Abstract
Objective
To assess the effects of a community-based exercise program on motor recovery and functional abilities of the paretic upper extremity in persons with chronic stroke.
Design
Randomized controlled trial.
Setting
Rehabilitation research laboratory and a community hall.
Participants
A sample of 63 people (≥ 50 years) with chronic deficits resulting from stroke (onset ≥ 1 year).
Interventions
The arm group underwent an exercise program designed to improve upper extremity function (1 hour per session, 3 sessions per week for 19 weeks). The leg group underwent a lower extremity exercise program.
Main outcome measures
(1) Wolf Motor Function Test (WMFT), (2) Fugl-Meyer Motor Assessment (FMA), (3) hand-held dynamometry (grip strength), and (4) Motor Activity Log.
Results
Multivariate analysis showed a significant group × time interaction (Wilk’s Lambda=0.726, P=0.017), indicating that overall, the arm group had significantly more improvement than the leg group. Post-hoc analysis demonstrated that gains in WMFT (functional ability) (P=0.001) and FMA (P=0.001) were significantly higher in the arm group. The amount of improvement was comparable to other novel treatment approaches such as constraint-induced movement therapy or robot-aided exercise training previously reported in chronic stroke. Participants with moderate arm impairment benefited more from the program.
Conclusions
The pilot study showed that a community-based exercise program can improve upper extremity function in persons with chronic stroke. This outcome justifies a larger clinical trial to further assess efficacy and cost-effectiveness.
Keywords: Cerebrovascular accident, rehabilitation, arm, exercise
Approximately 70–80% of people who sustain a stroke have upper extremity impairment.1,2 Many of them do not regain functional use of the paretic arm,2,3 which can lead to difficulties in activities of daily living (ADL) and engagement in community life. At 6 months post stroke, a substantial proportion (25–53%) of people remain dependent in at least one ADL task, which often involves the use of unilateral or bilateral arm movement.4–6
Different approaches have been used to improve upper extremity function following stroke, such as functional training,7–12 neurofacilitation techniques8,10,13,14 and strength training,9,11,15–17 and the results are mixed. The majority of these studies have a small sample size and only a few are devoted to studying persons with chronic stroke.10,15,16 While increase in strength has been reported following strength training in persons with chronic stroke, there is no indication of improvement in the functional use of the affected upper extremity.15,16 Similarly, there was no change in hand function following a 12-week program with functional training, muscle strengthening/facilitation for persons with chronic stroke.10
There is mounting evidence that both motor and functional changes in the paretic upper extremity can occur many years post stroke with forced use.18–23 Indeed, cortical reorganization has been demonstrated following intensive movement therapy in persons with chronic stroke.24 Novel approaches such as constraint-induced movement therapy (CIMT)18–23, repetitive bilateral arm training with rhythmic auditory cueing (BATRAC)25 and robot-aided exercise training26–31 have been developed to promote paretic upper extremity function following chronic stroke. However, these treatment approaches often involve one-to-one client-therapist ratio. In case of CIMT, extensive amount of daily therapy is also required.18–23 Considering the current limited health care resources, alternative rehabilitative programs are needed to reduce the long-term disability resulting from upper extremity hemiparesis.
A community-based group program may be a feasible alternative approach to upper extremity rehabilitation following a stroke. It is accessible to a large number of people in the community and does not require one-to-one supervision, thereby reducing the cost. The concept of community programs is also compatible with the emerging priority in health care policy to prevent secondary disabilities for persons living with chronic conditions.32,33 Community-based programs for promoting mobility and physical fitness in people with chronic stroke have been proposed and positive outcomes have been reported.34,35 An upper extremity group exercise program for people with chronic stroke has also been proposed by Dean et al.12 (used as the control group in their study). However, it failed to produce positive outcomes, probably due to the small sample size (n = 12).
A randomized controlled trial was conducted to examine the feasibility and efficacy of a community-based group exercise program. The participants were randomized into two different exercise groups: (1) arm exercise group and (2) leg exercise group. The trial was originally set out to test the effects of a leg exercise program on cardiorespiratory fitness, balance and leg muscle strength and the results have been reported in another article.36
However, the study design also allowed us to examine the results for the arm group. The purpose of the study was to determine (1) whether a community-based group exercise program for people with chronic stroke results in functional improvement in the paretic upper extremity, and (2) the relationship between severity of upper extremity impairment and gain from the treatment program. It was hypothesized that the arm group would have significantly more functional improvement in the paretic upper extremity than the leg group.
METHODS
Participants
Community-dwelling persons with stroke were recruited on a volunteer basis through a local rehabilitation hospital database, local stroke clubs and newspaper advertisements. All potential participants were screened by a telephone interview and had to fulfill the following inclusion criteria: (1) had one single stroke only, (2) was in chronic stage of stroke recovery (i.e. post-stroke duration of one year or more), (3) were independent in ambulation with or without an assistive device for at least 10m, (4) were 50 years of age or older, and (5) were living at home. Potential participants were excluded if they (1) had other neurological conditions in addition to stroke, (2) had unstable cardiovascular disease, or (3) had other serious diseases that precluded them from undergoing the exercise training. Potential participants gave informed, written consent to participate in the study. For those who were previously admitted to local hospitals, medical records were obtained to confirm the diagnosis of stroke based on imaging results (e.g. CT scan). In addition, the primary care physician was also required to complete a form to confirm the diagnosis of stroke and provide relevant medical history of the subject (i.e. characteristics of stroke, contraindications to exercise, co-morbid conditions).
For those persons who passed the initial interview, they were brought into the research laboratory for further screening. First, a Folstein Mini Mental Status Examination (MMSE) score was administered and a score of ≥22 was required for inclusion.37 Second, the ability to pedal the cycle ergometera was tested. The participant had to be able to pedal at 60rpm and raise the heart rate to at least 60% maximal heart rate. This was to ensure to us that the participant would be able to undergo cardiorespiratory fitness testing on the cycle ergometer, since the trial was originally set out to evaluate the leg exercise program on cardiorespiratory fitness. The study was approved by local research ethics committees. The study was conducted according to the Helsinki Declaration for human experiments.38
Randomization
The eligible participants were matched by sex and then randomly assigned to either the arm exercise group or the leg exercise group. This procedure was conducted to ensure an approximately equal number of men and women in each of the exercise groups. The randomization procedure was done by drawing ballots. The person who performed the randomization was not involved in enrolment or any of the screening and outcome assessments.
Blinding
This is a single-blind pilot randomized controlled trial. The research personnel who performed the outcome assessments were blinded to the group assignment. Participants, however, were not blinded to group assignment. To minimize systematic bias due to the non-blinding of the participants, participants were instructed not to tell the assessors about the group assignment or the treatment they received.
Interventions
Participants in both the arm and leg groups underwent an exercise program for 19 weeks (1-hour sessions, 3 sessions per week). A physical therapist, an occupational therapist and an exercise instructor supervised each exercise session consisting of 9–12 participants. The exercise programs took place in a community hall.
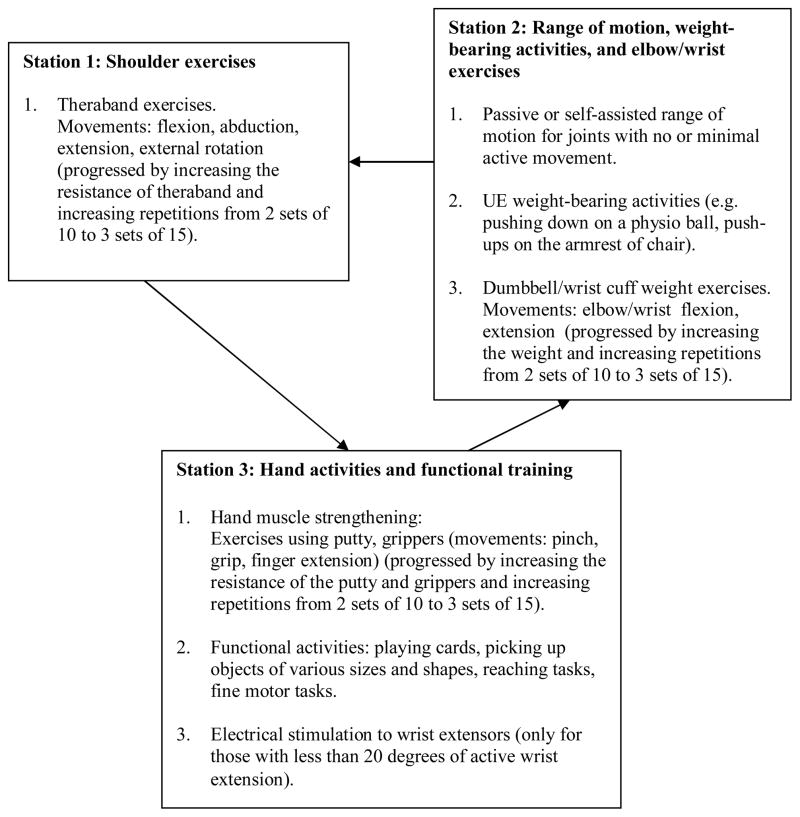
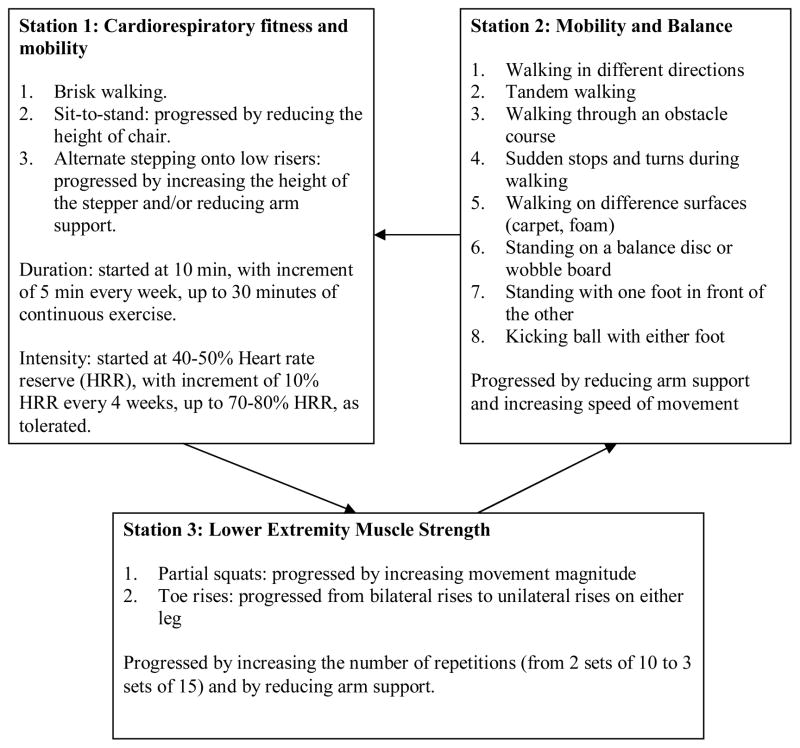
In the arm group, the participants underwent an exercise program designed to improve upper extremity function. The overall aim was to prevent learned non-use and to improve functional abilities of the paretic upper extremity through self-directed exercises. The participants were required to rotate through 3 exercise stations (Figure 1). In station 3, electrical stimulation of the wrist extensors was provided to those with less than 20 degrees of active wrist extension (n = 10), by using a electrical muscle stimulator (frequency: 100Hz, pulse duration: 150 μs, ON time: 10s, OFF time: 10s, ramp: 1s, treatment time: 10–15 minutes).b Each exercise session had a brief (5 minutes) warm up and cool down period in which the participants performed upper extremity stretches and active or self-assisted range of motion exercises. The specific exercises performed in each session were recorded in a log book provided for each participant. The participants were expected to be more self-directed as the trial progressed, by following the exercise protocol recorded in the log book. Any adverse symptoms (i.e. excessive fatigue, pain, injuries) were reported to the therapists. The leg exercise group participated in a lower extremity exercise program (Figure 2). No upper extremity exercises were performed in this group.
Figure 1. Exercise Training Protocol: Arm Group.
Participants in the arm group were required to rotate through 3 exercise stations to work on different upper extremity tasks.
Figure 2. Exercise Training Protocol: Leg Group.
Participants in the leg group underwent a leg exercise program.
Outcomes
All outcomes were measured by the same trained assessors immediately before the initiation of the program (week 0), and again immediately after the termination of the interventions (week 19). The assessors were not involved in conducting the training program.
Primary outcomes
The Wolf Motor Function Test (WMFT) was used to assess upper extremity function.39 It consists of 15 tasks and quantifies upper extremity movement through timed joint-segment movements (tasks 1–7) and functional tasks (tasks 8–15). The higher the score, the closer the movement appears to be normal. A score from 0 to 5 was given for each task (0: does not attempt with upper extremity being tested; 3: movement is influenced to some degree by synergy or is performed slowly or with effort; 5: movement appears to be normal). The scores for the 15 tasks were summed and then averaged to yield the mean functional ability score. In addition, the time (in seconds) required to complete each task was recorded. If the participant was unable to complete a particular task within 2 minutes, the task was considered unachievable for that participant and a time of +120 seconds was assigned. The final time score would be the median time required for all timed tasks executed, as per the WMFT protocol.40 Since the median time was used, all scores above the median have the same weight. Therefore, the inability to perform a particular task would not translate into a disproportionately large change in the final time. WMFT has been shown to have high interrater reliability [intraclass correlation coefficients (ICC)≥0.88], internal consistency (Cronbach’s α≥0.86) and test-retest reliability (r≥0.90) in people with stroke.39,40
The Fugl-Meyer Motor Assessment (FMA) was used to evaluate the severity of motor impairment in the paretic upper extremity.41 It was based on the performance of 33 tasks, which assessed the quality of movements, reflex activity and coordination. A score based on a 3-point ordinal scale (0–2) was given to each task, with a higher score indicating less impairment (maximum score = 66). FMA has been shown to have high interrater reliability (r=0.99).42
Secondary outcomes
Grip strength was measured by using the Jamar dynamometer.c Measurement of grip strength by Jamar dynamometer has been shown to have high test-retest reliability (r=0.88–0.93) and interrater reliability (r=0.99).43 The standard testing position recommended by the American Society of Hand Therapists was used.44 Each participant was seated in a chair with back support, with the shoulder adducted and neutrally rotated, elbow flexed at 90 degrees with neutral supination and pronation, and wrist in a neutral position. The participant was then instructed to squeeze the dynamometer as hard as she/he could for 5 seconds and the force value (in kg) was recorded. Three trials were performed and the force data were averaged and converted to Newtons (N).
The Motor Activity Log (MAL) was used to evaluate how much and how well a person used the paretic upper extremity in daily activities.45 MAL consisted of 30 functional tasks (e.g. putting on shoes, opening a drawer) and was administered as a semi-structured interview.45 Two scores, based on a 6-point ordinal scale, were given for each item, one for the amount of use (0: paretic arm not used; 3: paretic arm used about half as much as before the stroke; 5: paretic arm used as much as before the stroke), and one for the quality of movement (0: inability to use the paretic arm; 3: fair ability to use the paretic arm; 5: ability to use the paretic arm as well as before the stroke). The scores for the 30 items were averaged to obtain a mean score. The amount of use and quality of movement scores were summed and then averaged to yield a single MAL score for each participant. The MAL has been shown to have high internal consistency (Cronbach’s α≥0.88), and reasonable construct validity (Spearman’s ρ= 0.63) in persons with stroke.45
Patient satisfaction
Each participant was asked to fill out a questionnaire to rate the facility (location, accessibility) and the program (class size, frequency, duration, being beneficial) based on an ordinal scale of 1–5 (1= poor, 2 = satisfactory, 3 = good, 4 = very good, 5 = excellent). Participants were also required to indicate their likelihood of participating in a similar community program in the future (not at all likely, somewhat likely, likely, or very likely). Additional comments on the program were also recorded.
Statistical analysis
Baseline characteristics of the two groups were compared using independent t-tests (for continuous variables) and Chi-Square (for categorical variables). We found that the arm group had significantly greater impairment in the paretic upper extremity at baseline than the leg group indicated by the WMFT functional ability and FMA scores. To test the overall effect of treatment and to reduce the probability of type I error due to multiple comparisons, a multivariate analysis of co-variance (MANCOVA) incorporating all outcome measures (FMA, WMFT functional ability score, WMFT time score, grip strength, MAL) was performed (within-subject factor: time; between-subject factor: group). Baseline WMFT functional ability and FMA scores were entered as covariates because of the between-group difference in these measures.
If the MANOVA demonstrated a significant effect, follow-up analyses would be done using univariate 2-way ANCOVA.46 While the MANCOVA could detect whether there was a significant overall treatment effect, it could not indicate what particular outcome variable showed significant changes following the arm exercise therapy. In order to determine the effect of arm therapy on individual outcome variable, post-hoc univariate 2-way ANCOVA was performed for each outcome variable (within-subject factor: time; between-subject factor: group), with the baseline measurement of each respective outcome entered as the covariate. To specifically test the effect on performance of functional activities, a separate univariate 2-way ANCOVA based on the mean score from tasks 8–15 in the WMFT was also performed.
To calculate effect size, the mean change scores (posttest scores – pretest scores) for each outcome variable in the arm and leg groups were extracted. For each outcome, the baseline standard deviations (SDs) in the two groups were used to calculate the pooled population SD.47 The standardized effect size (SES) was established by calculating the difference between mean change scores of the arm and leg groups divided by the pooled population SD.47 The 95% confidence interval (CI) for each SES was also computed.
It was also of interest to determine whether the level of impairment at baseline had any impact on the amount of improvement in the arm group. The 30 participants in the arm group were thus stratified into 3 subgroups of 10 participants based on the FMA score at baseline (FMA score = 0–27: severely impaired; FMA score = 28–57: moderately impaired; FMA score = 58–66: mildly impaired). Pretest-posttest comparisons were made by using paired t-tests. For each participant, the pretest score was subtracted from the posttest score to obtain the change score for each outcome variable. These change scores were then entered into a MANOVA (between-subject factor: level of impairment) to assess the overall effect of initial upper extremity impairment on improvement obtained at the end of the trial. Tukey’s test was then used to analyse the data post-hoc. Within each of the 3 subgroups, the change score for each outcome was divided by the respective baseline SD to obtain the SES. A SES of 0.2–0.5 was considered as small, 0.5–0.8 as medium, and ≥0.8 as large.48 All statistical analyses were performed using SPSS11.5 software using a significance level of 0.05 (2-tailed).d
RESULTS
Subject characteristics, participation and retention
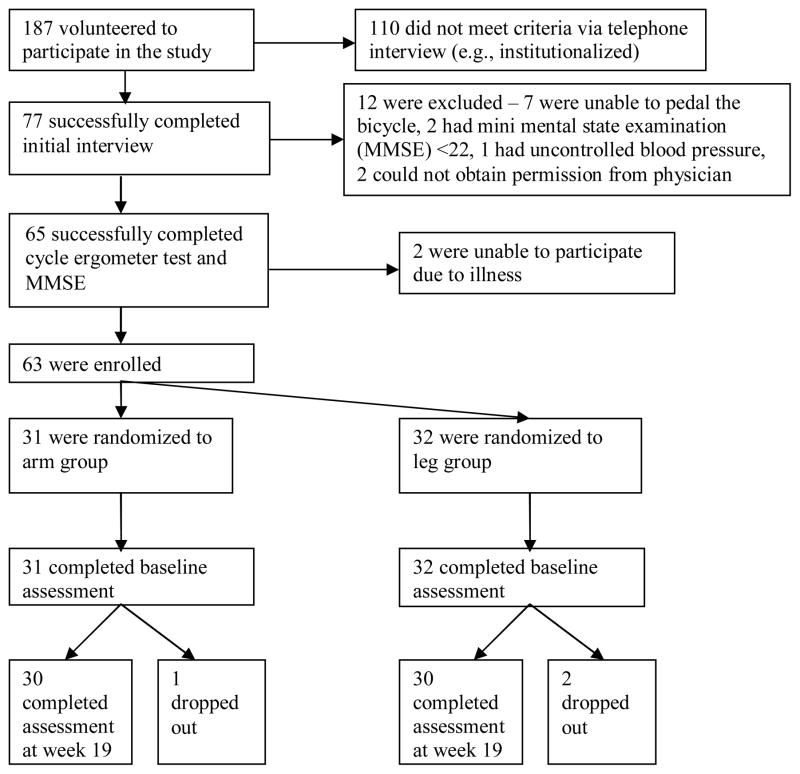
Sixty-three persons with chronic stroke (36 men, 27 women) participated in the study (Figure 3). Three participants dropped out during the course of the study and did not undergo the outcome assessments at the end of the trial (week 19). Of these 3 participants, one dropped out of the arm group after 8 sessions because of the inability to commit the time. Another two dropped out of the leg group: 1 withdrew after 6 sessions because of the inability to commit the time, and 1 withdrew after 9 sessions because he found the exercise too fatiguing. These 3 participants were excluded from data analysis.
Figure 3.
Study Flow Chart
Comparison of baseline characteristics (independent sample t-tests or Chi-squares) revealed that there was no significant difference in demographics, stroke characteristics, and presence of co-morbid conditions (Table 1). Regarding the primary outcomes, there was no significant difference in WMFT time score (P=0.733). However, the arm group had significantly lower WMFT functional ability score (P=0.042) and FMA (P=0.040). Participant attendance was similar in both groups (arm group: 82.6%; leg group: 85.8%; P=0.401).
Table 1.
Subject Characteristics at Baseline
| Arm Group (n=30) | Leg Group (n=30) | Dropouts (n=3) | |
|---|---|---|---|
| Demographics | |||
| Female sex, n | 12 | 12 | 2 |
| Age, years | 64.9±8.5 | 66.0± 8.7 | 61.7± 13.2 |
| White/Asian/Black, n | 18/12/0 | 18/11/1 | 2/1/0 |
| MMSE | 28.1±1.9 | 27.7±2.4 | 27.3±1.5 |
| Had inpatient rehabilitation after acute hospital stay, n | 26 | 22 | 2 |
| Had outpatient physical therapy after discharge, n | 21 | 20 | 0 |
| Had outpatient occupational therapy after discharge, n | 12 | 10 | 0 |
| Self-selected gait velocity (m/s) | 0.80±0.34 | 0.83±0.44 | 1.1±0.28 |
| Stroke characteristics | |||
| Paretic side (left), n | 21 | 19 | 1 |
| Paretic side (dominant side), n | 11 | 8 | 2 |
| Ischemic stroke, n | 19 | 17 | 1 |
| Post-stroke duration, years | 5.1±3.6 | 5.2±5.0 | 12.1±11.0 |
| Comorbidity | |||
| Hypertension, n | 19 | 17 | 1 |
| Diabetes, n | 5 | 4 | 1 |
| Arthritis, n | 6 | 6 | 0 |
| Depression, n | 8 | 6 | 1 |
Mean±SD
Treatment effect
All participants received treatment and control conditions as allocated. The results for all outcomes are presented in Table 2. MANCOVA (covariates: baseline WMFT functional ability and FMA scores) showed a significant time × group interaction (Wilk’s Lambda=0.729, P= 0.005). Post-hoc analysis (univariate 2-way ANCOVA) (Table 2) revealed that the arm group had significantly more improvements in WMFT functional ability (P=0.003) and FMA (P= 0.001) scores than the leg group. The improvement in the arm group remained significantly higher than the leg group when only tasks 8–15 (functional activities) in the WMFT were considered (univariate 2-way ANCOVA, P= 0.011). There was no significant time × group interaction for WMFT time score, grip strength and MAL.
Table 2.
Outcome Measurements
| Arm Group (n=30) | Leg Group (n=30) | Effect size (95%CI) | P | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Pre-test | Post-test | Pre-test | post-test | |||
| Primary Outcomes | ||||||
| WMFT (functional ability) | 2.9±1.6 | 3.1 ±1.8 | 3.7±1.5 | 3.7±1.5 | 0.13 (−0.38, 0.63) | 0.003* |
| WMFT (tasks 8–15 only) | 2.6±1.7 | 2.9±1.9 | 3.5±1.6 | 3.5±1.8 | 0.18 (−0.33, 0.69) | 0.011* |
| WMFT median time (s) | 29.7±47.1 | 26.9±47.5 | 25.5±48.4 | 25.3±48.2 | −0.05 (−0.56, 0.45) | 0.177 |
| FMA | 40.8±19.6 | 45.7± 19.3 | 51.3±19.2 | 52.5± 18.5 | 0.19 (−0.32, 0.70) | 0.001* |
| Secondary Outcomes | ||||||
| Grip strength (N) | 97.3±94.8 | 113.8± 96.7 | 166.6±128.8 | 170.7±131.6 | 0.11 (−0.4, 0.61) | 0.203 |
| MAL | 1.7±1.5 | 2.3±1.7 | 3.0±1.7 | 3.3±1.7 | 0.18 (−0.32, 0.69) | 0.274 |
p<0.005 (group × time interaction, post-hoc 2-way ANCOVA)
WMFT time score: a negative effect size means that the median time taken to complete the tasks in post-test was less when compared with pre-test, indicating improvement in arm function.
Impact of severity of impairment on changes in outcome measures
Another purpose of this study was to identify the participants who may benefit the most from this community-based upper extremity exercise program. The participants in the arm group were categorized into 3 different subgroups according to the baseline FMA score. Comparison between the pretest and posttest scores (paired t-tests) revealed that the mildly impaired group had significant change in WMFT functional ability (P=0.016), FMA (P=0.008) and MAL scores (P=0.012), but not in WMFT time score (P=0.100) and grip strength (P=0.165). The moderately impaired group had significant change in all outcomes (WMFT functional ability, P=0.002; FMA, P<0.001; grip strength, P=0.017; MAL, P=0.050) except WMFT time score (P=0.463). The severely impaired group had significant change in FMA (P=0.033) and MAL (P=0.035), but not others (WMFT functional ability, P=0.792; WMFT time score, P=0.233; grip strength, P=0.317).
The average change scores and effect sizes are presented in Table 3. There was an overall significant difference in change scores among the 3 subgroups of participants with different levels of impairment. (MANOVA; Wilk’s Lambda=0.347, P=0.003). Post-hoc analysis (Tukey’s test) revealed that the moderately impaired group had the most increase in FMA and this increase was significantly greater than that of the mildly impaired group (P=0.044). These participants also had significantly more improvement than the severely impaired group in WMFT functional ability score (P=0.011). On the other hand, there was a trend for the mildly impaired group to improve more in WMFT (P=0.065) and MAL (P=0.065) than the severely impaired group. The level of upper extremity impairment had no significant impact on subsequent improvement of WMFT time score and grip strength.
Table 3.
Impact of severity of impairment on changes in outcome measures
| Outcomes | Mild impairment (n=10) | Moderate impairment (n=10) | Severe impairment (n=10) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Change score ± SD | SES† | Change score ± SD | SES | Change score ± SD | SES | |
| WMFT functional ability | 0.3±0.3* | 0.67 | 0.4±0.3* | 0.56 | −0.0±0.3 | −0.04 |
| WMFT time (s) | −0.3±0.5 | −0.36 | −1.5±6.2 | −0.25 | −6.6±16.4 | −0.13 |
| FMA | 2.5±2.3* | 0.81 | 7.4±3.8* | 0.81 | 4.8±6.0* | 0.71 |
| Grip strength (N) | 15.5±32.5 | 0.19 | 22.0±23.9* | 0.32 | 12.0±36.0 | 0.48 |
| MAL | 1.0±1.0* | 0.65 | 0.5±0.7* | 0.40 | 0.2±0.3* | 0.50 |
p<0.05 (pretest-posttest comparisons of raw scores, paired t-test)
SES= standardized effect size.
Overall, the moderately impaired group improved most in the two primary outcomes (Table 3). Could the difference in results between the arm group and leg group be due to the fact that the leg group was on average less impaired and therefore had less room for improvement? To address this issue, pretest-posttest comparisons were also made for the moderately impaired participants in the leg group (n=7). Paired t-tests revealed that while the moderately impaired participants in the arm group had significant change in FMA (P<0.001) and WMFT functional ability scores (P=0.002), their counterparts in the leg group had no significant change in FMA (P= 0.094) and WMFT functional ability scores (P=0.229). Therefore, it is unlikely that the difference in baseline FMA and WFMT scores between the two groups could explain our results.
Participant satisfaction
The median scores for facility location and accessibility were 3 and 4, respectively. The upper extremity exercise program itself was also highly rated (median scores for class size: 4; frequency: 4.5; duration: 4.5; being beneficial: 5). Ninety-three percent of the participants in the arm group indicated that they would be likely or very likely to participate in a similar program in the future. When asked about what they liked the most about the arm program, 46% of the participants reported that the social interaction in the group setting was the most enjoyable.
Adverse symptoms
Two participants in the arm group reported muscle soreness in the shoulder on the paretic side during the first few weeks of training. The symptoms subsided after modifying the program by reducing the weight lifted in station 2. One participant fell once in the arm group during walking. Five falls (4 participants) occurred in the leg group. No injuries were reported.
DISCUSSION
Improvement in upper extremity function
The results indicated that the arm group had more gains in the WMFT functional ability (change from 40.8 to 45.7) and FMA scores (change from 2.9 to 3.1) than the leg group (Table 2). To our knowledge, no studies have examined the minimal clinically important difference for these outcome measures.39,40,49 However, the satisfaction survey has given us some insight into the clinical significance of these changes in FMA and WMFT. Some specific comments were “now able to switch on and off lights, curl my hair, and wash my face”, “now able to take bath myself”, “feel like the arm is part of my body again”, “reduced arm pain”, “now able to lift pop can or glass”, and “able to play tennis, use my weaker hand to drop ball and serve”. The changes in WMFT and FMA seem to be clinically significant from the participants’ perspective.
The effect sizes for primary outcomes such as FMA and WMFT functional ability scores were medium to large (i.e. SES>0.5), particularly for the mildly impaired and moderately impaired group (Table 3). The gains in primary outcomes in this study are at least comparable to other treatment methods used in chronic stroke. For example, our finding is comparable to the 3 to 5 point (SES = 0.29 – 0.52) increase in FMA found in studies involving robotic-aided training or BATRAC (1-hour sessions, 3 sessions per week for 6 weeks to 2 months) in participants with similar impairment levels.25,27,29,31 Our finding is also comparable to the results from studies on CIMT (for 12 to 14 days and between 6–7 hours per day), which has reported a gain of 0.5–0.6 (SES = 0.74 –0.93) in the WMFT functional ability score.18,19 Our treatment approach seems to be superior to using force feedback for muscle strengthening (3 sessions per week for 6 weeks), which resulted in no change in FMA.16 The lack of change in motor function may be due to the non-functional approach of the program. Thus, we have demonstrated that our community-based program is a feasible option to enhance arm motor function in chronic stroke.
Our study shows differential effects of treatment depending on the severity of upper extremity impairment. Those who are moderately impaired tend to benefit the most (Table 3). Those with severe impairment, on the other hand, did not improve in functional abilities despite a significant increase in FMA score. Previous studies in acute and chronic stroke also found that those with less severe impairment in the upper extremity benefit more from the treatment.31,50–52
Importance of community-based group program
Novel approaches such as CIMT18–23 and robot-assisted movement therapy26–31 have been devised to improve upper extremity function in persons with chronic stroke and positive outcomes have been reported. However, these approaches are not without their drawbacks. For example, robot-aided arm training26–31 and BATRAC25 involve the operation of expensive and sophisticated equipment. The constraint of the unaffected upper extremity for a large proportion (up to 90%) of waking hours in CIMT may impede the performance of many daily activities that require bilateral arm movements. The intensive training involved (up to 7 hours per day) may be too demanding for the participants and clinicians who may not have the available resources to administer the program.
We developed a community-based group upper extremity exercise program. The concept of community-based programs has many advantages. First, the target population is community-dwelling chronic stroke survivors. It is thus best to implement the program in a community center to increase the accessibility of rehabilitation service and to promote integration into the community. Second, the program does not require one-on-one supervision, which will be more economical when compared to other programs that require individual supervision. Third, our participants reported gains in various outcomes, regardless of their impairment levels (Table 3). Our community-based program may thus be able to reach a broader population of people with stroke, when compared with other treatment programs such as the CIMT. The eligibility criteria for CIMT are often limited to those who have significant recovery in the hand and wrist (i.e. 20 degrees of wrist extension).18–22 Based on these criteria, 20 of our 63 participants (32%; before randomization) would have been excluded. Although our participants with severe impairment had no change in WMFT functional ability score, they still benefited from the program (i.e. significant increase in FMA and MAL scores with effect sizes of 0.71 and 0.50, respectively). Finally, the participants enjoyed the program and found it beneficial. The group setting also provides ample opportunities for social interaction and many participants found the social aspect particularly enjoyable. This is important, given that the prevalence of post-stroke depression is quite high (around 20%).53
More people with stroke are returning home without undergoing a formal inpatient rehabilitation program.54 Consequently, more people recovering from a stroke may not be functioning at an optimal level when they first return to the community. This may translate into an increased risk of deterioration in function, activity level, and health. Secondary debilitating conditions attributable to physical inactivity have placed enormous burden on the health care system.55 If community-based programs are able to reduce or prevent these secondary disabilities, it would presumably lead to health care cost savings in the long run. The potential of community-based programs in reducing health care cost should interest health care funding agencies (i.e. government, insurance agencies). In fact, many federal agencies in the United States have taken the initiative in supporting projects in health promotion, in which community-based programs play a key role.33 The cost-effectiveness and long term effects of the proposed community-based program will require further investigations.
Limitations of the study
This is a pilot study to examine the effects of a community-based program for improving upper extremity function in chronic stroke. Some limitations were identified in this study, which could be used to improve the design of a large clinical trial in the future. First, the baseline characteristics of the two groups were different. However, such differences were accounted for by our statistical analysis (MANCOVA and ANCOVA) and differences in results between groups remained significant. Nevertheless, future trials may require further stratification of the sample according to severity of upper extremity impairment. Second, some of the variables (e.g., gains in grip strength and MAL) showed trends of greater improvement in the arm versus leg group, but the difference did not reach statistical significance. The study was probably underpowered to detect a significant difference for these variables between the two groups. A larger sample size is thus called for in the future study. Additionally, the training protocol may not have been optimal in producing a significant change in these variables. Training at a higher intensity (i.e. increasing resistance, repetitions, duration or frequency of exercise) may be required in the future. Third, future programs need to consider the diversity in functional abilities among the participants. The key is to keep the program challenging to promote improvement and maintain interest while ensuring safety. One possibility is to divide the participants into subgroups according to the level of impairment. Different activities, exercise intensity and progression can be implemented to optimize upper extremity function for each subgroup. Finally, we found that the outcome was somewhat dependent upon the initial level of impairment. Therefore, the overall treatment effect may have been diluted because of the inclusion of the severely impaired group.
CONCLUSION
This study shows that the community-based group upper extremity program is feasible and is beneficial for improving upper extremity function. It may provide an excellent model for upper extremity rehabilitation program for the stroke population in the community. Future study is required to further assess the efficacy and cost-effectiveness of the program.
Acknowledgments
Grant and financial support: M.Y.C.P. was supported by a post-doctoral fellowship from Natural Sciences and Engineering Research Council of Canada. This study was supported by a grant-in-aid from the Heart Stroke Foundation of New Brunswick and from career scientist awards to J.J.E. from Canadian Institute of Health Research (MSH-63617) and the Michael Smith Foundation for Health Research.
Footnotes
Excalibur, Lode B.V. Medical Technology, Zernikepark 16, 9747 AN Groningen, Netherlands.
NeuroTrac Sports; Verity Medical Ltd., Uplands Place, Drove Road, Chilbolton, Nr Stockbridge, Hampshire, England SO20 6AD.
Jamar hand dynamometer; Sammons Preston, 755 Queensway East, Unit 27, Mississauga, Ontario, Canada L4Y 4C5.
SPSS 11.5 for Windows; SPSS Inc, 233 S Wacker Drive, 11th Floor, Chicago, IL 60606, USA.
References
- 1.Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. Recovery of upper extremity function in stroke patients: The Copenhagen Stroke Study. Arch Phys Med Rehabil. 1994;75:394–98. doi: 10.1016/0003-9993(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 2.Parker VM, Wade DT, Hewer RL. Loss of arm function after stroke: measurement, frequency, and recovery. Int Rehabil Med. 1986;8:69–73. doi: 10.3109/03790798609166178. [DOI] [PubMed] [Google Scholar]
- 3.Heller A, Wade D, Wood V, Sunderland A, Hewer R, Ward E. Arm function after stroke: measurement and recovery over the first three months. J Neurol Neurosurg Psychiatry. 1987;50:714–9. doi: 10.1136/jnnp.50.6.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson C, Jamrozik K, Stewart-Wynne E. Physical disability after stroke in the Perth Community Stroke Study. Clin Exp Neurol. 1990;27:121–4. [PubMed] [Google Scholar]
- 5.Gresham GE, Fitzpatrick TE, Wolf PA, McNamara PM, Kannel WB, Dawber TR. Residual disability in survivors of stroke-the Framingham study. N Engl J Med. 1975;6:954–6. doi: 10.1056/NEJM197511062931903. [DOI] [PubMed] [Google Scholar]
- 6.O’Mahony PG, Thomson RG, Dobson R, Rodgers H, James OFW. The prevalence of stroke and associated disability. J Public Health Med. 1999;21:166–71. doi: 10.1093/pubmed/21.2.166. [DOI] [PubMed] [Google Scholar]
- 7.Jongbloed L, Stacey S, Brighton C. Stroke rehabilitation: sensorimotor integrative treatment versus functional treatment. Am J Occup Ther. 1989;43:391–7. doi: 10.5014/ajot.43.6.391. [DOI] [PubMed] [Google Scholar]
- 8.Gelber DA, Josefczyk PB, Herrman D, Good DC, Verhulst SJ. Comparison of two therapy approaches in the rehabilitation of pure motor hemiparetic stroke patients. J Neuro Rehabil. 1995;9:191–6. [Google Scholar]
- 9.Duncan P, Studenski S, Richards L, Gollub S, Lai SM, Reker D, et al. Randomized clinical trial of therapeutic exercise in subacute stroke. Stroke. 2003;34:2173–80. doi: 10.1161/01.STR.0000083699.95351.F2. [DOI] [PubMed] [Google Scholar]
- 10.Werner RA, Kessler S. Effectiveness of an intensive outpatient rehabilitation program for postacute stroke patients. Am J Phys Med Rehabil. 1996;75:114–20. doi: 10.1097/00002060-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Winstein CJ, Rose DK, Tan SM, Lewthwaite R, Chui HC, Azen SP. A randomized controlled comparison of upper-extremity rehabilitation strategies in acute stroke: A pilot study of immediate and long-term outcomes. Arch Phys Med Rehabil. 2004;85:620–8. doi: 10.1016/j.apmr.2003.06.027. [DOI] [PubMed] [Google Scholar]
- 12.Dean CM, Richards C, Malouin F. Task-related circuit training improves performance of locomotor tasks in chronic stroke: a randomized, controlled pilot trial. Arch Phys Med Rehabil. 2000;81:409–17. doi: 10.1053/mr.2000.3839. [DOI] [PubMed] [Google Scholar]
- 13.Langhammer B, Stanghelle JK. Bobath or motor relearning programme? A comparison of two different approaches of physiotherapy in stroke rehabilitation. Clin Rehabil. 2000;14:361–9. doi: 10.1191/0269215500cr338oa. [DOI] [PubMed] [Google Scholar]
- 14.Logigian MK, Samuels MA, Falconer J, Zagar R. Clinical exercise trial for stroke patients. Arch Phys Med Rehabil. 1983;64:364–7. [PubMed] [Google Scholar]
- 15.Carr M, Jones J. Physiological effects of exercise on stroke survivors. Top Stroke Rehabil. 2003;9:57–64. doi: 10.1310/0J2K-MDNX-1Q0L-8LX6. [DOI] [PubMed] [Google Scholar]
- 16.Bourbonnais D, Bilodeau S, Lepage Y, Beaudoin N, Gravel D, Forget R. Effect of force-feedback treatments in patients with chronic motor deficits after a stroke. Am J Phys Med Rehabil. 2002;81:890–7. doi: 10.1097/00002060-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Bütefisch C, Hummelsheima H, Denzlera P, Mauritz Repetitive training of isolated movements improves the outcome of motor rehabilitation of the centrally paretic hand. J Neurol Sci. 1995;103:59–68. doi: 10.1016/0022-510x(95)00003-k. [DOI] [PubMed] [Google Scholar]
- 18.Miltner WHR, Bauder H, Sommer M, Dettmers C, Taub E. Effects of constraint-induced movement therapy on patients with chronic motor deficits after stroke: a replication. Stroke. 1999;30:586–92. doi: 10.1161/01.str.30.3.586. [DOI] [PubMed] [Google Scholar]
- 19.Kunkel A, Kopp B, Muller G, Villringer K, Villringer A, Taub E, Flor H. Constraint-induced movement therapy for motor recovery in chronic stroke patients. Arch Phys Med Rehabil. 1999;80:624–8. doi: 10.1016/s0003-9993(99)90163-6. [DOI] [PubMed] [Google Scholar]
- 20.Sterr A, Elbert T, Berthold I, Kolbel S, Rockstroh B, Taub E. Longer versus shorter daily constraint-induced movement therapy of chronic hemiparesis: an exploratory study. Arch Phys Med Rehabil. 2002;83:1374–7. doi: 10.1053/apmr.2002.35108. [DOI] [PubMed] [Google Scholar]
- 21.Page SJ, Sisto S, Levine P, McGrath RE. Efficacy of modified constraint-induced movement therapy in chronic stroke: a single-blinded randomized controlled trial. Arch Phys Med Rehabil. 2004;85:14–8. doi: 10.1016/s0003-9993(03)00481-7. [DOI] [PubMed] [Google Scholar]
- 22.Taub E, Miller NE, Novack TA, Cook EW, III, Fleming WC, Nepomuceno CS, et al. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74:347–54. [PubMed] [Google Scholar]
- 23.Wolf SL, Lecraw DE, Barton LA, Jann BB. Forced use of hemiplegic upper extremities to reverse the effect of learned nonuse among chronic stroke and head-injured patients. Exp Neurol. 1989;104:125–32. doi: 10.1016/s0014-4886(89)80005-6. [DOI] [PubMed] [Google Scholar]
- 24.Liepert J, Bauder H, Miltner WHR, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31:1210–6. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- 25.Whitall J, Waller SMc, Silver KHC, Macko RF. Repetitive bilateral arm training with rhythmic auditory cueing improves motor function in chronic hemiparetic stroke. Stroke. 2000;31:2390–5. doi: 10.1161/01.str.31.10.2390. [DOI] [PubMed] [Google Scholar]
- 26.Aisen ML, Krebs HI, Hogan N, McDowell F, Volpe BT. The effect of robot-assisted therapy and rehabilitative training on motor recovery following stroke. Arch Neurol. 1997;54:443–6. doi: 10.1001/archneur.1997.00550160075019. [DOI] [PubMed] [Google Scholar]
- 27.Fasoli SE, Krebs HI, Stein J, Frontera WR, Hogan N. Effects of robotic therapy on motor impairment and recovery in chronic stroke. Arch Phys Med Rehabil. 2003;84:477–82. doi: 10.1053/apmr.2003.50110. [DOI] [PubMed] [Google Scholar]
- 28.Ferraro M, Palazzolo JJ, Krol J, Krebs HI, Hogan N, Volpe BT. Robot-aided sensorimotor arm training improves outcome in patients with chronic stroke. Neurology. 2003;61:1604–7. doi: 10.1212/01.wnl.0000095963.00970.68. [DOI] [PubMed] [Google Scholar]
- 29.Lum PS, Burgar CG, Shor PC, Majmundar M, Van der Loos M. Robot-assisted movement training compared with conventional therapy techniques for the rehabilitation of upper-limb motor function after stroke. Arch Phys Med Rehabil. 2002;83:952–9. doi: 10.1053/apmr.2001.33101. [DOI] [PubMed] [Google Scholar]
- 30.Volpe BT, Krebs HI, Hogan N, Edelstein L, Diels C, Aisen M. A novel approach to stroke rehabilitation: robot-aided sensorimotor stimulation. Neurology. 2000;54:1938–44. doi: 10.1212/wnl.54.10.1938. [DOI] [PubMed] [Google Scholar]
- 31.Stein J, Krebs HI, Frontera WR, Fasoli SE, Hogan N. Comparison of two techniques of robot-aided upper limb exercise training after stroke. Am J Phys Med Rehabil. 2004;83:720–8. doi: 10.1097/01.phm.0000137313.14480.ce. [DOI] [PubMed] [Google Scholar]
- 32.Rimmer JH. Health promotion for people with disabilities: the emerging paradigm shift from disability prevention to prevention of secondary conditions. Phys Ther. 1999;79:495–502. [PubMed] [Google Scholar]
- 33.Rimmer JH, Braddock D. Health promotion for people with physical, cognitive, and sensory disabilities: an emerging national priority. Am J Health Promot. 2002;16:220–4. doi: 10.4278/0890-1171-16.4.220. [DOI] [PubMed] [Google Scholar]
- 34.Eng JJ, Kelly SC, Kim CM, Dawson AS, Carswell A, Hepburn KE. A community-based group exercise program for persons with chronic stroke. Med Sci Sports Exerc. 2003;35:1271–8. doi: 10.1249/01.MSS.0000079079.58477.0B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rimmer JH, Riley B, Creviston T, Nicola T. Exercise training in a predominantly African-American group of stroke survivors. Med Sci Sports Exerc. 2000;32:1990–6. doi: 10.1097/00005768-200012000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Pang MYC, Eng JJ, Dawson AS, McKay HA, Harris JE. A community-based fitness and mobility exercise program for older adults with chronic stroke: a randomized, controlled trial. J Am Geriatr Soc. doi: 10.1111/j.1532-5415.2005.53521.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the state of patients for the clinician. J Psychiat Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 38.World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277:925–6. [PubMed] [Google Scholar]
- 39.Wolf SL, Catlin PA, Ellis M, Archer Al, Morgan B, Piacentino A. Assessing Wolf Motor Function Test as outcome measure for research in patients after stroke. Stroke. 2001;32:1635–9. doi: 10.1161/01.str.32.7.1635. [DOI] [PubMed] [Google Scholar]
- 40.Morris DM, Uswatte G, Crago JE, Cook EW, III, Taub E. The reliability of the Wolf Motor Function Test for assessing upper extremity function after stroke. Arch Phys Med Rehabil. 2001;82:750–5. doi: 10.1053/apmr.2001.23183. [DOI] [PubMed] [Google Scholar]
- 41.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post stroke hemiplegic patient. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:12–31. [PubMed] [Google Scholar]
- 42.Duncan PW, Propst M, Nelson SG. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther. 1983;63:1606–10. doi: 10.1093/ptj/63.10.1606. [DOI] [PubMed] [Google Scholar]
- 43.Mathiowetz V. Comparison of Rolyan and Jamar dynamometers for measuring grip strength. Occup Ther Int. 2002;9:201–9. doi: 10.1002/oti.165. [DOI] [PubMed] [Google Scholar]
- 44.Fess EE. Grip strength. In: Casanova JS, editor. Clinical assessment recommendations. 2. Chicago: American Society of Hand Therapists; 1992. pp. 41–5. [Google Scholar]
- 45.Van der Lee JH, Beckerman H, Knol DL, de Vet HCW, Bouter LM. Clinimetric properties of the motor activity log for the assessment of arm use in hemiparetic patients. Stroke. 2004;35:1410–4. doi: 10.1161/01.STR.0000126900.24964.7e. [DOI] [PubMed] [Google Scholar]
- 46.Portney LG, Watkins MP. Applications to practice. 2. Upper Saddle River, NJ: Prentice-Hall, Inc; 2000. Foundations of clinical research. [Google Scholar]
- 47.Hedges L, Olkin I. Statistical methods for meta-analysis. Orlando: Academic Press; 1985. [Google Scholar]
- 48.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1988. The t test for means; pp. 25–6. [Google Scholar]
- 49.Gladstone DJ, Danells CJ, Black SE. The fugl-meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16:232–40. doi: 10.1177/154596802401105171. [DOI] [PubMed] [Google Scholar]
- 50.Lincoln NB, Parry RH, Vass CD. Randomized, controlled trial to evaluate increased intensity of physiotherapy treatment of arm function after stroke. Stroke. 1999;30:573–9. doi: 10.1161/01.str.30.3.573. [DOI] [PubMed] [Google Scholar]
- 51.Parry RH, Lincoln NB, Vass CD. Effect of severity of arm impairment on response to additional physiotherapy early after stroke. Clin Rehabil. 1999;13:187–98. doi: 10.1177/026921559901300302. [DOI] [PubMed] [Google Scholar]
- 52.Sunderland A, Tinson DJ, Bradley EL, Fletcher D, Langton Hewer R, Wade DT. Enhanced physical therapy improves recovery of arm function after stroke. A randomized controlled trial. J Neurol Neurosurg Psychiatry. 1992;55:530–5. doi: 10.1136/jnnp.55.7.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robinson RG. Poststroke depression: prevalence, diagnosis, treatment, and disease progression. Biol Psychiatry. 2003;54:376–87. doi: 10.1016/s0006-3223(03)00423-2. [DOI] [PubMed] [Google Scholar]
- 54.Mayo NE, Wood-Dauphinee S, Ahmed S, Gordon C, Higgins J, McEwen S, et al. Disablement following stroke. Disability Rehabil. 1999;21:258–68. doi: 10.1080/096382899297684. [DOI] [PubMed] [Google Scholar]
- 55.Garrett NA, Brasure M, Schmitz KH, Schultz MM, Huber MR. Physical inactivity. Direct cost to a health plan. Am J Prev Med. 2004;27:304–9. doi: 10.1016/j.amepre.2004.07.014. [DOI] [PubMed] [Google Scholar]