Abstract
Fruits of the native South American tree Melicoccus bijugatus Jacq. (Sapindaceae) are consumed for both dietary and medicinal purposes, but limited information is available about the phytochemistry and health value of M. bijugatus fruits. Fruit tissues of the Florida Montgomery cultivar were assessed for sugars, using gas chromatography, and for total phenolics, using UV spectroscopy. Reverse phase high performance liquid chromatography (HPLC) fingerprints of crude methanolic pulp, embryo and seed coat extracts were obtained at 280 nm. Phenolics were characterised by both HPLC UV/vis analysis and HPLC electrospray ionization tandem mass spectrometry. Major sugars detected in the pulp and embryo extracts were sucrose, followed by glucose and fructose. The glucose:fructose ratio was 1:1 in the pulp and 0.1:1 in the embryo. Total phenolic concentrations of the fruit tissues were in the order: seed coat > embryo > pulp. Phenolic acids were identified mostly in pulp tissues. Phenolic acids, flavonoids, procyanidins and catechins were identified in embryo tissues, and higher molecular weight procyanidins were identified in seed coat tissues. This study provides new information about the phytochemistry and the potential health value of the Montgomery cultivar M. bijugatus fruit tissues.
Keywords: Melicoccus bijugatus, fruit phenolics, fruit sugars, HPLC, LC-MS/MS
1. Introduction
Melicoccus bijugatus Jacq., also known as Spanish lime, mamoncillo or genip, is a South American woody dicotyledenous tree in the Sapindaceae family, otherwise known as the Soapberry family (Acevedo-Rodriguez, 2003). M. bijugatus fruits are related to the more commonly known Asian fruit species longan (Dimocarpus longan Lam.), lychee (Litchi chinensis L.) and rambutan (Nephelium lappaceum L.) (Zomlefer, 1994). Although the fruits of M. bijugatus are consumed for both medicinal and dietary purposes, research on the fruit phytochemistry, especially the secondary metabolites and their associated biological activities, is nearly nonexistent.
M. bijugatus fruits have a green leathery skins, covering a fleshy salmon-coloured pulp (sarcotesta) layer that adheres to a crustaceous seed coat containing the embryo (Acevedo-Rodriguez, 2003). The sweet and astringent fruit pulp is usually consumed fresh and occasionally made into jelly, pies, or cold drinks (Morton, 1987). Analysis of the food value per 100 g of fresh fruit pulp from Cuba, Central America and Columbia, indicated 0.50–1.0 g protein, 0.08–0.2 g fat, 13.5–19.2 g carbohydrates, small amounts of phosphorus (9.8–23.9 mg) and calcium (3.4–15 mg), 0.47–1.19 mg of iron, 0.8–10 mg of ascorbic acid and 0.02–0.44 mg of carotene (70 IU) (Morton, 1987). Citric acid was the major organic acid and malic acid, succinic acid and acetic acids were minor constituents in fruit pulp of several cultivars in Puerto Rico (Sierra-Gómez, 2006).
In Cuba, pulp juice is reportedly used to treat hypertension (Beyra et al., 2004). Other traditional uses of the pulp include treatment for asthma or respiratory problems and constipation (Liogier, 1990). Seeds are noted for their astringent properties; they are used to treat diarrhoea, especially in children, and ground into a flour by indigenous people of the Orinoco region (i.e., Venezuela, Columbia) to make a bread used as a substitute for cassava (Vega, 1997; Liogier, 1990).
M. bijugatus fruits are usually obtained from the wild and sold at markets in northern South America and the Caribbean (Acevedo-Rodriguez, 2003; Sierra-Gómez, 2006). Several cultivars of M. bijugatus are grown in Puerto Rico and in Florida. Montgomery and Queen are the main cultivars (Morton, 1987). The especially popular Montgomery cultivar has several desirable fruit qualities, including large size, high pulp content (51.5%), good flavour and high yield (Morton, 1987).
Both phenolics and sugars were investigated because of the prevalence of these types of compounds in fruits and because of their reported health benefits. Plant phenolics are associated with the prevention and treatment of several health conditions, including diabetes (Johnston, Clifford & Morgan, 2003), gastrointestinal disorders (Schuier, Sies, Illek & Fischer, 2005) and cardiovascular disease (Jiang & Dusting, 2003). Sugar derivatives (e.g., cyclitols) found in some types of seeds, reportedly have anti-diabetic potential (Ortmeyer, Larner & Hansen, 1995) whilst other sugars (e.g., mannose derivatives) are reported to promote immune function (Campbell, Busbee & McDaniel, 1997), and certain sugar ratios prevent gastrointestinal problems (Hyams, Etienne, Leichtner & Theuer, 1988; Goldstein, Braverman & Stankiewicz, 2000). The objective of this study was to characterise phenolics and sugars in the M. bijugatus Montgomery cultivar fruits, especially compounds associated with medicinal uses or other beneficial health effects, and to compare edible (pulp, embryo) and nonedible (seed coat) fruit tissues.
2. Material and methods
2.1. Plant material
M. bijugatus ‘Montgomery’ cultivar fruits were harvested from Lara’s Farm in Homestead, Florida, during the summer, when they were ripe and edible. All fruits were rapidly transported in a cooler to Cornell University (Ithaca, NY) and were immediately separated into pulp, seed coat and embryo components. Duplicates of each tissue sample from four fruits of similar size were prepared for analysis.
2.2. Chemicals and materials
The Folin-Ciocalteu reagent (2.0 N), p-coumaric acid, caffeic acid, procyanidin B1 and procyanidin B2 were purchased from Sigma-Aldrich Chemicals (St. Louis, MO). Prunin and naringenin were purchased from Indofine Chemicals (Hillsborough, NJ). (−)-Epicatechin, (+)-catechin, (−)-epigallocatechin, gallic acid, ellagic acid and phloridzin were purchased from ChromaDex (Irvine, CA). Silica Gel 60 F254 aluminium plates were purchased from Merck (Darmstadt, Germany).
2.3. Sample extraction
The skin was peeled from the fruit and the pulp scraped off the seed into a beaker. The seed was left to air-dry overnight, and any remaining pulp material was removed before the seed coat was separated from the embryo. Eight seeds were roasted at 160 °C for 25 min. After cooling, the seed coats were separated and discarded. Embryos from roasted and unroasted seeds and seed coats from unroasted seeds were ground into a paste or powder. Approximately 15 g of fresh pulp or embryo material were weighed and extracted in 200 ml of 80% methanol or 70% acetone. Seed coats (3.0 g) were extracted in 80 ml of solvent. These mixtures were shaken for one hour, left overnight at 22 °C, and filtered the next day. The filtrate residue was re-extracted with 50 ml of the same solvent and filtered through Whatman No.1 filter paper.
Crude extracts were filtered with a Phenex (Phenomenex, Torrance, CA) nylon syringe filter (17 mm, 0.45 µm) and used directly for high performance liquid chromatography (HPLC) analysis or dried using a rotary evaporator below 40 °C to a syrup-like consistency, and dried in vacuo below 40 °C to constant weight. Dried extracts were stored at 4 °C and dissolved in 50% methanol solution for total phenolic analysis. Semi-purified extracts were prepared by evaporating alcohol from crude extracts and partitioning aqueous extracts, first with hexane, and then partitioning the aqueous layer with ethyl acetate. Semi-purified ethyl acetate extracts were dried under nitrogen to remove ethyl acetate, mixed with small amounts of 70% methanol and microfiltered before HPLC injection or mass spectrometry analysis.
2.4. GC analysis of sugars
High-resolution gas chromatography (GC) was used to determine sugar concentrations in M. bijugatus fruits, following the methods described by Horbowicz and Obendorf (1994). Phenyl α-d-glucoside was added as an internal standard to the fruit extracts. Samples were dried under N2 gas and stored overnight above P2O5 to remove traces of water. Dried residues were derivatised with 100 µl of 1-(trimethylsilyl)imidazole (TMSI): pyridine (1:1, v/v) and analysed on a Hewlett-Packard 6890 GC (Agilent Technologies, Palo Alto, CA, USA) equipped with a flame ionization detector, split-mode injector (1:50), and an HP-1MS capillary column (15 m length, 0.25 mm i.d., 0.25 µm film thickness). Quantities of soluble carbohydrates in pulp and embryo 70% methanol extracts were calculated from standard curves.
2.5. Total phenolics
Total phenolic content of each extract was measured as described by Emmons, Peterson and Paul (1999). Briefly, 1 ml of extract was mixed with 500 ml of Folin-Ciocalteu reagent (2.0 N) and 3 ml of Na2CO3 (200 g/l). The mixtures were vortexed, left at room temperature for 15 min, diluted with 10 ml deionised water and centrifuged. Absorbance was measured at 725 nm. Total phenolics were calculated from a gallic acid standard curve and reported as mg gallic acid equivalents (GAE)/g of dried extract.
2.6. LC-UV/Vis and TLC analyses of phenolics
Fruit extracts (20 µl) were analysed using an HP 1100 HPLC system (Hewlett-Packard, Palo Alto, CA) equipped with a variable wavelength UV detector (190–600 nm) and a LiCrospher RP-18 column (5-µm, 4.6 × 250 mm) connected to a guard column (Phenomenex, Torrance, CA). Mobile phases were 0.4% formic acid (solvent A) and methanol (Solvent B) at a flow rate of 1 ml/min with the detector set at 280 nm and at 320, 370 and 520 nm. The gradient system, according to the methods of Rangkadilok, Worasuttayangkurn, Bennett and Satayavivad (2005) was 0 min (100% A), 2 min (95% A), 5 min (70% A), 8 min (66% A), 11 min (45% A) and 17–20 min (100% A). Using these methods, 10 µl of concentrated extract from evaporated semi-purified ethyl acetate extracts were injected into the HPLC five times, and the appropriate peaks of interest were collected in a vial. Thin-layer chromatography (TLC) was used to confirm the identity of compounds in collected peak fractions by separating fractions on silica gel plates with ethyl acetate:methanol:H2O (77:15:8, v/v/v). Dried TLC plates were sprayed with vanillin-sulphuric acid solution and heated to observe the colour reaction.
2.7. LC-PDA analyses of isolated peaks from pulp extracts
A Waters HPLC system equipped with a 996 photodiode array detector (PDA), 600S controller, 626 quaternary gradient pulp, 717 autosampler, data acquisition software (Millennium ver. 4.0), and a Symmetry C18 reverse-phase column (5-µm, 4.6 × 250 mm) was used for chromatographic separations at 25°C, as described by Hu, Cheng, Heller, Krasnoff, Glahn and Welch (2006). Mobile phases were 50 mM (NH4)H2PO4 at pH 2.6 (solvent A), 80:20 (v/v) acetronitrile/solvent A (solvent B), and 200 mM H3PO4 at pH 1.5 (solvent C). The gradient system was 0 min (100% A), 4 min (92% A and 8% B), 10 min (14% B and 86% C), 22.5 min (16.5% B and 83.5% C), 27.5 min (25% B and 75% C), 50 min (80% B and 20% C), and 55–60 min (100% A).
2.8. LC-MS/MS analyses of fruit extracts
Extracts analysed by HPLC tandem mass spectrometry (LC-MS/MS) were prepared in 0.1% formic acid. An Agilent 1100 HPLC system was equipped with a UV detector and a Vydac C18 (5 µm/300 Å/10 × 150 mm) column attached to a Brownlee C18 guard column. Mobile phases were 0.2% formic acid in water (solvent A) and 0.2% formic acid in methanol (solvent B). The gradient was 0 min (15% A), 4 min (20% A), 7 min (45% A), 10 min (50% A), 13 min (80% A) and 19–45 min (15% A). Column effluent was monitored at 280 nm, and mass spectra data were acquired by electrospray ionization (ESI) in negative ion/positive ion mode with a Bruker Esquire Mass spectrometer. For semi-purified ethyl acetate extracts, the data collection time was extended from 20 min to 25 min. Three peaks isolated from semi-purified ethyl acetate pulp extracts were analysed by infusion mass spectrometry (2 µl/min) by ESI in negative and positive ion mode.
2.9. Statistical analysis
Data were reported as means ± SEM for duplicate samples. Statistical comparisons were calculated by analysis of variance, using transformed values (log of responses) for sugars and phenolics or an independent t test for comparison of the total phenolics in roasted and nonroasted seeds. Significant differences (p < 0.05), after a Tukey correction for multiple comparisons, were determined using JMP Statistical Discovery software for Windows Version 7.0, SAS Institute Inc. (Cary, NC).
3. Results and discussion
3.1. Sugar concentrations and total phenolics
Sweetness of the fruit pulp can be attributed to high sugar concentrations (Table 1). Sucrose, glucose and fructose were the major sugars detected in both pulp and embryo tissues, with sucrose concentrations significantly higher than glucose or fructose concentrations. All three sugar concentrations were significantly higher in pulp than in embryo tissues. The glucose: fructose ratio was 1:1 in the pulp and 0.1:1 in the embryo extracts. Raffinose family oligosaccharides, cyclitols or galactosyl cyclitols were not detected in pulp or embryo extracts, but small amounts of mannose and traces of raffinose were tentatively identified in pulp extracts (data not shown).
Table 1.
Sugar concentrations (g) of pulp and embryo extracts per 100 g of fresh fruit tissue (means ± SEM, n = 2)a
| Sugar | Pulp | Embryo |
|---|---|---|
| Sucrose | 7.72 ± 0.11 a | 2.26 ± 0.12 b |
| Glucose | 1.71 ± 0.08 c | 0.04 ± 0.00 e |
| Fructose | 1.72 ± 0.06 c | 0.32 ± 0.01 d |
| Glucose/fructose ratio | 0.99 ± 0.01 | 0.12 ± 0.01 |
Means not connected by the same letter are significantly different (p < 0.05) after a Tukey correction for multiple comparisons.
Total phenolics in the three fruit tissue extracts were all significantly different in both 70% acetone and 80% methanol extracts (Table 2). Total phenolic concentrations were, in decreasing order: seed coat > embryo > pulp. The 70% acetone extracts had significantly higher total phenolic concentrations than the 80% methanol extracts (Table 2). Total phenolics in the 80% methanol extracts were not significantly different between roasted and non-roasted embryos, suggesting that roasting, which is usually done for consumption, had minimal effect on extracted or soluble phenolic concentrations in 80% methanol extracts.
Table 2.
Total phenolics of fruit tissue extracts (means ± SEM, n = 2)a
| Tissue | 80% methanol extracts (mg GAE/g dried extract) | 70% acetone extracts (mg GAE/g dried extract) |
|---|---|---|
| Pulp | 12.2 ± 0.19 f | 32.3 ± 0.38 d |
| Embryo | 23.2 ± 0.03 e | 52.3 ± 1.92 c |
| Roasted embryo | 20.5 ± 1.5 e | ND |
| Seed coat | 184 ± 1.14 b | 214 ± 1.52 a |
Means not connected by the same letter are significantly different (P < 0.05) after a Tukey correction for multiple comparisons; GAE, gallic acid equivalents; ND , not determined.
3.2. LC-UV/Vis fingerprint analyses of phenolics in fruit tissue extracts
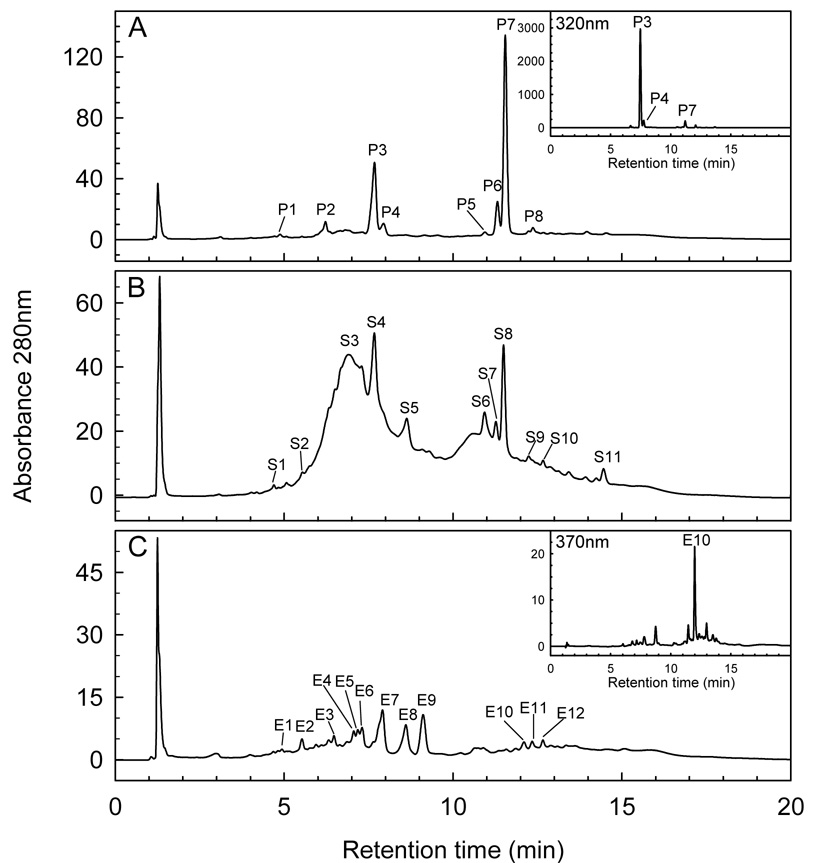
Pulp, embryo and seed coat crude extract fingerprints were obtained by HPLC with a UV/vis detector (LC-UV/vis) at 280 nm. Comparison of retention times and UV/vis data with those of standard compounds revealed several phenolic compounds (Fig. 1). A small gallic acid peak (4.9 min) was detected in all three tissues (P1, S1, E1) (Fig. 1A–C). Seed coat extracts showed a broad peak (S3; 6.3–7.2 min), characteristic of condensed tannins with catechin subunits (Guyot, Doco, Souquet, Moutounet & Drilleau, 1997). Catechin derivatives, including procyanidin B1 (epicatechin-(4β→8)catechin), (epi)gallocatechin, catechin and procyanidin B2 (epicatechin-(4β→8)-epicatechin), all had retention times within the range of peak S3 (Fig. 1B). These compounds were also identified by individual peaks from embryo extracts as procyanidin B1 (E3; 6.47 min), (epi)gallocatechin (E4; 7.06 min), catechin (E5; 7.17 min), and procyanidin B2 (E6; 7.3 min) (Fig. 1C).
Fig. 1.
Fingerprint HPLC chromatograms of crude 80% methanol fruit tissue extracts: (A) pulp; (B) seed coat; (C) embryo. Peaks and corresponding phenolic standards: P1,S1,E1 (gallic acid); S2,E3 (procyanidin B1); S3,E4 (epigallocatechin); S3,E5 (catechin); S3,E6 (procyanidin B2); S5,E8 (epicatechin), P5,S6 (p-coumaric acid); E10 (prunin); P8, S9, E11(phloridzin).
Peak P3 had the highest peak at 320 nm in pulp extracts (Fig. 1A insert) and similarly for peak S4 (Bystrom, 2007). Seed coat and embryo extracts showed peaks representative of epicatechin (S5, E8; 8.6 min) (Fig. 1B,C). Identification of epicatechin at 8.6 min is consistent with LC-UV/vis analysis of seed extracts of the related species longan (Dimocarpus longan Lam.) (Rangkadilok et al., 2005). Both pulp and seed coat extracts had peaks (P5, S6; 10.9 min) corresponding to p-coumaric acid (Fig. 1A,B). Embryo extracts indicated a peak (E10; 12.1 min) with the same retention time as the flavonoid prunin (naringenin-7-glucoside). Peak E10 had the highest absorbance at 370 nm (Fig. 1C insert), further confirming a flavonoid (Harborne, 1984). Peaks from pulp, seed coat and embryo extracts (P8, S9, E11) correspond to phloridzin (12.38 min), a type of flavonoid detected at lower wavelengths, i.e., UV/vis maxima are at 285, 230 nm (Fig. 1 A–C) (Hilt et al., 2003). Several peaks not identified by LC-UV/Vis analysis include: S2, E2 at 5.5 min; P2 at 6.2 min; P3, S4 at 7.6 min; P4, E7 at 7.9 min; E9 at 9.1 min; P6, S7 at 11.3 min; P7, S8 at 11.5 min; S10, E12 at 12.65 min and S11 at 14.45 min (Fig 1A–C).
3.3. LC-PDA and mass spectrometry of three major peaks in pulp extracts
The three highest peaks (P2, P3, P7) in chromatograms of pulp extracts at 280 nm (Fig 1A) did not match retention times of the phenolic standards tested. This was probably because many pulp phenolics are in the form of sugar derivatives that are not commercially available as standards. Therefore, LC-PDA analysis was used to identify UV/vis maxima, and mass spectrometry further characterised the compounds, of these peaks at higher concentrations in semi-purified ethyl acetate extracts (Table 3).
Table 3.
Identification of three major peaks isolated from pulp extracts at 280 nma
| Peak number | Rtb min | UV max | MS, m/z [M-H]− | MS/MS, m/z base ion | Phenolic identification |
|---|---|---|---|---|---|
| P2 | 6.2 | 261 | 299 | 137 | p-hydroxybenzoylhexose |
| P3 | 7.6 | 315 (228) | 325 | 163 | p-coumaroylhexose |
| P7 | 11.5 | 285 | 643 | 333 | unknown |
Semi-purified ethyl acetate extracts derived from crude 80% methanol pulp extracts
Retention times of these major peaks are based on the LC-UV/vis profile.
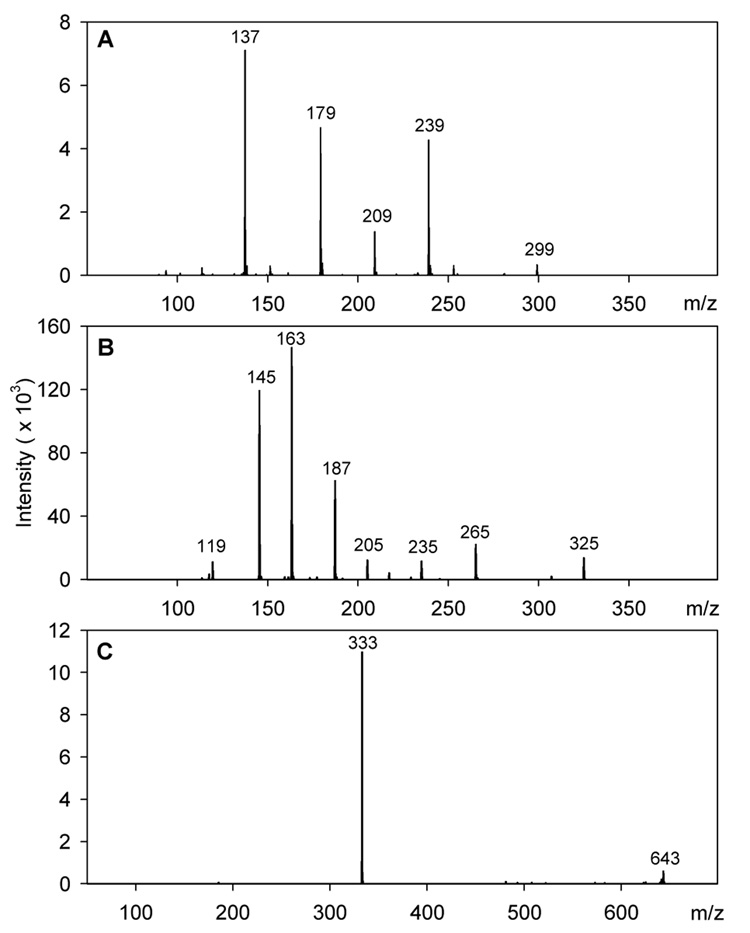
Mass spectra in negative ion mode (Fig. 2A) identified peak P2 as a hydroxybenzoic acid sugar derivative by the molecular ion [M–H]− m/z 299 and the MS/MS ion m/z 137 resulting from loss of 162 amu (glucose or galactose sugar moiety) (Moran, Klucas, Grayer, Abian, Harborne & Becana, 1998). TLC analysis demonstrated that both compound P2 and a hydroxybenzoic acid standard turned violet-blue after being sprayed with vanillin-sulphuric acid and heated. The UV/vis maxima of peak P2 (261 nm) corresponds to the sugar ester p-hydroxybenzoylglucose (262 nm) in ethyl acetate extracts of Ribes fruit species, and is different from the glycoside derivative (249 nm) or the aglycone (256 nm) (Määttä, Kamal-Eldin & Törrönen, 2003). Based on this information, compound P2 was identified as the phenolic acid sugar ester p-hydroxybenzoylglucose or galactose (Table 3).
Fig. 2.
MS/MS mass spectra: (A) peak P2 (m/z 299); (B) peak P3 (m/z 325); (C) peak P7 (m/z 643).
Mass spectra of peak P3 (Fig. 2B) in negative ion mode showed the molecular ion [M–H]− m/z 325, MS/MS base ion m/z 163 resulting from loss of 162 amu (glucose or galactose), and other fragment ions (m/z 145, m/z 119), all of which suggest that peak P3 is a coumaric acid sugar derivative (Määttä et al., 2003). TLC analysis demonstrated that both the standard p-coumaric acid and compound P3 had the same blue colour reaction with vanillin–sulphuric acid and a retention factor lower or more polar for compound P3 than that of the standard aglycone. The UV/vis maxima for peak P3 (315 nm) (Table 3) corresponds to the sugar ester of p-coumaric acid (314 nm) and is higher than the aglycone (310 nm) and glucoside derivative (296 nm) (Määttä et al., 2003). This indicates that compound P3 has a hexose sugar substituent attached to the carboxylic group, and therefore is identified as the phenolic sugar ester p-coumaroyl glucose or galactose (Table 3).
Peak P7 was the largest peak detected at 280 nm, with UV/vis maxima at 285 nm (Table 3). The mass spectral peak P7 was detected only in positive ion mode by the molecular ion [M+H]+ m/z 643 and MS/MS fragment ion m/z 333 (Fig. 2C). TLC analysis indicated that compound P7 had no colour reaction when sprayed with vanillin-sulphuric acid. To the best of the authors’ knowledge there are no reports of compounds identified fruit extracts that correspond to the UV/vis maxima and mass spectra of compound P7.
3.4. Characterization of fruit phenolics by HPLC and electrospray tandem mass spectrometry
3.4.1. General
LC-MS/MS was used to confirm the presence of compounds identified by LC-UV/vis fingerprint analysis (Fig. 1) and to identify and compare other compounds in fruit tissues. Identification was aided by comparisons with reference standards and by previous literature reports. Crude 80% methanol pulp, embryo and seed coat extracts, and semi-purified ethyl acetate extracts obtained from crude pulp and embryo extracts, were included in the analysis. To be concise, mass spectral data of semi-purified ethyl acetate extracts only included compounds that could be identified (embryo extracts), and were not detected in crude extracts, or if an isomer was present at a different retention time.
3.4.2. Phenolic acids
LC-MS/MS analysis did not indicate that hydroxybenzoic acids were prevalent in tissue extracts. Although gallic acid was detected by LC-UV/vis for all three tissue extracts P1, S1, E1 (Fig. 1A–C), the peaks were very small and may explain why LC-MS/MS analysis did not detect these compounds as free acids, but only as bound to other compounds such as (epi)gallocatechin in the seed coat and embryo extracts. Ellagic acid was not detected by LC-MS/MS or LC-UV/vis analysis, suggesting that this particular fruit cultivar or species contains little or no ellagic acid or ellago-tannins. This may differentiate M. bijugatus fruits from those of the related species longan (Dimocarpus longan Lam) with reportedly significant amounts of ellagic acid and ellago-tannins in pulp, seed and peel tissues (Rangkadilok et al., 2005). Benzyl alcohol pentose was detected by molecular ion [M–H]− m/z 401 and MS/MS ions (Moco et al., 2006) in pulp, embryo and seed coat embryo extracts (Table 4, Table 5, and Table 6). This indicates that benzoic acid derivatives are present, as confirmed by peak P2 (Table 3), but may not be detected as prominent molecular ions because they are at lower concentrations than other compounds at similar retention times.
Table 4.
Identification of phenolics and derivatives in 80% methanol pulp extracts by LC-MS/MSa
| Compound | Rt min | MW | MS, m/z M (+/−) | MS/MS ions m/z (relative intensity) | ref/std | |
|---|---|---|---|---|---|---|
| Hydroxycinnamic acids | ||||||
| p-Coumaric acid hexose | 4.3 | 326 | 325 (−) | 163(100) | 145(81), 119(7) | ref |
| Coumaric acid derivative | 6.8 | 488 | 487 (−) | 163(100) | 307(87), 265(67), 145(61), 325(50) | ref |
| Caffeic acid derivative | 8.6 | 378 | 377 (−) | 341(100) | 215(51), 179(21), 332(17) | ref |
| Ferulic acid derivative | 9.1 | 518 | 517 (−) | 193(100) | 295(61), 337(49) 355(43), 235(38), 175(32), 265(18) | ref |
| Ferulic acid derivative | 19.7 | 368 | 367 (−) | 295(100) | 235(8), 193(7) | ref |
| Ferulic acid derivative | 19.8 | 398 | 397(−) | 295(100) | 235(6) | ref |
| Benzyl alcohol derivatives | ||||||
| Benzyl alcohol hexose pentose | 5.7 | 402 | 401 (−) | 269(100) | 161(28), 293(13), 233(4) | ref |
| Stilbenes | ||||||
| Resveratrol derivativeb | 17.5 | 536 | 535 (−) | 227(100) | 163(15), 307(15), 389(7) | ref |
Rt, retention time; +/− indicates positive ion/negative ion mode; ref/std, compared to references/standards.
Molecular ions characteristic of resveratrol or resveratrol derivatives.
Table 5.
Identification of phenolics and derivatives in 80% methanol seed coat extracts by LC-MS/MSa
| Compound | Rt min | MW | MS, m/z M (+/−) | MS/MS, m/z (relative intensity) | ref/std | |
|---|---|---|---|---|---|---|
| Hydroxycinnamic acids | ||||||
| p-Coumaric acid hexose | 4.5 | 326 | 325 (−) | 145(100) | 163(94), 119(12) | ref |
| p-Coumaric acid | 8.4 | 164 | 163 (−) | 119(100) | ref | |
| Ferulic acid derivative | 19.8 | 398 | 397 (−) | 295(100) | 337(30), 325(18), 265(14), 193(3) | ref |
| Benzyl alcohol derivatives | ||||||
| Benzyl alcohol hexose pentose | 5.3 | 402 | 401 (−) | 269(100) | 161(25), 125(7), 293(3) | ref |
| Flavonoids | ||||||
| Procyanidin dimer (type B1 or B2) | 3.0 | 578 | 577 (−) | 425(100) | 407(81), 451(50), 289(34) | ref |
| (epi)catechin-(epi)gallocatechin | 3.4 | 594 | 593 (−) | 407(100) | 425(74), 305(73), 467(52), 289(50) | ref |
| Procyanidin trimer | 4.0 | 866 | 865 (−) | 695(100) | 577(55), 407(32), 287(22), 289 (11) | ref |
| Catechin | 6.6 | 290 | 289 (−) | 245(100) | 205(30) | std |
| Unknown tannin | 8.3 | 577 | 576 (−) | 289(100) | 451(74), 287(72), 559(71), 425(30) | - |
| Epicatechin | 8.6 | 290 | 289 (−) | 245(100) | 206(61), 205(41), 272 (17) | std |
| Procyanidin trimer | 8.7 | 866 | 865 (−) | 525(100) | 695(70), 577(54), 289(25) | ref |
| Procyanidin dimer | 8.8 | 578 | 577 (−) | 407(100) | 425(75), 289(52), 451(39), 559(35) | ref |
| Unknown tannin | 8.9 | 596 | 595 (−) | 545(100) | 287(62), 272(47), 461(45) | - |
| Procyanidin dimer (type A) | 9.1 | 576 | 575 (−) | 449(100) | 488(60), 433(59), 226(57), 177(32),423(26), 287(23) | ref |
| Procyanidin trimer | 10.2 | 866 | 865 (−) | 407(100) | 740(73), 577(69), 847(43), 467(39) | ref |
| Rutin | 19.4 | 610 | 609 (−) | 301(100) | 447(34), 373(20), 271(18), 255(14) | ref |
| Stilbenes | ||||||
| Resveratrol derivative b | 17.5 | 536 | 535 (−) | 227(100) | 307(4) | ref |
Rt, retention time; +/− indicates positive ion/negative ion mode; ref/std, compared to references/standards.
Molecular ions characteristic of resveratrol or resveratrol derivatives.
Table 6.
Identification of phenolics and derivatives in 80% methanol and semi-purified ethyl acetate embryo extracts by LC-MS/MSa,d
| Compound | Rt min | MW | MS m/z (+/−) | MS/MS ions m/z (relative intensity) | Ref/std | |
|---|---|---|---|---|---|---|
| Hydroxycinnamic acids | ||||||
| Sinapic acid hexose | 4.9 | 386 | 385 (−) | 223(100) | 179(1) | ref |
| Benzyl alcohol derivatives | ||||||
| Benzyl alcohol hexose pentose | 5.8 | 402 | 401 (−) | 269(100) | 161(28), 239(17), 293(16), 233(10) | ref |
| Flavonoids | ||||||
| (epi)Gallocatechin | 3.5 | 306 | 305 (−) | 179(100) | 261(22), 137(20) | ref |
| Procyanidin dimer (B1 or B2) | 4.7 | 578 | 579 (+) | 427(100) | 409(55), 291(49), 247(20) | ref |
| Catechinb | 6.2 | 290 | 289 (−) | 245(100) | 205(29), 179(16) | std |
| Naringenin pentosec | 10.2 | 404 | 405 (+) | 273(100) | - | |
| (epi)Catechin-(epi)gallocatechin | 11.5 | 594 | 593 (−) | 289(100) | 425(93), 407(48), 245(11) | ref |
| Phloretin hexoside | 14.7 | 436 | 435 (−) | 273(100) | 255(33) | - |
| Naringenin rhamnosidec | 14.2 | 418 | 419 (+) | 273(100) | - | |
| Naringenin | 15.4 | 272 | 271 (−) | 151(100) | std | |
| Naringenin hexoside | 15.5 | 434 | 433 (−) | 271(100) | std | |
| Myricetin rhamno-hexosided,e | 17.6 | 626 | 625 (−) | 316(100) | 317(91), 271(25), 607(9) | ref |
| Naringenin hexosidee | 17.5 | 434 | 433(−) | 271(100) | 151(12) | std |
| Myricetin rhamno-hexosided,e | 18.6 | 626 | 625 (−) | 316(100) | 317(78), 271(20), 287(11), 607(6), 463 (5) | ref |
| Myricetin rhamnosided,e | 19.2 | 464 | 463 (−) | 316(100) | 317 (65), 179(3) | ref |
| Myricetind,e | 19.3 | 317 | 316 (−) | 271(100) | 287(20), 179(13), 151(5) | ref |
| Phloretin hexosidee | 20.5 | 436 | 435 (−) | 273(100) | 167(2) | std |
| Quercetin rhamnosidee | 20.8 | 448 | 447 (−) | 301(100) | 179(2) | ref |
| Phloridzin (phloretin-2-glucoside)e | 21.5 | 436 | 435 (−) | 273(100) | 167(2) | std |
| Stilbenes | ||||||
| Resveratrol derivativec,e | 17.8 | 536 | 535 (−) | 227(100), | 241(43), 307(7) 185(5) | ref |
| Resveratrol derivativec,e | 23.1 | 522 | 521 (−) | 521(100) | 389(80), 227(6) | ref |
Rt, retention time; +/− indicates positive ion/negative ion mode; ref/stand., indicate if compared to references/standards.
Epicatechin detected at 8.7 min without MS/MS analysis.
fragmentation ions indicate compound but not compared to a standard/reference.
316/317 indicative derivative of myricetin or myrcetin derivative (Silva et al., 2005)
detected in semi-purified ethyl acetate extract
Several hydroxycinnamic acids were identified in the tissue extracts. p-Coumaric acid hexose (glucose or galactose) was detected in both pulp and seed coat extracts by molecular ion [M–H]− m/z 325 and the MS/MS ion m/z 163 (Määttä et al., 2003) (Table 4 and Table 5). Identification of this compound in crude pulp extracts is consistent with identification of peak P3, p-coumarylhexose (Table 3). Detection of this compound in both pulp and seed coat extracts (Table 4 and Table 5) further confirms that peaks P3 and S4 are the same compound (Fig. 1A,B). The aglycone p-coumaric acid was identified in crude seed coat extracts in negative ion mode (m/z 163), consistent with identification of peak S6 (Fig. 1B, Table 5). Coumaric and caffeic acid derivatives were detected in the pulp (Määttä et al., 2003) (Table 4). Sinapic acid hexose was detected by molecular ion [M–H]− m/z 385 and MS/MS ion m/z 223, representing the loss of 162 amu, and the aglycone sinapic acid (Ferreres et al., 2006) (Table 6). Molecular ions [M–H]− (m/z 517, m/z 367) in pulp extracts were identified as ferulic acid derivatives (Table 4) by the MS/MS ferulic acid fragment ion m/z 193 (Kammerer, Carle & Schieber, 2004). Both pulp and seed coat extracts indicated another ferulic derivative by molecular ion [M–H]− m/z 397, which produced MS/MS ions similar to the other derivatives (Table 4 and Table 5).
3.4.3. Flavonoids
Types of flavonoids known as flavanols were identified in both seed coat and embryo tissues. Flavanol monomers catechin/epicatechin were identified by molecular ion [M–H]− m/z 289 in seed coat and embryo extracts (Table 5 and Table 6), corresponding to peaks S3/S5 and E5/E8 (Fig. 1B,C), respectively. Catechin dimers identified in seed coat extracts include type B procyanidin dimmers, detected by molecular ion [M–H]− m/z 577 (Määttä et al., 2003) and a type A procyanidin dimmer, detected by molecular ion [M–H]− m/z 575 (Soong & Barlow, 2005) (Table 5). In embryo extracts, the molecular ion [M+H]+ m/z 579 identified as a type B procyanidin dimer (Määttä et al., 2003) (Table 6), was probably procyanidin B1, as indicated by peak E3 and based on retention time order of compounds identified by LC-UV/vis (Fig. 1C). Moreover, the dimer (epi)catechin-(epi)gallocatechin was identified in both seed coat and embryo extracts by molecular ion [M–H]− m/z 593 (Gu et al., 2003) (Table 5 and Table 6).
Catechin trimers were detected only in seed coat extracts by molecular ion [M–H]− m/z 865 (Sannomiya, Montoro, Piacente, Pizza, Brito & Vilegas, 2005) (Table 5). The trimer at 4.0 min (Table 5) probably contributes to the broad peak S3 because the trimer falls between retention times of (epi)gallocatechin (3.5 min) and catechin (6.6 min), all within the range of peak S3 (Fig. 1B). Other potential tannins were detected in the seed coat by molecular ions [M–H]− m/z 576 and m/z 595 (Table 5). Interestingly, no catechin or catechin derivatives were identified by LC-MS/MS or LC-UV/vis analyses in pulp extracts.
LC-MS/MS analysis detected flavanones and chalcones only in embryo extracts. Molecular ions [M–H]− m/z 271 and m/z 433 represented naringenin and prunin (naringenin-7-glucoside), respectively (Table 6). Although LC-UV/vis analysis indicated that peak E10 was prunin (Fig. 1C), LC-MS/MS analysis of two molecular ions at m/z 433 revealed retention times different from the standard, suggesting that these compounds may be chalcones or prunin isomers. Several molecular ions in positive ion mode also appear to be naringenin derivatives, including naringenin pentose (m/z 405) and naringenin rhamnoside (m/z 419) (Table 6).
Several compounds indicated mass spectra similar to phloridzin (phloretin-2-glucoside) by the molecular ion [M–H]− m/z 435. Compounds in embryo crude (14.7 min) and ethyl acetate extracts (20.5 min) were identified as phloretin hexosides (galactoside or glucoside) due to different retention times from the standard, and a compound in the embryo ethyl acetate extracts (21.5 min) was confirmed as phloridzin (Table 6). Identification of phloridzin in embryo extracts is consistent with the identification of peak E11 (Fig. 1C), but phloridzin or phloretin derivatives were not detected in the other tissues by LC-MS/MS analysis.
The flavonol quercetin was detected in ethyl acetate embryo extracts in the form of quercetin rhamnoside by molecular ion [M–H]− m/z 447 and MS/MS ions (m/z 301, m/z 179) (Soong et al., 2005) (Table 6). Quercetin-3-rutinoside (rutin) was identified in crude seed coat extracts by molecular ion [M–H]− m/z 609 with characteristic MS/MS fragment ions (Määttä et al., 2003; Soong et al., 2005) (Table 5). Additionally, the molecular ion [M–H]− m/z 625 was present in both embryo crude and ethyl acetate extracts, with MS/MS fragment ions m/z 271 and m/z 316/317 (both with similar intensity relative to other ions), indicating a derivative of the flavonol myricetin (Silva, Matias, Nunes, Duarte, Coelho & Bronze, 2005) (Table 6).
3.4.4. Other phenolics and unidentified compounds
Several compounds yielded the fragment ion m/z 227 in negative ion mode in pulp and seed coat (m/z 535, 17.5 min), and in embryo extracts (m/z 535, 17.8 min; m/z 521, 23.1 min) (Table 4, Table 5 and Table 6). In negative ion mode, the molecular ion m/z 227 indicates the stilbene resveratrol (Urpi-Sarda et al., 2007), suggesting that these compounds are resveratrol or stilbene derivatives. Pulp, embryo and seed coat extracts all showed the unknown molecular ion [M–H]− m/z 329 and MS/MS ions (m/z 191, m/z 161) (Bystrom, 2007). A prominent but unidentified compound in pulp extracts was indicated by molecular ion [M+H]+ m/z 333, a fragment ion of compound P7 (Table 3), and MS/MS ions (m/z 185, m/z 171) (Bystrom, 2007). All crude tissue extracts indicated the presence of m/z 451 as either a molecular ion in embryo extracts or as the prominent MS/MS ion for molecular ion [M–H]− m/z 497 in seed coat and pulp extracts (Bystrom, 2007). Pulp and seed coat extracts had many unidentified compounds in common with similar or identical molecular and MS/MS ions.
Phenolics and sugars characterised in M. bijugatus Montomgery cultivar fruit extracts may be associated with beneficial health effects and traditional uses of this fruit species. p-Coumaric acid hexose (glucose or galactose) was the major phenolic detected in pulp extracts and may explain the use of M. bijugatus fruits for treatment of hypertension; the aglycone p-coumaric acid is a systemic antioxidant with anti-platelet activity in humans at doses that can be obtained with dietary intervention (Luceri et al., 2007). Laxative effects of the fruit pulp may be due to ferulic acid derivatives. Both ferulic acid and its polar derivatives are reported to cause laxative activity in Wistar rats (Mitra, Badu & Ranganna, 2002). The equal glucose-to-fructose ratios in pulp tissues may prevent gastrointestinal problems in sensitive individuals, including children and people with irritable bowel syndrome (Hyams et al.,1988; Goldstein et al., 2000).
Several catechins identified in embryo extracts may validate the usage of seeds for treatment of diarrhoea. Specific catechins inhibit over-activated chloride transport, associated with bacterial infections that cause diarrhoea, by blocking the cystic fibrosis transmembrane conductance regulator (CFTR) in human colon epithelial cells (Schuier et al., 2005). This suggests that catechins in embryo extracts, especially epicatechin, may prevent dehydration and nutrient loss associated with diarrhoea. Moreover, seed coats, although not edible, have high concentrations of total phenolics and therefore may be a good source of different types of phenolic compounds, especially tannins. Further analysis of seed coat phenolics and confirmation of their biological activities may indicate that these compounds are valuable for natural health care products or as less toxic natural preservatives for the food industry.
4. Conclusions
This study provides, for the first time, information about phenolics and sugars in different tissues of M. bijugatus Montgomery cultivar fruits. LC-UV/vis fingerprint profiles were different for the pulp, embryo and seed coat tissues, but some of the same compounds were detected in the different tissues. Phenolics and sugars characterised in the fruits may be associated with medicinal uses or have positive effects on human health. Results from this study suggest that the Montgomery cultivar fruits may have more commercial potential due to their favourable physical traits and potential health-promoting properties. Additional studies on biological activities and phytochemistry of M. bijugatus fruits are warranted to confirm their therapeutic effects and the phytochemicals responsible for these effects.
Supplementary Material
Appendix Fig 1.
Photo of Melicoccus bijugatus Jacq. (Sapindaceae), ‘Montgomery’ cultivar, fruits.
Acknowledgements
L.M.B. was funded by NIH training grant no. 5 T32 DK-007158 31. Results presented herein were part of a PhD thesis (Bystrom, 2007). A preliminary report (poster/abstract) of this research was presented at the Plant Biology and Botany Joint Congress 2007 in Chicago. We thank Robert Sherwood for help with the LC-MS/MS analysis, Ying Hu and Magnolia Ariza-Nieto for help with the HPLC, and Pedro Acevedo for information about this fruit species.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acevedo-Rodríguez P. Meliococceae (Sapindaceae): Melicoccus and Talisia. Bronx, N.Y.: Published for Organization for Flora Neotropica by the New York Botanical Garden; 2003. pp. 3–49. [Google Scholar]
- Beyra A, León MC, Iglesias E, Ferrándiz D, Herrera R, Volpato G, Godínez D, Guimarais M, Álvarez R. Estudios etnobotánicos sobre plantas medicinales en la provincial de Camagüey (Cuba) Anales de Jardín Botánico de Madrid. 2004;61:185–204. [Google Scholar]
- Bystrom LM. Ph.D. thesis, Phenolics and sugars in Melicoccus bijugatus Jacq. fruits in relation to medicinal and dietary uses. Ithaca, NY: Cornell University; 2007. pp. 1–189. [Google Scholar]
- Campbell BD, Busbee DL, McDaniel HR. Enhancement of immune function in rodents using a proprietary complex mixture of glyconutritionals. Proceedings of the Fisher Institute for Medical Research. 1997;1:34–37. [Google Scholar]
- Emmons CL, Peterson DM, Paul GL. Antioxidant capacity of oat (Avena sativa L.) extracts. 2. In vitro antioxidant activity and contents of phenolic and tocol antioxidants. Journal of Agricultural and Food Chemistry. 1999;47:4894–4898. doi: 10.1021/jf990530i. [DOI] [PubMed] [Google Scholar]
- Ferreres F, Sousa C, Vrchovska V, Valentao P, Pereira J, Seabra RM, Andrade PB. Chemical composition and antioxidant activity of tronchunda cabbage internal leaves. European Food Research and. Technology. 2006;222:88–98. [Google Scholar]
- Goldstein R, Braverman D, Stankiewicz H. Carbohydrate malabsorption and the effect of dietary restriction on symptoms of irritable bowel syndrome and functional bowel complaints. The Israel Medical Association Journal. 2000;2:583–587. [PubMed] [Google Scholar]
- Gu L, Kelm MA, Hammerstone JF, Beecher G, Holden J, Haytowitz D, Prior RL. Screening of foods containing proanthocyanidins and their structural characterization using LC-MS/MS and thiolytic degradation. Journal of Agricultural and Food Chemistry. 2003;51:7513–7521. doi: 10.1021/jf034815d. [DOI] [PubMed] [Google Scholar]
- Guyot S, Doco T, Souquet JM, Moutounet M, Drilleau JF. Characterization of highly polymerized procyanidins in cider apple (Malus sylvestris var. Kermerrien) skin and pulp. Pytochemistry. 1997;44:351–357. [Google Scholar]
- Harborne JB. Phytochemical methods : a guide to modern techniques of plant analysis. London; New York: Chapman and Hall; 1984. pp. 1–288. [Google Scholar]
- Hilt P, Schieber A, Yildirim C, Arnold G, Klaiber I, Conrad J, Beifuss U, Carle R. Detection of phloridzin in strawberries (Fragaria x ananassa Duch.) by HPLC-PDA-MS/MS and NMR spectroscopy. Journal of Agricultural and Food Chemistry. 2003;51:2896–2899. doi: 10.1021/jf021115k. [DOI] [PubMed] [Google Scholar]
- Horbowicz M, Obendorf RL. Seed desiccation tolerance and storability: Dependence on flatulence-producing oligosaccharides and cyclitols -- review and survey. Seed Science Research. 1994;4:385–405. [Google Scholar]
- Hu Y, Cheng Z, Heller LI, Krasnoff SB, Glahn RP, Welch RM. Kaempferol in red and pinto bean seed (Phaseolus vulgaris L.) coats inhibits iron bioavailability using an in vitro digestion/human caco-2 cell model. Journal of Agricultural and Food Chemistry. 2006;54:9254–9261. doi: 10.1021/jf0612981. [DOI] [PubMed] [Google Scholar]
- Hyams JS, Etienne NL, Leichtner AM, Theuer RC. Carbohydrate malabsorption following fruit juice ingestion in young children. Pediatrics. 1988;82:64–68. [PubMed] [Google Scholar]
- Jiang F, Dusting GJ. Natural phenolic compounds as cardiovascular therapeutics: potential role of their antiinflammatory effects. Current Vascular Pharmacology. 2003;1:135–156. doi: 10.2174/1570161033476736. [DOI] [PubMed] [Google Scholar]
- Johnston KL, Clifford MN, Morgan LM. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: glycemic effects of chlorogenic acid and caffeine. The American Journal of Clinical Nutrition. 2003;78:728–733. doi: 10.1093/ajcn/78.4.728. [DOI] [PubMed] [Google Scholar]
- Kammerer D, Carle R, Schieber A. Characterization of phenolic acids in black carrots (Daucus carota ssp. sativus var. atrorubens Alef.) by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Communications in Mass Spectrometry. 2004;18:1331–1340. doi: 10.1002/rcm.1496. [DOI] [PubMed] [Google Scholar]
- Liogier AH. Plantas medicinales de Puerto Rico y del Caribe. San Juan, P.R.: Iberoamericana de Ediciones; 1990. [Google Scholar]
- Luceri C, Giannini L, Lodovici M, Antonucci E, Abbate R, Masini E, Dolara P. p-Coumaric acid, a common dietary phenol, inhibits platelet activity in vitro and in vivo. The British Journal of Nutrition. 2007;97:458–463. doi: 10.1017/S0007114507657882. [DOI] [PubMed] [Google Scholar]
- Määttä KR, Kamal-Eldin A, Törrönen AR. High-performance liquid chromatography (HPLC) analysis of phenolic compounds in berries with diode array and electrospray ionization mass spectrometric (MS) detection: ribes species. Journal of Agricultural and Food Chemistry. 2003;51:6736–6744. doi: 10.1021/jf0347517. [DOI] [PubMed] [Google Scholar]
- Mitra SK, Badu UV, Ranganna MV. Herbal Laxative Preparation. 20,020,150,639. US Patent. 2002
- Moco S, Bino RJ, Vorst O, Verhoeven HA, de Groot J, van Beek TA, Vervoort J, de Vos CH. A liquid chromatography-mass spectrometry-based metabolome database for tomato. Plant Physiology. 2006;141:1205–1218. doi: 10.1104/pp.106.078428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton JF. Fruits of warm climates. Miami, FL: Creative Resources Systems; 1987. Mamoncillo; pp. 267–269. [Google Scholar]
- Ortmeyer HK, Larner J, Hansen BC. Effect of d-chiroinositol added to a meal on plasma glucose and insulin in hyperinsulinemic rhesus monkeys. Obesity Research. 1995;3:605S–608S. doi: 10.1002/j.1550-8528.1995.tb00232.x. [DOI] [PubMed] [Google Scholar]
- Rangkadilok N, Worasuttayangkurn L, Bennett RN, Satayavivad J. Identification and quantification of polyphenolic compounds in Longan (Euphoria longana Lam.) fruit. Journal of Agricultural and Food Chemistry. 2005;53:1387–1392. doi: 10.1021/jf0403484. [DOI] [PubMed] [Google Scholar]
- Sannomiya M, Montoro P, Piacente S, Pizza C, Brito AR, Vilegas W. Application of liquid chromatography/electrospray ionization tandem mass spectrometry to the analysis of polyphenolic compounds from an infusion of Byrsonima crassa Niedenzu. Rapid Communications in Mass Spectrometry. 2005;19:2244–2250. doi: 10.1002/rcm.2053. [DOI] [PubMed] [Google Scholar]
- Schuier M, Sies H, Illek B, Fischer H. Cocoa-related flavonoids inhibit CFTR-mediated chloride transport across T84 human colon epithelia. The Journal of Nutrition. 2005;135:2320–2325. doi: 10.1093/jn/135.10.2320. [DOI] [PubMed] [Google Scholar]
- Silva S, Matias AA, Nunes A, Duarte C, Coelho AV, Bronze RM. Identification of flavanol glycosides in winemaking by-products by HPLC with different detectors and hyphenated with mass spectrometry. Ciencia e Tecnica Vitivinicola. 2005;20:17–33. [Google Scholar]
- Sierra-Gómez MP. Master’s thesis, Physical-chemical analysis of selected quenepa (Melicoccus bijugatus Jacq.) varieties. Mayagüez, Puerto Rico: University of Puerto Rico; 2006. pp. 1–49. [Google Scholar]
- Soong YY, Barlow PJ. Isolation and structure elucidation of phenolic compounds from longan (Dimocarpus longan Lour.) seed by high-performance liquid chromatography-electrospray ionization mass spectrometry. Journal of chromatography. A. 2005;1085:270–277. doi: 10.1016/j.chroma.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Urpi-Sarda M, Zamora-Ros R, Lamuela-Raventos R, Cherubini A, Jauregui O, de la Torre R, Covas MI, Estruch R, Jaeger W, Andres-Lacueva C. HPLC-tandem mass spectrometric method to characterize resveratrol metabolism in humans. Clinical Chemistry. 2007;53:292–299. doi: 10.1373/clinchem.2006.071936. [DOI] [PubMed] [Google Scholar]
- Vega B. Las frutas de los taínos. Santo Domingo, República Dominicana: Fundación Cultural Dominicana; 1997. pp. 132–206. [Google Scholar]
- Zomlefer WB. Guide to flowering plant families. Chapel Hill: University of North Carolina Press; 1994. pp. 153–155. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.