Abstract
We describe the creation of a transgenic zebrafish expressing GFP driven by a 7.5 kb promoter region of the tbx16 gene. This promoter segment is sufficient to recapitulate early embryonic expression of endogenous tbx16 in the presomitic mesoderm, the polster and, subsequently, in the hatching gland. Expression of GFP in the transgenic lines later in development diverges to some extent from endogenous tbx16 expression with the serendipitous result that one line expresses GFP specifically in commissural primary ascending (CoPA) interneurons of the developing spinal cord. Using this line we demonstrate that the gene mafba (valentino) is expressed in CoPA interneurons.
Introduction
The production of transgenic organisms for research purposes has been a powerful method for accelerating the study of cell lineages in embryo development. This fact in combination with the genetic amenability and accessibility of zebrafish (Danio rerio) has stimulated production of an increased number of germline transgenic fish stocks available for such investigations. In particular, using zebrafish promoters linked to green fluorescent protein (GFP) has enabled recapitulation of the expression of various genes and the tracing of cells in embryos as they develop [1], [2], [3].
The zebrafish spadetail (spt) mutation was first discovered in a screen for recessive mutations affecting neuronal development [4] The gene affected by the spt mutation (tbx16) was subsequently found to be required for normal morphogenetic cell movement during mesoderm development [5]. The disruption to early development caused by the spt mutation results in a lack of trunk mesoderm with cells normally directed to this region accumulating to create a mass of cells at the distal end of the extending tail, the “spade" structure characteristic of these mutants [6], [7].
tbx16 encodes a member of the T-box family of transcription factors [8], [9]. tbx16 expression is found in presomitic paraxial mesoderm, the polster and hatching gland cells [9] and a subset of spinal cord cells (first reported by Ruvinsky et. al. (1998) but mistakenly identified as Rohon-Beard neurons). We later demonstrated that these cells are dorsal longitudinal ascending (DoLA) interneurons [10].
DoLAs have a seemingly irregular distribution along the rostrocaudal axis of the spinal cord, a pattern that is particularly difficult to dissect. We have recently shown that there is an underlying cryptic organisation to the rostrocausal and contralateral distribution of these cells [11]. Furthermore, we have shown that this distribution is, to some extent, created by the rostralwards migration of these cells shortly after their birth in the developing spinal cord [11]. Subsequently, we endeavoured to use these neurons to examine the molecules and mechanisms that establish irregular distributions of cells along the rostrocaudal axis in the spinal cord. The creation of a GFP transgenic zebrafish line under the control of the tbx16 promoter might allow us to examine aspects of DoLA neuron development and migration and examine paraxial mesoderm development in real time.
In this paper we describe an attempt to track the developmental expression of tbx16-expressing cells using a transgene possessing 7.5 kb of DNA sequence surrounding the site of transcription initiation of the tbx16 gene. Four transgenic lines of fish all displayed expression of GFP in the hatching gland progenitors, presomitic mesoderm, newly formed somites, and the hatching gland similar to endogenous tbx16. However no expression was observed in DoLA interneurons. Surprisingly one transgenic line of fish expressed GFP in commissural primary ascending (CoPA) interneurons and these cells are shown to be marked by transcripts of the gene v-maf musculoaponeurotic fibrosarcoma oncogene family, protein B-avian (mafba)/valentino.
Materials and Methods
Ethics Statement
The work was carried out under the auspices of the Animal Ethics Committee and the Institutional Biosafety Committee of the University of Adelaide (Permit number S-033-2006).
Embryos and staging
Zebrafish were maintained as described [12]. Embryos were collected and allowed to develop at 28.5°C to the required stage. Morphological features of embryos were consistent with the zebrafish staging guide [13].
Generation of tbx16: GFP transgenic lines
We obtained tbx16 genomic DNA by screening a zebrafish BAC construct library (Genome Systems, Inc., St. Louis, MO, USA) using a tbx16 cDNA probe. The BAC clone was analysed by Southern blot, and an EcoRI-BamHI fragment containing 5 kb of 5′ promoter DNA, the first exon and part of the first intron was cloned in frame upstream of GFP in the vector pEGFP-N1 (Clontech, Mountain View, CA, USA). The region for injection was excised from vector sequence, gel purified and was microinjected into the cytoplasm of embryos at the 1-cell stage. GFP expression was analysed before 24 hpf by observation under a fluorescence dissection microscope and embryos positive for GFP expression were raised to sexual maturity. Germline transgenic founders were identified by screening their F1 progeny for GFP fluorescence. Four founders (192A, 512B, 812A, 812C) were isolated, mated with wild-type fish, and their GFP positive offspring were raised to adulthood.
Detailed investigation of developing embryos by fluorescence microscopy was undertaken using a Zeiss Axioplan 2 deconvolution microscope with an AxioCam MRm camera (Carl Zeiss Jena GmbH, Jena, Germany).
Whole mount in-situ transcript hybridisation
The clones for production of probes against tbx16 and mafba transcripts have been described previously [10]. The GFP sequence was excised from pIRES2-EGFP (Clontech) and subcloned into pBluescript (Stratagene Products Division, La Jolla, CA, USA). The insert containing regions from these plasmids were amplified by PCR with M13 primers and then transcribed with T7 or T3 RNA polymerases to produce digoxigenin- (Sigma-Aldrich Corp., St. Louis, MO, USA) or fluorescein- (Sigma) labelled antisense riboprobes. Whole mount in situ transcript hybridisation was carried out essentially as described [14] but the two-colour staining reactions were undertaken initially with BCIP/NBT (F. Hoffmann-La Roche Ltd, Basel, Switzerland), and subsequently, the second staining reaction used the Vector Red Alkaline Phosphatase Substrate Kit I (SK-5100, Vector Laboratories Inc., Burlingame, CA, USA). Inactivation of the first alkaline phosphatase reaction was achieved by heating to 65°C for 1 hour in phosphate buffered saline. Older embryos were treated in proteinase K as described [14]. Light field observations were conducted under a Zeiss Axiophot microscope (Carl Zeiss) with a 20× objective using differential interference contrast (DIC) optics.
Results
Generation of tbx16:GFP transgenic zebrafish
We have previously shown that tbx16-expressing neurons are migratory and this migration contributes to their distribution in the developing spinal cord [11]. To examine further these cells including their origins and interaction with other cells of the spinal cord we attempted to label them with GFP by creating transgenic fish expressing GFP under the control of the tbx16 promoter. A 7.5 kb genomic DNA fragment including 5 kb of 5′ promoter (Fig. 1a) and 2.5 kb of transcribed tbx16 gene sequence (Fig. 1b) – the “tbx16 promoter" (Fig. 1c) was cloned upstream of EGFP in the pEGFP-N1 vector (Fig. 1d, see Materials and Methods). This promoter region included the site of insertion in the enhancer trap line CLGY6 that was shown to recapitulate tbx16 expression at 24 hpf [15]. Independent lines of transgenic zebrafish were generated by injecting linearised construct into single-cell embryos. Germline transmission was found in 2.6% of examined surviving adults (not all mosaic adults were screened). Four founders (192A, 512B, 812A, 812C) were isolated, mated with wild-type fish, and their GFP positive offspring were raised to adulthood. Founder germline mosaicism was determined to be between 4 and 40%. To confirm germline integration of the transgene, heterozygous GFP expressing F1 embryos were raised to sexual maturity and outbred to wild-type fish. Transgenic F2 progeny were generated at approximately 50% (49–52%, n>870 for each line) indicating a single insertion site in each founder, and a typical Mendelian inheritance pattern. Each line has consistently retained its pattern of GFP expression for at least 3 generations.
Figure 1. Genomic region of the tbx16 gene and transgenic construct.
(a) EcoRI and BamHI restriction sites in the genomic region of the tbx16 gene surrounding the transcription initiation site. The interval marked “CLGY6" indicates the published enhancer trap insertion site resulting in GFP expression that recapitulates tbx16 gene expression [15]. (b) Boxes indicate the first three exons and filled boxes are protein-coding regions. The start of translation is shown (ATG). (c) The EcoRI/BamHI fragment of the tbx16 promoter used in the transgenic construct includes the translation start site, the first exon, and part of the second exon of the tbx16 gene. (d) The promoter fragment was cloned in-frame upstream of enhanced GFP (EGFP) and a SV40 polyadenylation signal (A) in the multiple cloning site of the pEGFP-N1 vector.
Consistent expression of tbx16:GFP in embryonic zebrafish
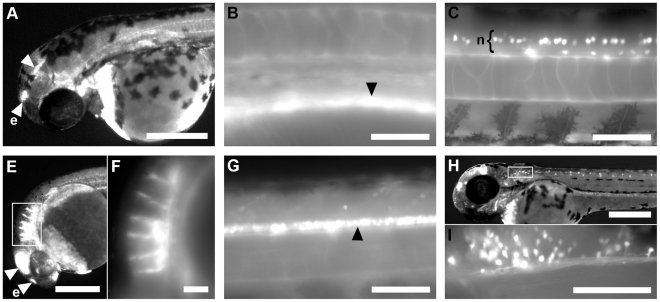
We examined transgenic lines for temporal and spatial expression of GFP. All examined transgenic lines displayed extensive GFP fluorescence consistent with the expression of tbx16 transcript in wild type embryos. Transgene expression was first detected at 12 hpf in the hatching gland progenitors, presomitic mesoderm and the tail bud (Fig. 2a). GFP expression at this stage was also noted throughout the somitic mesoderm indicating that the protein persists longer in the developing mesoderm than endogenous tbx16 transcripts. Expression at 24 hpf is seen in the hatching gland and in the posterior somites while expression has faded from the earliest formed somites (Fig. 2b). By 48 hpf GFP expression in somitic mesoderm has diminished in all lines except 192A, allowing cells of the spinal cord to be viewed (Fig. 2c). It is unknown when spinal cord neurons commence expression of GFP due to the masking effect of overlying somitic mesoderm obscuring more medial tissues. At 48 hpf all lines strongly express GFP in the hatching gland. Peculiarly, all lines show some level of expression in the notochord (Fig. 2d). Line 192A displays persistent low-level somitic mesodermal expression at 96 hpf (not shown).
Figure 2. GFP expression in stable transgenic lines consistent with tbx16 expression in wild type embryos.
(a) At 12 hpf strong expression is seen in the polster (arrowhead), and the presomitic mesoderm (p). Expression persists in mesoderm that has formed somites (s). (b) At 24 hpf GFP expression is seen in the hatching gland (arrowhead) and in posterior somites, while it has faded from the earliest formed somites. (c) At 48 hpf expression remains in the hatching gland that has formed from the polster (arrowhead) and persists in the posterior somites. (d) The same embryo as in (c). GFP expression is observed throughout the notochord (n) highlighting cell extremities. Lateral views of embryos, anterior is left and dorsal is up. Scale bars in a–c = 250 µm, in d = 100 µm.
Tissue specific GFP fluorescence patterns
The earliest GFP expression appears to be consistent among the lines, with line-specific expression beginning later in development. At 24 hpf line 192A displays GFP expression in cells in the midbrain (not shown). At 48 hpf (and 96 hpf) this expression pattern has coalesced into two distinct regions that appear to consist of distinct cells in the midbrain and the epiphysis (Fig. 3a). At 24 hpf line 512B expresses GFP in the dorsal aorta (Fig. 3b) and later at 48 hpf in ventrally projecting neurons in the ventral spinal cord (Fig. 3c). These neural cells persist until at least 96 hpf (not shown). 24 hpf embryos of the 812A line have a large portion of the midbrain expressing GFP along with the epiphysis and show a distinct banding pattern in the hindbrain (Fig. 3d) that appears to be related to the rhombomeres (Fig. 3e). This pattern disappears by 48 hpf. At 96 hpf the line 812A expresses GFP in the floor plate of the spinal cord (Fig. 3f). In line 812C at 48 hpf we observe expression of GFP in the epiphysis and regions of the midbrain. This line also shows expression in dorsal neurons in the spinal cord in an apparently irregular rostrocaudal distribution that appears similar to that of DoLA interneurons (Fig. 3g). These embryos display expression in distinct neural cells in the hindbrain (Fig. 3h). Tissue specific expression is summarised in Table 1.
Figure 3. Expression unique to individual stable transgenic lines.
(a) Line 192A at 48 hpf. GFP expression is observed in regions of the midbrain (arrowhead) and in the epiphysis (e). Line 512B at 24 hpf (b) and 48 hpf (c). Expression is seen in the dorsal aorta and in ventral neurons (n) of the spinal cord. Line 812A at 24 hpf (d–e) and at 48 hpf (f). Expression can be seen in the epiphysis, the midbrain and in a banding pattern in rhombomeres (boxed). White box indicates the area enlarged in (e). Later expression is observed in the floor plate of the spinal cord (f). (g–h) Line 812C at 48 hpf. Expression is seen in the epiphysis and the midbrain. GFP is also noted in a specific subpopulation of spinal cord neurons and neurons posterior of the otic vesicle in the hindbrain (boxed). White box indicates the area enlarged in (h). Lateral views of embryos, anterior is left and dorsal is up. Scale bars in a, d, g = 250 µm, in b, c, e, f, h = 50 µm.
Table 1. Tissue specific GFP fluorescence patterns.
| 24 hpf | 48 hpf | 96hpf | |
| 192A | midbrain | midbrainepiphysis | midbrainepiphysis |
| 512B | dorsal aorta | dorsal aortaventral spinal cord neurons | ventral spinal cord neurons |
| 812A | midbrainepiphysishindbrain/rhombomeres | N/A | floor plate |
| 812C | N/A | epiphysismidbrain neuronsCoPA interneurons | N/A |
GFP expression diverges between the transgenic lines at later developmental time points. Expression of GFP is noted in numerous tissues over the first 96 hpf (N/A: not applicable).
Neurons expressing GFP in the spinal cord of the 812C line are comissural primary ascending interneurons (CoPAs)
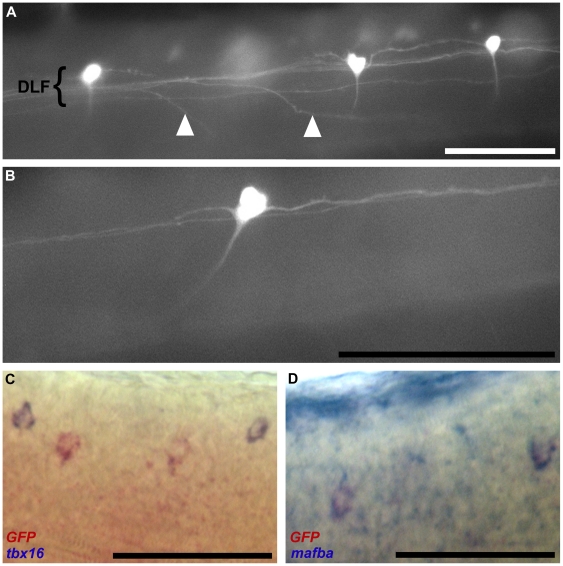
We have previously shown that the tbx16-expressing cells of the spinal cord are the dorsal longitudinal ascending (DoLA) interneurons. GFP positive spinal cord cells observed in the 812C line (Fig. 3g) exhibit characteristics consistent with DoLA cells; both cell types show an apparent irregular rostrocaudal distribution of approximately 20–25 cells per embryo with similar dorsoventral positioning and they occur at similar developmental times. Upon closer examination, the GFP expressing cells have properties differing from the published morphology of DoLA cells [16], [17]. Although the GFP expressing neurons are distributed in an apparently irregular rostrocaudal pattern and can be found along the dorsal longitudinal fasciculus (Fig. 4a) they have ascending and descending longitudinal projections, and a single ventral projecting axon that crosses the midline and ascends in the DLF contralateral to the cell body (Fig. 4a, b). The morphology and distribution of these cells is consistent with them being commissural primary ascending (CoPA) interneurons.
Figure 4. Identification of spinal cord neurons in line 812C at 48 hpf.
(a) Lateral view of three GFP-positive neural cells situated in a non-uniform distribution along the dorsal longitudinal fasiculus (DLF) indicated by a tight grouping of neuronal projections. Axons projecting from neurons located in the contralateral DLF can be seen to rise from the ventral midline before joining the in-focus DLF (arrowheads). (b) Lateral view of a single GFP-positive neuron illustrating the projections originating from the cell. Two projections extend within the DLF – one ascending and one descending – and a third ventrally extending axon turns anteriorly and fades from view as it approaches the ventral midline before ascending in the contralateral DLF (not shown). (c) Two-colour in situ transcript hybridisation indicating that GFP-positive cells (stained red) are not the tbx16-positive dorsal longitudinal ascending interneurons (stained blue). (d) Two-colour in situ transcript hybridisation showing that neurons expressing GFP (stained red) also express mafba (stained blue). All embryos are positioned anterior left and dorsal up. Scale bars indicate 100 µm.
To confirm that the 812C transgenic line is not expressing GFP in the DoLA neurons, we undertook in situ transcript hybridisation using probes against GFP and tbx16 simultaneously. This showed that the neurons expressing GFP do not simultaneously express tbx16, although the two cell types are found at the same dorsoventral level of the developing spinal cord (Fig. 4c). We examined the expression of another transcript, mafba known to be expressed in a similar distribution in the developing spinal cord but not in spt-expressing DoLA neurons {Tamme, 2002 #8} and discovered that the mafba-expressing cells show coexpression of GFP (Fig. 4d). Thus expression of mafba appears to mark CoPA interneurons.
Discussion
In this paper we have described an attempt to produce transgenic zebrafish to track the developmental expression of tbx16 by using GFP fused to tbx16 promoter sequence.
We used a 7.5 kb segment of genomic DNA that encompassed 5 kb of the 5′ promoter and includes the translation start site, the first exon, and part of the second exon of the tbx16 gene. Extensive outbreeding has shown that these lines are all single insertion site transgenic lines that show typical Mendelian inheritance.
The four lines of fish examined display expression of GFP in the hatching gland progenitors (the polster), presomitic mesoderm, newly formed somites, and the hatching gland consistent with endogenous tbx16 expression and perdurance of GFP from expression in progenitor tissues. However, none of the lines express GFP in the DoLA neurons which was the original purpose for which they were created. Later in development expression of GFP in the four lines diverges somewhat from endogenous tbx16 expression and from each other. Persistent expression of enhanced GFP in lateral tissues can mask expression in deeper medial tissues, suggesting that destabilised GFP might have been a better choice for creating these particular transgenic zebrafish.
The common differences in expression between the transgenic lines and endogenous tbx16 expression (e.g. absence of expression in DoLAs in the transgenic fish) are most likely due to the absence of some tbx16 promoter regulatory sequences from the construct. Our construct includes 5 kb of 5′ promoter sequence, an amount that has been shown to be sufficient to replicate the expression patterns of many endogenous genes in transgenic fish [18], [19], [20]. However, the work of Kikuta et. al. [21] has shown that genes can be classified into two broad types depending on whether their transcription is regulated by elements close to the transcription initiation site (“bystander genes") or dispersed over relatively large genetic distances defining “genomic regulatory blocks" (GRBs). Typically, the genes regulated by elements dispersed over GRBs are those regulating embryo development such as tbx16.
The particular differences in transgene expression between the individual lines are more likely due to the influence upon their transcription of different regulatory elements flanking the transgene insertion sites in the genome. Interestingly, one transgenic line, 812C, uniquely expresses GFP in a subset of spinal interneurons. These cells are distributed in a seemingly irregular pattern and can be found along the dorsal longitudinal fasciculus (Fig. 4a). They have ascending and descending longitudinal projections, and a single ventral projecting axon that crosses the midline and ascends in the DLF contralateral to the cell body (Fig. 4a, b). The morphology and distribution of these cells is consistent with them being commissural primary ascending (CoPA) interneurons. Furthermore these cells have been shown to be marked by transcripts of the mafba gene. The 812C transgenic line may be useful for studies of the birth and development of CoPAs and their projections.
The zebrafish spinal cord consists of a limited number of discrete neuronal cell types. Although it is possible to distinguish each of these types by their spatiotemporal position and morphology few unique molecular markers of these cells have been discovered. Circumferential Ascending (CiA) interneurons express Eng1b transcripts [22]. alx is expressed in Circumferential Descending (CiD) interneurons although it appears, perhaps, that not all CiD neurons express this gene [23]. We previously showed that DoLA cells express tbx16 [10]. We believe that the gene mafba – the expression pattern of which superficially resembles that of tbx16 that marks DoLAs in the spinal cord - may be a unique marker of another zebrafish spinal neuron, the CoPA interneuron. Since DoLAs and CoPAs share very similar dorsoventral positions and rostrocaudal distributions in the spinal cord, it is interesting to speculate that regulatory elements within our transgene are cooperating with elements flanking the transgene insertion site to alter activation of transcription away from DoLAs so that it occurs instead in CoPAs. Identification of the GRB in which our 812C insertion has occurred may help to explain this phenomenon. However, the idea that some elements required for neuronal expression occur within the 7.5 kb of DNA from the tbx16 locus within our transgene is supported by the fact that all of our transgenic lines show expression at some stage in neurons.
Acknowledgments
We thank Juliane Cohen for fluorescence microscopy work.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by funds from the Australian Research Council via its Special Research Centre for the Molecular Genetics of Development (S00001531). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chen JY, Chiou MJ. Molecular cloning and functional analysis of the zebrafish luteinizing hormone beta subunit (LH<beta>) promoter. Fish Physiol Biochem. 2010;2010:5. doi: 10.1007/s10695-010-9405-8. [DOI] [PubMed] [Google Scholar]
- 2.Gong Z, Ju B, Wang X, He J, Wan H, et al. Green fluorescent protein expression in germ-line transmitted transgenic zebrafish under a stratified epithelial promoter from keratin8. Dev Dyn. 2002;223:204–215. doi: 10.1002/dvdy.10051. [DOI] [PubMed] [Google Scholar]
- 3.Mione M, Baldessari D, Deflorian G, Nappo G, Santoriello C. How neuronal migration contributes to the morphogenesis of the CNS: insights from the zebrafish. Dev Neurosci. 2008;30:65–81. doi: 10.1159/000109853. [DOI] [PubMed] [Google Scholar]
- 4.Streisinger G, Walker C, Dower N, Knauber D, Singer F. Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nature. 1981;291:293–296. doi: 10.1038/291293a0. [DOI] [PubMed] [Google Scholar]
- 5.Kimmel CB, Kane DA, Walker C, Warga RM, Rothman MB. A mutation that changes cell movement and cell fate in the zebrafish embryo. Nature. 1989;337:358–362. doi: 10.1038/337358a0. [DOI] [PubMed] [Google Scholar]
- 6.Ho RK, Kane DA. Cell-autonomous action of zebrafish spt-1 mutation in specific mesodermal precursors. Nature. 1990;348:728–730. doi: 10.1038/348728a0. [DOI] [PubMed] [Google Scholar]
- 7.Molven A, Wright CV, Bremiller R, De Robertis EM, Kimmel CB. Expression of a homeobox gene product in normal and mutant zebrafish embryos: evolution of the tetrapod body plan. Development. 1990;109:279–288. doi: 10.1242/dev.109.2.279. [DOI] [PubMed] [Google Scholar]
- 8.Griffin KJ, Amacher SL, Kimmel CB, Kimelman D. Molecular identification of spadetail: regulation of zebrafish trunk and tail mesoderm formation by T-box genes. Development. 1998;125:3379–3388. doi: 10.1242/dev.125.17.3379. [DOI] [PubMed] [Google Scholar]
- 9.Ruvinsky I, Silver LM, Ho RK. Characterization of the zebrafish tbx16 gene and evolution of the vertebrate T-box family. Dev Genes Evol. 1998;208:94–99. doi: 10.1007/s004270050158. [DOI] [PubMed] [Google Scholar]
- 10.Tamme R, Wells S, Conran JG, Lardelli M. The identity and distribution of neural cells expressing the mesodermal determinant spadetail. BMC Dev Biol. 2002;2:9. doi: 10.1186/1471-213X-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wells S, Conran JG, Tamme R, Gaudin A, Webb J, et al. Cryptic organisation within an apparently irregular rostrocaudal distribution of interneurons in the embryonic zebrafish spinal cord. Exp Cell Res. 2010;2010:1. doi: 10.1016/j.yexcr.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Westerfield M. The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio) Eugene: University of Oregon Press; 2000. [Google Scholar]
- 13.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 14.Jowett T. Tissue In Situ Hybridization: Methods in Animal Development. New York: John Wiley & Sons, Inc; 1997. 128 [Google Scholar]
- 15.Ellingsen S, Laplante MA, Konig M, Kikuta H, Furmanek T, et al. Large-scale enhancer detection in the zebrafish genome. Development. 2005;132:3799–3811. doi: 10.1242/dev.01951. [DOI] [PubMed] [Google Scholar]
- 16.Kuwada JY, Bernhardt RR, Nguyen N. Development of spinal neurons and tracts in the zebrafish embryo. J Comp Neurol. 1990;302:617–628. doi: 10.1002/cne.903020316. [DOI] [PubMed] [Google Scholar]
- 17.Roberts A. Early functional organization of spinal neurons in developing lower vertebrates. Brain Res Bull. 2000;53:585–593. doi: 10.1016/s0361-9230(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 18.Ju B, Chong SW, He J, Wang X, Xu Y, et al. Recapitulation of fast skeletal muscle development in zebrafish by transgenic expression of GFP under the mylz2 promoter. Dev Dyn. 2003;227:14–26. doi: 10.1002/dvdy.10273. [DOI] [PubMed] [Google Scholar]
- 19.Park HC, Kim CH, Bae YK, Yeo SY, Kim SH, et al. Analysis of upstream elements in the HuC promoter leads to the establishment of transgenic zebrafish with fluorescent neurons. Dev Biol. 2000;227:279–293. doi: 10.1006/dbio.2000.9898. [DOI] [PubMed] [Google Scholar]
- 20.Yeo SY, Kim M, Kim HS, Huh TL, Chitnis AB. Fluorescent protein expression driven by her4 regulatory elements reveals the spatiotemporal pattern of Notch signaling in the nervous system of zebrafish embryos. Dev Biol. 2007;301:555–567. doi: 10.1016/j.ydbio.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 21.Kikuta H, Laplante M, Navratilova P, Komisarczuk AZ, Engstrom PG, et al. Genomic regulatory blocks encompass multiple neighboring genes and maintain conserved synteny in vertebrates. Genome Res. 2007;17:545–555. doi: 10.1101/gr.6086307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higashijima S, Masino MA, Mandel G, Fetcho JR. Engrailed-1 expression marks a primitive class of inhibitory spinal interneuron. J Neurosci. 2004;24:5827–5839. doi: 10.1523/JNEUROSCI.5342-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura Y, Okamura Y, Higashijima S. alx, a zebrafish homolog of Chx10, marks ipsilateral descending excitatory interneurons that participate in the regulation of spinal locomotor circuits. J Neurosci. 2006;26:5684–5697. doi: 10.1523/JNEUROSCI.4993-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]