Abstract
Mammary myoepithelial cells are specialized smooth musclelike epithelial cells that express the smooth muscle actin isoform: smooth muscle alpha-actin (ACTA2). These cells contract in response to oxytocin to generate the contractile force required for milk ejection during lactation. It is believed that ACTA2 contributes to myoepithelial contractile force generation; however, this hypothesis has not been directly tested. To evaluate the contribution of ACTA2 to mammary myoepithelial cell contraction, Acta2 null mice were utilized and milk ejection and myoepithelial cell contractile force generation were evaluated. Pups suckling on Acta2 null dams had a significant reduction in weight gain starting immediately postbirth. Cross-fostering demonstrated the lactation defect is with the Acta2 null dams. Carmine alum whole mounts and conventional histology revealed no underlying structural defects in Acta2 null mammary glands that could account for the lactation defect. In addition, myoepithelial cell formation and organization appeared normal in Acta2 null lactating mammary glands as evaluated using an Acta2 promoter-GFP transgene or phalloidin staining to visualize myoepithelial cells. However, mammary myoepithelial cell contraction in response to oxytocin was significantly reduced in isolated Acta2 null lactating mammary glands and in in vivo studies using Acta2 null lactating dams. These results demonstrate that lack of ACTA2 expression impairs mammary myoepithelial cell contraction and milk ejection and suggests that ACTA2 expression in mammary myoepithelial cells has the functional consequence of enhancing contractile force generation required for milk ejection.
Keywords: cytoskeleton, lactation, mammary glands, milk ejection, myoepithelial cell, pregnancy, smooth muscle alpha-actin, transgenic/knockout model
Female mice lacking smooth muscle alpha-actin have a lactation defect that results from the inability of myoepithelial cells to generate sufficient contractile force in response to oxytocin to promote milk ejection.
INTRODUCTION
Mammary myoepithelial cells are contractile epithelial cells that express smooth muscle α-actin (ACTA2) [1, 2]. The primary function of mammary myoepithelial cells is to contract in response to oxytocin released by the hypothalamus upon suckling by pups during lactation [3]. Myoepithelial cell contraction reduces the diameter of the alveolar lumen, resulting in milk ejection, which is the transfer of milk through the ductules toward the teats to provide pups with milk. The contraction of myoepithelial cells in response to oxytocin is essential because mice lacking oxytocin are unable to nurse their offspring [4, 5]. While it is clear that contraction of myoepithelial cells is necessary for milk ejection during lactation, the role of ACTA2 in promoting myoepithelial cell contraction is not known.
There are six actin isoforms: two ubiquitous cytoplasmic actins, β- and γ-actin found in all epithelial and mesenchymal cells, and four muscle actins, ACTA2, smooth muscle γ-actin, skeletal muscle α-actin, and cardiac muscle α-actin. Typically nonmuscle cells, such as epithelial cells and fibroblasts, express only the β- and γ-cytoplasmic actin isoforms. These cytoplasmic actin isoforms can assemble into contractile structures in nonmuscle cells, termed stress fibers, to generate contractile force [6, 7]. Nonmuscle cells such as myoepithelial cells and myofibroblasts are highly contractile and express ACTA2 in addition to the cytoplasmic actin isoforms. Previous studies have demonstrated that increased ACTA2 expression in fibroblasts will enhance their ability to generate contractile force [8], while either decreasing ACTA2 expression with anti-sense mRNA [9] or disassembling ACTA2 from stress fibers with a synthetic peptide homologous to the amino-terminal end of ACTA2 [10] reduces myofibroblast force generation. These studies suggest that increased expression of ACTA2 in fibroblasts results in increased contractile force generation. Based upon these studies, it has been proposed that ACTA2 expression in myoepithelial cells plays a role in generation of contractile force required for milk ejection; however, no study has directly tested this hypothesis.
In addition to ACTA2, myoepithelial cells express other smooth muscle-specific contractile proteins, including smooth muscle myosin heavy chain (MYH11), calponin 1 (CNN1), and tropomyosin 2 (TPM2) [2, 11]. Recently it has been demonstrated that mice lacking the transcription factor myocardin-related transcription factor-A (MRTF-A; official symbol Mkl1, megakaryoblastic leukemia 1, or Mal, megakaryocytic acute leukemia) are unable to effectively nurse their offspring due to a failure in maintenance of the differentiated state of mammary myoepithelial cells during lactation [11, 12]. MKL1 is a member of a three-protein family that includes MRTF-B (official symbol MKL2) and myocardin. These myocardin/MRTF proteins serve as serum response factor (SRF) coactivators that bind to SRF and strongly activate SRF target genes [13]. Recent studies have demonstrated that MKL/SRF in nonmuscle cells can activate early response genes as well as smooth muscle contractile genes [14, 15]. Myoepithelial cells in Mkl1 null mice have decreased expression of Acta2, Myh11, Cnn1, and Tpm2 along with a large number of other proteins [11, 12]. These studies suggest a potential role for these smooth muscle proteins in myoepithelial contraction; however, the role of individual contractile proteins, particularly ACTA2, was not determined, and the generation of contractile force was not evaluated.
To determine the role of ACTA2 in myoepithelial cell function in mammary tissue, we utilized Acta2 null (Acta2−/−) mice. Previous publications have described that while Acta2−/− mice are viable and can reproduce successfully, they have defects in the generation of contractile force by vascular smooth muscle [16] and bladder smooth muscle [17]. In this study, we describe the effect of the lack of Acta2 expression on mammary myoepithelial cell function. Postpartum female mice homozygous for a null mutation in the Acta2 gene are unable to productively nurse their offspring. Mammary tissue and myoepithelial cells develop normally in pregnant Acta2−/− mice and appear structurally normal; however, myoepithelial cells in Acta2−/− mice contract significantly less in response to oxytocin than do myoepithelial cells in wild-type (WT) mice. These results demonstrate that Acta2−/− dams have impaired myoepithelial cell contraction and milk ejection. We conclude that ACTA2 expression in mammary myoepithelial cells has the functional consequence of enhancing contractile force generation required for milk ejection.
MATERIALS AND METHODS
Animals
The Acta2−/− mice used in this study were generated by inserting the Pol2NeobpA cassette into the +1 start site of the Acta2 gene [16]. Acta2−/− and WT mice were obtained from a breeding colony maintained at the University of Oklahoma Health Sciences Center. Animals were genotyped as previously described [17].
The transgenic mice carrying the Acta2 promoter conjugated to green fluorescent protein (GFP), that is, Tg(Acta2:GFP), used in this study were generated by Dr. J.Y. Tsai [18]. These mice express GFP under the control of the Acta2 promoter. The regulatory sequence of Acta2 promoter contains −1074 bp of the 5′-flanking region, the transcription start site, 48 bp of exon 1, the 2.5 kb intron 1, and the 15 bp exon 2 of the mouse Acta2 gene [19]. GFP expression has been described in both vascular and nonvascular smooth muscle cells as well as meningeal cells [18, 20]. These mice were the generous gift of Dr. J.Y. Tsai (National Eye Institute, Bethesda, MD). Tg(Acta2:GFP) mice were obtained from a breeding colony maintained at the University of Oklahoma Health Sciences Center. All of the mice in this breeding colony were homozygous for the Acta2-GFP transgene. To produce Acta2−/−/Tg(Acta2:GFP) mice, Acta2−/− male and Acta2+/+/Tg(Acta2:GFP) female mice were bred producing pups heterozygous for both Acta2 and Acta2:GFP. These heterozygous females were bred with Acta2−/− males to produce experimental female Acta2−/−/Tg(Acta2:GFP) mice. Genotypes were determined by immunofluorescence staining for ACTA2 and visualization of GFP as follows. Ear punches were obtained from pups generated from Acta2+/−/Tg(Acta2:GFP) × Acta2−/− crosses at the time of weaning. Punches were fixed in 4% neutral buffered formalin, rinsed in phosphate buffered saline (PBS; 2.4 mM NaH2PO4*H2O, 7.1 mM Na2HPO4*7H2O, 154 mM NaCl, adjusted to pH 7.45), and dissected using fine forceps to expose the dermis and epidermis. Punches were placed in 1% Triton-X 100/PBS plus 0.05% Na azide (PBS-azide) for 1 h at room temperature, washed three times for 5 min in PBS, blocked for 1 h with 10% goat serum in PBS, and stained overnight at 4°C with anti-ACTA2 antibody directly conjugated to Cy3 (C6198; Sigma-Aldrich) diluted 1:400 in PBS. Punches were rinsed three times for 5 min, mounted on slides in 80% glycerin/20% PBS, and examined by fluorescence microscopy. The blood vessels in punches positive for GFP fluoresce green and vessels positive for ACTA2 fluoresce red. Blood vessels in Acta2−/−/Tg(Acta2:GFP) mice will fluoresce green but not red.
To produce lactating female mice needed for the study, female mice approximately 6 wk of age of the appropriate genotype were caged with male mice and allowed to mate naturally. Females were observed daily and removed to individual cages when visibly pregnant to allow for delivery and nursing of pups.
All the experimental procedures involving animals were reviewed by the Institutional Animal Care and Use Committee of the University of Oklahoma Health Sciences Center, and animals were cared for in accordance with its guidelines under protocol 06–073.
Mammary Tissue Analysis
Lactating females were euthanized by CO2 asphyxiation, and mammary tissue was immediately dissected and prepared for microscopic analysis as described below. Females were removed from the pups either 6 h before euthanasia or removed from actively nursing pups immediately prior to euthanasia.
For histological analysis, mammary tissue was dissected from euthanized female mice, placed in PBS, cut into 2 mm3 pieces, fixed in 4% neutral buffered formalin overnight at 4°C, embedded in paraffin, and sectioned at 4-μm intervals. After graded alcohol dehydration and two xylene-clearing stages, the sections were stained with conventional hematoxylin-eosin reagents.
For carmine alum-stained whole mount analysis, mammary tissue was dissected from euthanized female mice, rinsed in PBS, placed on ProbeOn Plus slides, and allowed to stand for several minutes. Tissue was fixed in Carnoy fixative (60% ethanol, 30% chloroform, 10% glacial acetic acid) for 16 h, rehydrated through graded ethanol, stained in carmine alum stain (0.2% carmine alum, 10.5 mM aluminum potassium sulfate) for 16 h, and dehydrated in graded ethanol. The slides were cleared for 4 h in xylene and mounted in permount under a glass cover slip. Whole mount tissues were examined using an Olympus MVX10 Research Macro Zoom System microscope.
For fluorescence whole mount analysis, mammary tissue was dissected from euthanized female mice, placed in PBS, cut into 2 mm3 pieces, fixed in 4% neutral buffered formalin for 30 min, rinsed three times in PBS, and stored in PBS-azide until use. Tissue from Tg(Acta2:GFP) mice was further dissected into 1- × 2-mm pieces and mounted on slides in 80% glycerin/20% PBS-azide. For visualization of ACTA2, mammary tissue was opened with 1% Triton-X 100/PBS-azide for 1 h at room temperature, rinsed three times with PBS, blocked for 1 h with 10% goat serum in PBS-azide, and stained with anti-ACTA2 antibody directly conjugated to Cy3 (diluted 1:400 in PBS) for 24 h at 4°C. For visualization of myoepithelial cells using rhodamine phalloidin, mammary tissue was treated with 1% Triton-X 100/PBS-azide for 18 h, rinsed three times with PBS, and stained with rhodamine phalloidin diluted 1:40 in PBS (R415; Invitrogen) for 24 h at 4°C [21]. Whole mount tissues were examined by either wide-field fluorescence microscope using an Olympus AX 20 wide-field fluorescence microscope or by confocal microscope using an Olympus IX81-FV500 epifluorescence/confocal laser-scanning microscope with a UApo 20x water immersion lens and excitation with either 488- or 546-nm wavelength lasers.
In Vitro Mammary Tissue Contraction Assay
Day 2–3 postpartum lactating WT or Acta2−/− dams were removed from nursing pups for 6 h and subsequently euthanized as described above. Mammary tissue was immediately removed, rinsed in contraction buffer (136 mM NaCl, 1.2 mM NaH2PO4, 1.2 mM MgSO4, 5 mM KCl, 1.7 mM CaCl2, 1.1 mM glucose, 0.03 mM Na2 ethylenediaminetetraacetic acid, 10 mM Hepes, pH 7.4) and dissected into 1-mm3 pieces [22]. Tissue pieces were incubated in contraction buffer for 10 min on a slide warmer at 37°C. Oxytocin (0–3251; Sigma) was added to the dishes to give a final concentration of 0.005 nM, 0.05 nM, 0.5 nM, or 5nM. The tissue pieces were incubated in oxytocin for 2 min at 37°C, immediately fixed, stained with rhodamine phalloidin, and mounted as described above. To quantify the contraction of myoepithelial cells, we developed a 1–5 scoring system, with 1 being no contraction and 5 being extreme contraction. Five tissue pieces exposed to each oxytocin concentration were analyzed from each of three WT and three Acta2−/− females. Each tissue piece was mounted individually on a labeled slide. The labels were then covered, and the slides were randomly assigned letters; two independent observers scored the slides. The labels were then uncovered, and the scores tabulated and analyzed.
In Vivo Mammary Tissue Contraction Assay
Day-2 postpartum lactating WT or Acta2−/− females were removed from nursing pups for 6 h. Females were injected i.p. with 0.1 IU oxytocin in 100 μl PBS for 15 min before euthanasia. Mammary tissue was removed and immediately fixed and stained with rhodamine phalloidin as described above.
RESULTS
Pups Suckling on Acta2−/− Mothers Fail to Thrive
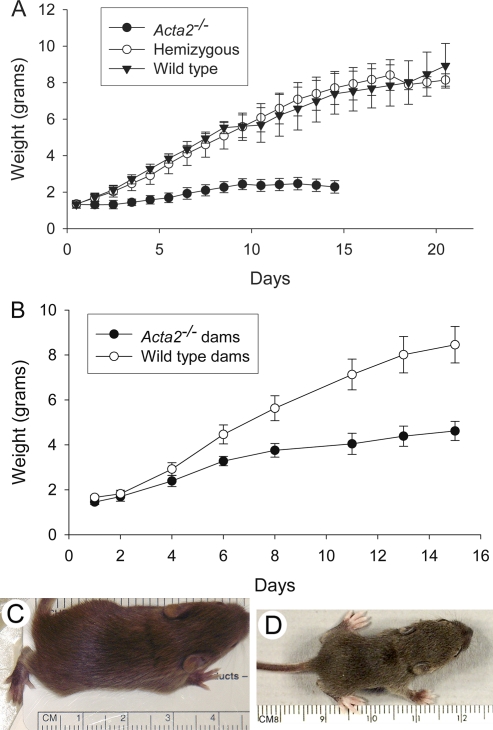
The phenotype of Acta2−/− mice has been previously described [16]; the Acta2−/− mice have no gross anatomical defects and appear normal. Acta2+/− hemizygote intercrosses gave the expected Mendelian frequency of offsprings (1 Acta2−/−:2 Acta2+/−:1 Acta2+/+). No significant differences were observed in numbers of pups per litter born to intercrosses between Acta2−/− females and males with a WT, hemizygous, or Acta2−/− genotype. Thus, Acta2−/− mice were fertile and did not possess any inherent gestational or parturition defects. However, pups born to Acta2−/− dams failed to thrive. These pups gained little weight from the time of birth; in contrast, pups born to hemizygous or WT dams gained weight normally and thrived (Fig. 1A). Upon gross examination, it was observed that little if any milk could be seen in the stomach of Day-1 pups born to Acta2−/− dams (not illustrated). Pups suckling on Acta2−/− dams began dying several days after birth with greater than 50% dying by Day 5; usually all the pups in the litter were dead by Day 16 in contrast to pups born to hemizygous or WT dams (data not shown). Interestingly, pups that did survive the first several postpartum days with an Acta2−/− dam gained weight but at a much slower rate than pups born to a hemizygous or WT dam (Fig. 1A); they were visibly stunted in their growth at Day 14 compared to pups suckling on WT dams (Fig. 1, C and D).
FIG. 1.
Pups suckling on Acta2−/− dams fail to thrive. A) Graph illustrates daily weight of pups born to Acta2−/−, hemizygous (Acta2+/−), or WT (Acta2+/+) dams. Pups born to Acta2−/− dams failed to gain weight compared to pups born to Acta2+/− or WT dams starting at Postnatal Day 1 (P ≤ 0.001). Each time point represents the average weight of pups from three litters at the time points indicated during the postnatal period. WT and Acta2−/− litters are the same genotype as the dam; hemizygous litters are a mixed genotype. Litter size was initially between six and eight pups. B) Graph illustrating daily weight of pups cross-fostered with WT or Acta2−/− dams starting at Postnatal Day 1. Weight gain is dependent on the genotype of the dam and not the pups. Each time point represents the average weight of pups from three litters at the time points indicated during the postnatal period. Litters prior to cross-fostering were the same genotype as the dam. Litter size was initially between six and eight pups. C, D) Images of Postnatal Day 14 pups suckled on WT or Acta2−/− dams. Pup born to and suckling on an Acta2−/− dam (D) is visibly stunted compared to pup born to and suckling on a WT dam (C).
Failure to thrive was not the result of poor mothering by Acta2−/− dams. Acta2−/− dams built nests for pups and would retrieve pups when they were removed from the nest. Dams could be observed allowing pups to nurse; in fact, the nipples of Acta2−/− dams were noted to be inflamed several days postpartum, presumably due to the excess amount of time the pups spent suckling.
To determine whether the genotype of the dam was responsible for the failure to thrive, we cross-fostered pups between Acta2−/− and WT mothers. Day-1 pups born to Acta2−/− dams were placed with Day-1 postpartum WT dams, and Day-1 pups born to WT dams were placed with Day-1 postpartum Acta2−/− dams. The WT pups placed with the Acta2−/− dams grew significantly less than Acta2−/− pups placed with WT dams and all died by Day 16, while pups placed with WT dams survived to weaning and adulthood (Fig. 1B). These results demonstrate that the failure to thrive of the pups was independent of the pup's genotype but was related to the genotype of the mother. Interestingly, pups born to WT dams and placed with Acta2−/− dams gained more weight than did pups that were born to and stayed with an Acta2−/− dam. It was observed that pups removed from WT dams had milk in their stomachs, which may explain why these cross-fostered pups gained more weight. Together these results suggest that Acta2−/− dams are unable to provide the nourishment required by suckling pups.
Milk Is Accumulated in Mammary Glands of Acta2−/− Dams
To determine whether mammary glands of Acta2−/− dams contained milk, we examined their gross appearance and histological structure. Gross dissection of mammary tissue of Acta2−/− and hemizygous dams suckling pups for 2 days demonstrated the presence of milk in the mammary glands from dams of both genotypes (Supplemental Fig. S1, available online at www.biolreprod.org). The overall abundance of the mammary tissue was similar, or even increased, in the Acta2−/− dams compared with WT dams. The mammary tissue of Acta2−/− dams appeared engorged compared to WT dams, which is indicative of the accumulation of milk, possibly due to insufficient milk ejection. To determine whether milk could be expressed from mammary glands of Acta2−/− dams, Day-2 postpartum WT and Acta2−/− dams had their pups removed for 6 h and then were injected i.p. with 0.1 IU oxytocin in 100 μl PBS. Light manipulation of the nipple in WT dams resulted in ejection of milk; in contrast, similar manipulation of the nipple of Acta2−/− dams did not result in ejection of milk. Milk could be ejected from the nipple of Acta2−/− dams by simultaneous manipulation of the mammary gland and nipple (not illustrated). These results are consistent with the hypothesis that mammary glands of Acta2−/− dams contain milk; however, normal milk ejection does not occur.
Nonlactating and Lactating Mammary Gland Structure Is Similar in Acta2−/− and WT Female Mice
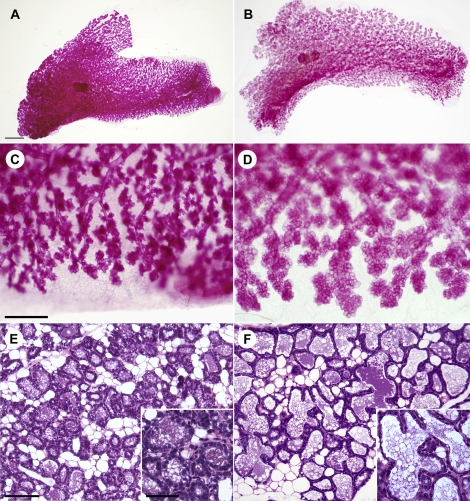
No differences in mammary gland structure were observed in 4- and 9-wk-old virgin WT and Acta2−/− mice examined by carmine alum-stained whole mounts and in 9-wk-old mice examined by hematoxylin-eosin-stained tissue sections. In carmine alum-stained whole mounts of abdominal No. 4 mammary glands of 4- and 9-wk-old mice, the branching pattern and density of ducts appeared similar in both genotypes, and in the 4-wk-old mice, the formation of terminal end buds appeared similar (4-wk old, not illustrated; 9-wk old, Fig. 2, A–D). Similarly, hematoxylin-eosin-stained sections from mammary tissue of 9-wk-old virgin mice, comparable to the carmine alum-stained whole mount mammary tissue, demonstrated no structural differences between WT and Acta2−/− mammary gland tissue (Fig. 2, E and F).
FIG. 2.
Structural appearance of 9-wk-old virgin WT and Acta2−/− abdominal No. 4 mammary gland tissue. A–D) Carmine alum-stained mammary glands from virgin WT (A, C) and Acta2−/− (B, D) females appear similar. Low-magnification images demonstrate that the branching pattern and density of ducts appear similar (A, B). High-magnification images of similar regions in the glands demonstrate that the ducts appear similar (C, D). E, F) Hematoxylin and eosin-stained paraffin sections from virgin WT (E) and Acta2−/− (F) females appear similar. Bars = 2 mm (A, B), 500 μm (C, D), and 100 μm (E, F).
Similarly, no major structural differences were observed in mammary gland tissue from postpartum WT and Acta2−/− lactating females. Carmine alum-stained whole mounts of abdominal No. 4 mammary gland tissue from Day-1 postpartum WT and Acta2−/− mice showed similar extensive development of the ductal network and formation of terminal alveoli (Fig. 3, A and B); however, the alveoli in Acta2−/− mammary tissue appeared larger compared with WT mammary tissue (Fig. 3, C and D). Hematoxylin and eosin-stained sections of postpartum Day-2 mammary tissue from WT and Acta2−/− lactating dams, whose pups were actively nursing just prior to euthanasia, demonstrated well-developed secretory epithelium and alveoli in both genotypes (Fig. 3, E and F); however, the alveolar cavities appeared more distended and secretory epithelium appeared thinned in Acta2−/− dams. These results suggest that mammary glands in Acta2−/− females develop normally and undergo normal differentiation in response to pregnancy consistent with our observations that the lactating mammary glands in Acta2−/− dams produce milk; however, the milk that is secreted into the alveoli may not be ejected normally in response to suckling.
FIG. 3.
Structural appearance of postpartum WT and Acta2−/− abdominal No. 4 mammary gland tissue. A–D) Carmine alum-stained mammary glands from Day 1 postpartum WT (A, C) and Acta2−/− (B, D) dams. Low-magnification images demonstrate similar extensive development of the ductal network and terminal alveoli in both WT and Acta2−/− mammary glands. Alveoli in Acta2−/− mammary tissue appear larger than in WT (A, B). High-magnification images suggest that the glandular structure looks similar; however, the alveoli in Acta2−/− appear larger compared with alveoli in WT mammary gland (C, D). E, F) Hematoxylin and eosin-stained paraffin sections from mammary glands of Day-2 WT (E) and Acta2−/− (F) dams whose pups were actively nursing just prior to euthanasia. The general histological appearance of the mammary tissue from these two mice appears similar; however, the alveolar lumina are larger and the alveolar epithelial cells appear thinned in Acta2−/− dams (F) compared with WT dams (E). High-magnification images of alveolar secretory epithelium of WT (E inset) and Acta2−/− (F inset) dams illustrating differences between the two genotypes. Bars = 2 mm (A, B), 500 μm (C, D), 100 μm (E, F), and 50 μm (insets).
Myoepithelial Cells Assume a Normal Structure and Organization in Mammary Glands of Postpartum Acta2−/− Dams
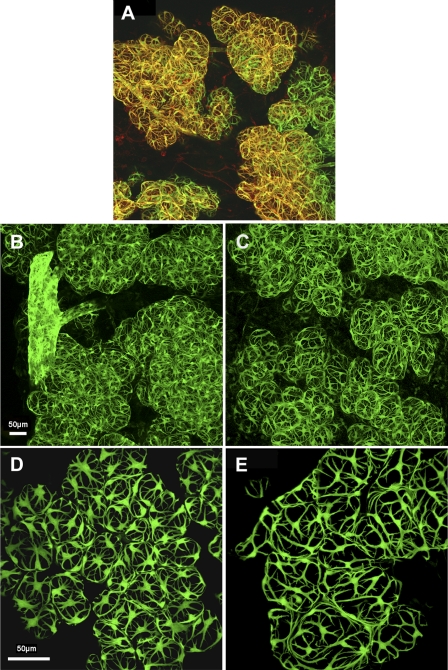
Myoepithelial cells are proposed to be responsible for generating the force required for milk ejection in response to suckling [3]. The organization of these cells into a basketlike meshwork located at the basal region of mammary alveoli and the presence of the actin isoform ACTA2 are both believed to assist alveolar contraction responsible for milk ejection. It was not possible to examine the three-dimensional organization of myoepithelial cells in mammary tissue of postpartum Acta2−/− mice using an anti-ACTA2 antibody; therefore, transgenic mice carrying the Acta2 promoter conjugated to GFP—that is, Tg (Acta2:GFP)—mice were mated with Acta2−/− and WT mice, as described in Materials and Methods to obtain Acta2+/+/Tg(Acta2:GFP) and Acta2−/−/Tg(Acta2:GFP) female mice. To demonstrate that the Acta2-GFP transgene labeled mammary myoepithelial cells, lactating postpartum Day-2 Acta2+/+/Tg(Acta2:GFP) mammary tissue was obtained and stained as a whole mount with anti-ACTA2 antibody and examined for colocalization of anti-ACTA2 antibody and GFP (Fig. 4A). ACTA2 immunostaining and GFP were colocalized in myoepithelial cells near the surface of the mammary tissue in a basketlike organization demonstrating that the Acta2-GFP transgene is expressed in these cells and can be used as a marker for myoepithelial cells. Myoepithelial cells deeper in the tissue did not demonstrate ACTA2 immunostaining, presumably due to lack of antibody penetration; however, these myoepithelial cells could be observed because of the expression of the Acta2-GFP transgene.
FIG. 4.
Myoepithelial cell structure and organization. A) Confocal image of mammary tissue from a lactating postpartum Day-2 Acta2+/+/Tg(Acta2:GFP) dam stained with anti-ACTA2 antibody conjugated to rhodamine. Colocalization of ACTA2 immunostaining and GFP can be observed in myoepithelial cells (yellow) predominantly at the surface of the tissue; deeper myoepithelial cells do not show ACTA2 immunostaining presumably due to lack of antibody penetration. Myoepithelial cells are organized into a basketlike meshwork around the mammary alveoli. B–E) Confocal images of mammary tissue from lactating postpartum Day-2 Acta2+/+/Tg(Acta2:GFP) dams (B, D) and Acta2−/−/Tg(Acta2:GFP) dams (C, E) in which pups were removed 6 h prior to euthanasia (B, C) or were suckling at the time of euthanasia (D, E). A similar basketlike organization and numbers of myoepithelial cells can be observed in mammary tissue from both genotypes. Alveoli are larger and myoepithelial cells less contracted in Acta2−/− mammary tissue where pups were removed at the time of suckling (D, E). Bars = 50 μm (A–C and D, E).
Myoepithelial cell organization and structure was compared in mammary tissue from Acta2+/+/Tg(Acta2:GFP) and Acta2−/−/Tg(Acta2:GFP) Day-2 postpartum dams in which pups were removed 6 h prior to euthanasia (Fig. 4, B and C) or were suckling at the time of euthanasia (Fig. 4, D and E). The number of myoepithelial cells and their basketlike organization appeared similar in Acta2−/−/Tg(Acta2:GFP) and Acta2+/+/Tg(Acta2:GFP) mammary tissue. The only noticeable difference was that the alveoli consistently appeared larger in mammary tissue from lactating Acta2−/− dams compared with WT dams. This was most obvious in dams where the pups were actively suckling at the time of euthanasia (Fig. 4, D and E), presumably due to the ability of WT alveoli to eject milk with suckling in contrast to Acta2−/− alveoli. In addition, myoepithelial cells in these WT dams appeared thicker and more contracted than did the myoepithelial cells in Acta2−/− dams (Fig. 4, D and E). These results are consistent with the hypothesis that Acta2−/− mammary tissue cannot undergo normal milk ejection despite a normal structural appearance and presence of myoepithelial cells, suggesting that Acta2−/− myoepithelial cells are unable to generate sufficient contractile force to bring about milk ejection.
Myoepithelial Cells in Mammary Tissue of Postpartum Acta2−/− Mice Contract Less in Response to Oxytocin Than Do Myoepithelial Cells in WT Mice
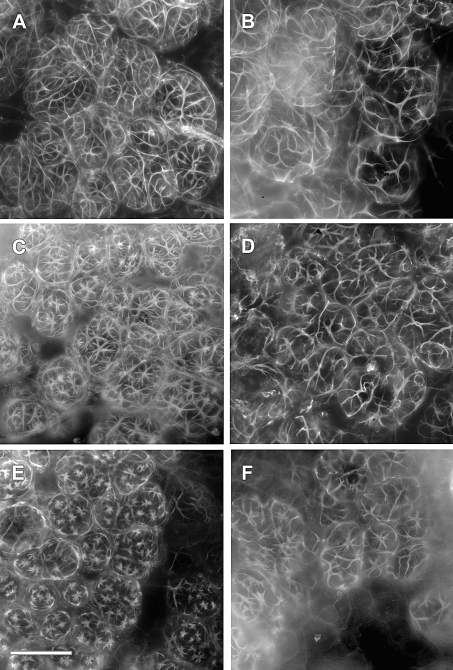
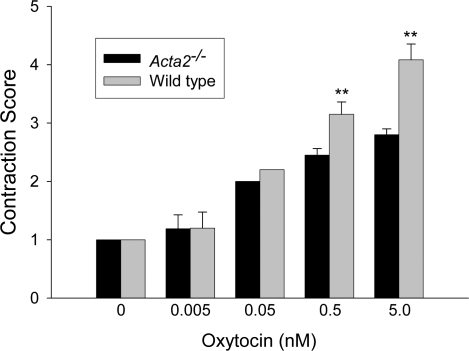
Previous studies have demonstrated that isolated mammary glandular tissue will contract in vitro in a dose-dependent manner in response to oxytocin [22]. Mammary glandular tissue from Day 2–3 postpartum WT and Acta2−/− dams, in which pups were removed for 6 h, was isolated, incubated in increasing doses of oxytocin for 2 min, and fixed and stained with rhodamine phalloidin to visualize myoepithelial cells. Myoepithelial cells in WT tissue were observed to contract in response to oxytocin in a dose-dependent manner, dramatically reducing their size and the size of the alveoli they surround (Fig. 5, A, C, and E). In contrast, while myoepithelial cells in Acta2−/− tissue contracted in response to oxytocin, these cells contracted considerably less than myoepithelial cells in WT tissue, especially at the higher concentrations of oxytocin (Fig. 5, B, D, and F). These observations were quantified using a contraction scoring system (Fig. 6). Interestingly, Acta2−/− myoepithelial cells contracted only slightly less than WT myoepithelial cells at the lower concentrations of oxytocin; however, at higher concentrations of oxytocin, Acta2−/− myoepithelial cells contracted significantly less than WT myoepithelial cells (P < 0.01; Fig. 6), suggesting that the total amount of contractile force Acta2−/− myoepithelial cells can generate is significantly less than WT myoepithelial cells.
FIG. 5.
WT myoepithelial cells will contract more than Acta2−/− myoepithelial cells in response to oxytocin in an isolated mammary tissue contraction assay. Mammary tissue from Day 2–3 postpartum WT and Acta2−/− dams, in which pups were removed for 6 h, was isolated and exposed to increasing doses of oxytocin for 2 min, fixed, and stained with rhodamine phalloidin. A, B) Control tissue from WT dams (A) and Acta2−/− dams (B) appears similar. C, D) Tissue exposed in vitro to 0.5 nM oxytocin for 2 min exhibited shorter myoepithelial cells and smaller lobules. This reduction is much more visible in WT (C) compared to Acta2−/− (D) mammary tissue. E, F) WT tissue exposed to 5.0 nM oxytocin shows a dramatic shortening in myoepithelial cells and reduction in size of the lobules (E) compared to Acta2−/− (F) mammary tissue. Bar = 100 μm.
FIG. 6.
Contraction of myoepithelial cells in response to oxytocin was quantified. Mammary tissue obtained and visualized as described in Fig. 5 was examined in a blind fashion and given a contraction score ranging from 1 to 5 with no contraction = 1 and maximum contraction = 5. Acta2−/− myoepithelial cells contracted only slightly less than WT myoepithelial cells at lower concentrations of oxytocin; however, at higher concentrations of oxytocin (0.5 and 5.0 nM) WT myoepithelial cells contracted significantly greater than did Acta2−/− myoepithelial cells (**P < 0.01).
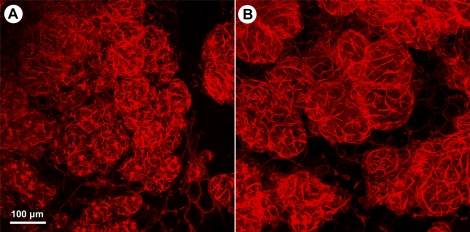
To determine whether myoepithelial cells in mammary glandular tissue in vivo respond similarly to oxytocin, Day-2 postpartum WT and Acta2−/− dams were injected i.p. with 0.1 IU oxytocin in 100 μl PBS 6 h after removal of the pups. Fifteen minutes after the injection of oxytocin, mammary tissue was removed, immediately fixed, and stained with rhodamine phalloidin to visualize myoepithelial cells in mammary tissue as whole mounts. Similar to isolated mammary tissue incubated with oxytocin, i.p. injection of oxytocin resulted in a dramatic contraction of myoepithelial cells in WT mammary tissue along with a reduction in the size of the alveoli (Fig. 7A); in contrast, myoepithelial cells in Acta2−/− tissue demonstrated only a slight contraction and reduction in alveolar size (Fig. 7B). These results demonstrate that lack of ACTA2 results in a dramatic reduction in contraction of myoepithelial cells in response to oxytocin in both isolated mammary glands and in vivo, suggesting that decreased milk ejection observed in Acta2−/− dams is the result of decreased contractile force generation in Acta2−/− myoepithelial cells.
FIG. 7.
Oxytocin promotes greater contraction of WT than Acta2−/− myoepithelial cells in mammary tissue of postpartum mice. Day-2 postpartum WT dams (A) and Acta2−/− dams (B) had their pups removed, and 6 h later received an i.p. injection of oxytocin. Fifteen minutes after the injections, mammary tissue was removed and fixed and stained with rhodamine phalloidin. Myoepithelial cells in WT mammary tissue contracted strongly with a concomitant reduction in alveoli in response to oxytocin (A). In contrast, myoepithelial cells in Acta2−/− mammary tissue contracted dramatically less, and the alveoli were much larger (B). Bar = 100 μm.
DISCUSSION
Mammary myoepithelial cells are specialized smooth musclelike epithelial cells that contract in response to oxytocin, generating the contractile force required for milk ejection during lactation [2, 3]. The results from this study demonstrate that ACTA2 contributes to the contractile function of these mammary myoepithelial cells. In this study, we demonstrate that Acta2−/− dams are unable to productively nurse their offspring. This lactation defect does not appear to be a result of an underlying structural defect in the Acta2−/− mammary glands as both virgin and lactating Acta2−/− mammary gland tissue appear structurally similar to WT mammary tissue. Acta2−/− mammary myoepithelial cells do generate significantly less contractile force in response to oxytocin than do WT mammary myoepithelial cells. It should be emphasized that Acta2−/− myoepithelial cells do contract in response to oxytocin; however, the overall contractile force that is generated is significantly less than in WT myoepithelial cells, suggesting that the level of force generated in Acta2−/− myoepithelial cells is not sufficient to promote milk ejection and successful lactation. These findings demonstrate the importance of ACTA2 in myoepithelial cells for functional contraction in response to oxytocin required for milk ejection and successful lactation. In addition, these results demonstrate that Acta2 expression and complete myoepithelial cell contractile force generation are not necessary for myoepithelial cell and mammary gland development.
The noticeable difference between mammary tissue in Acta2−/− and WT postnatal dams was the size of the alveoli lumen. Acta2−/− alveoli were significantly larger than WT alveoli when observed both as whole mounts and by histology. Previous studies have demonstrated that reduced milk ejection results in increased alveoli size, either through sealing of the teat [23], subcutaneous ligation of the teat ducts [24], or by increasing the viscosity of the milk [25]. While it is possible that other factors, such as increased proliferation, could contribute to increased alveoli size, the increase in alveoli size we observe in Acta2−/− mammary tissue of postnatal dams is consistent with reduced milk ejection as a result of decreased contractile function of the myoepithelial cells.
Previous studies have demonstrated the importance of ACTA2 expression in the contractile function of vascular smooth muscle cells [16], bladder smooth muscle cells [17], and myofibroblasts [7–10]. Decreased contractile activity was observed in aortic vascular smooth muscle cells and bladder smooth muscle cells in Acta2−/− mice [16, 17]; however, for both cell types sufficient contractile force generation can occur to allow for survival of the Acta2−/− mice. Myofibroblasts are specialized fibroblasts found in granulation tissue and tissue contractures and have acquired a smooth musclelike contractile phenotype, including the expression of ACTA2. Overexpression of Acta2 in fibroblasts results in increased force generation [8], while a peptide homologous to the amino-terminal end of ACTA2 can compete with ACTA2 from stress fibers in myofibroblasts and result in decreased force generation [10]. Similarly, decreasing the expression of ACTA2 with anti-sense mRNA decreases myofibroblast contractile force generation [9]. Our findings of decreased contractile force generation in myoepithelial cells from Acta2−/− mice are consistent with these results and suggest that expression of ACTA2 in myoepithelial cells has the functional consequence of increasing contractile force generation. It should be stressed that currently the mechanism by which expression of this specific muscle actin isoform can increase contractile force generation is unknown.
Myoepithelial cells lacking ACTA2 do generate some contractile force in response to oxytocin as evidenced by limited growth and survival of the pups and by some contraction in response to oxytocin in the assays. These results demonstrate that lack of ACTA2 does not totally alleviate myoepithelial cell contractile force generation; rather, loss of ACTA2 reduces the level of force generation below that needed for milk ejection in vivo. The extent of contraction of Acta2−/− myoepithelial cells in the in vitro assay is much greater than that observed in myoepithelial cells in Acta2−/− mice in response to the i.p. injection of oxytocin. This could be the result of the much higher levels of oxytocin in the in vitro assay than the in vivo assay; however, myoepithelial cells in WT mice in the in vivo assay appeared to be fully contracted, which is similar to their appearance upon using higher levels of oxytocin in the in vitro assay. More likely, the isolation and dissection of the mammary tissue for the in vitro assay resulted in less resistance to myoepithelial cell contraction than was seen in vivo; therefore, a greater amount of contraction by Acta2−/− myoepithelial cells is observed in the in vitro assay than would be expected in vivo.
In addition to expressing ACTA2, myoepithelial cells also express the β- and γ-cytoplasmic actin isoforms. Fibroblasts that express only these two cytoplasmic actin isoforms can assemble these actin isoforms along with actin-binding proteins and myosin into contractile elements that are called stress fibers [7]. Based upon the strong phalloidin staining observed in Acta2−/− myoepithelial cells, it is likely that these cytoplasmic actin isoforms can assemble into f-actin bundles with associated actin-binding proteins and myosin to form contractile elements; however, the level of contractile force generated is significantly less than in myoepithelial cells expressing ACTA2. Future studies using electron microscopy will be necessary to determine whether the structural organization of contractile elements is altered in myoepithelial cells upon the loss of ACTA2 expression. Vascular smooth muscle cells in Acta2−/− mice appear to respond to the loss of ACTA2 expression by expressing the skeletal muscle α-actin isoform [16], a muscle actin isoform not normally expressed in vascular smooth muscle cells. Whether other muscle actin isoforms are expressed in response to the loss of ACTA2 in mammary myoepithelial cells is unclear; however, even if other muscle actin isoforms are expressed, they cannot completely compensate functionally for the lack of ACTA2.
Two recent studies have demonstrated that Mkl1 null dams are unable to productively nurse their offspring [11, 12]. Due to the lack of MKL1, myoepithelial cells in Mkl1 null dams have reduced expression of ACTA2, smooth muscle myosin heavy chain, calponin, and tropomyosin 2. MKL2, another member of the MKL/myocardin transcription family, was found to be upregulated in myoepithelial cells from Mkl1 null mice and may be responsible for the low level of expression observed for these proteins. Interestingly, Acta2−/− and Mkl1 null dams have different phenotypes with respect to their ability to maintain offspring. Pups born to Acta2−/− dams show reduced weight gain starting from the time of birth; in contrast, pups born to Mkl1 null mice do not begin to show declined weight gain until between Days 4 and 7 [11, 12]. Consistent with ACTA2 expression being important for myoepithelial cell function, ACTA2 expression in myoepithelial cells from Mkl1 null dams is almost at the same level as that seen in myoepithelial cells in WT dams at Postpartum Day 1; however, expression of ACTA2 declines with increasing postpartum time [11]. It should be pointed out that in Mkl1 null dams the other smooth muscle cytoskeletal proteins regulated by MKL1 also show a similar pattern of decreased expression; therefore, decreased myoepithelial cell function in Mkl1 null mice cannot be demonstrated to be the result of the loss of expression of one specific smooth muscle cytoskeletal protein.
In the Mkl1 null mice, there is decreased expression of the smooth muscle contractile proteins expressed in myoepithelial cells, including ACTA2, smooth muscle myosin heavy chain, calponin, and tropomyosin 2 [11, 12]. In the Acta2−/− myoepithelial cells, it is clear that ACTA2 expression is reduced; however, the effect this has upon expression of other smooth muscle contractile proteins is not known. MKL1 transcriptional activity is regulated by actin dynamics [26]. It is possible that the lack of ACTA2 expression and resulting reduction of contractile force generation alters the actin dynamics, increasing G-actin and thereby decreasing transcriptional activity of MKL1 and decreasing expression of other smooth muscle contractile proteins. However, no difference in stress fiber organization was observed in phalloidin-stained WT and Acta2−/− myoepithelial cells. Future studies will need to focus on the effect lack of expression of ACTA2 has upon expression of other smooth muscle contractile proteins in myoepithelial cells.
The results from this study demonstrate that myoepithelial cell contraction is essential for milk ejection. In addition, they demonstrate that ACTA2 is necessary for myoepithelial cells to function normally and generate the contractile force required for milk ejection. These results suggest that there are functional differences between ACTA2 and the other actin isoforms; however, the molecular basis for these differences is not clear. Future studies should be able to identify the structural basis for ACTA2 function in myoepithelial cell contractile force generation.
ACKNOWLEDGMENTS
The authors wish to thank Dr. J.Y. Tsai of the National Eye Institute, Bethesda, MD, for providing the Acta2-GFP transgenic mice used in this study. The authors also wish to thank Joel McRae for technical assistance.
Footnotes
Supported by a grant from the National Institutes of Health (R01 GM060651) to J.J.T.
REFERENCES
- Bremel RD, Shaw ME. Actomyosin from mammary myoepithelial cells and phosphorylation by myosin light chain kinase. J Dairy Sci 1978; 61: 1561 1566 [DOI] [PubMed] [Google Scholar]
- Deugnier MA, Moiseyeva EP, Thiery JP, Glukhova M. Myoepithelial cell differentiation in the developing mammary gland: progressive acquisition of smooth muscle phenotype. Dev Dyn 1995; 204: 107 117 [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev 2001; 81: 629 683 [DOI] [PubMed] [Google Scholar]
- Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR, Matzuk MM. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc Natl Acad Sci U S A 1996; 93: 11699 11704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KU, Young WS, III., Liu X, Ginns EI, Li M, Furth PA, Hennighausen L. Oxytocin and milk removal are required for post-partum mammary-gland development. Genes Funct 1997; 1: 233 244 [DOI] [PubMed] [Google Scholar]
- Byers HR, White GE, Fujiwara K. Organization and function of stress fibers in cells in vitro and in situ. A review. Cell Muscle Motil 1984; 5: 83 137 [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 2002; 3: 349 363 [DOI] [PubMed] [Google Scholar]
- Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell 2001; 12: 2730 2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnov-Jessen L, Petersen OW. A function for filamentous alpha-smooth muscle actin: retardation of motility in fibroblasts. J Cell Biol 1996; 134: 67 80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Gabbiani G, Chaponnier C. The NH2-terminal peptide of alpha-smooth muscle actin inhibits force generation by the myofibroblast in vitro and in vivo. J Cell Biol 2002; 157: 657 663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Boyd K, Xu W, Ma J, Jackson CW, Fu A, Shillingford JM, Robinson GW, Hennighausen L, Hitzler JK, Ma Z, Morris SW. Acute myeloid leukemia-associated Mkl1 (Mrtf-a) is a key regulator of mammary gland function. Mol Cell Biol 2006; 26: 5809 5826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Chang S, Qi X, Richardson JA, Olson EN. Requirement of a myocardin-related transcription factor for development of mammary myoepithelial cells. Mol Cell Biol 2006; 26: 5797 5808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DZ, Li S, Hockemeyer D, Sutherland L, Wang Z, Schratt G, Richardson JA, Nordheim A, Olson EN. Potentiation of serum response factor activity by a family of myocardin-related transcription factors. Proc Natl Acad Sci U S A 2002; 99: 14855 14860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cen B, Selvaraj A, Prywes R. Myocardin/MKL family of SRF coactivators: key regulators of immediate early and muscle specific gene expression. J Cell Biochem 2004; 93: 74 82 [DOI] [PubMed] [Google Scholar]
- Morita T, Mayanagi T, Sobue K. Reorganization of the actin cytoskeleton via transcriptional regulation of cytoskeletal/focal adhesion genes by myocardin-related transcription factors (MRTFs/MAL/MKLs). Exp Cell Res 2007; 313: 3432 3445 [DOI] [PubMed] [Google Scholar]
- Schildmeyer LA, Braun R, Taffet G, Debiasi M, Burns AE, Bradley A, Schwartz RJ. Impaired vascular contractility and blood pressure homeostasis in the smooth muscle alpha-actin null mouse. FASEB J 2000; 14: 2213 2220 [DOI] [PubMed] [Google Scholar]
- Zimmerman RA, Tomasek JJ, McRae J, Haaksma CJ, Schwartz RJ, Lin HK, Cowan RL, Jones AN, Kropp BP. Decreased expression of smooth muscle alpha-actin results in decreased contractile function of the mouse bladder. J Urol 2004; 172: 1667 1672 [DOI] [PubMed] [Google Scholar]
- Tsai JY, Yamamoto T, Fariss R, Hickman F, Pagan-Mercado G. Using SMAA-GFP mice to study pericyte coverage of retinal vessels. Invest Ophthalmol Vis Sci 2002; 43E. Abstract 1929
- Wang J, Niu W, Nikiforov Y, Naito S, Chernausek S, Witte D, LeRoith D, Strauch A, Fagin JA. Targeted overexpression of IGF-I evokes distinct patterns of organ remodeling in smooth muscle cell tissue beds of transgenic mice. J Clin Invest 1997; 100: 1425 1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T, Kawakami Y, Nagai Y, Ma JX, Tsai JY, Kincade PW, Sato S. Bone marrow lacks a transplantable progenitor for smooth muscle type alpha-actin-expressing cells. Stem Cells 2006; 24: 13 22 [DOI] [PubMed] [Google Scholar]
- Emerman JT, Vogl AW. Cell size and shape changes in the myoepithelium of the mammary gland during differentiation. Anat Rec 1986; 216: 405 415 [DOI] [PubMed] [Google Scholar]
- Moore DM, Vogl AW, Baimbridge K, Emerman JT. Effect of calcium on oxytocin-induced contraction of mammary gland myoepithelium as visualized by NBD-phallacidin. J Cell Sci 1987; 88: 563 569 [DOI] [PubMed] [Google Scholar]
- Hanwell A, Linzell JL. The effects of engorgement with milk and of suckling on mammary blood flow in the rat. J Physiol 1973; 233: 111 125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross BA, Silver IA. Milk ejection and mammary engorgement. Proc R Soc Med 1956; 49: 978 979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwertfeger KL, McManaman JL, Palmer CA, Neville MC, Anderson SM. Expression of constitutively activated Akt in the mammary gland leads to excess lipid synthesis during pregnancy and lactation. J Lipid Res 2003; 44: 1100 1112 [DOI] [PubMed] [Google Scholar]
- Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell 2003; 113: 329 342 [DOI] [PubMed] [Google Scholar]