP
C4b-binding protein
- CP
classical pathway
- DCM
dichloromethane
- fH1–4
N-terminal factor H fragment (domains 1–4)
- fH19–20
C-terminal factor H fragment (domains 19–20)
- HSA
human serum albumin
- RCA
regulator of complement activation
- SCR
short consensus repeats
- SPR
surface plasmon resonance
- TFA
trifluoroacetic acid
- VBS
veronal-buffered saline
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Huebsch N, Mooney DJ. Inspiration and application in the evolution of biomaterials. Nature. 2009;462:426–432. doi: 10.1038/nature08601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams DF. On the nature of biomaterials. Biomaterials. 2009;30:5897–5909. doi: 10.1016/j.biomaterials.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 3.Korsgren O, Nilsson B. Improving islet transplantation: a road map for a widespread application for the cure of persons with type I diabetes. Curr Opin Organ Transplant. 2009;14:683–687. doi: 10.1097/MOT.0b013e328332c44c. [DOI] [PubMed] [Google Scholar]
- 4.Nilsson B, Korsgren O, Lambris JD, Ekdahl KN. Can cells and biomaterials in therapeutic medicine be shielded from innate immune recognition? Trends Immunol. 2010;31:32–38. doi: 10.1016/j.it.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ratner BD. The catastrophe revisited: blood compatibility in the 21st century. Biomaterials. 2007;28:5144–5147. doi: 10.1016/j.biomaterials.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sahu A, Kay BK, Lambris JD. Inhibition of human complement by a C3-binding peptide isolated from a phage-displayed random peptide library. J Immunol. 1996;157:884–891. [PubMed] [Google Scholar]
- 7.Ricklin D, Lambris JD. Compstatin: a complement inhibitor on its way to clinical application. Adv Exp Med Biol. 2008;632:273–292. doi: 10.1007/978-0-387-78952-1_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lappegård KT, Riesenfeld J, Brekke OL, Bergseth G, Lambris JD, Mollnes TE. Differential effect of heparin coating and complement inhibition on artificial surface-induced eicosanoid production. Ann Thorac Surg. 2005;79:917–923. doi: 10.1016/j.athoracsur.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt S, Haase G, Csomor E, Lütticken R, Peltroche-Llacsahuanga H. Inhibitor of complement, compstatin, prevents polymer-mediated Mac-1 up-regulation of human neutrophils independent of biomaterial type tested. J Biomed Mater Res A. 2003;66:491–499. doi: 10.1002/jbm.a.10031. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson B, Larsson R, Hong J, Elgue G, Ekdahl KN, Sahu A, Lambris JD. Compstatin inhibits complement and cellular activation in whole blood in two models of extracorporeal circulation. Blood. 1998;92:1661–1667. [PubMed] [Google Scholar]
- 11.Engstad CS, Gutteberg TJ, Osterud B. Modulation of blood cell activation by four commonly used anticoagulants. Thromb Haemost. 1997;77:690–696. [PubMed] [Google Scholar]
- 12.Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD. The role of complement in biomaterial-induced inflammation. Mol Immunol. 2007;44:82–94. doi: 10.1016/j.molimm.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 13.Sperling C, Maitz MF, Talkenberger S, Gouzy MF, Groth T, Werner C. In vitro blood reactivity to hydroxylated and non-hydroxylated polymer surfaces. Biomaterials. 2007;28:3617–3625. doi: 10.1016/j.biomaterials.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 14.Mollnes TE, Brekke OL, Fung M, Fure H, Christiansen D, Bergseth G, Videm V, Lappegård KT, Köhl J, Lambris JD. Essential role of the C5a receptor in E coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood. 2002;100:1869–1877. [PubMed] [Google Scholar]
- 15.Kourtzelis I, Markiewski MM, Doumas M, Rafail S, Kambas K, Mitroulis I, Panagoutsos S, Passadakis P, Vargemezis V, Magotti P, et al. Complement anaphylatoxin C5a contributes to hemodialysis-associated thrombosis. Blood. 2010;116:631–639. doi: 10.1182/blood-2010-01-264051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosengren A, Pavlovic E, Oscarsson S, Krajewski A, Ravaglioli A, Piancastelli A. Plasma protein adsorption pattern on characterized ceramic biomaterials. Biomaterials. 2002;23:1237–1247. doi: 10.1016/s0142-9612(01)00244-7. [DOI] [PubMed] [Google Scholar]
- 17.Collier TO, Jenney CR, DeFife KM, Anderson JM. Protein adsorption on chemically modified surfaces. Biomed Sci Instrum. 1997;33:178–183. [PubMed] [Google Scholar]
- 18.Andersson J, Ekdahl KN, Lambris JD, Nilsson B. Binding of C3 fragments on top of adsorbed plasma proteins during complement activation on a model biomaterial surface. Biomaterials. 2005;26:1477–1485. doi: 10.1016/j.biomaterials.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Andersson J, Ekdahl KN, Larsson R, Nilsson UR, Nilsson B. C3 adsorbed to a polymer surface can form an initiating alternative pathway convertase. J Immunol. 2002;168:5786–5791. doi: 10.4049/jimmunol.168.11.5786. [DOI] [PubMed] [Google Scholar]
- 20.Liu L, Elwing H. Complement activation on solid surfaces as determined by C3 deposition and hemolytic consumption. J Biomed Mater Res. 1994;28:767–773. doi: 10.1002/jbm.820280703. [DOI] [PubMed] [Google Scholar]
- 21.Lhotta K, Würzner R, Kronenberg F, Oppermann M, König P. Rapid activation of the complement system by cuprophane depends on complement component C4. Kidney Int. 1998;53:1044–1051. doi: 10.1111/j.1523-1755.1998.00836.x. [DOI] [PubMed] [Google Scholar]
- 22.Tengvall P, Askendal A, Lundström I. Complement activation by 3-mercapto-1,2-propanediol immobilized on gold surfaces. Biomaterials. 1996;17:1001–1007. doi: 10.1016/0142-9612(96)84675-8. [DOI] [PubMed] [Google Scholar]
- 23.Lappegård KT, Fung M, Bergseth G, Riesenfeld J, Lambris JD, Videm V, Mollnes TE. Effect of complement inhibition and heparin coating on artificial surface-induced leukocyte and platelet activation. Ann Thorac Surg. 2004;77:932–941. doi: 10.1016/S0003-4975(03)01519-4. [DOI] [PubMed] [Google Scholar]
- 24.Harboe M, Ulvund G, Vien L, Fung M, Mollnes TE. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clin Exp Immunol. 2004;138:439–446. doi: 10.1111/j.1365-2249.2004.02627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambris JD, Ricklin D, Geisbrecht BV. Complement evasion by human pathogens. Nat Rev Microbiol. 2008;6:132–142. doi: 10.1038/nrmicro1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu J, Wu YQ, Ricklin D, Janssen BJ, Lambris JD, Gros P. Structure of complement fragment C3b-factor H and implications for host protection by complement regulators. Nat Immunol. 2009;10:728–733. doi: 10.1038/ni.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt CQ, Herbert AP, Kavanagh D, Gandy C, Fenton CJ, Blaum BS, Lyon M, Uhrín D, Barlow PN. A new map of glycosaminoglycan and C3b binding sites on factor H. J Immunol. 2008;181:2610–2619. doi: 10.4049/jimmunol.181.4.2610. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt CQ, Herbert AP, Hocking HG, Uhrín D, Barlow PN. Translational mini-review series on complement factor H: structural and functional correlations for factor H. Clin Exp Immunol. 2008;151:14–24. doi: 10.1111/j.1365-2249.2007.03553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pickering MC, Cook HT. Translational mini-review series on complement factor H: renal diseases associated with complement factor H: novel insights from humans and animals. Clin Exp Immunol. 2008;151:210–230. doi: 10.1111/j.1365-2249.2007.03574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Józsi M, Zipfel PF. Factor H family proteins and human diseases. Trends Immunol. 2008;29:380–387. doi: 10.1016/j.it.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Ross GD, Lambris JD, Cain JA, Newman SL. Generation of three different fragments of bound C3 with purified factor I or serum. I. Requirements for factor H vs CR1 cofactor activity. J Immunol. 1982;129:2051–2060. [PubMed] [Google Scholar]
- 33.Schmidt CQ, Herbert AP, Mertens HD, Guariento M, Soares DC, Uhrin D, Rowe AJ, Svergun DI, Barlow PN. The central portion of factor H (modules 10–15) is compact and contains a structurally deviant CCP module. J Mol Biol. 2010;395:105–122. doi: 10.1016/j.jmb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okemefuna AI, Nan R, Gor J, Perkins SJ. Electrostatic interactions contribute to the folded-back conformation of wild type human factor H. J Mol Biol. 2009;391:98–118. doi: 10.1016/j.jmb.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Prosser BE, Johnson S, Roversi P, Herbert AP, Blaum BS, Tyrrell J, Jowitt TA, Clark SJ, Tarelli E, Uhrín D, et al. Structural basis for complement factor H linked age-related macular degeneration. J Exp Med. 2007;204:2277–2283. doi: 10.1084/jem.20071069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oppermann M, Manuelian T, Józsi M, Brandt E, Jokiranta TS, Heinen S, Meri S, Skerka C, Götze O, Zipfel PF. The C-terminus of complement regulator factor H mediates target recognition: evidence for a compact conformation of the native protein. Clin Exp Immunol. 2006;144:342–352. doi: 10.1111/j.1365-2249.2006.03071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nilsson PH, Engberg AE, Bäck J, Faxälv L, Lindahl TL, Nilsson B, Ekdahl KN. The creation of an antithrombotic surface by apyrase immobilization. Biomaterials. 2010;31:4484–4491. doi: 10.1016/j.biomaterials.2010.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krishna OD, Kiick KL. Protein- and peptide-modified synthetic polymeric biomaterials. Biopolymers. 2010;94:32–48. doi: 10.1002/bip.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andersson J, Larsson R, Richter R, Ekdahl KN, Nilsson B. Binding of a model regulator of complement activation (RCA) to a biomaterial surface: surface-bound factor H inhibits complement activation. Biomaterials. 2001;22:2435–2443. doi: 10.1016/s0142-9612(00)00431-2. [DOI] [PubMed] [Google Scholar]
- 40.Watkins NJ, Braidley P, Bray CJ, Savill CM, White DJ. Coating of human decay accelerating factor (hDAF) onto medical devices to improve biocompatibility. Immunopharmacology. 1997;38:111–118. doi: 10.1016/s0162-3109(97)00068-4. [DOI] [PubMed] [Google Scholar]
- 41.Andersson J, Bexborn F, Klinth J, Nilsson B, Ekdahl KN. Surface-attached PEO in the form of activated Pluronic with immobilized factor H reduces both coagulation and complement activation in a whole-blood model. J Biomed Mater Res A. 2006;76:25–34. doi: 10.1002/jbm.a.30377. [DOI] [PubMed] [Google Scholar]
- 42.Engberg AE, Sandholm K, Bexborn F, Persson J, Nilsson B, Lindahl G, Ekdahl KN. Inhibition of complement activation on a model biomaterial surface by streptococcal M protein-derived peptides. Biomaterials. 2009;30:2653–2659. doi: 10.1016/j.biomaterials.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dodds AW. Small-scale preparation of complement components C3 and C4. Methods Enzymol. 1993;223:46–61. doi: 10.1016/0076-6879(93)23037-n. [DOI] [PubMed] [Google Scholar]
- 44.Lambris JD, Ross GD. Assay of membrane complement receptors (CR1 and CR2) with C3b- and C3d-coated fluorescent microspheres. J Immunol. 1982;128:186–189. [PubMed] [Google Scholar]
- 45.Scholle MD, Kehoe JW, Kay BK. Efficient construction of a large collection of phage-displayed combinatorial peptide libraries. Comb Chem High Throughput Screen. 2005;8:545–551. doi: 10.2174/1386207054867337. [DOI] [PubMed] [Google Scholar]
- 46.Sahu A, Soulika AM, Morikis D, Spruce L, Moore WT, Lambris JD. Binding kinetics, structure-activity relationship, and biotransformation of the complement inhibitor compstatin. J Immunol. 2000;165:2491–2499. doi: 10.4049/jimmunol.165.5.2491. [DOI] [PubMed] [Google Scholar]
- 47.Alsenz J, Lambris JD, Schulz TF, Dierich MP. Localization of the complement-component-C3b-binding site and the cofactor activity for factor I in the 38kDa tryptic fragment of factor H. Biochem J. 1984;224:389–398. doi: 10.1042/bj2240389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Menendez A, Scott JK. The nature of target-unrelated peptides recovered in the screening of phage-displayed random peptide libraries with antibodies. Anal Biochem. 2005;336:145–157. doi: 10.1016/j.ab.2004.09.048. [DOI] [PubMed] [Google Scholar]
- 49.Barilla-LaBarca ML, Liszewski MK, Lambris JD, Hourcade D, Atkinson JP. Role of membrane cofactor protein (CD46) in regulation of C4b and C3b deposited on cells. J Immunol. 2002;168:6298–6304. doi: 10.4049/jimmunol.168.12.6298. [DOI] [PubMed] [Google Scholar]
- 50.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9:729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 51.Schneider MC, Prosser BE, Caesar JJ, Kugelberg E, Li S, Zhang Q, Quoraishi S, Lovett JE, Deane JE, Sim RB, et al. Neisseria meningitidis recruits factor H using protein mimicry of host carbohydrates. Nature. 2009;458:890–893. doi: 10.1038/nature07769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fernando AN, Furtado PB, Clark SJ, Gilbert HE, Day AJ, Sim RB, Perkins SJ. Associative and structural properties of the region of complement factor H encompassing the Tyr402His disease-related polymorphism and its interactions with heparin. J Mol Biol. 2007;368:564–581. doi: 10.1016/j.jmb.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 53.Sperling C, Houska M, Brynda E, Streller U, Werner C. In vitro hemocompatibility of albumin-heparin multilayer coatings on polyethersulfone prepared by the layer-by-layer technique. J Biomed Mater Res A. 2006;76:681–689. doi: 10.1002/jbm.a.30519. [DOI] [PubMed] [Google Scholar]
- 54.Kopp R, Bernsberg R, Kashefi A, Mottaghy K, Rossaint R, Kuhlen R. Effect of hirudin versus heparin on hemocompatibility of blood contacting biomaterials: an in vitro study. Int J Artif Organs. 2005;28:1272–1277. doi: 10.1177/039139880502801211. [DOI] [PubMed] [Google Scholar]
- 55.Andersson J, Sanchez J, Ekdahl KN, Elgue G, Nilsson B, Larsson R. Optimal heparin surface concentration and antithrombin binding capacity as evaluated with human non-anticoagulated blood in vitro. J Biomed Mater Res A. 2003;67:458–466. doi: 10.1002/jbm.a.10104. [DOI] [PubMed] [Google Scholar]
- 56.Mirow N, Zimmermann B, Maleszka A, Knobl H, Tenderich G, Koerfer R, Herberg FW. Plasma protein binding properties to immobilized heparin and heparin-albumin conjugate. Artif Organs. 2007;31:466–471. doi: 10.1111/j.1525-1594.2007.00386.x. [DOI] [PubMed] [Google Scholar]
- 57.Yu H, Muñoz EM, Edens RE, Linhardt RJ. Kinetic studies on the interactions of heparin and complement proteins using surface plasmon resonance. Biochim Biophys Acta. 2005;1726:168–176. doi: 10.1016/j.bbagen.2005.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wahba A, Rothe G, Lodes H, Barlage S, Schmitz G, Birnbaum DE. Effects of extracorporeal circulation and heparin on the phenotype of platelet surface antigens following heart surgery. Thromb Res. 2000;97:379–386. doi: 10.1016/s0049-3848(99)00181-4. [DOI] [PubMed] [Google Scholar]
- 59.Videm V. Heparin in clinical doses ‘primes’ granulocytes to subsequent activation as measured by myeloperoxidase release. Scand J Immunol. 1996;43:385–390. doi: 10.1046/j.1365-3083.1996.d01-57.x. [DOI] [PubMed] [Google Scholar]
- 60.Keil LB, Jimenez E, Guma M, Reyes MD, Liguori C, DeBari VA. Biphasic response of complement to heparin: fluid-phase generation of neoantigens in human serum and in a reconstituted alternative pathway amplification cycle. Am J Hematol. 1995;50:254–262. doi: 10.1002/ajh.2830500406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.