Abstract
Cells respond to extracellular cues through a variety of receptors on the surface. These signals once transduced across the cell membrane, activate protein tyrosine kinases, which through phosphorylation of substrates on key tyrosine residues, are able to control cellular growth, activation and differentiation pathways. Recent data suggest that protein tyrosine kinases are critical in integrating signals from various cellular receptors, including pathogen detection receptors that mediate the host innate immune response. In this article, we have reviewed the roles of tyrosine kinases of the Tec, FAK, Fps, Fer, Syk, Src and TAM-receptor families in toll-like receptor signaling. The shared roles of these tyrosine phosphorylation mediators in host defense, inflammation, autoimmune disease and oncogenesis provides promising avenues for the use of their inhibitors in multiple disorders.
Keywords: Toll-like receptors, tyrosine kinases, innate immunity, phosphorylation, cytokine release
Introduction
Pathogen detection by toll-like receptors (TLRs) is crucial for the initiation and regulation of innate immune responses. Engagement of these receptors signals the activation of transcription factors, such as NFκB, via activation of canonical IκB kinases (IKKs) and IKK-related kinases (ex. TANK- binding kinase 1, TBK1 and IKKi) (reviewed in [1, 2]). This leads to the induction of molecules, such as TNFα, and IL6, that are required for pathogen clearance. Significantly, these molecules are also implicated in cell survival, proliferation and angiogenesis signaling pathways. Inflammatory events can promote tumorigenesis. NFκB activation is a hallmark of most cancers.
Protein tyrosine kinases (PTKs) are compelling targets in human cancer. These enzymes regulate multiple cellular processes that contribute to tumor development and progression; such as cell growth, differentiation, migration and apoptosis. Roles for multiple PTKs in early innate immune signaling have recently emerged. This article reviews our current knowledge of regulation of TLR signaling by PTKs. Many of these are targets for therapeutic intervention of immune disorders.
A role for PTKs in early innate immune response was first suggested by the observation that they are required for the induction of pro-inflammatory cytokines IL-1β, IL-6 and TNFα in response to endotoxin in murine macrophages [3]. Conflictingly, inhibition of LPS-mediated tyrosine kinase activity with broad spectrum PTK inhibitor, genistein, failed to inhibit LPS-mediated NFκB translocation [4]. In the past three years, roles for multiple PTKs have emerged in TLR signaling (summarized in Table 1).
Table 1. Tyrosine Kinases Implicated in TLR Signaling. Tyrosine Kinases, their Substrates, Binding Partners, Relevant Cell Types and Activities in TLR Signaling.
| Kinase | Substrate(s) | Binding Partners | Reported Expression/Activity in Cell Type | Regulation/Function | TLR Signaling | Reference |
|---|---|---|---|---|---|---|
| Btk | Mal | TLR4, MyD88, Mal | Mouse macrophages, myeloid cells | NO, IL1β, TNFα | TLR2, TLR4 | [5-11] |
| Bmx | Macrophages and rheumatoid arthritis synovial cells | IL6 | TLR4 | [12,13] | ||
| Etk | FAK, MyD88, Mal | Epithelial, endothelial cells and fibroblasts | IL6 | TLR4 | [14, 15] | |
| FAK | Etk | Etk, | Human monocytes, epithelial cells, endothelial cells, fibroblasts | IL6, IL8 | TLR2, TLR4 | [16-20] |
| Pyk2 | Paxillin | Paxillin, vinculin, FAK | Human T cells | TLR2, TLR4 | [21, 23] | |
| Fps | TLR4 internalization, leucocyte recruitment. neutrophil rolling, adhesion and extravasation, TNFα | TLR4 | [24, 27, 28] | |||
| Syk | TLR4 | TLR4, 9 Dectin-1 | Macrophages, dendritic cells | macropinocytosis and lipid accumulation, IL12p70 IL10 IL2 |
TLRs 2, 4, 5, 7, 9, Dectin-1 | [29, 30, 34-37] |
| Lyn | TLR4 | B cells | IL6, IL12 SLE |
TLR4 | [41, 42, 44] [51] |
|
| c-Src, Fyn, Yes | Zonal adherens proteins | endothelial paracellular permeability | TLR4 | [46] | ||
| c-Src | TLR3 | Dendritic cells | IRF-3 and STAT-1 | TLR3 | [47] | |
| Csk | Mouse macrophages | IL6, TNFα | TLR4 | [54] | ||
| Tyro3, Axl and Mer | Broad spectrum autoimmune disease. Cytokine release Type I IFNR | TLR4 | [55, 56, 58-60] | |||
| SHP-1 | Kinase domain of IRAK-1 | Inflammatory cytokines and type I IFN | [61] | |||
| SHP-2 | TBK-1 | Inflammatory cytokines and IFNβ | TLR3 | [62] |
Tec Tyrosine Kinases
A role for bruton's tyrosine kinase (Btk), the prototypical member of the tec family of tyrosine kinases, in LPS signaling was first suggested by studies from xid mice that lack a functional Btk [5]. Macrophages from xid mice show poor nitric oxide induction and reduced production of inflammatory cytokines IL1β and TNFα in response to LPS challenge. LPS induces tyrosine phosphorylation of Btk, presumably via Src family kinases [6], and activates its tyrosine kinase activity. Btk interacts with TIR domains of many TLRs including TLR4 [7], and adaptor proteins MyD88 and Mal [8, 9]. Upon TLR2 and TLR4 stimulation, Mal is phosphorylated by Btk [9] and then interacts with SOCS-1, which results in Mal polyubiquitination and subsequent degradation [10]. Deficient Btk function selectively impairs dendritic cell cytokine induction in response to viral single-stranded RNAs that activate TLR8 [11].
A second tec family member, Bmx, is required for TLR4 induced IL6 production in macrophages and rheumatoid arthritis synovial cells, via mRNA stabilization [12, 13]. A third tec family kinase member, Etk, is present in epithelial and endothelial cells and fibroblasts. Etk activation is regulated by focal adhesion kinase (FAK) through interaction between the PH domain of Etk and the FERM domain of FAK [14]. Etk is implicated in crosstalk between integrin α5β1/FAK and MyD88 pathways in fibroblast-like synoviocytes and plays a role in IL-6 synthesis [15]. In coimmunoprecipitation experiments, Etk is associated with MyD88, FAK and Mal, and Etk phosphorylation is dependent on FAK kinase activity.
Focal Adhesion Kinases
FAK can trigger inflammatory responses [16]. Protein I/II, a cell wall component from oral streptococci, upon binding to integrin α5β1, induces inflammatory mediators such as IL-6 and IL-8 in human monocytes, epithelial cells, endothelial cells and fibroblasts [16,17], and the signaling leading to cytokine release involves FAK. FAK is also involved in invasin-mediated bacterial uptake [18]. There is considerable cross-talk between integrin/FAK and TLR pathways; LPS induces FAK phosphorylation in a murine monocytic cell line [19]. TLR2 ligand Pam3CSK4 also induces FAK phosphorylation at Tyr397, and requires FAK for cytokine release [20]. TLR2 and TLR4 agonists also induce tyrosine phosphorylation of the proline-rich tyrosine kinase 2 (Pyk2), which in turn increases tyrosine phosphorylation of the adaptor protein paxillin. Paxillin is involved in binding to Pyk2, vinculin and FAK through MyD88-dependent and independent pathways [21]. MyD88 is however not essential for FAK autophosphorylation, as it is phosphorylated in MyD88-/- macrophages that were activated with LPS or proteinI/II. MyD88 and FAK, but not TLRs 2, 4 or 6 are involved in cytokine release mediated by proteinI/II. Furthermore, LPS-induced cytokine release also depends on presence of both FAK and MyD88, suggesting that FAK may play a general role in proinflammatory cytokine release [20].
Pyk2 acts as a molecular switch to overcome suppression of leukocyte oxidant generation by cell adhesion [22]. Prior exposures of neutrophils to cytokines and inflammatory mediators (e.g. TNF-α, GM-CSF), overcomes the adhesion-mediated suppression of reactive oxygen species formation. LPS can induce human T cells to adhere to fibronectin via TLR4 signaling, and the human T cell response to LPS depends on protein kinase C and involves the phosphorylation of Pyk2 within 10 min [23] of challenge.
Fps/Fes and Fer
Knockout mouse models have provided evidence for the involvement of tyrosine kinase Fps [24] and Fer [25] in the innate immune response. Fps/Fes knockout mice are hypersensitive to systemic LPS challenge. Fer-deficient mice display increased leukocyte recruitment at sites of localized LPS challenge, and enhanced intestinal barrier dysfunction in response to LPS [26]. Fps null mice display increased mortality in response to intraperitoneal challenge with LPS. There is an increase in circulating TNFα levels in LPS-challenged Fps-null mice, which show an enhanced NFκB signaling response. These mice have a defective down-regulation of TLR4, which correlates with a general role for Fps in internalization [27]. A role for Fps has also been determined in leukocyte recruitment to areas of inflammation [28]; and fps null mice display increased neutrophil rolling, adhesion and extravasation subsequent to LPS challenge.
Spleen Tyrosine Kinase (Syk)
Tyrosine kinase Syk plays a role in TLR4 dependent macropinocytosis and lipid accumulation in macrophages [29]. Minimally modified LDL induces Syk association with TLR4, as well as Syk phosphorylation. Syk-deficient dendritic cells (DCs) show increased inflammatory cytokine production, maturation and antigen presentation [30]. Immunoreceptor tyrosine-based activation motif (ITAM) containing adaptors, DAP12 and FcεRIγ-chain (FcRγ), are both required for negative regulation of TLR responses in bone marrow derived DC's [30], and DAP12 negatively regulates TLR signaling in macrophages [31, 32] and plasmacytoid DC's [33].
Syk is implicated in direct modulation of multiple TLR responses. Syk associates with TLRs 4 [34] and 9 [35]. Stimulation of TLR9 with CpG, for example, induces IL12p70 production, that is dependent on Syk [36]. Similarly, stimulation of TLR4 with LPS induces release of IL-10 and IL12-p70, which is dependent on Syk [34]. More significantly, emerging data implicates a role for Syk in collaborative TLR signaling. The fungal β-glucan receptor, Dectin-1, a C-type lectin, binds to yeast and signals through the kinase Syk and the adaptor CARD9 to induce production of IL-10 and IL-2 in DCs [36]. The interaction between Syk and Dectin-1 occurs through a novel mechanism [37] and Syk-/- DCs do not make IL-10 or IL-2 upon yeast stimulation but produce IL-12, indicating that the Dectin-1/Syk and Dectin-1/TLR2 pathways can operate independently. Dectin-1 induced Syk activation is essential for the production of pro-inflammatory cytokines and the release of reactive oxygen by Mycobacterium abscessus infected macrophages. M. abscessus activation also requires a physical interaction between TLR2 and dectin-1 [38]. Collaborative cytokine production (TNFα, MIP-1α and MIP-2) induced through dectin-1 and TLRs 2, 4, 5, 7 and 9 [39] requires Syk. Deficiency of either Syk or MyD88 abolishes collaborative responses. In neutrophils, an adhesion receptor (CEACAM3), acts to capture and engulf invasive bacteria. Bacterial binding to CEACAM3 also causes recruitment of Syk, and its phosphorylation [40].
Src Family Kinases (SFK)
A role for PTKs in LPS signaling was first established when it was reported that myeloid cells stimulated with LPS showed enhanced expression of Src kinases Hck and Lyn [41, 42]. Src kinases were more directly implicated in LPS signaling to TNFα and iNOS in murine macrophages, based on effects seen with Src inhibitor PP1 [43]. Endotoxin tolerant cells show suppressed LPS-mediated TLR4 tyrosine phosphorylation, and recruitment of Lyn kinase to TLR4. However, their importance in LPS signaling in macrophages has been questioned by the studies from Hck-/-Fgr-/-Lyn-/- mice, which show no major impairment in LPS-mediated activation [44].
A systemic inhibition of Src kinases can attenuate LPS-induced lung injury, suggesting a role for SFK's in acute lung injury [45]. Src inhibitors, PP2, SU6656 and tyrphostin A1, block LPS-dependent cytokine and chemokine production in the lung and in serum. In human lung microvascular endothelial cells, LPS recognition by TLR4 activates c-Src, Fyn and Yes, which contribute to tyrosine phosphorylation of zonula adherens proteins and enhance endothelial paracellular permeability [46]. These three SFKs play redundant but not totally compensatory roles in LPS-induced barrier dysfunction. c-Src is also activated by dsRNA in human monocyte derived dendritic cells, and is recruited to TLR3 in a dsRNA dependent manner [47]. Src kinase-deficient cells show no activation of dsRNA induced IRF3 and STAT1, suggesting a critical role in antiviral immunity. Additionally, PP2 inhibits TNF production upon engagement of TLRs 1, 2, 4, 5 and 6; and marginally to TLR7/8 indicating SFKs may be common regulators of many TLR signaling in primary human monocyte derived macrophages [48].
SFKs play a key role in autoimmunity. Lyn-deficient mice develop systemic lupus erythematosus (SLE) [49, 50]. Additionally, lower levels of Lyn have been discovered in B cells of human SLE patients [51]. A recent correlation suggests that autoimmune disease in Lyn-deficient mice is MyD88-dependent [52]. TLR signaling plays an essential role in the development of pathogenic autoantibodies and kidney disease in Lyn-/- mice. The autoimmune phenotyes are completely eliminated in MyD88-/- Lyn-/- mice. A role for TLR signaling in the development of autoimmune diseases is still in its early stages. Notably, a single nucleotide polymorphism in TLR5 has been associated with SLE resistance [53].
C-terminal src kinase (Csk), a negative regulator of Src kinases, also plays a role in LPS response in mouse macrophages. Csk knockdown cells show reduced protein expression levels of tyrosine kinase Fgr, and reduced secretion of cytokines IL6 and TNFα [54].
Tyro, Axl and Mer (TAM) Receptor Tyrosine Kinases
Mer receptor tyrosine kinase is involved in apoptotic cell clearance, by mediating an engulfment pathway in concert with αvβ5 integrin. It also acts as a negative regulator for inflammation by down-modulating pro-inflammatory signals (IL1β, TNFα and IL-12) mediated from LPS-TLR4 signaling [55, 56]. Significantly, the trans-inhibition of NFκB activation by Mer occurs independent of cytosolic IκB phosphorylation and p65/RelA sequesteration [57]. Mice that express mutant Mer (MerKD), when backcrossed into the C57/bl/6 background develop an age-dependent autoimmune disease characterized by the loss of ability to ingest apoptotic cells [58].
Loss of function of three TAM receptors, Tyro3, Axl and Mer, is associated with dysregulation of the immune response [59]. Additionally, Tyro3-/-Axl-/-Mer-/- triple mutant mice display broad spectrum autoimmune disease. Mer-/- single mutants are hypersensitive to LPS-induced endotoxic shock [56]. Tyro3, Axl and Mer broadly inhibit both TLR and TLR-induced cytokine receptor cascades [60]. TAM inhibition of inflammation is transduced through the type I interferon (IFN) receptor and its associated transcription factor STAT1, and this pathway controls the phased attenuation of antigen presenting cell activation.
Tyrosine Phosphatases
SHP-1 (A002156) and SHP-2 (A002157) are intracellular protein tyrosine phosphatases that contain two tandemly linked Src homology 2 domains at their amino termini, followed by a catalytic domain. Recently, SHP-1 was shown to differentially regulate the production of proinflammatory cytokines and type I IFN [61]. SHP-1 inhibits TLR-mediated production of cytokines by suppressing the activation of MAPs and NFκB, but it increased TLR and RIG-1 activated type I IFN by directly binding to the kinase domain of IRAK1 and inhibiting its activity. Significantly, the phosphatase activity of SHP-1 is not required for inhibition of IRAK1 activation. This study suggests role for SHP1 in immune homeostasis. Its relative SHP2 more narrowly regulates TLR3 induced inflammatory cytokines and IFNβ, without affecting TLR2 or 9 signaling. SHP2 targets TBK-1, the kinase that phosphorylates IRF3 [62].
Tyrosine Kinase Inhibitors in Autoimmune Disease
Drugs targeting kinases e.g. Lck and Syk are currently undergoing clinical trials for the treatment of diseases related to inflammation and autoimmunity (reviewed in [63]). In human peripheral blood macrophages and monocytes, imatinib, a small molecule PTK inhibitor, inhibits LPS –induced production of TNFα through a c-Fms independent mechanism. TNFα is a driver of autoimmune tissue injury in a spectrum of autoimmune diseases including rheumatoid arthritis, Crohn disease, psoriasis and multiple sclerosis [64]. Imatinib can inhibit a narrow spectrum of PTKs (Abl, c-Fms, c-Kit, PDGFR α̃β and Lck) at submicromolar concentrations [65]. This ability of Imatinib and other PTK inhibitors to inhibit multiple kinases, could actually be beneficial for treatment of autoimmune diseases, where multiple unmutated kinases (unlike mutations associated with malignancy) play a role.
Summary
Emerging data indicate that PTKs integrate signals from various receptors to TLRs, thereby defining critical regulatory mechanisms based on recognition of multiple ligands in tandem (see Fig. (1)). The Tec family kinases have been studied in some detail, and not only play direct roles in transduction of TLR signals via interactions with TIR domains; but like the FAK family, interface signals from adhesion receptors such as the integrins to TLRs. Tyrosine kinase Syk integrates collaborative signals between fungal receptors and multiple TLRs. SFKs play a role in tolerance, membrane permeability, antiviral immunity, and autoimmunity. TAM receptor tyrosine kinases feed in regulatory signals from adjacent apoptotic cells, playing a critical role in control of autoimmune disorders. In summary, tyrosine mediators of innate immunity are key regulators of an uncontrolled immune response as they interface signals from multiple cellular receptors.
Fig. (1).
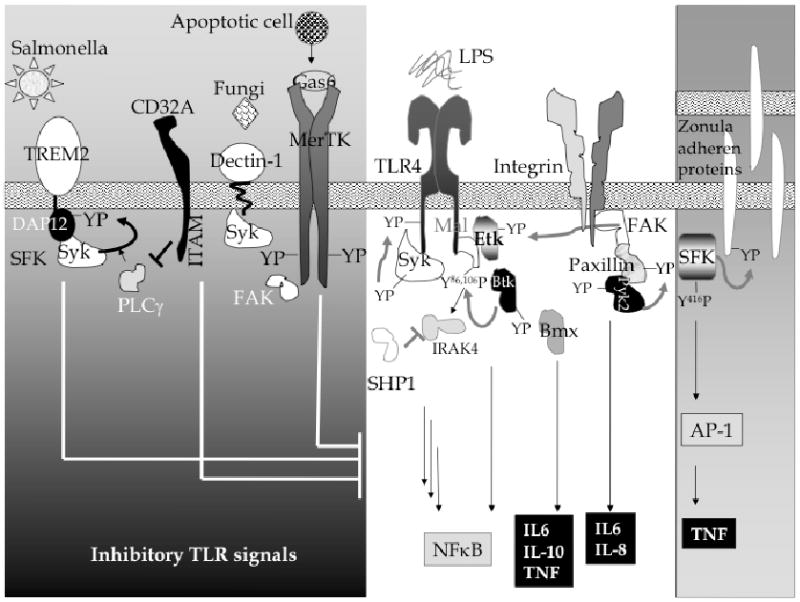
Tyrosine phosphorylation plays a role in regulation of the innate immune response through TLRs. Signals from TLR-mediated PTK activation couple with signals from recognition of other stimuli, such as the detection of fungi, apoptotic cells (grey box on the left) to determine the net host response. YP represents tyrosine phosphorylation.
Acknowledgments
The authors thank Dr. William S. Hlavacek for useful discussions and critical reading of the manuscript. Dr. Chaudhary was supported in part by a grant from the Defense Threat Reduction Agency and the LANL Laboratory Office of Directed Research and Development. Ms. Nag was supported by NIH RO1 grant GM076570 to WSH.
References
- 1.Clement JF, Meloche S, Servant MJ. The IKK-related kinases: from innate immunity to oncogenesis. Cell Res. 2008 Aug 12; doi: 10.1038/cr.2008.273. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 2.Karin M. The IkappaB kinase - a bridge between inflammation and cancer. Cell Res. 2008;18:334–42. doi: 10.1038/cr.2008.30. [DOI] [PubMed] [Google Scholar]
- 3.Geng Y, Zhang B, Lotz M. Protein tyrosine kinase activation is required for lipopolysaccharide induction of cytokines in human blood monocytes. J Immunol. 1993;151:6692–700. [PubMed] [Google Scholar]
- 4.Delude RL, Fenton MJ, Savedra R, Jr, et al. CD14-mediated translocation of nuclear factor-kappa B induced by lipopolysaccharide does not require tyrosine kinase activity. J Biol Chem. 1994;269:22253–60. [PubMed] [Google Scholar]
- 5.Mukhopadhyay S, Mohanty M, Mangla A, et al. Macrophage effector functions controlled by Bruton's tyrosine kinase are more crucial than the cytokine balance of T cell responses for microfilarial clearance. J Immunol. 2002;168:2914–21. doi: 10.4049/jimmunol.168.6.2914. [DOI] [PubMed] [Google Scholar]
- 6.Rawlings DJ, Scharenberg AM, Park H, et al. Activation of BTK by a phosphorylation mechanism initiated by SRC family kinases. Science. 1996;271:822–5. doi: 10.1126/science.271.5250.822. [DOI] [PubMed] [Google Scholar]
- 7.Jefferies CA, Doyle S, Brunner C, et al. Bruton's tyrosine kinase is a Toll/interleukin-1 receptor domain-binding protein that participates in nuclear factor kappaB activation by Toll-like receptor 4. J Biol Chem. 2003;278:26258–64. doi: 10.1074/jbc.M301484200. [DOI] [PubMed] [Google Scholar]
- 8.Jefferies CA, O'Neill LA. Bruton's tyrosine kinase (Btk)-the critical tyrosine kinase in LPS signalling? Immunol Lett. 2004;92:15–22. doi: 10.1016/j.imlet.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Gray P, Dunne A, Brikos C, Jefferies CA, Doyle SL, O'Neill LA. MyD88 adapter-like (Mal) is phosphorylated by Bruton's tyrosine kinase during TLR2 and TLR4 signal transduction. J Biol Chem. 2006;281:10489–95. doi: 10.1074/jbc.M508892200. [DOI] [PubMed] [Google Scholar]
- 10.Mansell A, Smith R, Doyle SL, et al. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat Immunol. 2006;7(2):148–55. doi: 10.1038/ni1299. [DOI] [PubMed] [Google Scholar]
- 11.Sochorová K, Horváth R, Rozková D, et al. Impaired Toll-like receptor 8-mediated IL-6 and TNF-alpha production in antigen-presenting cells from patients with X-linked agammaglobulinemia. Blood. 2007;109:2553–6. doi: 10.1182/blood-2006-07-037960. [DOI] [PubMed] [Google Scholar]
- 12.Palmer CD, Mutch BE, Page TH, Horwood NJ, Foxwell BM. Bmx regulates LPS- induced IL-6 and VEGF production via mRNA stability in rheumatoid synovial fibroblasts. Biochem Biophys Res Commun. 2008;370:599–602. doi: 10.1016/j.bbrc.2008.03.142. [DOI] [PubMed] [Google Scholar]
- 13.Palmer CD, Mutch BE, Workman S, McDaid JP, Horwood NJ, Foxwell BM. Bmx tyrosine kinase regulates TLR4-induced IL-6 production in human macrophages independently of p38 MAPK and NFkapp{B activity. Blood. 2008;111:1781–8. doi: 10.1182/blood-2007-07-102343. [DOI] [PubMed] [Google Scholar]
- 14.Chen R, Kim O, Li M, et al. Regulation of the PH-domain- containing tyrosine kinase Etk by focal adhesion kinase through the FERM domain. Nat Cell Biol. 2001;3:439–44. doi: 10.1038/35074500. [DOI] [PubMed] [Google Scholar]
- 15.Semaan N, Alsaleh G, Gottenberg JE, Wachsmann D, Sibilia J. Etk/BMX, a Btk family tyrosine kinase, and Mal contribute to the cross-talk between MyD88 and FAK pathways. J Immunol. 2008;180:3485–91. doi: 10.4049/jimmunol.180.5.3485. [DOI] [PubMed] [Google Scholar]
- 16.Neff L, Zeisel M, Druet V, et al. ERK 1/2- and JNKs-dependent synthesis of interleukins 6 and 8 by fibroblast-like synoviocytes stimulated with protein I/II, a modulin from oral streptococci, requires focal adhesion kinase. J Biol Chem. 2003;278:27721–8. doi: 10.1074/jbc.M212065200. [DOI] [PubMed] [Google Scholar]
- 17.Al-Okla S, Chatenay-Rivauday C, Klein JP, Wachsmann D. Involvement of alpha5beta1 integrins in interleukin 8 production induced by oral viridans streptococcal protein I/IIf in cultured endothelial cells. Cell Microbiol. 1999;1:157–68. doi: 10.1046/j.1462-5822.1999.00016.x. [DOI] [PubMed] [Google Scholar]
- 18.Alrutz MA, Isberg RR. Involvement of focal adhesion kinase in invasin-mediated uptake. Proc Natl Acad Sci USA. 1998;95:13658–63. doi: 10.1073/pnas.95.23.13658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monick MM, Powers L, Butler N, Yarovinsky T, Hunninghake GW. Interaction of matrix with integrin receptors is required for optimal LPS-induced MAP kinase activation. Am J Physiol Lung Cell Mol Physiol. 2002;283:L390–402. doi: 10.1152/ajplung.00437.2001. [DOI] [PubMed] [Google Scholar]
- 20.Zeisel MB, Druet VA, Sibilia J, Klein JP, Quesniaux V, Wachsmann D. Cross talk between MyD88 and focal adhesion kinase pathways. J Immunol. 2005;174:7393–7. doi: 10.4049/jimmunol.174.11.7393. [DOI] [PubMed] [Google Scholar]
- 21.Hazeki K, Masuda N, Funami K, et al. Toll-like receptor-mediated tyrosine phosphorylation of paxillin via MyD88-dependent and -independent pathways. Eur J Immunol. 2003;33:740–7. doi: 10.1002/eji.200323375. [DOI] [PubMed] [Google Scholar]
- 22.Zhao T, Bokoch GM. Critical role of proline-rich tyrosine kinase 2 in reversion of the adhesion-mediated suppression of reactive oxygen species generation by human neutrophils. J Immunol. 2005;174:8049–55. doi: 10.4049/jimmunol.174.12.8049. [DOI] [PubMed] [Google Scholar]
- 23.Zanin-Zhorov A, Tal-Lapidot G, Cahalon L, et al. Cutting edge: T cells respond to lipopolysaccharide innately via TLR4 signaling. J Immunol. 2007;179:41–4. doi: 10.4049/jimmunol.179.1.41. [DOI] [PubMed] [Google Scholar]
- 24.Zirngibl RA, Senis Y, Greer PA. Enhanced endotoxin sensitivity in fps/fes-null mice with minimal defects in hematopoietic homeostasis. Mol Cell Biol. 2002;22:2472–86. doi: 10.1128/MCB.22.8.2472-2486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCafferty DM, Craig AW, Senis YA, Greer PA. Absence of Fer protein-tyrosine kinase exacerbates leukocyte recruitment in response to endotoxin. J Immunol. 2002;168:4930–5. doi: 10.4049/jimmunol.168.10.4930. [DOI] [PubMed] [Google Scholar]
- 26.Qi W, Ebbert KV, Craig AW, Greer PA, McCafferty DM. Absence of Fer protein tyrosine kinase exacerbates endotoxin induced intestinal epithelial barrier dysfunction in vivo. Gut. 2005;54:1091–7. doi: 10.1136/gut.2004.061887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parsons SA, Greer PA. The Fps/Fes kinase regulates the inflammatory response to endotoxin through down-regulation of TLR4, NF-kappaB activation, and TNF-alpha secretion in macrophages. J Leukoc Biol. 2006;80:1522–8. doi: 10.1189/jlb.0506350. [DOI] [PubMed] [Google Scholar]
- 28.Parsons SA, Mewburn JD, Truesdell P, Greer PA. The Fps/Fes kinase regulates leucocyte recruitment and extravasation during inflammation. Immunology. 2007;122:542–50. doi: 10.1111/j.1365-2567.2007.02670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi S, Almazan F, Miller Y. Spleen tyrosine kinase regulates TLR4 dependent macropinocytosis and lipid accumulation in macrophages. Circulation. 2007;116:S179. [Google Scholar]
- 30.Chu CL, Yu YL, Shen KY, Lowell CA, Lanier LL, Hamerman JA. Increased TLR responses in dendritic cells lacking the ITAM-containing adapters DAP12 and FcRgamma. Eur J Immunol. 2008;38:166–73. doi: 10.1002/eji.200737600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamerman JA, Tchao NK, Lowell CA, Lanier LL. Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nat Immunol. 2005;6:579–86. doi: 10.1038/ni1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamerman JA, Jarjoura JR, Humphrey MB, Nakamura MC, Seaman WE, Lanier LL. Cutting edge: inhibition of TLR and FcR responses in macrophages by triggering receptor expressed on myeloid cells (TREM)-2 and DAP12. J Immunol. 2006;177:2051–5. doi: 10.4049/jimmunol.177.4.2051. [DOI] [PubMed] [Google Scholar]
- 33.Sjolin H, Robbins SH, Bessou G, et al. DAP12 signaling regulates plasmacytoid dendritic cell homeostasis and down-modulates their function during viral infection. J Immunol. 2006;177:2908–16. doi: 10.4049/jimmunol.177.5.2908. [DOI] [PubMed] [Google Scholar]
- 34.Chaudhary A, Fresquez TM, Naranjo MJ. Tyrosine kinase Syk associates with toll-like receptor 4 and regulates signaling in human monocytic cells. Immunol Cell Biol. 2007;85:249–56. doi: 10.1038/sj.icb7100030. [DOI] [PubMed] [Google Scholar]
- 35.Sanjuan MA, Rao N, Lai KT, et al. CpG-induced tyrosine phosphorylation occurs via a TLR9-independent mechanism and is required for cytokine secretion. J Cell Biol. 2006;172:1057–68. doi: 10.1083/jcb.200508058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LeibundGut-Landmann S, Gross O, Robinson MJ, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–8. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 37.Rogers NC, Slack EC, Edwards AD, et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. 2005;22:507–17. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Shin DM, Yang CS, Yuk JM, et al. Mycobacterium abscessus activates the macrophage innate immune response via a physical and functional interaction between TLR2 and dectin-1. Cell Microbiol. 2008 Apr;10:1608–21. doi: 10.1111/j.1462-5822.2008.01151.x. [DOI] [PubMed] [Google Scholar]
- 39.Dennehy KM, Ferwerda G, Faro-Trindade I, et al. Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur J Immunol. 2008;38:500–6. doi: 10.1002/eji.200737741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarantis H, Gray-Owen SD. The specific innate immune receptor CEACAM3 triggers neutrophil bactericidal activities via a Syk kinase-dependent pathway. Cell Microbiol. 2007;9:2167–80. doi: 10.1111/j.1462-5822.2007.00947.x. [DOI] [PubMed] [Google Scholar]
- 41.Staples KJ, Smallie T, Williams LM, et al. IL-10 induces IL-10 in primary human monocyte-derived macrophages via the transcription factor Stat3. J Immunol. 2007;178:4779–85. doi: 10.4049/jimmunol.178.8.4779. [DOI] [PubMed] [Google Scholar]
- 42.Boulet I, Ralph S, Stanley E, et al. Lipopolysaccharide- and interferon-gamma-induced expression of hck and lyn tyrosine kinases in murine bone marrow-derived macrophages. Oncogene. 1992;7:703–10. [PubMed] [Google Scholar]
- 43.Orlicek SL, Hanke JH, English BK. The src family-selective tyrosine kinase inhibitor PP1 blocks LPS and IFN-gamma-mediated TNF and iNOS production in murine macrophages. Shock. 1999;12:350–4. doi: 10.1097/00024382-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Meng F, Lowell CA. Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr, and Lyn. J Exp Med. 1997;185:1661–70. doi: 10.1084/jem.185.9.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Severgnini M, Takahashi S, Tu P, et al. Inhibition of the Src and Jak kinases protects against lipopolysaccharide-induced acute lung injury. Am J Respir Crit Care Med. 2005;171:858–67. doi: 10.1164/rccm.200407-981OC. [DOI] [PubMed] [Google Scholar]
- 46.Gong P, Angelini DJ, Yang S, et al. TLR4 signaling is coupled to SRC family kinase activation, tyrosine phosphorylation of zonula adherens proteins, and opening of the paracellular pathway in human lung microvascular endothelia. J Biol Chem. 2008;283:13437–49. doi: 10.1074/jbc.M707986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnsen IB, Nguyen TT, Ringdal M, et al. Toll-like receptor 3 associates with c-Src tyrosine kinase on endosomes to initiate antiviral signaling. EMBO J. 2006;25:3335–46. doi: 10.1038/sj.emboj.7601222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smolinska MJ, Horwood NJ, Page TH, Smallie T, Foxwell BM. Chemical inhibition of Src family kinases affects major LPS-activated pathways in primary human macrophages. Mol Immunol. 2008;45:990–1000. doi: 10.1016/j.molimm.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 49.Hibbs ML, Tarlinton DM, Armes J, et al. Multiple defects in the immune system of Lyn- deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–11. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 50.Nishizumi H, Taniuchi I, Yamanashi Y, et al. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity. 1995;3:549–60. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 51.Flores-Borja F, Kabouridis PS, Jury EC, Isenberg DA, Mageed RA. Decreased Lyn expression and translocation to lipid raft signaling domains in B lymphocytes from patients with systemic lupus erythematosus. Arthritis Rheum. 2005;52:3955–65. doi: 10.1002/art.21416. [DOI] [PubMed] [Google Scholar]
- 52.Silver KL, Crockford TL, Bouriez-Jones T, Milling S, Lambe T, Cornall RJ. MyD88- dependent autoimmune disease in Lyn-deficient mice. Eur J Immunol. 2007;37:2734–43. doi: 10.1002/eji.200737293. [DOI] [PubMed] [Google Scholar]
- 53.Hawn TR, Wu H, Grossman JM, Hahn BH, Tsao BP, Aderem A. A stop codon polymorphism of Toll-like receptor 5 is associated with resistance to systemic lupus erythematosus. Proc Natl Acad Sci USA. 2005;102:10593–7. doi: 10.1073/pnas.0501165102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aki D, Mashima R, Saeki K, Minoda Y, Yamauchi M, Yoshimura A. Modulation of TLR signalling by the C-terminal Src kinase (Csk) in macrophages. Genes Cells. 2005;10:357–68. doi: 10.1111/j.1365-2443.2005.00839.x. [DOI] [PubMed] [Google Scholar]
- 55.Sen P, Wallet MA, Yi Z, et al. Apoptotic cells induce Mer tyrosine kinase-dependent blockade of NF-kappaB activation in dendritic cells. Blood. 2007;109:653–60. doi: 10.1182/blood-2006-04-017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Camenisch TD, Koller BH, Earp HS, Matsushima GK. A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. J Immunol. 1999;162:3498–503. [PubMed] [Google Scholar]
- 57.Tibrewal N, Wu Y, D'Mello V, et al. Autophosphorylation docking site Tyr-867 in Mer receptor tyrosine kinase allows for dissociation of multiple signaling pathways for phagocytosis of apoptotic cells and down-modulation of lipopolysaccharide-inducible NF-kappaB transcriptional activation. J Biol Chem. 2008;283:3618–27. doi: 10.1074/jbc.M706906200. [DOI] [PubMed] [Google Scholar]
- 58.Cohen PL, Caricchio R, Abraham V, et al. Delayed apoptotic cell clearance and lupus- like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J Exp Med. 2002;196:135–40. doi: 10.1084/jem.20012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293:306–11. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- 60.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–36. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 61.An H, Hou J, Zhou J, et al. Phosphatase SHP-1 promotes TLR- and RIG-I-activated production of type I interferon by inhibiting the kinase IRAK1. Nat Immunol. 2008;9:542–50. doi: 10.1038/ni.1604. [DOI] [PubMed] [Google Scholar]
- 62.An H, Zhao W, Hou J, et al. SHP-2 phosphatase negatively regulates the TRIF adaptor protein-dependent type I interferon and proinflammatory cytokine production. Immunity. 2006;25:919–28. doi: 10.1016/j.immuni.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 63.Bhagwat SS. Kinase inhibitors for the treatment of inflammatory and autoimmune disorders. Purinergic Signal. 2008 Jun 21; doi: 10.1007/s11302-008-9117-z. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davidson A, Diamond B. Autoimmune diseases. N Engl J Med. 2001;345:340–50. doi: 10.1056/NEJM200108023450506. [DOI] [PubMed] [Google Scholar]
- 65.Paniagua RT, Robinson WH. Imatinib for the treatment of rheumatic diseases. Nat Clin Pract Rheumatol. 2007;3:190–1. doi: 10.1038/ncprheum0465. [DOI] [PubMed] [Google Scholar]


