Abstract
Objectives
Single nucleotide allelic variants in the promoter region of the chromosome 15 alpha-7 acetylcholine nicotinic receptor gene (CHRNA7) are associated with both schizophrenia and the P50 auditory evoked potential sensory gating deficit. The purpose of this study was to determine if CHRNA7 promoter allelic variants are also associated with abnormal P50 ratios in persons with schizoaffective disorder, bipolar type.
Methods
P50 auditory evoked potentials were recorded in a paired stimulus paradigm in 17 subjects with schizoaffective disorder, bipolar type. The P50 test to conditioning ratio was used as the measure of sensory gating. Mutation screening of the CHRNA7 promoter region was performed on the subjects’ DNA samples. Comparisons to previously obtained data from persons with schizophrenia and controls were made.
Results
Subjects with schizophrenia, regardless of allele status, had an abnormal mean P50 ratio. Subjects with schizoaffective disorder, bipolar type and a variant allele had an abnormal mean P50 ratio, whereas those schizoaffective subjects with the common alleles had a normal mean P50 ratio. Normal control subjects had a normal mean ratio, but controls with variant alleles had higher P50 ratios.
Conclusions
In persons with bipolar type schizoaffective disorder, CHRNA7 promoter region allelic variants are linked to the capacity to inhibit the P50 auditory evoked potential and thus are associated with a type of illness genetically and biologically more similar to schizophrenia.
Keywords: schizophrenia, bipolar disorder, chromosomes, human, pair 15, receptors, nicotinic, evoked potentials, auditory
INTRODUCTION
Linkage and/or association between chromosome 15q14 and schizophrenia has been found in 30 North American families of African descent [Kaufmann et al., 1998], 12 German families [Stöber et al., 2000], 20 South African families [Riley et al., 2000], 30 Azorean families [Xu et al., 2001], 166 North American families of European descent [Tsuang et al., 2001], 52 Taiwanese families [Liu et al., 2001], and 82 Canadian families [De Luca et al., 2004], but not in 5 Canadian families [Neves-Pereira et al., 1998] or 54 families within the United States of America [Curtis et al., 1999]. Most of these studies have involved polymorphic markers in or near the chromosome 15q14 locus of CHRNA7, the gene for the alpha-7-nicotinic acetylcholine receptor subunit.
The P50 auditory evoked potential recorded in a paired stimulus paradigm is a biological marker that has been used to characterize sensory gating deficits in schizophrenia. The P50 ratio, the amplitude of response to the second stimulus divided by the amplitude of the response to the first stimulus, is a measure of the ability to inhibit the auditory evoked response to the second of two paired stimuli. Persons with schizophrenia and approximately 50% of their first-degree relatives have abnormally high P50 ratios [Siegel et al., 1984]. Associations between the P50 ratio and CHRNA7 promoter region variant alleles have been reported in persons with and without schizophrenia [Leonard et al., 2002; Houy et al., 2004].
The mean P50 ratio in persons with schizoaffective disorder, bipolar type is intermediate between healthy controls and individuals with schizophrenia [Olincy and Martin, 2005]. It is not known whether variant alleles in CHRNA7 contribute to this intermediate impairment in persons with schizoaffective disorder. Genetic studies of association between schizophrenia and chromosome 15 often include subjects with schizoaffective disorder, with the type unspecified [Gejman et al., 2001; Liu et al., 2001] or the depressed type [Kaufmann et al., 1998; Tsuang et al., 2001]. Linkage at chromosome 15 has also been demonstrated using an affected definition including both bipolar type I and schizoaffective bipolar type diagnoses with a LOD of 3.5 [Turecki et al., 2001], a LOD of 3.12 [Faraone et al., 2004], a MOD of 2.37 [Edenberg et al., 1997], and a LOD of 1.5 [Bailer et al., 2002]. A comparison between patients and controls using the D15S1360 marker, within intron 2 of CHRNA7, found that patients with schizophrenia, bipolar disorder and schizoaffective disorder all contributed to the difference between controls and patients [Stassen et al., 2000]. However, a recent genome wide scan in 24 families of persons with schizoaffective disorder, bipolar type found no evidence for linkage at chromosome 15 [Hamshere et al., 2005].
No published studies have examined the relationship between the P50 ratio and CHRNA7 allelic variants in a schizoaffective population. Within our sample, we divided each of the subject groups into two subgroups. The first subgroup included those subjects with the most common alleles at each of the promoter region sites. The second subgroup included those subjects with less common or variant alleles occurring in the promoter region. We then sought to test the hypothesis that the mean P50 ratio in subjects with schizoaffective disorder, bipolar type could reflect 2 distinct groups of patients: those persons with the common CHRNA7 promoter region alleles and normal sensory gating, and those persons with one of the variant CHRNA7 promoter region alleles and impaired sensory gating.
MATERIALS AND METHODS
Subjects
Seventeen subjects with schizoaffective disorder, bipolar type (43.9 ± 9 years old and 42% male) were selected from a previous study of bipolar disorder [Olincy and Martin, 2005]. Subjects received a complete description of the study and then gave their written informed consent as required by the Colorado Multiple Institute Review Board. Cognitive capacity was informally assessed by asking the subjects to explain the risks of the study as well as to describe what would be expected of them during the study day. Diagnostic and symptom measures included the Structured Clinical Interview for DSM-IV [First et al., 1997] the Beck Depression Inventory [BDI; Beck et al., 1961], the Young Mania Rating Scale [YMRS; Young et al., 1978], and the Positive and Negative Symptom Scales [PANSS; Kay et al., 1987; Supplementary Table 1]. Groups were compared with 37 subjects with schizophrenia (43.0 ± 10 years old and 60% male) and 149 normal subjects (44.2 ± 13 years old and 34% male) for whom a diagnosis had been ascertained using the Structured Clinical Interview for DSM IV [First et al., 1996, 1997]. Genotyping and P50 data had been previously reported [Leonard et al., 2002]. All subjects were also asked if they currently smoked cigarettes.
Electrophysiological Recordings
The methodology for recording and analysis of the P50 auditory evoked potential is previously described [Olincy and Martin, 2005]. A conditioning testing paradigm presented auditory stimuli with an intrapair interval of 0.5-sec and interstimulus interval of 10-sec. Please see the supplementary materials for more details. Figure 1 in the supplementary materials demonstrates representative examples of the P50 evoked potentials for each of the diagnostic groups.
Genotyping
DNA was isolated from blood, collected by venipuncture on the day of testing, as described [Miller et al., 1988; Leonard et al., 2002]. A fragment of 270 bp, containing sequence of the proximal promoter of the CHRNA7 gene, was generated from each DNA by PCR, utilizing 500 ng DNA, and 0.6 µM each of the following primer sets: forward primer, 5′ AGTACCTCCCGCTCACACCTCG 3′; reverse primer, 5′ ATGTTGAGTCCCGGAGCTGCAG 3′. The PCR reactions also included 0.2 mM dNTPs, DNA polymerase and 5× buffer from the GC-Rich PCR kit (Roche Applied Sciences, Indianapolis, IN), adjusted to 0.2 mM final MgCl2. The PCR program was: 95°C, 3 min; 38 cycles of 95°C, 30 sec; 56°, 30 sec; 72°, 1 min, then a 4°C hold. The PCR fragments were screened for variant alleles utilizing a Transgenomic WAVE™ denaturing high performance liquid chromatography (DHPLC) system. The methodology for this instrument is described in [Leonard et al., 2002; Gault et al., 2003]. Fragments showing a pattern different from the common allele by DHPLC were sequenced for identification of the specific mutation (see Fig. 2 in the supplementary materials for the variant allele locations). Patterns similar to the common allele were mixed with a common allele sample to ensure that any homozygotic mutations were not missed.
Statistical Analyses
Chi-square and Fisher’s Exact Tests were used to evaluate differences in frequencies of allelic variants and categorical clinical variables between groups. t tests were used to evaluate differences in electrophysiological and clinical variables between subjects with and without the common alleles. If the variances of the two populations were not equal, a separate variance t-test for means rather than a pooled variance t-test was used, thereby decreasing the degrees of freedom. Cohen’s d was calculated to estimate effect sizes (abbreviated as “es”). Pearson’s correlations were used to assess the relationship between clinical variables and electrophysiological measures.
RESULTS
Eleven of the 37 subjects with schizophrenia (30%), 6 of the 17 subjects with schizoaffective disorder (35%) and 41 of 149 normal control subjects (28%) had a variant CHRNA7 promoter region allele (χ2 = 0.48, df = 2, P = 0.79; Supplementary Table 2).
The mean P50 ratio differed among subject groups (F = 74.0, df = 2,200, P < 0.001). Post hoc analyses revealed that the subjects with schizophrenia had a greater mean P50 ratio than both the subjects with schizoaffective, bipolar type disorder (103.9 ± 64.6 vs. 50.5 ± 41.1; P < 0.001; es 0.93) and the normal control subjects (21.8 ± 25.5; P < 0.001; es 2.26). Subjects with schizoaffective disorder, bipolar type also had a mean P50 ratio greater than the normal control subjects (P = 0.008; es 1.05). Group differences emerged for the conditioning wave amplitude (F = 6.6, df = 2,200, P = 0.002) with the schizophrenia group having a smaller mean conditioning wave amplitude than the normal control group (2.1 ± 1.1 mV vs. 3.0 ± 1.6 mV; P = 0.004; es 0.6) but not the schizoaffective, bipolar type group (2.2 ± 1.1 mV; P = 0.969; es 0.09). The schizoaffective group trended toward a difference from the normal control group (P = 0.099; es 0.52). The test wave amplitudes also differed among groups (F = 37.7, df = 2,200, P < 0.001). The schizophrenia group had a larger mean test wave amplitude than both the schizoaffective, bipolar type (1.9 ± 1.1 mV vs. 1.1 ± 0.8, P = 0.001; es 0.8) and normal control groups (0.7 ± 0.7 mV, P < 0.001; es 1.52). The schizoaffective group trended toward a difference from the normal control group (P = 0.125; es 0.57).
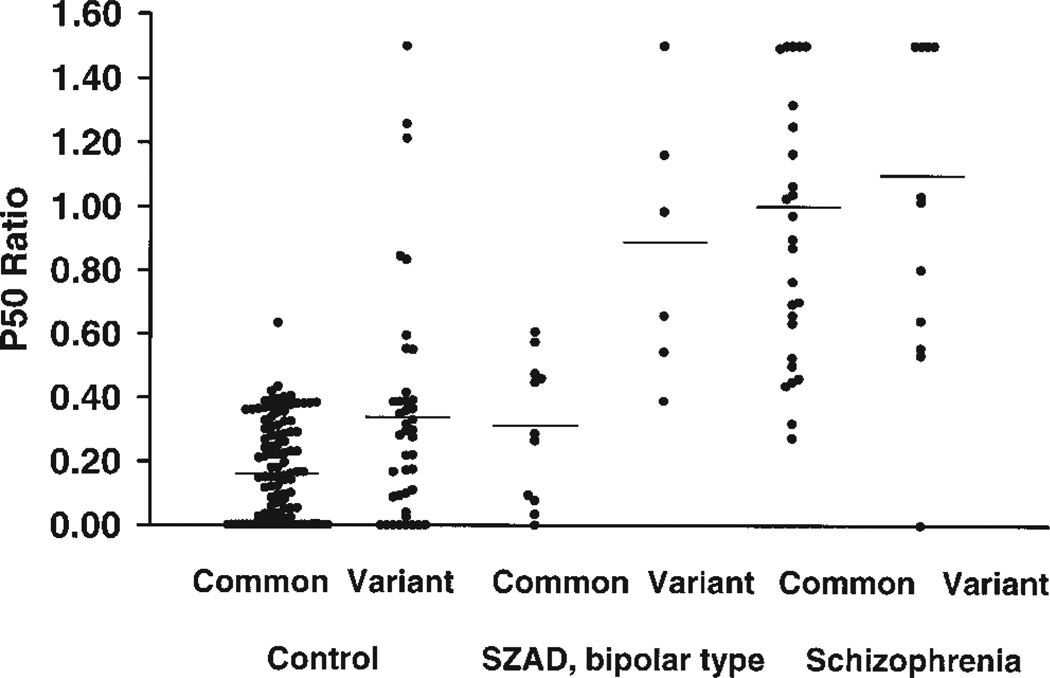
Subjects with schizophrenia and with a variant allele had a mean P50 ratio indistinguishable from those subjects with schizophrenia and with the common alleles (Table I; Fig. 1; es 0.16). In contrast, subjects with schizoaffective disorder, bipolar type who carry a variant allele had a significantly larger mean P50 ratio than those subjects with schizoaffective disorder and with the common alleles (es 2.01).Control subjects with a variant allele had a higher mean P50 ratio when compared to those control subjects with the common alleles (es 0.71).
TABLE I.
Physiologic Variables
| Subject group | Conditioning wave latency |
Conditioning wave amplitude |
Test wave amplitude |
P50 ratio |
|---|---|---|---|---|
| Control, common allele N = 108 | 62.0 ± 6.8 (42.0–83.0) | 3.1 ± 1.7 (0.3–9.1) | 0.6 ± 0.7 (0.0–4.0) | 0.17 ± 0.15a (0.00–0.63) |
| Control, variant allele N = 41 | 63.1 ± 7.5 (46.0–78.0) | 2.8 ± 1.3 (0.7–5.5) | 0.8 ± 0.7 (0.0–2.0) | 0.34 ± 0.39 (0.00–1.91) |
| SZAD, bipolar common allele N = 11 | 63.1 ± 8.5 (47.0–75.0) | 1.9 ± 0.5 (1.2–2.7) | 0.6 ± 0.5b (0.0–2.0) | 0.30 ± 0.22c (0.00–0.61) |
| SZAD, bipolar variant allele N = 6 | 64.5 ± 11.2 (57.0–87.0) | 2.7 ± 1.8 (0.8–5.7) | 1.9 ± 0.7 (1.0–3.0) | 0.88 ± 0.43 (0.39–1.53) |
| Schizophrenia common allele N = 26 | 62.5 ± 7.7 (45.0–81.0) | 2.1 ± 0.9 (0.7–4.2) | 1.8 ± 0.9 (1.0–4.0) | 1.01 ± 0.63 (0.27–2.75) |
| Schizophrenia variant allele N = 11 | 62.7 ± 10.0 (38.0–72.0) | 2.2 ± 1.5 (0.8–5.6) | 2.1 ± 1.5 (0.0–5.0) | 1.11 ± 0.72 (0.00–2.31) |
Data are presented as mean ± standard deviation, range.
SZAD, schizoaffective.
common allele group P50 ratio < variant allele group (t44.7 = −2.69, P = 0.01).
common allele group test wave amplitude < variant allele group (t6.5 = −3.07, P = 0.02).
common allele group P50 ratio < variant allele group (t15 = −3.69, P = 0.002).
Fig. 1.
Individual values (circles) and means (lines) for the P50 ratio. Control subjects with a variant allele had a significantly higher mean P50 ratio than control subjects with the common alleles. Likewise, schizoaffective, bipolar type subjects with a variant allele had a higher mean P50 ratio than those subjects with the common alleles. SZAD = Schizoaffective, bipolar type; Common = common CHRNA7 promoter region alleles; Variant = variant CHRNA7 promoter region alleles.
Additional P50 wave characteristics including conditioning wave latency, conditioning wave amplitude and test wave amplitude did not differ between those subjects with schizophrenia and with a variant or common allele (Table I; es 0.02, 0.09, and 0.28, respectively). Subjects with schizoaffective disorder and a variant allele had a significantly smaller mean test wave amplitude than those subjects with schizoaffective disorder and with the common alleles (es 2.41). There were no differences in conditioning wave amplitude or latency (es 0.16, 0.76 respectively). The mean test wave amplitude in the control subjects with a variant allele trended toward being larger than the test wave amplitude in control subjects with the common alleles (es 0.29). There were no differences in conditioning wave latency or amplitude between these two normal groups (es 0.14 and 0.19, respectively).
Clinical symptoms on the day of testing within the schizoaffective, bipolar type group were not correlated with any of the electrophysiological measures. Furthermore, they did not differ between those subjects with variant or common CHRNA7 promoter region alleles. The number of subjects taking a mood stabilizer, antidepressant or antipsychotic medication also did not differ between those subjects with the variant or common alleles. Finally, within the normal, schizoaffective and schizophrenic groups, smoking status was no different between these subjects with the variant or common alleles.
DISCUSSION
Persons with schizoaffective disorder, bipolar type and allelic variants in the CHRNA7 promoter region have an abnormally high mean P50 ratio, similar to persons with schizophrenia. Those subjects with the common CHRNA7 promoter alleles have a normal mean P50 ratio, similar to controls. This genetic finding sheds some light on the difficulties inherent in biological studies of schizoaffective disorder. Despite the ascertainment of one specific type of schizoaffective disorder (DSM-IV bipolar type), an initial biological measurement resulted in an intermediately impaired group [Olincy and Martin, 2005]. The current study, however, was able to parse out this still heterogeneous group into two distinct subgroups based on allelic variants in the alpha-7 nicotinic receptor gene promoter region. The impaired mean P50 ratio in the schizoaffective group with the variant alleles was driven by a significantly larger mean test wave amplitude, consistent with impaired inhibitory processing.
Houy et al. [2004] replicated the finding that persons with schizophrenia have a larger mean P50 ratio than healthy controls, regardless of genetic makeup. They next described the finding of an association between the −194 G→C variant allele of the CHRNA7 promoter region and the P50 ratio. In contrast to the previously reported impairment of P50 ratios in both persons with and without schizophrenia associated with allelic variants in the promoter region of this gene [Leonard et al., 2002], Houy et al. reported a protective effect of the C variant of this allele for a mixed group of control and schizophrenic subjects. However, there were fewer individuals with schizophrenia in this variant group (4 of 20 individuals) than in the G, or normal genotype, group (107 of 176). Given both our current and their finding that persons with schizophrenia, regardless of genotype, have a mean impaired P50 ratio, and that control subjects, regardless of genotype, have a normal mean P50 ratio, the Houy et al. analysis (variant versus normal allele carriers) is confounded. It may also be that the −194 variant allele is not a particularly pathogenic allele and that it is actually in linkage disequilibrium with another allele that has a different distribution among populations. Although the distribution of this allelic variant is different between the American and French groups, the present study along with the Leonard et al. study and the Houy et al. study find association of P50 ratio and schizophrenia with the CHRNA7 promoter region.
The small number of individuals with schizoaffective disorder in this study may have limited the conclusions that we can draw from our secondary measures. The schizoaffective, bipolar type group trended toward having smaller mean conditioning wave and test wave amplitudes than the control groups. The medium effect sizes for each of these analyses indicate that a larger N may have resulted in a significant finding that further supports similarity in neurophysiology between persons with schizoaffective disorder and schizophrenia. This might also explain why there was a failure to find a difference in the distribution of variant alleles in these groups. However, the number of subjects studied was sufficient to answer our primary hypothesis concerning sensory gating. Further study of this subgroup is warranted as they may have a type of illness genetically and biologically more similar to people with schizophrenia.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Veterans Affairs Medical Research Service and MH 38321. The authors would like to thank Jamey Ellis for his technical assistance in creating Supplementary Figure 1.
Footnotes
This article contains supplementary material, which may be viewed at the American Journal of Medical Genetics website at http://www.interscience.wiley.com/jpages/1552-4841/suppmat/index.html.
REFERENCES
- Bailer U, Leisch F, Meszaros K, Lenzinger E, Willinger U, Strobl R, Heiden A, Gebhardt C, Döge E, Fuchs K, et al. Genome scan for susceptibility loci for schizophrenia and bipolar disorder. Biol Psychiatry. 2002;52:40–52. doi: 10.1016/s0006-3223(02)01320-3. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory of measuring depression. Arch Gen Psychiatry. 1961;4:563–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Curtis L, Blouin J-L, Radhakrishna U, Gehrig C, Lasseter VK, Wolyniec P, Nestadt G, Dombroski B, Kazazian HH, Pulver AE, et al. No evidence for linkage between schizophrenia and markers at chromosome 15q13-14. Am J Med Genet. 1999;88:109–112. doi: 10.1002/(sici)1096-8628(19990416)88:2<109::aid-ajmg1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- De Luca V, Wang H, Squassina A, Wong GW, Yeomans J, Kennedy JL. Linkage of M5 muscarinic and alpha7-nicotinic receptor genes on 15q13 to schizophrenia. Neuropsychobiology. 2004;50:124–127. doi: 10.1159/000079102. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Foroud T, Conneally PM, Sorbel JJ, Carr K, Crose C, Willig C, Zhao J, Miller M, Bowman E, et al. Initial genomic scan of the NIMH genetics initiative bipolar pedigrees: Chromosomes 3,5,15,16,17, and 22. Am J Med Genet (Neuropsychiatr Genet) 1997;74:238–246. [PubMed] [Google Scholar]
- Faraone SV, Glatt SJ, Su J, Tsuang MT. Three potential susceptibility loci shown by a genome-wide scan for regions influencing the age at onset of mania. Am J Psychiatry. 2004;161:625–630. doi: 10.1176/appi.ajp.161.4.625. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders- non-patient edition (SCID-I/NP, Version 2.0) New York City: NYSPI/Biometrics Research Department; 1996. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders-patient edition. New York: New York State Psychiatric Institute, Biometrics Research Department; 1997. [Google Scholar]
- Gault J, Hopkins J, Berger R, Drebing C, Logel J, Walton K, Short M, Vianzon R, Olincy A, Ross RG, et al. Comparison of polymorphisms in the alpha7 nicotinic receptor gene and its partial duplication in schizophrenic and control subjects. Am J Med Genet Part B. 2003;123B:39–49. doi: 10.1002/ajmg.b.20061. [DOI] [PubMed] [Google Scholar]
- Gejman PV, Sanders AR, Badner JA, Cao Q, Zhang J. Linkage Analysis of Schizophrenia to Chromosome 15. Am J Med Genet. 2001;105:789–793. doi: 10.1002/ajmg.1552. [DOI] [PubMed] [Google Scholar]
- Hamshere ML, Bennett P, Williams N, Segurado R, Cardno A, Norton N, Lambert D, Williams H, Kirov G, Corvin A, et al. Genomewide linkage scan in schizoaffective disorder. Arch Gen Psychiatry. 2005;62:1081–1088. doi: 10.1001/archpsyc.62.10.1081. [DOI] [PubMed] [Google Scholar]
- Houy E, Raux G, Thibaut F, Belmont A, Demily C, Allio G, Haouzir S, Fouldrin G, Petit M, Frebourg T, et al. The promoter −194 C polymorphism of the nicotinic alpha 7 receptor gene has a protective effect against the P50 sensory gating deficit. Mol Psychiatry. 2004;9:320–322. doi: 10.1038/sj.mp.4001443. [DOI] [PubMed] [Google Scholar]
- Kaufmann CA, Suarez B, Malaspina D, Pepple J, Svrakic DM, Markel PD, Meyer J, Zambuto CT, Schmitt K, Matise TC, et al. NIMH genetics initiative millenium schizophrenia consortium: Linkage analysis of African-American pedigrees. Am J Med Genet. 1998;81:282–289. [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Leonard S, Gault J, Hopkins J, Logel J, Vianzon R, Short M, Drebing C, Berger R, Venn D, Sirota P, et al. Association of promoter variants in the alpha-7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch Gen Psychiatry. 2002;59:1085–1096. doi: 10.1001/archpsyc.59.12.1085. [DOI] [PubMed] [Google Scholar]
- Liu C-M, Hwu H-G, Lin M-W, Ou-Yang W-C, Lee SF-C, Fann CSJ, Wong S-H, Hsieh S-H. Suggestive evidence for linkage of schizophrenia to markers at chromosome 15q13-14 in Taiwanese families. Am J Med Genet. 2001;105:658–661. doi: 10.1002/ajmg.1547. [DOI] [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedures for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves-Pereira M, Bassett AS, Honer WG, Lang D, King NA, Kennedy JL. No evidence for linkage of the CHRNA7 gene region in Canadian schizophrenia families. Am J Med Genet. 1998;81:361–363. doi: 10.1002/(sici)1096-8628(19980907)81:5<361::aid-ajmg3>3.0.co;2-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olincy A, Martin L. Diminished suppression of the P50 auditory evoked potential in bipolar subjects with a history of psychosis. Am J Psychiatry. 2005;162:43–49. doi: 10.1176/appi.ajp.162.1.43. [DOI] [PubMed] [Google Scholar]
- Riley BP, Makoff A, Mogudi-Carter M, Jenkins T, Williamson R, Collier D, Murray R. Haplotype transmission disequilibrium and evidence for linkage of the CHRNA7 gene region to schizophrenia in Southern African Bantu families. Am J Med Genet. 2000;96:196–201. doi: 10.1002/(sici)1096-8628(20000403)96:2<196::aid-ajmg15>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Siegel C, Waldo MC, Mizner G, Adler LE, Freedman R. Deficits in Sensory Gating in Schizophrenic Patients and their Relatives. Arch Gen Psychiatry. 1984;41:607–612. doi: 10.1001/archpsyc.1984.01790170081009. [DOI] [PubMed] [Google Scholar]
- Stassen HH, Bridler R, Hägele S, Hergersberg M, Mehmann B, Schinzel A, Weisbrod M, Scharfetter C. Schizophrenia and smoking: Evidence for a common neurobiological basis? Am J Med Genet. 2000;96:173–177. [PubMed] [Google Scholar]
- Stöber G, Saar K, Rüschendorf F, Meyer J, Nürnberg G, Jatzke S, Franzek E, Reis A, Lesch K-P, Wienker TF, et al. Splitting schizophrenia: Periodic catatonia-susceptibility locus on chromosome 15q15. Am J Hum Genet. 2000;67:1201–1207. doi: 10.1016/s0002-9297(07)62950-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang DW, Skol AD, Faraone SV, Bingham S, Young KA, Prabhudesai S, Haverstock SL, Mena F, Menon AS, Bisset D, et al. Examination of genetic linkage of chromosome 15 to schizophrenia in a large veterans affairs cooperative study sample. Am J Med Genet. 2001;105:662–668. [PubMed] [Google Scholar]
- Turecki G, Grof P, Grof E, D’Souza V, Lebuis L, Marineau C, Cavazzoni P, Duffy A, Bétard C, Zvolský P, et al. Mapping susceptibility genes for bipolar disorder: A pharmacogenetic approach based on excellent response to lithium. Mol Psychiatry. 2001;6:570–578. doi: 10.1038/sj.mp.4000888. [DOI] [PubMed] [Google Scholar]
- Xu J, Pato MT, Dalla Torre C, Medeiros H, Carvalho C, Basile VS, Bauer A, Dourado A, Valente J, Soares MJ, et al. Evidence of linkage disequilibrium between the alpha 7-nicotinic receptor gene (CHRNA7) locus, and schizophrenia in azorean families. Am J Med Genet. 2001;105:669–674. doi: 10.1002/ajmg.1549. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale of mania: Reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.