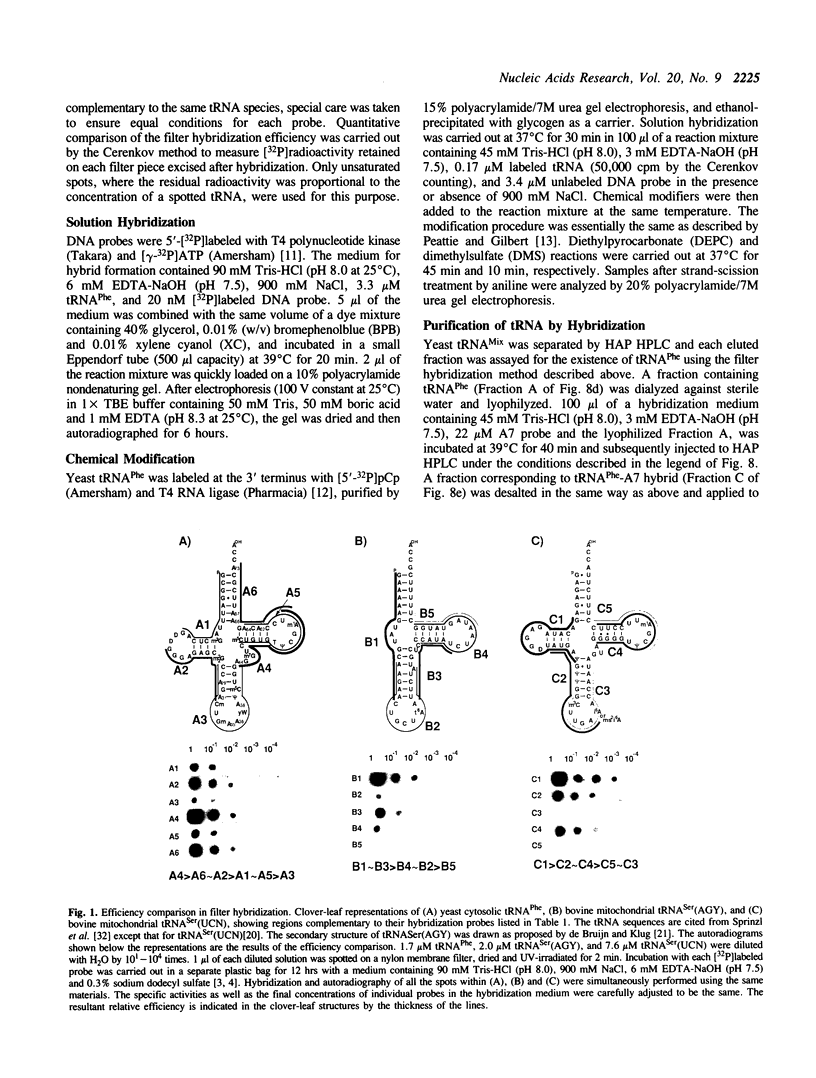
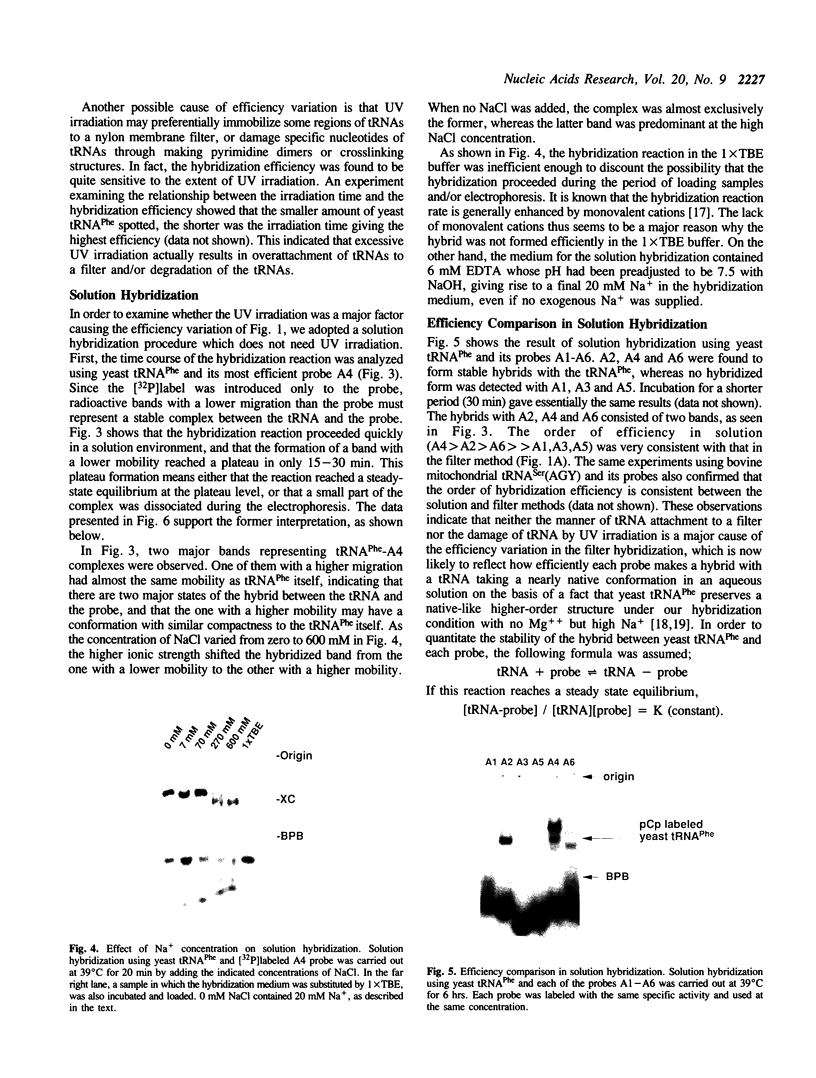
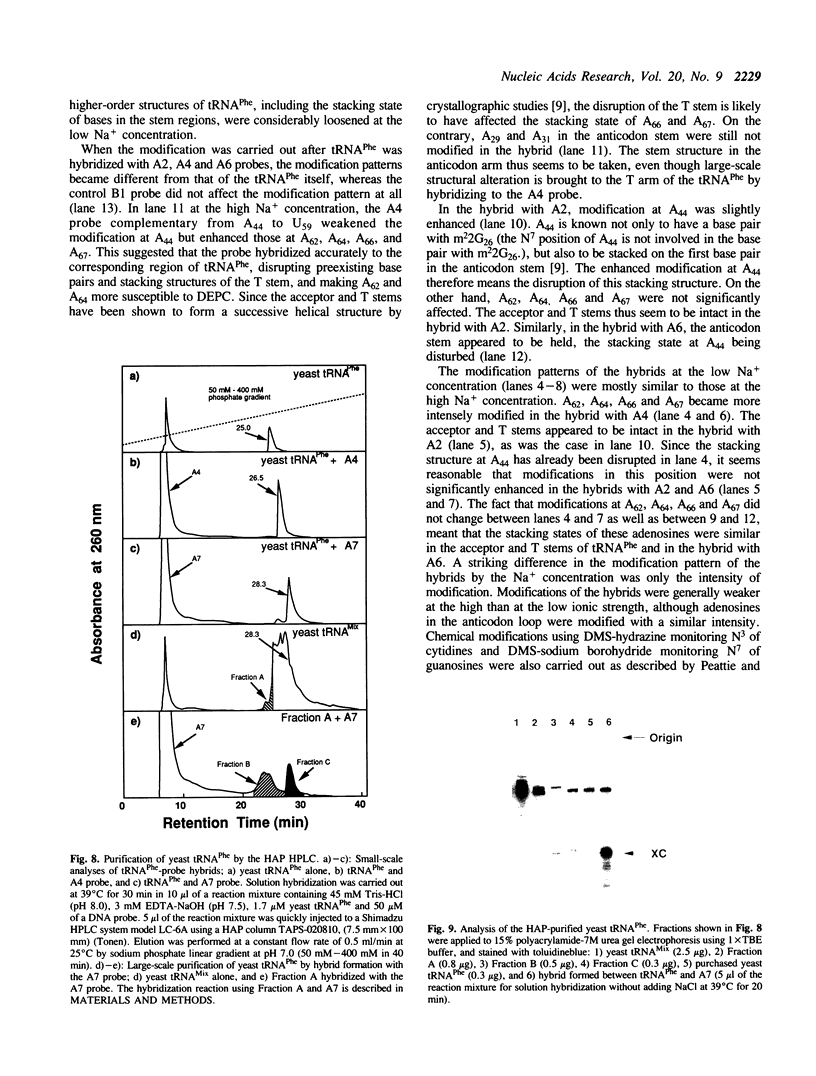
Abstract
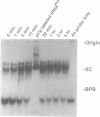
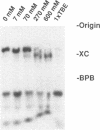
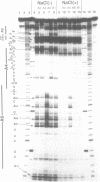
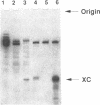
In the course of developing a method to purify a single tRNA species efficiently, we have examined hybridization efficiencies between some tRNAs and short oligodeoxyribonucleotide probes both by the filter and solution hybridization methods without denaturants. The hybridization efficiencies varied considerably among probes which are complementary to different regions of the tRNAs, although there was little efficiency variation in the probes toward DNA substrates including the same nucleotide sequence. This efficiency variation was shown to be due to tRNA-specific higher-order structures as well as a hypermodified nucleotide in the anticodon loop. Characterization of the tRNA-probe hybrids by both nondenaturing gel electrophoresis and chemical modification showed the existence of two stable hybridizing states as a function of ionic strength. Our results indicate that RNA molecules with a number of intramolecular base pairings are able to form stable hybrids with complementary sequences under nondenaturing conditions. On the basis of these data, an appropriate probe was designed to successfully purify yeast tRNA(Phe) by making a tRNA(Phe)-probe hybrid, which has a longer retention time in hydroxyapatite high performance liquid chromatography than the tRNA(Phe) itself.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andachi Y., Yamao F., Iwami M., Muto A., Osawa S. Occurrence of unmodified adenine and uracil at the first position of anticodon in threonine tRNAs in Mycoplasma capricolum. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7398–7402. doi: 10.1073/pnas.84.21.7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcari P., Brownlee G. G. The nucleotide sequence of a small (3S) seryl-tRNA (anticodon GCU) from beef heart mitochondria. Nucleic Acids Res. 1980 Nov 25;8(22):5207–5212. doi: 10.1093/nar/8.22.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslauer K. J., Frank R., Blöcker H., Marky L. A. Predicting DNA duplex stability from the base sequence. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3746–3750. doi: 10.1073/pnas.83.11.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce A. G., Uhlenbeck O. C. Reactions at the termini of tRNA with T4 RNA ligase. Nucleic Acids Res. 1978 Oct;5(10):3665–3677. doi: 10.1093/nar/5.10.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole P. E., Yang S. K., Crothers D. M. Conformational changes of transfer ribonucleic acid. Equilibrium phase diagrams. Biochemistry. 1972 Nov 7;11(23):4358–4368. doi: 10.1021/bi00773a024. [DOI] [PubMed] [Google Scholar]
- Crothers D. M., Cole P. E., Hilbers C. W., Shulman R. G. The molecular mechanism of thermal unfolding of Escherichia coli formylmethionine transfer RNA. J Mol Biol. 1974 Jul 25;87(1):63–88. doi: 10.1016/0022-2836(74)90560-9. [DOI] [PubMed] [Google Scholar]
- Ghosh S. S., Musso G. F. Covalent attachment of oligonucleotides to solid supports. Nucleic Acids Res. 1987 Jul 10;15(13):5353–5372. doi: 10.1093/nar/15.13.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingeras T. R., Kwoh D. Y., Davis G. R. Hybridization properties of immobilized nucleic acids. Nucleic Acids Res. 1987 Jul 10;15(13):5373–5390. doi: 10.1093/nar/15.13.5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayase Y., Inoue H., Ohtsuka E. Secondary structure in formylmethionine tRNA influences the site-directed cleavage of ribonuclease H using chimeric 2'-O-methyl oligodeoxyribonucleotides. Biochemistry. 1990 Sep 18;29(37):8793–8797. doi: 10.1021/bi00489a041. [DOI] [PubMed] [Google Scholar]
- Hitaka T., Mizutani T., Watanabe K., Totsuka T. The high content of natural suppressor serine tRNA in dystrophic mouse muscle. Biochem J. 1990 Feb 15;266(1):201–206. doi: 10.1042/bj2660201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremsky J. N., Wooters J. L., Dougherty J. P., Meyers R. E., Collins M., Brown E. L. Immobilization of DNA via oligonucleotides containing an aldehyde or carboxylic acid group at the 5' terminus. Nucleic Acids Res. 1987 Apr 10;15(7):2891–2909. doi: 10.1093/nar/15.7.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazawa Y., Yokogawa T., Hasegawa E., Miura K., Watanabe K. The aminoacylation of structurally variant phenylalanine tRNAs from mitochondria and various nonmitochondrial sources by bovine mitochondrial phenylalanyl-tRNA synthetase. J Biol Chem. 1989 Aug 5;264(22):13005–13011. [PubMed] [Google Scholar]
- Manzini G., Xodo L. E., Gasparotto D., Quadrifoglio F., van der Marel G. A., van Boom J. H. Triple helix formation by oligopurine-oligopyrimidine DNA fragments. Electrophoretic and thermodynamic behavior. J Mol Biol. 1990 Jun 20;213(4):833–843. doi: 10.1016/S0022-2836(05)80267-0. [DOI] [PubMed] [Google Scholar]
- Mizutani T., Maruyama N., Hitaka T., Sukenaga Y. The detection of natural opal suppressor seryl-tRNA in Escherichia coli by the dot blot hybridization and phosphorylation by a tRNA kinase [corrected] . FEBS Lett. 1989 Apr 24;247(2):345–348. doi: 10.1016/0014-5793(89)81367-5. [DOI] [PubMed] [Google Scholar]
- Peattie D. A., Gilbert W. Chemical probes for higher-order structure in RNA. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4679–4682. doi: 10.1073/pnas.77.8.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilch D. S., Levenson C., Shafer R. H. Structural analysis of the (dA)10.2(dT)10 triple helix. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1942–1946. doi: 10.1073/pnas.87.5.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal P., Feigon J. NMR studies of triple-strand formation from the homopurine-homopyrimidine deoxyribonucleotides d(GA)4 and d(TC)4. Biochemistry. 1989 Sep 19;28(19):7859–7870. doi: 10.1021/bi00445a048. [DOI] [PubMed] [Google Scholar]
- Rich A., Kim S. H. The three-dimensional structure of transfer RNA. Sci Am. 1978 Jan;238(1):52–62. doi: 10.1038/scientificamerican0178-52. [DOI] [PubMed] [Google Scholar]
- Tsurui H., Suyama A., Wada A. Temperature gradient DNA-probe column chromatography: as tools for detection and purification of particular DNAs and RNAs. Nucleic Acids Symp Ser. 1988;(19):49–52. [PubMed] [Google Scholar]
- Wallace R. B., Shaffer J., Murphy R. F., Bonner J., Hirose T., Itakura K. Hybridization of synthetic oligodeoxyribonucleotides to phi chi 174 DNA: the effect of single base pair mismatch. Nucleic Acids Res. 1979 Aug 10;6(11):3543–3557. doi: 10.1093/nar/6.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokogawa T., Kumazawa Y., Miura K., Watanabe K. Purification and characterization of two serine isoacceptor tRNAs from bovine mitochondria by using a hybridization assay method. Nucleic Acids Res. 1989 Apr 11;17(7):2623–2638. doi: 10.1093/nar/17.7.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn M. H., Klug A. A model for the tertiary structure of mammalian mitochondrial transfer RNAs lacking the entire 'dihydrouridine' loop and stem. EMBO J. 1983;2(8):1309–1321. doi: 10.1002/j.1460-2075.1983.tb01586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]