Abstract
The prevalence of maternal obesity is increasing rapidly in recent decades. We previously showed that maternal obesity affected skeletal muscle development during the fetal stage. The objective of this study was to evaluate the effects of maternal obesity on the skeletal muscle properties of offspring. Ewes were fed a control diet (100% energy requirement, Con) or an obesogenic diet (150% energy requirement, OB) from 2 mo before pregnancy to weaning. After weaning, the offspring lambs were fed a maintenance diet until 19 mo of age and then ad libitum for 12 wk to measure feed intake. At 22 mo old, the longissimus dorsi (LD) muscle was biopsied. The downstream insulin signaling was lower in OB than Con lambs as shown by reduction in the phosphorylation of protein kinase B, mammalian target of rapamycin, and 4-E binding protein 1. On the other hand, the phosphorylation of protein kinase C and insulin receptor substrate 1 was higher in OB compared to Con lambs. More intramuscular adipocytes were observed in OB compared to Con offspring muscle, and the expression of peroxisome proliferator-activated receptor gamma, an adipocyte marker, was also higher, which was consistent with the higher intramuscular triglyceride content. Both fatty acid transport protein 1 and cluster of differentiation 36 (also known as fatty acid translocase) were increased in the OB group. In addition, higher collagen content was also detected in OB compared to Con offspring. In conclusion, our data show that offspring from obese mothers had impaired insulin signaling in muscle compared with control lambs, which correlates with increased intramuscular triglycerides and higher expression of fatty acid transporters. These data clearly show that maternal obesity impairs the function of the skeletal muscle of offspring, supporting the fetal programming of adult metabolic diseases.
Keywords: adipocytes, collagen, fetus, insulin resistance, maternal obesity, offspring, pregnancy, signal transduction, skeletal muscle
Maternal obesity has long-lasting effects on the properties of offspring muscle; this may provide a key mechanism for the fetal programming of adult diseases.
INTRODUCTION
Obesity is increasing at an alarming rate. According to a recent National Health and Nutrition Examination Survey (1999–2002), 29% of nonpregnant women between 20 and 39 years of age are obese. Meanwhile, obesity in children and teenagers is also increasing [1]. Maternal obesity predisposes offspring to obesity, hypertension, and insulin resistance [2–4]. However, the underlying mechanisms responsible for such a link remain missing.
Skeletal muscle constitutes about 40%–50% of body mass [5] and is the main peripheral tissue responsive to insulin-stimulated uptake of glucose and fatty acids [6]. Development of insulin resistance in skeletal muscle is an essential step in the development of type 2 diabetes [7–9]. The fetal period is crucial for skeletal muscle development because no net increase in the number of muscle fibers occurs after birth [10, 11]. In addition, its lower priority in nutrient partitioning during fetal development compared to organs such as the brain, heart, and liver renders skeletal muscle particularly vulnerable to nutrient alteration [12]. Furthermore, the late fetal stage is also very important for adipogenesis, which forms intramuscular adipocytes [13]. Increase of adipogenesis in fetal skeletal muscle will induce muscle insulin resistance as a result of the paracrine effect of intramuscular adipocytes [14–16]. Fibrogenesis, which forms endomysium and perimysium, is also actively ongoing during the fetal stage, and an increase of fibrogenesis leads to impaired muscle functions [17]. Therefore, any change in fetal skeletal muscle development has important negative physiological consequences for the offspring, including predisposing offspring to obesity and type 2 diabetes and muscle weakness [18–21].
During aging, a progressive loss of muscle mass occurs accompanied by increased adiposity and fibrosis [17, 22], resulting in a decline in muscle structural integrity and functional capacity [23]. Our previous studies demonstrated that maternal obesity induces changes in fetal skeletal muscle at an early developmental stage, including an increase in intramuscular adipocytes and fibrosis and insulin resistance [24, 25], that typically are not observed until later in life. Here, we report that the effect of maternal obesity on fetal skeletal muscle development is maintained in offspring skeletal muscle, which might provide a reason for the increased incidence of obesity and diabetes in offspring born to obese mothers.
MATERIALS AND METHODS
Care and Use of Animals
All the animal procedures were approved by the University of Wyoming Animal Care and Use Committee. Animal procedures have been previously described in detail [26]. Briefly, multiparous Rambouillet/Columbia ewes were mated to a single ram. From 60 days before conception to weaning (first day of mating = d0), ewes were individually fed either a highly palatable diet at 100% (Con) of National Research Council recommendations for energy [27] or 150% (OB) of recommended energy requirements [19]. Ewes were housed in individual pens within a temperature-controlled room (∼20°C). All ewes were weighed at weekly intervals, and the rations were adjusted for weekly changes in metabolic body weight (BW0.75) [28, 29]. The body condition was scored at monthly intervals to evaluate changes in fatness. A body condition score of 1 (emaciated) to 9 (obese) was assigned by two trained observers after palpation of the transverse and vertical processes of the lumbar vertebrae (L2 through L5) and the region around the tail head [30].There was no difference in body weight between the two groups of ewes before the diet treatment; after 60 days of diet treatment, the body weight of maternal sheep in the OB group was more than 30% higher than that in the Con group (92.1 ± 3.0 and 70.3 ± 2.8 kg, respectively; P < 0.05), while the body condition score was about 40% higher in OB maternal sheep (OB vs. Con = 7.1 ± 0.2 vs. 4.8 ± 0.2, respectively; P < 0.05). At d135, the body weight and body condition score of ewes remained higher in OB compared to the Con group (109.1 ± 4.3 vs. 73.1 ± 4.2 kg and 8.7 ± 0.2 vs. 5.1 ± 0.3, respectively; P < 0.05). More information can be found in a previous publication using the same pool of sheep [26].
Eight offspring lambs from the Con ewes (three males and five females) and nine from the OB ewes (four males and five females) were randomly selected for further studies. All the male lambs were castrated after birth. After weaning at 4 mo of age, a standard commercially available creep diet (Lamb Creep B30 w/Bovatec; Ranch-Way Feeds) that meets 100% of their nutrient requirement was given for 19 mo. Then, the lambs were placed in individual pens so that the daily feed intake could be measured, and a concentrated diet was fed ad libitum for three additional months. The ration consisted of corn, soy hulls, wheat midds, alfalfa meal, and distillers byproducts, and when it was analyzed, it contained 71.05% total digestible nutrients, 13.5% crude protein, and 4.05% fat (ADM Alliance Nutrition, Inc.). Detailed feed composition has been previously described [26]. Before biopsy, we examined the total percentage of body fat in offspring sheep using dual energy x-ray absorptiometry. The ratio of fat content to body weight was significantly higher in OB offspring (Con vs. OB = 16.5% ± 1.2% vs. 20.8% ± 1.1%, P < 0.05), while the total fat mass tended to be higher in the OB group (by 23.4% ± 9.0%, P < 0.10) [26].
Following overnight fasting, the lambs were anesthetized and the longissimus dorsi (LD) muscle (2 g) was sampled at the 12th rib of the left side. The muscle was cut into two pieces with one piece snap frozen for biochemical analyses and the other piece fixed and paraffin embedded for histochemical staining.
Antibodies
The following were purchased from Cell Signaling: antibodies against protein kinase B (AKT) (cat. no. 9272), phospho-AKT modified at Ser-473 (cat. no. 4060S), insulin-like growth factor 1 (IGF1) receptor (cat. no. 3027), phospho-IGF1 receptor modified at Tyr-1131/insulin receptor β modified at Tyr-1146 (cat. no. 3021S), insulin receptor substrate 1 (IRS1) (cat. no. 2382), phospho-IRS1 modified at Ser-1101 (cat. no. 2385S), nuclear factor κ-light-chain-enhancer of activated B cells (NFKB) subunit p65 (cat. no. 4764), phospho-p65 modified at Ser-536 (cat. no. 3033), phospho-protein kinase C (PRKC) δ/θ (Ser-643/Ser-676) (cat. no. 9376), mammalian target of rapamycin (MTOR) (cat. no. 2972), phospho-MTOR modified at Ser-2448 (cat. no. 2971), eukaryotic translation initiation factor 4E-binding protein (EIF4EBP) 1 (cat. no. 9452), and phospho-EIF4EBP1 modified at Thr-37/Thr-46 (cat. no. 9459S). Antibody to β-tubulin was from Sigma (cat. no. T4026), antibody to fatty acid transport protein 1 (FATP1, SLC27A1) was from Santa Cruz biotechnology (cat. no. sc-25541), antibody to peroxisome proliferator-activated receptor γ (PPARG) was from Delta Biolabs (cat. no. DB134), and IRDye 800CW goat anti-rabbit secondary antibody and IRDye 680 goat anti-mouse secondary antibody were purchased from LI-COR Biosciences. For use, primary antibodies were diluted 1:1000 and secondary antibodies were diluted 1:10 000.
Histochemical Analyses
LD muscle samples were fixed in 4% (w/v) paraformaldehyde in 0.12 M phosphate buffer, pH 7.4, embedded in paraffin, and sectioned at 10 μm. Every fifth section was rehydrated by a series of incubations in xylene and ethanol solutions and then used for Masson trichrome staining [31]. With this staining, muscle fibers were stained red, nuclei black, and collagen blue. Five fields per section and five sections per sample were randomly selected for quantification of fat area and collagen area using the Image J 1.30v software (National Institutes of Health). The averaged data were used for the calculations [19].
Total Triglyceride Analysis
Total triglycerides were extracted using the Folch method [32]. Briefly, around 60 mg of LD muscle powder was weighed into 2-ml Eppendorf tubes, and 1.5 ml of chloroform-methanol 2:1 (v/v) was added; the samples were then kept at 4°C for 48 h. After that, a quarter volume (in this case, 375 μl) of 0.9% NaCl was added, and the tubes were mixed by shaking. The mixture was kept at room temperature overnight and then centrifuged at 10 000 × g for 5 min at 4°C. Twenty microliters of the lower phase was transferred into a new 1.5-ml Eppendorf tube and dried for 1 h under the hood. The total triglycerides were measured using a kit from Sigma following the manufacturer's instructions (cat. no. TR0100). The results were divided by the initial muscle powder weight to calculate the content of triglycerides per gram of muscle.
Collagen Measurement Using Chemical Method
Ground muscle powders (0.1 g) were dried in a convection oven at 60°C, and samples were weighed and then hydrolyzed in 6 N HCl at 105°C for 16 h. An aliquot was removed for hydroxyproline determination as previously described [33]. Collagen concentration (% dry muscle weight) was calculated assuming collagen weight = 7.25 × hydroxyproline measured weight [34].
Real-Time Quantitative PCR (RT-PCR)
Total mRNA was extracted from the LD muscle using TRI reagent (Sigma) and reverse transcribed into cDNA by using a kit (Qiagen). Reverse-transcribed cDNAs were used for real-time PCR analyses using a SYBR Green RT-PCR kit from Bio-Rad. The primers used were: PPARG forward, 5′-CCGCATCTTCCAGGGGTGTC-3′ and reverse, 5′-CAAGGAGGCCAGCATCGTGAAAT-3′; SLC27A1 forward, 5′-ACTGTCTGCCCCTGTACCAC-3′ and reverse, 5′-GGCTGGCTGAAAACTTCTTG-3′; CD36 forward, 5′-GGTGATTTGACCCAGCACTT-3′ and reverse, 5′-AATGCTGGTTGGAGGACAAC-3′; CD14 forward, 5′-CTCAGCGTGCTTGATCTCAG-3′ and reverse, 5′-AAGGGATTTCCGTCCAGAGT-3′; TLR2 forward, 5′-CAAGAGGAAGCCCAGGAAG-3′ and reverse, 5′-TGGACCATGAGGTTCTCCA-3′; TLR4 forward, 5′-TGCTGGCTGCAAAAAGTATG-3′ and reverse, 5′-CCCTGTAGTGAAGGCAGAGC-3′; and tubulin forward, 5′-CGAGAGCTGTGACTGTCTGC-3′ and reverse, 5′-GGCATGACGCTAAAGGTGTT-3′. Each reaction yielded amplicons between 80 and 200 bp. PCR conditions were as follows: 10 sec at 95°C followed by 30 sec at 55°C for 40 cycles. After amplification, a melting curve (0.01°C/sec) was used to confirm product purity. In addition, electrophoresis was conducted to confirm that only one fragment with the right size was amplified. The results are expressed relative to tubulin [25].
Immunoblotting Analysis
Immunoblotting analyses were conducted according to the procedures previously described [12, 19]. Membranes were visualized by the Odyssey Infrared Imaging System (LI-COR Biosciences). The density of the bands of all the animals was quantified and then normalized to the tubulin content. Only representative bands are shown in the figures.
Statistical Analysis
Statistical analyses were conducted according to the methods outlined in our previous studies on sheep [12, 19, 25, 35]. Briefly, each animal was considered as an experimental unit. Because there were no differences in measured parameters between female and castrated male offspring, the data from the different sexes were combined. Data were analyzed as a complete randomized design using a general linear model of statistical analysis system (SAS, 2000). Differences in mean values were compared by the Tukey multiple comparison test, and mean ± SEM are reported. Statistical significance was considered as P < 0.05.
RESULTS
Insulin Resistance Was Detected in Skeletal Muscle of the Progeny of OB Sheep
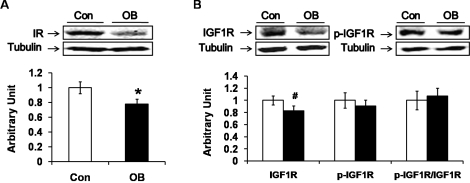
The muscle content of insulin receptor protein decreased by 22.5% ± 7.1% (P < 0.05) in the progeny of OB lambs compared to Con lambs (Fig. 1A), and the IGF1 receptor tended to decrease (by 20.2% ± 8.8%; P < 0.10) in OB compared to Con muscle (Fig. 1B). Because the sequences surrounding the phosphorylation sites of insulin and IGF1 receptor are identical, the antibody recognizes the phosphorylated receptors of both insulin and IGF1. No difference in insulin and IGF1 receptor phosphorylation was observed between the two types of sheep (Fig. 1B).
FIG. 1.
Insulin receptor content and receptor phosphorylation in LD muscle of progeny of obese (OB ▪) versus control group (Con □). A) Western blot showed less insulin receptor in OB than in Con muscle. B) The total protein content of IGF1 displayed a decreasing trend in OB compared to Con muscle. No significant differences were observed in the phosphorylation of IGF1 receptor (IGF1R) and the ratio of phospho-IGF1R to total IGF1R. (*P < 0.05, #P < 0.10; mean ± SEM; n = 8 in Con and n = 9 in OB group).
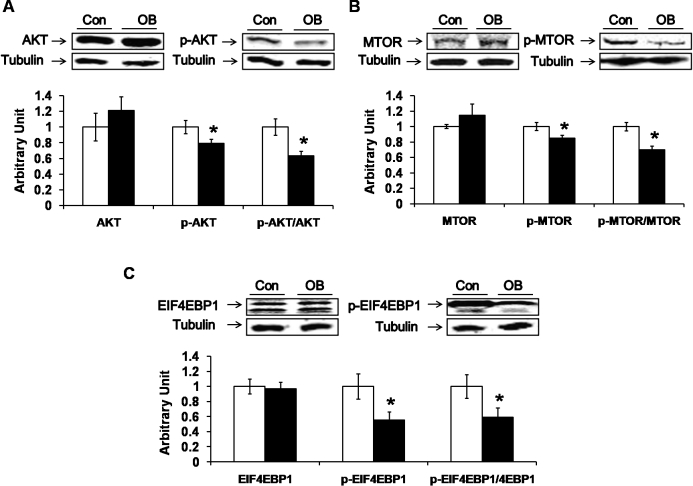
AKT is the main downstream mediator of the insulin-signaling pathway. Despite a lack of difference in the phosphorylation of insulin and IGF1 receptors, there was a decrease in the phosphorylation of AKT at Ser-473 (21.0% ± 5.3%; P < 0.05) as well as a lower ratio of phospho-AKT to total AKT (26.6% ± 6.0%; P < 0.05) in the muscle of the progeny of OB compared to Con lambs (Fig. 2A). In addition, both MTOR and EIF4EBP1 are downstream effectors of AKT; the phosphorylation of both MTOR (by 19.9% ± 5.5%; P < 0.05) and EIF4EBP1 (by 55.4% ± 10.7%, P < 0.05) decreased in the muscle of the progeny of OB compared to Con lambs (Fig. 2, B and C). These data show that the downstream signaling of insulin/IGF1 was attenuated despite a lack of difference in receptor phosphorylation, indicating insulin/IGF1 resistance.
FIG. 2.
Down-regulation of downstream insulin signaling in LD muscle of progeny of obese (OB ▪) versus control group (Con □). A) Decreased phosphorylation in AKT at Ser-473 in OB compared to Con muscle. B) Phosphorylation of MTOR as well as the ratio of phospho-MTOR to total MTOR were decreased in OB compared to Con muscle. C) Phosphorylation of EIF4EBP1 was decreased in OB muscle without a change in the total protein content of EIF4EBP1. (*P < 0.05; mean ± SEM; n = 8 in Con and n = 9 in OB group).
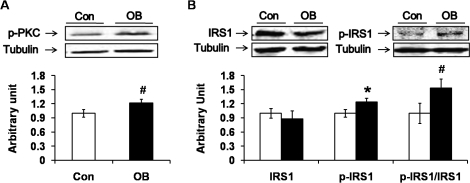
PRKC θ (PRKCQ) phosphorylates IRS1 at Ser-1101, which attenuates downstream insulin signaling, leading to insulin resistance. The phosphorylation of PRKC appeared to increase in the muscle of the progeny of OB sheep (P < 0.10) (Fig. 3A). In addition, the phosphorylation of IRS1 at Ser-1101 was higher in the OB group (by 23.8% ± 8.4%; P < 0.05) (Fig. 3B), which further confirmed that there was insulin resistance in the muscle of the progeny of the OB sheep.
FIG. 3.
Protein kinase C (PRKC) and insulin substrate 1 (IRS1) phosphorylation in LD muscle of progeny of obese (OB ▪) versus control group (Con □). A) Phosphorylation of PRKC tended to increase in OB compared to Con muscle. B) Phosphorylation of IRS1 at Ser-1101 was increased in OB muscle. (*P < 0.05, #P < 0.10; mean ± SEM; n = 8 in Con and n = 9 in OB group).
Skeletal Muscle of the Progeny of Obese Sheep Had Enhanced Intramuscular Lipid Accumulation and Fibrosis
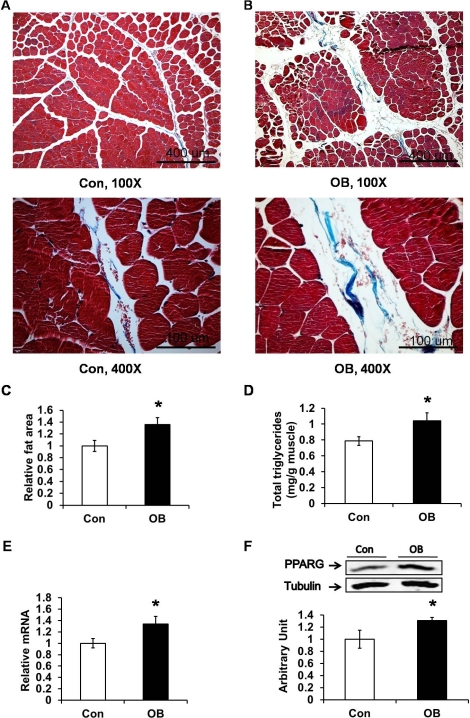
Intramuscular lipid accumulation activates PRKC, which induces insulin resistance. More intramuscular adipocytes were observed in the muscle of the offspring of OB than Con lambs (Fig. 4, A and B). We quantified the fat area in muscle sections and found it to be higher in muscle of the progeny of the OB sheep (by 35.6% ± 11.8%; P < 0.05) (Fig. 4C). Furthermore, chemical analysis showed that there were more triglycerides in the OB muscle (by 20.1% ± 2.8%; P < 0.05) (Fig. 4D). In addition, both the mRNA and protein levels of PPARG, a marker of adipocytes, were higher in the OB group compared to the Con group (by 33.6% ± 13.6% and 31.1% ± 5.1%, respectively; P < 0.05) (Fig. 4, E and F).
FIG. 4.
Masson trichrome staining showed more intramuscular adipocytes and collagen in LD muscle of progeny of obese (OB ▪) versus control group (Con □). Using Masson trichrome staining, muscle cells stained red, collagen stained blue, while adipocytes were colorless. A) LD muscle of Con sheep (top: 100× magnification; bottom: 400× magnification). B) LD muscle of OB sheep (top: 100× magnification; bottom: 400× magnification). C) Larger fat area in muscle tissue of OB sheep. D) Total triglycerides content in Con and OB LD muscle. The results showed a higher content of total triglycerides in OB muscle than in Con muscle, displayed in mg of total triglycerides per gram of muscle powder. E) Increased expression of PPARG mRNA in OB muscle determined by quantitative PCR. F) PPARG protein was enhanced in OB muscle as shown by Western blot analysis (*P < 0.05; mean ± SEM; n = 8 in Con and n = 9 in OB group).
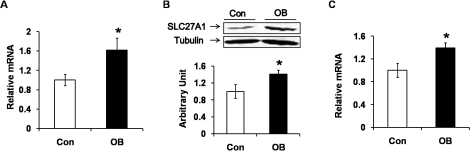
Compared to muscle of the progeny of Con sheep, the mRNA and protein levels of SLC27A1 (FATP1) were enhanced in the muscle of the OB group (by 61.8% ± 24.8% and 40.8% ± 9.3%, respectively; P < 0.05) (Fig. 5, A and B). In addition, the level of CD36 (cluster of differentiation 36 or fatty acid translocase, FAT) mRNA was increased by 39.4% ± 8.8% (P < 0.05) (Fig. 5C). Taken together, our results indicated that there were more adipocytes and enhanced lipid accumulation in offspring skeletal muscle of OB compared to Con sheep.
FIG. 5.
Expression of fatty acid transport protein 1 (SLC27A1) and fatty acid translocase (CD36) in LD muscle of progeny of obese (OB ▪) versus control group (Con □). A) Quantitative PCR showed an enhanced expression of SLC27A1 mRNA in OB muscle compared to Con muscle. B) An increase in expression of SLC27A1 protein as shown by using Western blot analysis. C) FAT/CD36 mRNA was higher in OB muscle as shown by quantitative PCR. (*P < 0.05; mean ± SEM; n = 8 in Con and n = 9 in OB group).
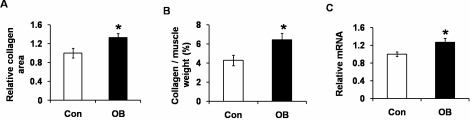
As shown in Figure 4, A and B, the muscle of the offspring of OB sheep also appeared to have more connective tissue. Indeed, the collagen area was larger in the muscle tissue of the progeny of OB sheep (by 32.8% ± 8.8%; P < 0.05) (Fig. 6A); chemical analysis also showed that the collagen content was higher in the progeny of OB lambs compared to Con lambs (by 50.6% ± 15.3%; P < 0.05) (Fig. 6B). Transforming growth factor β (TGFB) signaling stimulates fibrogenesis. The level of TGFB mRNA was 27.0% ± 9.1% higher in OB compared to Con muscle (P < 0.05) (Fig. 6C). Combining these results, we concluded that fibrogenesis was increased in LD muscle of the progeny of OB sheep.
FIG. 6.
Collagen content and TGFB signaling in LD muscle of progeny of obese (OB ▪) versus control group (Con □). A) More collagen was observed in the muscle tissue of OB sheep. B) Higher collagen content was detected in OB than in Con muscle. C) Quantitative PCR showed an increased mRNA level of TGFB. (*P < 0.05; mean ± SEM; n = 8 in Con and n = 9 in OB group).
Inflammatory Signaling Was Enhanced in LD Muscle of the Progeny of Obese Sheep
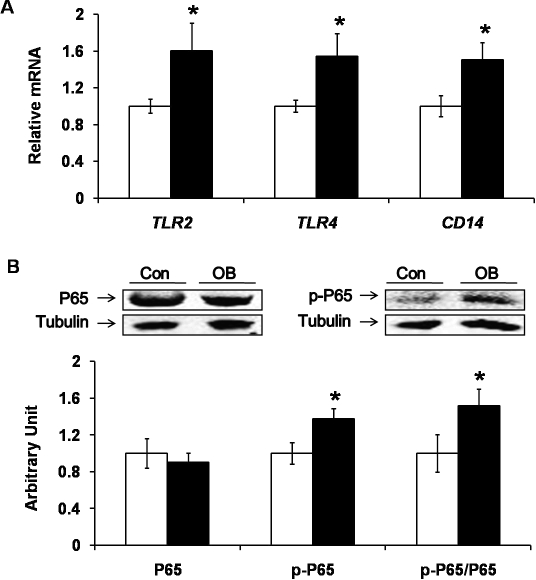
Inflammatory responses are linked with obesity, enhanced lipid accumulation, fibrosis, and insulin resistance. Our previous results showed that toll-like receptor 4 (TLR4) signaling was induced in the fetal muscle of OB mothers. In the current study, we detected an increase of TLR2 and TLR4 mRNA expression (by 60.6% ± 29.7% and 54.5% ± 24.1%, respectively; P < 0.05) (Fig. 7A) in LD muscle of the progeny of OB sheep compared to Con sheep. The mRNA level of CD14 (cluster of differentiation 14, mainly expressed in macrophages), which functions as a coreceptor of TLR4, was 50.6% ± 18.6% (P < 0.05) higher in the progeny of the OB group (Fig. 7A). TLR2 and TLR4 initiate the inflammatory signaling pathway involving NFKB. The phosphorylation of p65, a key component of the NFKB-signaling pathway, was increased by 37.4% ± 11.5% (P < 0.05) in the muscle of the progeny of OB sheep compared to Con sheep (Fig. 7B).
FIG. 7.
Toll-like receptor 4 (TLR4) and its downstream signaling nuclear factor κB (NFKB) in LD muscle of progeny of obese (OB ▪) versus control group (Con □). A) Higher mRNA expression of TLR2 and TLR4, as well as CD14 (a coreceptor of TLR4), was detected in OB compared to Con muscle. B) Phosphorylation of NFKB subunit p65 was increased in OB muscle. (*P < 0.05; mean ± SEM; n = 8 in Con and n = 9 in OB group).
DISCUSSION
Sheep are one of the most commonly used models for pregnancy studies. Using the same sheep model, we previously showed that maternal obesity increases intramuscular adipocytes and connective tissue in fetal muscle at late gestation and that insulin resistance was detected in the fetal muscle of OB sheep [24, 25]. In this study, we further analyzed whether such changes in OB fetal muscle persist in the musculature of the adult offspring. Consistent with our observations in fetal muscle, we detected similar changes in offspring muscle. For instance, the phosphorylation of AKT was lower in muscle of the progeny of OB sheep to an extent that was very similar to the data in fetal muscle. The expression of PPARG and selected inflammatory cytokines in the skeletal muscle of OB offspring was also comparable to those seen in OB fetal muscle. Even though we cannot rule out the possible effects of lactation and postnatal feed intake on the changes of muscle properties, it seems that most of the changes in fetal muscle of OB sheep were maintained postnatally, indicating that maternal obesity generates persistent effects on the properties of offspring muscle.
IRS1 is a key mediator of insulin signaling. When IRS1 is activated by tyrosine phosphorylation, it serves as a docking center for the recruitment and activation of downstream pathways, of which the most important is the phosphoinositide 3-kinases/AKT (PIK3/AKT) pathway [36]. PRKC-induced IRS1 serine phosphorylation reduces the ability of IRS1 to serve as a docking center for the recruitment and activation of downstream pathways, leading to insulin resistance [36]. In the current study, although the circulatory insulin concentration was higher in OB [26], the PIK3/AKT pathway, a major insulin downstream signaling pathway, was down-regulated in the skeletal muscle of the progeny of OB sheep. The phosphorylation of key mediators of insulin signaling, including AKT, MTOR, and EIF4EBP1, was attenuated in the LD muscle of the offspring of the OB group, showing insulin resistance. This observation was in agreement with the lower insulin sensitivity observed in the same pool of OB lambs [26].
To analyze possible reasons for the observed insulin resistance in OB offspring muscle, we analyzed the phosphorylation of PRKC and the content of intramuscular lipids, both of which were higher in OB offspring muscle, consistent with our previous observation in fetal skeletal muscle [25]. Higher intramuscular lipid content might be responsible for the activation of PRKC. Increasing intracellular fatty acyl-CoA is known to activate the PRKC pathway [37, 38].
Fatty acid transporters mediate the cellular uptake of fatty acids. SLC27A1 is a plasma membrane protein that facilitates the uptake of fatty acids into cells [39, 40]. SLC27A1 up-regulation in skeletal muscle of the progeny of OB sheep might be associated with the higher intramuscular fat content and insulin resistance in skeletal muscle of the progeny of OB sheep. In addition, the expression of FAT/CD36 was also higher in OB offspring muscle, consistent with a previous report showing FAT/CD36 up-regulation under obese and type 2 diabetic conditions [41].
Obesity induces chronic low-grade inflammation, which alone can induce insulin resistance [42]. TLRs recognize fatty acids and play an important role in inducing inflammation associated with obesity [43]. TLRs activate the NFKB pathway to mediate the inflammatory response [44, 45]. Consistent with our observation of insulin resistance and enhanced lipid accumulation in OB fetal muscle [25], enhanced TLR4 level and inflammation signaling were also observed. In a recent study, enhanced expression of TLR4 was linked to insulin resistance and diabetes [46].
Inflammation induces the expression of anti-inflammatory factors, one of which is TGFB [47]. By analyzing the mRNA level, we observed a higher expression of TGFB in muscle of the offspring of OB sheep. TGFB signaling induces fibrogenesis [48]. Using Masson trichrome staining, we observed a higher collagen content in OB muscle, which was further confirmed by chemical analyses. During aging, a progressive loss of muscle mass occurs accompanied by increased adiposity and fibrosis [17, 22], resulting in a decline in muscle structural integrity and functional capacity [23]. Our observation that maternal obesity induces changes in offspring skeletal muscle characterized by increased intramuscular adiposity and fibrosis clearly shows that the properties of skeletal muscle in OB offspring were negatively affected by maternal obesity.
In conclusion, we observed intramuscular adiposity and fibrosis in the offspring of overnourished mothers, which was associated with attenuated insulin signaling and enhanced inflammatory signaling when compared with Con muscle. We propose that maternal obesity enhances adipogenesis and fibrogenesis in fetal and offspring muscle, which is expected to impair the physiological function of skeletal muscle. The possible consequences include: 1) the higher fat content in offspring muscle impairs insulin signaling, which limits the utilization of fatty acids by periphery tissues, leading to further accumulation of lipids; 2) enhanced adipogenesis and fibrogenesis limit the oxidative capacity of skeletal muscle, reducing lipid oxidation; and 3) excessive lipid accumulation and obesity in offspring lead to inflammation, further deteriorating insulin signaling, forming a vicious circle. In brief, our data show that maternal obesity has long-lasting effects on the properties of offspring muscle, which may provide a key mechanism for the fetal programming of adult metabolic diseases such as obesity and type 2 diabetes.
Footnotes
Supported by NIH 1R01HD067449 and Wyoming INBRE P20RR016474, and by National Research Initiative grant 2008-35206-18826 from the USDA National Institute of Food and Agriculture Animal Growth and Nutrient Utilization Program.
REFERENCES
- Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA 2010; 303: 242 249 [DOI] [PubMed] [Google Scholar]
- Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension 2008; 51: 383 392 [DOI] [PubMed] [Google Scholar]
- Shankar K, Harrell A, Liu X, Gilchrist JM, Ronis MJ, Badger TM. Maternal obesity at conception programs obesity in the offspring. Am J Physiol Regul Integr Comp Physiol 2008; 294: R528 R538 [DOI] [PubMed] [Google Scholar]
- White CL, Purpera MN, Morrison CD. Maternal obesity is necessary for programming effect of high-fat diet on offspring. Am J Physiol Regul Integr Comp Physiol 2009; 296: R1464 R1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RC, Wang ZM, Heymsfield SB. Skeletal muscle mass and aging: regional and whole-body measurement methods. Can J Appl Physiol 2001; 26: 102 122 [DOI] [PubMed] [Google Scholar]
- Tong JF, Yan X, Zhu MJ, Ford SP, Nathanielsz PW, Du M. Maternal obesity downregulates myogenesis and beta-catenin signaling in fetal skeletal muscle. Am J Physiol Endocrinol Metab 2009; 296: E917 E924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science 2005; 307: 384 387 [DOI] [PubMed] [Google Scholar]
- Ozanne SE, Jensen CB, Tingey KJ, Storgaard H, Madsbad S, Vaag AA. Low birthweight is associated with specific changes in muscle insulin signalling protein expression. Diabetologia 2005; 48: 547 552 [DOI] [PubMed] [Google Scholar]
- Selak MA, Storey BT, Peterside I, Simmons RA. Impaired oxidative phosphorylation in skeletal muscle of intrauterine growth-retarded rats. Am J Physiol Endocrinol Metab 2003; 285: E130 E137 [DOI] [PubMed] [Google Scholar]
- Nissen PM, Danielsen VO, Jorgensen PF, Oksbjerg N. Increased maternal nutrition of sows has no beneficial effects on muscle fiber number or postnatal growth and has no impact on the meat quality of the offspring. J Anim Sci 2003; 81: 3018 3027 [DOI] [PubMed] [Google Scholar]
- Greenwood PL, Hunt AS, Hermanson JW, Bell AW. Effects of birth weight and postnatal nutrition on neonatal sheep: II. Skeletal muscle growth and development. J Anim Sci 2000; 78: 50 61 [DOI] [PubMed] [Google Scholar]
- Zhu MJ, Ford SP, Means WJ, Hess BW, Nathanielsz PW, Du M. Maternal nutrient restriction affects properties of skeletal muscle in offspring. J Physiol 2006; 575: 241 250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M, Yan X, Tong JF, Zhao J, Zhu MJ. Maternal obesity, inflammation, and fetal skeletal muscle development. Biol Reprod 2010; 82: 4 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Shulman GI. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am J Cardiol 2002; 90: 11G 18G [DOI] [PubMed] [Google Scholar]
- Kim JK, Michael MD, Previs SF, Peroni OD, Mauvais-Jarvis F, Neschen S, Kahn BB, Kahn CR, Shulman GI. Redistribution of substrates to adipose tissue promotes obesity in mice with selective insulin resistance in muscle. J Clin Invest 2000; 105: 1791 1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguiari P, Leo S, Zavan B, Vindigni V, Rimessi A, Bianchi K, Franzin C, Cortivo R, Rossato M, Vettor R, Abatangelo G, Pozzan T, et al. High glucose induces adipogenic differentiation of muscle-derived stem cells. Proc Natl Acad Sci U S A 2008; 105: 1226 1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahoute C, Sotiropoulos A, Favier M, Guillet-Deniau I, Charvet C, Ferry A, Butler-Browne G, Metzger D, Tuil D, Daegelen D. Premature aging in skeletal muscle lacking serum response factor. PLoS ONE 2008; 3: e3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayol SA, Macharia R, Farrington SJ, Simbi BH, Stickland NC. Evidence that a maternal “junk food” diet during pregnancy and lactation can reduce muscle force in offspring. Eur J Nutr 2009; 48: 62 65 [DOI] [PubMed] [Google Scholar]
- Zhu MJ, Han B, Tong J, Ma C, Kimzey JM, Underwood KR, Xiao Y, Hess BW, Ford SP, Nathanielsz PW, Du M. AMP-activated protein kinase signalling pathways are down regulated and skeletal muscle development impaired in fetuses of obese, over-nourished sheep. J Physiol 2008; 586: 2651 2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen QW, Zhu MJ, Tong J, Ren J, Du M. Ca2+/calmodulin-dependent protein kinase kinase is involved in AMP-activated protein kinase activation by alpha-lipoic acid in C2C12 myotubes. Am J Physiol Cell Physiol 2007; 293: C1395 C1403 [DOI] [PubMed] [Google Scholar]
- Bakay M, Wang Z, Melcon G, Schiltz L, Xuan J, Zhao P, Sartorelli V, Seo J, Pegoraro E, Angelini C, Shneiderman B, Escolar D, et al. Nuclear envelope dystrophies show a transcriptional fingerprint suggesting disruption of Rb-MyoD pathways in muscle regeneration. Brain 2006; 129: 996 1013 [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 2007; 317: 807 810 [DOI] [PubMed] [Google Scholar]
- Beggs ML, Nagarajan R, Taylor-Jones JM, Nolen G, Macnicol M, Peterson CA. Alterations in the TGFbeta signaling pathway in myogenic progenitors with age. Aging Cell 2004; 3: 353 361 [DOI] [PubMed] [Google Scholar]
- Huang Y, Yan X, Zhu MJ, McCormick RJ, Ford SP, Nathanielsz PW, Du M. Enhanced transforming growth factor-beta signaling and fibrogenesis in ovine fetal skeletal muscle of obese dams at late gestation. Am J Physiol Endocrinol Metab 2010; 298: E1254 E1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Zhu MJ, Xu W, Tong JF, Ford SP, Nathanielsz PW, Du M. Up-regulation of Toll-like receptor 4/nuclear factor-kappaB signaling is associated with enhanced adipogenesis and insulin resistance in fetal skeletal muscle of obese sheep at late gestation. Endocrinology 2010; 151: 380 387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long NM, George LA, Uthlaut AB, Smith DT, Nijland MJ, Nathanielsz PW, Ford SP. Maternal obesity and increased nutrient intake before and during gestation in the ewe results in altered growth, adiposity, and glucose tolerance in adult offspring. J Anim Sci 2010; 88: 3546 3553 [DOI] [PubMed] [Google Scholar]
- National Research Council Committee on Animal Nutrition. Nutrient Requirements of Sheep. Washington, DC: National Academy Press; 1985. [Google Scholar]
- Corbett SW, Keesey RE. Energy balance of rats with lateral hypothalamic lesions. Am J Physiol 1982; 242: E273 E279 [DOI] [PubMed] [Google Scholar]
- Ford SP, Zhang L, Zhu M, Miller MM, Smith DT, Hess BW, Moss GE, Nathanielsz PW, Nijland MJ. Maternal obesity accelerates fetal pancreatic beta-cell but not alpha-cell development in sheep: prenatal consequences. Am J Physiol Regul Integr Comp Physiol 2009; 297: R835 R843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanson DW, West TR, Tatman WR, Riley ML, Judkins MB, Moss GE. Relationship of body composition of mature ewes with condition score and body weight. J Anim Sci 1993; 71: 1112 1116 [DOI] [PubMed] [Google Scholar]
- Carson FL. American Society for Clinical Pathology; Chicago, IL:: 2007. Histotechnology. [Google Scholar]
- Frayn KN, Maycock PF. Skeletal muscle triacylglycerol in the rat: methods for sampling and measurement, and studies of biological variability. J Lipid Res 1980; 21: 139 144 [PubMed] [Google Scholar]
- Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this amino acid. Arch Biochem Biophys 1961; 93: 440 447 [DOI] [PubMed] [Google Scholar]
- Zimmerman SD, Thomas DP, Velleman SG, Li X, Hansen TR, McCormick RJ. Time course of collagen and decorin changes in rat cardiac and skeletal muscle post-MI. Am J Physiol Heart Circ Physiol 2001; 281: H1816 H1822 [DOI] [PubMed] [Google Scholar]
- Zhu MJ, Ford SP, Nathanielsz PW, Du M. Effect of maternal nutrient restriction in sheep on the development of fetal skeletal muscle. Biol Reprod 2004; 71: 1968 1973 [DOI] [PubMed] [Google Scholar]
- Vary TC. IGF-I stimulates protein synthesis in skeletal muscle through multiple signaling pathways during sepsis. Am J Physiol Regul Integr Comp Physiol 2006; 290: R313 R321 [DOI] [PubMed] [Google Scholar]
- Ragheb R, Shanab GM, Medhat AM, Seoudi DM, Adeli K, Fantus IG. Free fatty acid-induced muscle insulin resistance and glucose uptake dysfunction: evidence for PKC activation and oxidative stress-activated signaling pathways. Biochem Biophys Res Commun 2009; 389: 211 216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey D, Basu D, Roy SS, Bandyopadhyay A, Bhattacharya S. Involvement of novel PKC isoforms in FFA induced defects in insulin signaling. Mol Cell Endocrinol 2006; 246: 60 64 [DOI] [PubMed] [Google Scholar]
- Hall AM, Smith AJ, Bernlohr DA. Characterization of the acyl-CoA synthetase activity of purified murine fatty acid transport protein 1. J Biol Chem 2003; 278: 43008 43013 [DOI] [PubMed] [Google Scholar]
- Kim JK, Gimeno RE, Higashimori T, Kim HJ, Choi H, Punreddy S, Mozell RL, Tan G, Stricker-Krongrad A, Hirsch DJ, Fillmore JJ, Liu ZX, et al. Inactivation of fatty acid transport protein 1 prevents fat-induced insulin resistance in skeletal muscle. J Clin Invest 2004; 113: 756 763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonen A, Tandon NN, Glatz JF, Luiken JJ, Heigenhauser GJ. The fatty acid transporter FAT/CD36 is upregulated in subcutaneous and visceral adipose tissues in human obesity and type 2 diabetes. Int J Obes (Lond) 2006; 30: 877 883 [DOI] [PubMed] [Google Scholar]
- Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007; 132: 2169 2180 [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2001; 2: 675 680 [DOI] [PubMed] [Google Scholar]
- Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science 2001; 293: 1673 1677 [DOI] [PubMed] [Google Scholar]
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 2005; 11: 191 198 [DOI] [PubMed] [Google Scholar]
- Reyna SM, Ghosh S, Tantiwong P, Meka CS, Eagan P, Jenkinson CP, Cersosimo E, Defronzo RA, Coletta DK, Sriwijitkamol A, Musi N. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes 2008; 57: 2595 2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur JP, Gjata B, Lafont H, Sebille A. Angiogenic and inflammatory responses following skeletal muscle injury are altered by immune neutralization of endogenous basic fibroblast growth factor, insulin-like growth factor-1 and transforming growth factor-beta 1. J Neuroimmunol 1996; 70: 37 44 [DOI] [PubMed] [Google Scholar]
- Franklin TJ. Therapeutic approaches to organ fibrosis. Int J Biochem Cell Biol 1997; 29: 79 89 [DOI] [PubMed] [Google Scholar]