Abstract
Objective
The risk of cardiovascular disease increases after menopause. Recent evidence suggests that it is possible for HDL to become proatherogenic or dysfunctional in certain situations. Our objective was to evaluate whether the relationship of HDL-C to subclinical cardiovascular disease differed across the menopausal transition, which would provide insight for this increased risk.
Methods
Aortic calcification (AC), coronary artery calcification (CAC), carotid plaque and intima media thickness (IMT) were measured in the Study of Women’s Health Across the Nation (SWAN Heart). Women, not using hormone therapy, were stratified into premenopausal or early-perimenopausal (Pre/EP, n=316) and late-perimenopausal or postmenopausal (LP/Post, n=224).
Results
The inverse relationship of HDL-C to subclinical atherosclerosis measures among Pre/EP women was weaker or reversed among LP/post women, adjusted for age, site, race, SBP, glucose, BMI, smoking, menopausal status and LDL-C. Specifically: Multivariable modeling demonstrated an inverse association between HDL-C and AC and IMT, among Pre/EP women; however, the protective effect of HDL-C for AC, left main CAC, carotid plaque and IMT was not seen in LP/Post women. In a small subset (n=53), LP/Post women had more total and small HDL particles, higher triglycerides and more total LDL particles compared to Pre/EP women (p<0.05).
Conclusion
These results suggest that the protective effect of HDL may be diminished as women transition the menopause. Future studies should examine whether this may be due to changes in HDL size, functionality, or related changes in other lipids or lipoproteins.
Keywords: subclinical cardiovascular disease, lipids, lipoproteins, menopause
Introduction
Cardiovascular disease (CVD) is the leading cause of mortality for women. As women age they are increasingly exposed to high levels of major CVD risk factors, including a poor lipid profile and weight gain. In addition to adverse changes associated with chronological aging, women experience the biological changes of the menopausal transition. Decreasing estrogen levels during the transition have been linked to endothelial dysfunction and larger vessel diameters, markers of early adverse vascular changes 1-5. Therefore, decreased estrogen following the menopausal transition leaves the vasculature vulnerable to CVD risk factors, such as lipids.
Lipids are known to change in association with both age and the menopausal transition 6-8. Understanding these changes, including how they contribute to early vascular disease, may shed light onto the underlying mechanisms that increase CVD risk following the transition. During the transition, low-density lipoprotein cholesterol (LDL-C) levels increase, while high-density lipoprotein cholesterol (HDL-C) levels tend to remain stable or increase slightly 9-13. It is widely accepted that higher levels of LDL-C promote CVD while higher levels of HDL-C are cardioprotective. However, no studies have yet evaluated whether or not these associations are consistent across the stages of menopause. One study has suggested that the protective effects of HDL on IMT may diminish over time in middle aged-women 14. Very recently, some reports have attempted to qualify “dysfunctional” HDL, and suggest that the anti-atherogenic, anti-inflammatory properties of HDL may be lost in certain inflammatory conditions 15-20. However, one time measures of lipids may not fully evaluate the complex nature of triglyceride exchanged between HDL-C and LDL-C, and its potential effect on subclinical CVD, therefore exploration beyond HDL-C and LDL-C is required 21.
The Study of Women’s Health Across the Nation (SWAN) provides a unique opportunity to determine if the relationship between lipids and subclinical CVD varies by menopausal status. SWAN explored biological changes as women have transitioned through menopause. SWAN Heart, an ancillary study of SWAN, collected subclinical CVD measures in two of the seven parent study sites. Subclinical CVD measures are reliable measures of the atherosclerotic process 22. Accordingly, the purpose of this study was to evaluate whether the menopausal transition modifies the contribution of lipids to subclinical CVD risk. Understanding mechanisms that promote early CVD in women may lead to new preventive efforts or treatments to delay CVD in the postmenopausal years.
Methods
Study population
SWAN is a multi-center, community-based, longitudinal study. Baseline enrollment of 3302 women between the ages of 42 and 52 years occurred during 1996 to 1997. A detailed description of study design and recruitment has previously been published 23. Only women who had an intact uterus and at least one ovary, menstruated in the prior 3 months and not taken hormone therapy or oral contraceptives in the prior 3 months were eligible for the parent project. Women who were pregnant or breast-feeding were excluded. The ancillary study, SWAN Heart, evaluated 608 women for subclinical CVD from two SWAN sites (Pittsburgh and Chicago). Baseline enrollment occurred between 2001 and 2003 (corresponding to the 4th - 7th annual SWAN visits). Women were excluded from this analysis for CVD history (n=2), hysterectomy/oophorectomy (n=16), diabetes (n=1) and hormone therapy (n=32). An additional 17 women were excluded due to missing menopausal status or subclinical CVD data, resulting in a final sample size of 540 (89%) for this analysis. The site-specific institutional review boards approved research protocols and all women provided informed consent prior to enrollment.
Subclinical Measures
Aortic and coronary artery calcification (AC and CAC) were quantified using electron beam computed tomography (GE Imatron C-150 Ultrafast CT Scanner, San Francisco, CA). Participants were exposed to 2.45 rads during the aortic scan and 0.783 rads for the coronary scan. All scans were saved to optical disc and read centrally at the University of Pittsburgh using a DICOM workstation and Acuimage software (South San Francisco, CA). Using the Agatston scoring method, 3 contiguous pixels > 130 Hounsfield units was used to define the presence of calcified lesions within the vessel. A total calcium score was calculated summing the calcium scores in the four major coronary arteries (left main, left anterior descending, left circumflex and right coronary artery), while the aorta total score was from the whole aortic vessel. One blinded physician, centrally trained in EBCT with a standardized protocol, analyzed all scans from the two sites. A reproducibility study conducted by the Pittsburgh, PA EBCT image reading center in 40 consecutive subjects previously reported an intrareader intraclass correlation (ICC) of 0.98 for AC and 0.99 for CAC 24.
Carotid intima media thickness (IMT) and carotid plaque assessment were made using B-mode ultrasound (Pittsburgh site: Toshiba American Medical Systems, Tustin, CA and Chicago site: Hewlett Packard, Andover, MA). Sonographers were centrally trained with a standardized protocol. Right and left carotid arteries were scanned to obtain a total of 8 images: near and far wall of common carotid (1 cm proximal to bulb), far wall of carotid bulb (starting from the point where the common carotid walls are no longer parallel and ending at the flow divider) and far wall of internal carotid artery (distal 1 cm from flow divider). Semi-automated reading software (AMS system developed in Sweden by Dr. Thomas Gustavsson) generated approximately 140 measurements of IMT from the leading edge of the intima to the trailing edge of the media for each 1 cm segment. The mean IMT of each of the 8 carotid segments was determined, and the average of these 8 mean measures was computed for the outcome variable in this analysis. Carotid plaques were identified as discrete focal protrusions into the lumen > 50% of the surrounding wall thickness. Images obtained from the comparable ultrasound machines were read centrally at the University of Pittsburgh, Ultrasound Research Laboratory (Pittsburgh, PA). Interreader reproducibility of IMT and carotid plaque during annual recertifications for the Ultrasound Research Laboratory were as follows: ICC of 0.98 for IMT and 0.86-0.93 for plaque 25-27.
Covariate Measures
During annual visits, SWAN administered standard questionnaires, collected fasting blood samples and obtained anthropometric measures and blood pressure readings. In this cross-sectional analysis, data from these annual SWAN clinic visits (4th visit: n=272, 5th visit: n=231, 6th visit: n=20 and 7th visit: n=17) were matched to participant’s baseline SWAN heart subclinical measures. Based on self-reported bleeding history, menopausal status was classified as premenopausal (Pre; menses in the last 3 months with no irregularity), early-perimenopausal (EP; menses in the last 3 months with irregularity), late-perimenopausal (LP; no menses for at least 3 months, but less than 12 months) and postmenopausal (Post; no menses for at least 12 months).
Fasting blood samples were analyzed at the Medical Research Laboratories (Lexington, KY). EDTA treated plasma was used to analyze lipids; isolation of HDL-C (mg/dL) was done with heparin-2M manganese chloride and LDL-C (mg/dL) was estimated with the Friedewald equation 28.
For a subset of Pittsburgh SWAN Heart women, lipoprotein subclasses were available from blood samples collected in 1997 to 2001. Lipoprotein subclasses were determined using stored EDTA plasma using an automated nuclear magnetic resonance spectroscopic assay (LipoScience Inc., NC), with a modification of the method previously reported 29, 30. The following lipoprotein concentrations were analyzed: total LDL and HDL particle concentrations (LDL-P in nmol/L and HDL-P in μmol/L), large LDL and HDL particle concentration (large LDL-P and large HDL-P), small LDL and HDL particle concentration (small LDL-P and small HDL-P). Large LDL particle diameters are defined as 21.2-23 nm, small LDL particles as 18-21.2 nm, large HDL particles as 8.8-13 nm and small HDL particles as 7.3-8.2 nm. The total number of HDL particles = prebeta HDL-P + small HDL-P + large HDL-P and NMR technology cannot quantify prebeta HDL-P and thus total HDL-P derived by adding small HDL-P +medium HDL-P+ large HDL-P may underestimate total HDL-P, however, prebeta HDL-P is usually less that 5% of total HDL-P and thus most studies do not look at it. Total LDL-P and total HDL-P are the sum of small + large LDL-P + IDL-P and small + large HDL-P respectively. Mean LDL and HDL particle sizes are weighted-averages (i.e. the diameter of each subclass multiplied by its relative concentration). A total of 71 SWAN Heart women had both lipoprotein subclass data and subclinical CVD measures available. Those with lipoprotein data collected more than 16 months apart from their subclinical measures were excluded from this analysis. Of the remaining 53 women, average collection time from subclinical measures was 184.5 days for Pre/EP and 236.3 days for LP/Post women.
Statistical Analysis
The distribution of each continuous variable was examined and variables were log transformed or categorized if the distribution was not approximately normal. Glucose and triglycerides were log transformed, however, medians (IQR) are presented in Table 1 and 3 for descriptive purposes. Alcohol consumption, AC, CAC and carotid plaque were identified as having skewed distributions and large proportions of zero values. Alcohol consumption was grouped into zero drinks and ≥ one drink. Single vessel CAC (left main, left anterior descending, left circumflex and right coronary artery) and carotid plaque were dichotomized into none or any given the small sample of women with any disease, and AC and total CAC were classified as none, moderate and high calcification scores (AC: 0, 1-74 and 75+, and CAC: 0, 1-7.54 and 7.55+ respectively). Due to low levels of calcification, high AC and CAC were defined as the ≥ 75th percentile or greater, as reported by prior SWAN manuscripts, to allow for analysis between high and low calcification, which is similar to studies with low calcification burden 31, 32. Menopausal status was dichotomized into Pre plus EP menopause (Pre/EP) and LP plus Postmenopausal (LP/Post) to maintain a moderate sample size as previously reported 4. All remaining variables met the guidelines for normality and were treated as continuous. Two sample t-test and chi-square test were utilized to compare participant characteristics between Pre/EP and LP/Post.
Table 1.
Participant Characteristics by Menopausal Status
| Characteristic | n | Total | Pre/EP n=316 |
LP/Post n=224 |
p-value | Age Adjusted p-value |
|---|---|---|---|---|---|---|
| Age (years) | 540 | 50.2 (2.9) | 48.9 (2.2) | 52.1 (2.7) | < .0001 | – |
| African American † | 540 | 209 (38.7) | 114 (36.1) | 95 (42.4) | 0.14 | 0.31 |
| Alcohol (yes/no) † | 537 | 331 (61.6) | 193 (61.5) | 138 (61.9) | 0.92 | 0.50 |
| Smoker (ever regular) † | 488 | 78 (16.0) | 47 (16.6) | 31 (15.2) | 0.69 | 0.32 |
| HDL-C (mg/dL) | 472 | 57.5 (15.0) | 56.2 (13.8) | 59.3 (16.4) | 0.04 | 0.23 |
| LDL-C (mg/dL) | 474 | 119.0 (32.8) | 113.5 (28.7) | 126.6 (36.5) | < .0001 | < .0001 |
| Triglycerides | 477 | 117 (74, 137) | 91 (71, 121) | 111 (82, 158) | < .0001 | < .0001 |
| SBP (mmHg) | 519 | 119.7 (16.7) | 118.2 (16.3) | 121.7 (17.0) | 0.017 | 0.94 |
| Glucose (mg/dL) * | 480 | 88 (83, 96) | 87 (81, 95) | 91 (84, 98) | 0.0003 | 0.01 |
| BMI (kg/m2) | 518 | 29.4 (6.4) | 29.1 (6.5) | 29.7 (6.3) | 0.35 | 0.85 |
| Aortic Calcification † None (0) Moderate (1-74) High (75 +) |
515 |
156 (30.3) 230 (44.7) 129 (25.0) |
104 (34.3) 136 (44.9) 63 (20.8) |
52 (24.5) 94 (44.4) 66 (31.1) |
0.0096 | 0.28 |
| Coronary Artery Calcification † None (0) Moderate (1-7.54) High (7.55 +) |
517 |
271 (52.4) 116 (22.4) 130 (25.2) |
167 (54.9) 73 (24.0) 64 (21.1) |
104 (48.8) 43 (20.2) 66 (31.0) |
0.037 | 0.83 |
| Any Left Main CAC | 518 | 39 (7.5) | 24 (7.9) | 15 (7.0) | 0.71 | 0.53 |
| Any Carotid Plaque † | 535 | 81 (15.1) | 36 (11.5) | 45 (20.4) | 0.0047 | 0.04 |
| Intima Media Thickness (mm) | 529 | 0.67 (0.1) | 0.66 (0.1) | 0.68 (0.1) | 0.040 | 0.77 |
Values are presented as mean (SD); p-values generated with t-test
Values are presented as n (%); p-values generated with chi-square test
Non-normally distributed; values presented as median (IQR); p-values generated with t-test with log transformed values.
Table 3.
Lipids and lipoprotein levels by menopausal status and correlations with triglycerides for Pittsburgh SWAN Heart sub-sample
| Lipid/Lipoprotein Variables | Pre/EP (n=19) |
LP/Post (n=34) |
p-value | Age Adjusted p-value |
Correlation with Triglyceride * (p-value) |
|---|---|---|---|---|---|
| HDL cholesterol (mg/dL) | 58.2 (20.0) | 55.8 (13.4) | 0.62 | 0.51 | −0.57 (<0.0001) |
| Small HDL Particles (μmol/L) | 18.7 (5.6) | 23.6 (5.9) | 0.0044 | 0.0094 | 0.50 (0.0002) |
| Large HDL Particles (μmol/L) | 6.7 (2.9) | 6.1 (3.1) | 0.49 | 0.51 | −0.27 (0.065) |
| Total HDL Particles (μmol/L) | 28.3 (6.4) | 32.7 (7.6) | 0.037 | 0.078 | 0.29 (0.041) |
| HDL Particle Size (nm) | 9.4 (0.5) | 9.1 (0.4) | 0.0078 | 0.015 | −0.71 (<0.0001) |
| Triglycerides (mg/dL) ** | 91 (66, 124) | 124 (84, 203) | 0.028 | 0.082 | -- |
| LDL cholesterol (mg/dL) | 103 (20.8) | 139 (36) | 0.0004 | 0.0003 | 0.42 (0.0031) |
| Small LDL Particles (nmol/L) | 478.2 (247.1) | 620.5 (351.7) | 0.13 | 0.26 | 0.34 (0.018) |
| Large LDL Particles (nmol/L) | 575.4 (193.7) | 620.3 (227.1) | 0.47 | 0.28 | 0.030 (0.84) |
| Total LDL Particles (nmol/L) | 1095.6 (189.3) | 1311.6 (325) | 0.011 | 0.020 | 0.45 (0.0011) |
| LDL Particle Size (nm) | 21.6 (0.5) | 21.5 (0.7) | 0.50 | 0.71 | −0.25 (0.080) |
Values are presented as mean (SD); p-values generated with t-test.
Spearman correlations are rho (p-value) for entire lipoprotein subset.
Non-normally distributed; values presented as median (IQR); p-values generated with t-test with log transformed values.
Interactions between lipids and menopausal status were tested in multivariable models with and without adjustments for other covariates. Further explorations of the lipid interactions were done using a reparameterized model to include the effects of the lipid among Pre/EP and LP/Post women in a single multivariable model (subclinical measure = menopausal status + effect of the lipid for Pre/EP women + effect for of the lipid for LP/Post women + covariates). Multivariable regression models were used to assess the association between traditional CVD risk factors and AC, CAC, single vessel CAC, carotid plaque and IMT. For AC and CAC, multinomial logistic regression rather than ordinal logistic regression was preferred due to unmet assumptions of proportional odds 33. No AC and no CAC were the reference groups for the multinomial logistic regression. No single vessel CAC and no carotid plaque were the reference groups for the binary logistic regression. Preliminary analyses used stepwise regression selection to evaluate the inclusion of traditional CVD risk factors in the multivariable linear and logistic regression models with default p-value=0.15 entry and exit thresholds. Final models were adjusted for known CVD risk factors and confounders: age, site, race, SBP, glucose, BMI, smoking, menopausal status and lipids (HDL-C, LDL-C or triglycerides). The sub-sample of Pittsburgh women (n=53) with available lipoprotein subclass data was correlated with triglycerides using spearman correlations and compared across menopausal status using a two-sample t-test. The lipoprotein data were not used in the regression analysis due to small sample size and inability to adjust for known confounders. All statistical analyses were completed with SAS 9.1. Two-sided p-values ≤0.05 were considered statistically significant.
Results
Of the 540 SWAN Heart participants included in this analysis, 316 (59%) were Pre/EP and 224 (41%) were LP/Post women (Table 1). Average age was 50 years, with Pre/EP women being 3 years younger than LP/Post women. LP/Post women had higher mean HDL-C, LDL-C, triglycerides, SBP and glucose compared to Pre/EP. Following age-adjustment, mean HDL-C and SBP no longer differed significantly. In addition, LP/Post women had more AC, CAC and carotid plaque, and higher IMT on average, however, once adjusted for age, only carotid plaque differed between the two groups.
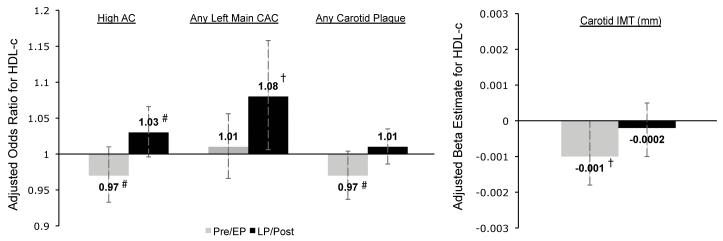
The effect of menopausal status on the relationships between lipids (HDL-C, triglyceride and LDL-C) and subclinical atherosclerosis are shown in Table 2. To better visualize the multiple interactions found with HDL-C, the data are also presented in Figure 1. For CAC, only the results for total and left main vessel are included in this manuscript because the effect of lipids on calcification did not vary by menopausal status in the other three vessels measured. In the multivariable models adjusted for age, site, race, HDL-C, LDL-C, SBP, glucose, BMI and smoking, the effect of HDL-C varied by menopausal status for high AC, any left main CAC, any carotid plaque and IMT. The relationship of triglyceride to AC was stronger for Pre/EP women while LDL-C was more strongly related to carotid plaque and IMT among LP/Post women. For every 1 mg/dL increase in HDL-C the odds of high AC among Pre/EP women was decreased by 3%, while the odds for LP/Post women was increased by 3%. Similarly, among LP/Post women, the odds of any left main CAC was increased by 8% for every 1mg/dL of HDL-C. The pattern of HDL-C being protective for Pre/EP women, but not for LP/Post women was also seen in any carotid plaque and IMT. The pattern for log of triglycerides was reversed with an increased risk among Pre/EP for AC. Whereas LDL-C was associated with higher odds of any carotid plaque among LP/Post women as compared to Pre/EP, and an increase of 0.0004mm in IMT was observed among LP/Post women for every 1mg/dL of LDL-C. Of note, similar to LDL-C, non-HDL-C and ApoB were also associated with thicker IMT among LP/Post women, but not Pre/EP (data not shown).
Table 2.
Multivariable association between lipids and subclinical measures by menopausal status
| Subclinical Measure | HDL-C | Triglyceride * | LDL-C | |
|---|---|---|---|---|
|
High Aortic
Calcification |
Pre/EP
LP/Post Interaction p-value |
0.97 (0.93, 1.01) # 1.03 (1.00, 1.07) # 0.012 |
1.01 (1.00, 1.02) † 1.00 (0.99, 1.01) 0.084 |
1.02 (1.00, 1.04) † 1.03 (1.01, 1.05) † 0.51 |
|
High Coronary
Artery Calcification |
Pre/EP
LP/Post Interaction p-value |
0.99 (0.95, 1.03) 0.99 (0.96, 1.02) 1.00 |
1.00 (0.99, 1.01) 1.00 (1.00, 1.01) 0.51 |
1.01 (1.00, 1.03) 1.00 (0.99, 1.02) 0.58 |
|
Any Left Main
Coronary Artery Calcification |
Pre/EP
LP/Post Interaction p-value |
1.01 (0.96, 1.05) 1.08 (1.00, 1.16) † 0.11 |
1.00 (0.99, 1.01) 1.00 (0.99, 1.01) 0.32 |
0.99 (0.97, 1.01) 0.99 (0.97, 1.01) 0.75 |
|
Any Carotid
Plaque |
Pre/EP
LP/Post Interaction p-value |
0.97 (0.94, 1.01) # 1.01 (0.98, 1.03) 0.080 |
1.00 (0.99, 1.01) 1.00 (0.99, 1.00) † 0.20 |
1.00 (0.99, 1.01) 1.01 (1.00, 1.02) † 0.18 |
|
Intima Media
Thickness |
Pre/EP
LP/Post Interaction p-value |
−.001 (−.002, −.0004) † −.0002 (−.001, .0006) 0.064 |
.00002 (−.0002, .0003) −.0001 (−.0003, .0001) 0.37 |
−.0001 (−.0005, .0003) .0004 (.0001, .0008) † 0.045 |
High Aortic Calcification - values are OR (95% CI) of high aortic calcification for HDL-C; Any Left Main Coronary Artery Calcification - values are odds ratios (95% CI) of any left main coronary artery calcification for HDL-C; Any Carotid Plaque - values are odds ratios (95% CI) of any carotid plaque for HDL-C; Carotid IMT - values are β coefficient estimates (95% CI) of IMT for HDL-C.
Models adjusted for age, site, race, SBP, glucose, BMI, smoking, menopausal status and lipids.
Analyses used the log transformed triglyceride variable.
p <0.05
p <0.1
Figure 1.
Multivariable association between HDL-C and subclinical measures by menopausal status
High AC - values are OR (95% CI) of high AC for HDL-C; Any Left Main CAC - values are odds ratios (95% CI) of any left main CAC for HDL-C; Any Carotid Plaque - values are odds ratios (95% CI) of any carotid plaque for HDL-C; Carotid IMT - values are β coefficient estimates (95% CI) of IMT for HDL-C.
Models adjusted for age, site, race, SBP, glucose, BMI, smoking, menopausal status and lipids.
Abbreviations: AC (Aortic Calcification), CAC (Coronary Artery Calcification), IMT (Intima Media Thickness), CCAIMT (Common Carotid Intima Media Thickness). Gray bars: Pre/EP - Premenopausal & Early-Perimenopausal; Black bars: LP/Post - Late-Perimenopausal & Postmenopausal.
† p <0.05, # p <0.1 for Pre/EP versus LP/Post.
An exploratory analysis using the lipoprotein subclass data for a subset of the study population evaluated potential mechanisms for differences in lipid effects with menopause. LP/Post women (n=34) had significant differences in their lipoprotein subclass profile compared to Pre/EP women (n=19) (Table 3). LP/Post women had more small HDL-P, more total HDL-P and a smaller mean HDL particle size on average. Additionally, LP/Post women had significantly higher triglycerides and LDL-C and more total LDL-P than Pre/EP women. Finally, the correlations between lipoproteins and triglycerides among SWAN heart women were tested given the known dynamic exchange of triglyceride during HDL-C and LDL-C remodeling. All of the lipoprotein subclass measures except large HDL-P (borderline), large LDL-P and mean LDL size (borderline) were significantly correlated with triglycerides.
Discussion
Evaluation of the interactions between menopausal status and HDL-C for AC (p = 0.01), left main CAC (0.1), carotid plaque (0.08) and IMT (0.06) suggests that the atheroprotective effect of HDL may be weaker in postmenopausal women, whereas the effect of LDL-C was stronger among postmenopausal women for IMT (0.05). Higher HDL-C was associated with higher levels of high AC and any left main CAC in LP/Post women after adjustments for traditional risk factors; whereas higher HDL-C had a traditionally protective effect in Pre/EP women for high AC, any carotid plaque and IMT. These results are in accord with Fan et al. who report IMT progression in postmenopausal women was directly associated with HDL-C, adjusted for CVD risk factors 14. Among men in that study, there was the expected inverse association between IMT progression and HDL-C, suggesting the phenomenon for LP/Post women in this SWAN Heart analysis may be due to changes occurring with the menopausal transition rather than just age. A detrimental effect of HDL-C among postmenopausal women was also reported in the EUROSTROKE study, which found that among women pooled from the Novosibirsk and Rotterdam cohorts, higher HDL-C was associated with increased odds of stroke 34. The average age of the cohorts were 51.6 and 72.8 years respectively; therefore these effects were seen among women who have recently transitioned through menopause and those who have been postmenopausal for years.
The reduced or reversed CVD risk association with HDL-C postmenopause may be explained by functional and compositional changes in HDL 29. SWAN is one of the few studies that has a large enough sample size with sufficient follow-up to differentiate the effects of menopause from aging, and it has recently shown that although triglycerides increased with age, only total cholesterol, LDL-C and apoB showed a substantial increase specifically with menopause 8. In contrast, HDL-C increased acutely before menopause, and decreased following menopause. Given that conventional methods of measuring HDL-C and LDL-C reflect the concentration of cholesterol carried by the lipoprotein particles rather than the concentration of the particles themselves, and that lipoprotein particles are believed to provide more information in predicting CVD, evaluation of lipoproteins and lipoprotein subclasses may provided additional insight into the association between lipids and CVD 35, 36. Data on menopause-specific effects on lipoproteins and lipoprotein subclass distribution are very sparse. Cross-sectional data from the Framingham Offspring Study (FOS) did not specifically look at menopause, but demonstrates the complex interrelationships between lipids, lipoproteins, lipoprotein subclasses and mean particle sizes 29. The FOS data also show that these relationships differ with age and for women compared to men. For example, among women, LDL-C increases with age surpassing LDL-C among men by about age 60, but total LDL-P does not equal men until age about 75, and small LDL-P levels remain substantially lower compared with men throughout the age span. Among women, HDL-C shows a slight decline with age, similar to large HDL-P while total HDL particles (HDL-P), intermediate and small HDL-P increase substantially with age, possibly accelerating around menopause. For women both large and small LDL-P and medium and small HDL-P appear to increase more around age 50 than at other ages 29. In epidemiological studies CVD often, but not always, has a stronger inverse association with larger HDL particles or the cholesterol carried by larger HDL particles 37, 38. The large Women’s Health Study (WHS) also found that large HDL-P was significantly associated with reduced CVD incidence, whereas small HDL-P was not 39. However, the WHS was a mixture of pre and postmenopausal women, and the CVD incidence was also statistically significantly related to all LDL and VLDL subclass concentrations, highlighting the difficulty of attributing risk to any one lipid or lipoprotein measure given their dynamic metabolic interrelationships. Among postmenopausal women, the Healthy Women Study reported a significant protective association between CAC and large HDL-P and HDL particle size, whereas large VLDL-P, small and total LDL-P were associated with higher CAC levels, but small HDL-P levels were not correlated with CAC 40. In addition, postmenopausal women with AC or CAC had lower levels of (larger) HDL2 cholesterol and higher levels of (smaller) HDL3 cholesterol 41. In our subset with lipoprotein subclass data, LP/Post women had more small HDL-P and smaller HDL particle size on average compared with Pre/EP women, but they also had higher levels of total LDL-P and LDL-C. The inverse correlation between triglycerides and large HDL-P and the direct correlation with small HDL-P suggest that as triglycerides increase, as seen with the menopausal transition, fewer large HDL-P and more small HDL-P are circulating. Additionally, more total LDL-P are observed with higher triglyceride levels. These results are consistent with the WHS 39. Any or all of these interrelated changes in the lipoprotein profile around menopause could potentially explain our findings that HDL-C was not inversely associated with subclinical atherosclerosis among postmenopausal women.
Interpretation of HDL particle size may not be the only explanation for these observed associations given the dynamic relationship between HDL-C, LDL-C and triglyceride exchange 21. A recent study exploring the relationship between HDL-C and the risk of CVD in postmenopausal women found that subpopulations of triglyceride-rich lipoproteins and HDL were better predictors of disease than triglycerides and HDL-C concentrations, suggesting differences in HDL lipoproteins may be directly related to triglycerides. The triglyceride interaction with menopausal status for AC in this SWAN Heart suggested that triglyceride may be a stronger risk factor among Pre/EP women compared to LP/Post. The relationship between the lipoproteins and triglycerides showed that higher triglycerides, as seen in LP/Post women, is correlated with lower HDL-C, more small HDL-P, less large HDL-P and smaller HDL size. Additionally, triglycerides were positively correlated with LDL-C, total and small LDL-P. Therefore changes in triglycerides may be the driving force for the reduced or reversed HDL-C associations with subclinical CVD and lipoprotein profile among postmenopausal women observed in our study. Furthermore, this relationship may also be due to concurrent and related changes in LDL-C. The association of CVD risk and reduced large HDL-P implies causality when it may simply be an indicator for elevated apoB or total LDL-P. In this study, the association of LDL-C with carotid plaque and IMT was larger for LP/Post women, potentially indicating LDL-C as a stronger risk factor among postmenopausal women. LP/Post women had higher triglycerides, LDL-C and total LDL-P compared with Pre/EP women. This agrees with our recent report from the entire SWAN study, which used longitudinal data to demonstrate that menopause increases apoB, a measure highly correlated with total LDL-P, along with total cholesterol and LDL-C 8. Differences in the dynamic flux between lipids and lipoproteins across the menopausal transition status need further exploration.
Another potential mechanism responsible for these lipoprotein profile changes is enzymatic activity. Large HDL-P are converted to small HDL-P by increased hepatic lipase 42. Estrogen inhibits hepatic lipase; therefore decreases in estrogen with menopause should lead to a higher ratio of small HDL-P to large HDL-P and smaller mean HDL particle size. In support of this theory, postmenopausal women have been found to have higher hepatic lipase activity that covaried inversely with large HDL-P 42. In addition, increasing insulin resistance with age may contribute to lower lipoprotein lipase (LPL) activity, which is a determinant of lower HDL-C, and in concert with hepatic lipase as a determinant of lower levels of cholesterol carried by the larger HDL2 particles 43.
In addition to increasing hepatic lipase, decreased estrogen levels with menopause, hinders several protective vascular mechanisms. Estrogen is believed to accelerate reendothelialization in the face of vascular injury thereby inhibiting smooth muscle proliferation and subsequently medial thickening 44. Vascular tone is maintained by estrogen through several pathways, including nitric oxide and prostacyclin 2. Therefore, decreased estrogen following the menopausal transition leaves the vasculature vulnerable to CVD risk factors, such as lipids. SWAN Heart, has previously shown that lower estrogens are directly related to larger carotid arterial diameter among women not taking exogenous estrogen therapy 4. Larger vessel diameters lead to less ability to compensate for hemodynamic changes due to blood pressure or arterial wall thickening and thus leave the vessel more vulnerable 4, 5. Thus, not only are postmenopausal women at increased risk for CVD as estrogen decreases, they are exposed to poorer lipid profiles, which may add to their risk.
The lipoprotein subclass data presented here provide initial evidence in support of lipoprotein compositional changes as a potential mechanism underlying the observed associations between HDL-C and subclinical CVD measures. Additionally, this study supports other research showing functional changes in HDL, such as HDL can become pro-inflammatory or pro-atherogenic in some circumstances 15-19. However, this is a preliminary, cross-sectional analysis, and thus a causal relationship between change in menopause status and HDL cannot be inferred. Furthermore, evaluating lipoprotein-associated risk is difficult because of the interrelationships between lipids and lipoprotein levels 21. It is plausible that the lipid changes attributed to the menopausal transition may instead be explained by altered lipid and lipoprotein patterns due to premature and early menopause 45. Premature and early menopause ranged from 1 - 1.4% and 2.9 - 3.7%, respectively, in SWAN Caucasian and African American women 46. Despite the modest sample size for detecting interactions, the HDL-C by menopausal status interaction was significant for AC and borderline for left main CAC, carotid plaque and IMT; borderline for triglyceride and menopausal status for AC; and significant for LDL-C and menopausal status for IMT, suggesting differential effects by menopausal status. Left main coronary artery stenosis is known to be an independent risk factor for individuals with coronary artery disease, and therefore may be the reason a difference in the effect of HDL-C was only observed in the left main coronary artery 47, 48. The low prevalence of moderate to high CAC burden (2.4% with CAC score > 100) observed in the SWAN Heart cohort may explain the non-significant findings for CAC 31. Further studies need to be conducted in a population with more extensive coronary artery disease. The mean carotid artery IMT across carotid segments rather than the mean common carotid artery IMT was used since it may better capture early atherosclerosis among these relatively young women, but this should be considered when comparing these data to other published results 49. Lastly, since all participants did not have lipoprotein subclass data, lipoproteins could not be used in the regression models, and associations between lipoprotein and subclinical disease could not be directly analyzed.
Conclusion
In conclusion, these results suggest that the protective effect of HDL is reduced among postmenopausal women, possibly, related to changes in the lipoprotein subclass profile seen with the menopausal transition. This, in addition to a more vulnerable vessel caused by aging, decreased estrogen and adipose tissue redistribution, may partially explain the increased CVD risk seen after menopause. Future studies evaluating lipid and lipoprotein profiles longitudinally through the menopause transition are needed.
Acknowledgements
Clinical Centers: University of Michigan, Ann Arbor - MaryFran Sowers, PI; Massachusetts General Hospital, Boston, MA - Robert Neer, PI 1994 - 1999; Joel Finkelstein, PI 1999- present; Rush University, Rush University Medical Center, Chicago, IL - Lynda Powell, PI 1994 – 2009; Howard Kravitz, PI 2009; University of California, Davis/Kaiser - Ellen Gold, PI; University of California, Los Angeles - Gail Greendale, PI; University of Medicine and Dentistry - New Jersey Medical School, Newark –Gerson Weiss, PI 1994 – 2004; Nanette Santoro, PI 2004 – present; and the University of Pittsburgh, Pittsburgh, PA - Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD - Marcia Ory 1994 – 2001; Sherry Sherman 1994 – present; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor - Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001; University of Pittsburgh, Pittsburgh, PA – Kim Sutton-Tyrrell, PI 2001 – present.
Steering Committee: Chris Gallagher, Chair; Susan Johnson, Chair
We thank the study staff at each site and all the women who participated in SWAN.
Sources of Funding
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). SWAN Heart was supported by grants from the NIH through the National Heart, Lung, and Blood Institute (HL065581, HL065591). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Footnotes
Disclosures
The authors have no personal or financial conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mendelsohn ME. Protective effects of estrogen on the cardiovascular system. Am J Cardiol. 2002;89(12A):12E–17E. doi: 10.1016/s0002-9149(02)02405-0. discussion 17E-18E. [DOI] [PubMed] [Google Scholar]
- 2.Kublickiene K, Luksha L. Gender and the endothelium. Pharmacol Rep. 2008;60(1):49–60. [PubMed] [Google Scholar]
- 3.Jensen-Urstad K, Johansson J. Gender difference in age-related changes in vascular function. J Intern Med. 2001;250(1):29–36. doi: 10.1046/j.1365-2796.2001.00843.x. [DOI] [PubMed] [Google Scholar]
- 4.Wildman RP, Colvin AB, Powell LH, Matthews KA, Everson-Rose SA, Hollenberg S, Johnston JM, Sutton-Tyrrell K. Associations of endogenous sex hormones with the vasculature in menopausal women: the Study of Women’s Health Across the Nation (SWAN) Menopause. 2008;15(3):414–421. doi: 10.1097/gme.0b013e318154b6f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polak JF, Kronmal RA, Tell GS, O’Leary DH, Savage PJ, Gardin JM, Rutan GH, Borhani NO. Compensatory increase in common carotid artery diameter. Relation to blood pressure and artery intima-media thickness in older adults. Cardiovascular Health Study. Stroke. 1996;27(11):2012–2015. doi: 10.1161/01.str.27.11.2012. [DOI] [PubMed] [Google Scholar]
- 6.Bakx JC, van den Hoogen HJ, Deurenberg P, van Doremalen J, van den Bosch WJ. Changes in serum total cholesterol levels over 18 years in a cohort of men and women: The Nijmegen Cohort Study. Prev Med. 2000;30(2):138–145. doi: 10.1006/pmed.1999.0608. [DOI] [PubMed] [Google Scholar]
- 7.de Aloysio D, Gambacciani M, Meschia M, Pansini F, Bacchi Modena A, Bolis PF, Massobrio M, Maiocchi G, Peruzzi E, The Icarus Study Group The effect of menopause on blood lipid and lipoprotein levels. Atherosclerosis. 1999;147(1):147–153. doi: 10.1016/s0021-9150(99)00315-9. [DOI] [PubMed] [Google Scholar]
- 8.Matthews KA, Crawford SL, Chae CU, Everson-Rose SA, Sowers MF, Sternfeld B, Sutton-Tyrrell K. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54(25):2366–2373. doi: 10.1016/j.jacc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Do KA, Green A, Guthrie JR, Dudley EC, Burger HG, Dennerstein L. Longitudinal study of risk factors for coronary heart disease across the menopausal transition. Am J Epidemiol. 2000;151(6):584–593. doi: 10.1093/oxfordjournals.aje.a010246. [DOI] [PubMed] [Google Scholar]
- 10.Berg G, Mesch V, Boero L, Sayegh F, Prada M, Royer M, Muzzio ML, Schreier L, Siseles N, Benencia H. Lipid and lipoprotein profile in menopausal transition. Effects of hormones, age and fat distribution. Horm Metab Res. 2004;36(4):215–220. doi: 10.1055/s-2004-814450. [DOI] [PubMed] [Google Scholar]
- 11.Kim CJ, Kim TH, Ryu WS, Ryoo UH. Influence of menopause on high density lipoprotein-cholesterol and lipids. J Korean Med Sci. 2000;15(4):380–386. doi: 10.3346/jkms.2000.15.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews KA, Wing RR, Kuller LH, Meilahn EN, Plantinga P. Influence of the perimenopause on cardiovascular risk factors and symptoms of middle-aged healthy women. Arch Intern Med. 1994;154(20):2349–2355. [PubMed] [Google Scholar]
- 13.Derby CA, Crawford SL, Pasternak RC, Sowers M, Sternfeld B, Matthews KA. Lipid changes during the menopause transition in relation to age and weight: the Study of Women’s Health Across the Nation. Am J Epidemiol. 2009;169(11):1352–1361. doi: 10.1093/aje/kwp043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan AZ, Dwyer JH. Sex differences in the relation of HDL cholesterol to progression of carotid intima-media thickness: the Los Angeles Atherosclerosis Study. Atherosclerosis. 2007;195(1):e191–196. doi: 10.1016/j.atherosclerosis.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 15.Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fogelman AM. Mechanisms of disease: proatherogenic HDL--an evolving field. Nat Clin Pract Endocrinol Metab. 2006;2(9):504–511. doi: 10.1038/ncpendmet0245. [DOI] [PubMed] [Google Scholar]
- 16.Ansell BJ, Fonarow GC, Fogelman AM. High-density lipoprotein: is it always atheroprotective? Curr Atheroscler Rep. 2006;8(5):405–411. doi: 10.1007/s11883-006-0038-4. [DOI] [PubMed] [Google Scholar]
- 17.Keidar S, Bogner I, Gamliel-Lazarovich A, Leiba R, Fuhrman B, Kouperberg E. High plasma high-density lipoprotein levels, very low cardiovascular risk profile, and subclinical carotid atherosclerosis in postmenopausal women. Journal of Clinical Lipidology. 2009;3(5):345–350. doi: 10.1016/j.jacl.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Corsetti JP, Ryan D, Rainwater DL, Moss AJ, Zareba W, Sparks CE. Cholesteryl Ester Transfer Protein Polymorphism (TaqIB) Associates With Risk in Postinfarction Patients With High C-Reactive Protein and High-Density Lipoprotein Cholesterol Levels. Arterioscler Thromb Vasc Biol. 2010 doi: 10.1161/ATVBAHA.110.207977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ansell BJ, Fonarow GC, Fogelman AM. The paradox of dysfunctional high-density lipoprotein. Curr Opin Lipidol. 2007;18(4):427–434. doi: 10.1097/MOL.0b013e3282364a17. [DOI] [PubMed] [Google Scholar]
- 20.Dullaart RP. Increased Coronary Heart Disease Risk Determined by High High-Density Lipoprotein Cholesterol and C-Reactive Protein: Modulation by Variation in the CETP Gene. Arterioscler Thromb Vasc Biol. 2010;30(8):1502–1503. doi: 10.1161/ATVBAHA.110.209544. [DOI] [PubMed] [Google Scholar]
- 21.Barter PJ, Brewer HB, Jr., Chapman MJ, Hennekens CH, Rader DJ, Tall AR. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23(2):160–167. doi: 10.1161/01.atv.0000054658.91146.64. [DOI] [PubMed] [Google Scholar]
- 22.Kuller LH, Sutton-Tyrrell K. Aging and cardiovascular disease. Use of subclinical measurements. Cardiol Clin. 1999;17(1):51–65. viii. doi: 10.1016/s0733-8651(05)70056-4. [DOI] [PubMed] [Google Scholar]
- 23.Sowers MF, Sternfeld B, Morganstein D, Gold EB, Greendale G, Evans D, Neer R, Matthews K, Sherman S, Lo A, Weiss G, Kelsey J. Design, survey, sampling and recruitment methods of SWAN: a multi-center, multi-ethnic, community based cohort study of women and the menopausal transition. In: Lobo RA, Marcus M, editors. Menopause: biology and pathobiology. Academic Press; San Diego: 2000. pp. 175–188. CS. KJ. [Google Scholar]
- 24.Sutton-Tyrrell K, Kuller LH, Edmundowicz D, Feldman A, Holubkov R, Givens L, Matthews KA. Usefulness of electron beam tomography to detect progression of coronary and aortic calcium in middle-aged women. Am J Cardiol. 2001;87(5):560–564. doi: 10.1016/s0002-9149(00)01431-4. [DOI] [PubMed] [Google Scholar]
- 25.Sutton-Tyrrell K, Wolfson SK, Jr., Thompson T, Kelsey SF. Measurement variability in duplex scan assessment of carotid atherosclerosis. Stroke. 1992;23(2):215–220. doi: 10.1161/01.str.23.2.215. [DOI] [PubMed] [Google Scholar]
- 26.Thompson T, Wildman R. Continuous quality assessment programs can improve carotid duplex scan quality. J Vasc Technol. 2001;25:33–39. S-TK. [Google Scholar]
- 27.Everson-Rose SA, Lewis TT, Karavolos K, Matthews KA, Sutton-Tyrrell K, Powell LH. Cynical hostility and carotid atherosclerosis in African American and white women: the Study of Women’s Health Across the Nation (SWAN) Heart Study. Am Heart J. 2006;152(5):982, e987–913. doi: 10.1016/j.ahj.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 29.Freedman DS, Otvos JD, Jeyarajah EJ, Shalaurova I, Cupples LA, Parise H, D’Agostino RB, Wilson PW, Schaefer EJ. Sex and age differences in lipoprotein subclasses measured by nuclear magnetic resonance spectroscopy: the Framingham Study. Clin Chem. 2004;50(7):1189–1200. doi: 10.1373/clinchem.2004.032763. [DOI] [PubMed] [Google Scholar]
- 30.Otvos JD. Measurement of lipoprotein subclass profiles by nuclear magnetic resonance spectroscopy. Clin Lab. 2002;48(3-4):171–180. [PubMed] [Google Scholar]
- 31.Farhat GN, Cauley JA, Matthews KA, Newman AB, Johnston J, Mackey R, Edmundowicz D, Sutton-Tyrrell K. Volumetric BMD and vascular calcification in middle-aged women: the Study of Women’s Health Across the Nation. J Bone Miner Res. 2006;21(12):1839–1846. doi: 10.1359/jbmr.060903. [DOI] [PubMed] [Google Scholar]
- 32.Schwarz EB, McClure CK, Tepper PG, Thurston R, Janssen I, Matthews KA, Sutton-Tyrrell K. Lactation and maternal measures of subclinical cardiovascular disease. Obstet Gynecol. 2010;115(1):41–48. doi: 10.1097/AOG.0b013e3181c5512a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lunt M. Prediction of ordinal outcomes when the association between predictors and outcome differs between outcome levels. Stat Med. 2005;24(9):1357–1369. doi: 10.1002/sim.2009. [DOI] [PubMed] [Google Scholar]
- 34.Bots ML, Elwood PC, Nikitin Y, Salonen JT, Freire de Concalves A, Inzitari D, Sivenius J, Benetou V, Tuomilehto J, Koudstaal PJ, Grobbee DE, EUROSTROKE: a collaborative study among research centres in Europe Total and HDL cholesterol and risk of stroke. J Epidemiol Community Health. 2002;56(Suppl 1):i19–24. doi: 10.1136/jech.56.suppl_1.i19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otvos JD, Jeyarajah EJ, Cromwell WC. Measurement issues related to lipoprotein heterogeneity. Am J Cardiol. 2002;90(8A):22i–29i. doi: 10.1016/s0002-9149(02)02632-2. [DOI] [PubMed] [Google Scholar]
- 36.Lamon-Fava S, Herrington DM, Reboussin DM, Sherman M, Horvath KV, Cupples LA, White C, Demissie S, Schaefer EJ, Asztalos BF. Plasma levels of HDL subpopulations and remnant lipoproteins predict the extent of angiographically-defined coronary artery disease in postmenopausal women. Arterioscler Thromb Vasc Biol. 2008;28(3):575–579. doi: 10.1161/ATVBAHA.107.157123. [DOI] [PubMed] [Google Scholar]
- 37.Asztalos BF, Collins D, Horvath KV, Bloomfield HE, Robins SJ, Schaefer EJ. Relation of gemfibrozil treatment and high-density lipoprotein subpopulation profile with cardiovascular events in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Metabolism. 2008;57(1):77–83. doi: 10.1016/j.metabol.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Otvos JD, Collins D, Freedman DS, Shalaurova I, Schaefer EJ, McNamara JR, Bloomfield HE, Robins SJ. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation. 2006;113(12):1556–1563. doi: 10.1161/CIRCULATIONAHA.105.565135. [DOI] [PubMed] [Google Scholar]
- 39.Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119(7):931–939. doi: 10.1161/CIRCULATIONAHA.108.816181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mackey RH, Kuller LH, Sutton-Tyrrell K, Evans RW, Holubkov R, Matthews KA. Lipoprotein subclasses and coronary artery calcium in postmenopausal women from the healthy women study. Am J Cardiol. 2002;90(8A):71i–76i. doi: 10.1016/s0002-9149(02)02636-x. [DOI] [PubMed] [Google Scholar]
- 41.Kuller LH, Matthews KA, Sutton-Tyrrell K, Edmundowicz D, Bunker CH. Coronary and aortic calcification among women 8 years after menopause and their premenopausal risk factors: the healthy women study. Arterioscler Thromb Vasc Biol. 1999;19(9):2189–2198. doi: 10.1161/01.atv.19.9.2189. [DOI] [PubMed] [Google Scholar]
- 42.Berg GA, Siseles N, Gonzalez AI, Ortiz OC, Tempone A, Wikinski RW. Higher values of hepatic lipase activity in postmenopause: relationship with atherogenic intermediate density and low density lipoproteins. Menopause. 2001;8(1):51–57. doi: 10.1097/00042192-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Borggreve SE, De Vries R, Dullaart RP. Alterations in high-density lipoprotein metabolism and reverse cholesterol transport in insulin resistance and type 2 diabetes mellitus: role of lipolytic enzymes, lecithin:cholesterol acyltransferase and lipid transfer proteins. Eur J Clin Invest. 2003;33(12):1051–1069. doi: 10.1111/j.1365-2362.2003.01263.x. [DOI] [PubMed] [Google Scholar]
- 44.Iwakura A, Luedemann C, Shastry S, Hanley A, Kearney M, Aikawa R, Isner JM, Asahara T, Losordo DW. Estrogen-mediated, endothelial nitric oxide synthase-dependent mobilization of bone marrow-derived endothelial progenitor cells contributes to reendothelialization after arterial injury. Circulation. 2003;108(25):3115–3121. doi: 10.1161/01.CIR.0000106906.56972.83. [DOI] [PubMed] [Google Scholar]
- 45.Knauff EA, Westerveld HE, Goverde AJ, Eijkemans MJ, Valkenburg O, van Santbrink EJ, Fauser BC, van der Schouw YT. Lipid profile of women with premature ovarian failure. Menopause. 2008;15(5):919–923. doi: 10.1097/gme.0b013e31816b4509. [DOI] [PubMed] [Google Scholar]
- 46.Luborsky JL, Meyer P, Sowers MF, Gold EB, Santoro N. Premature menopause in a multi-ethnic population study of the menopause transition. Hum Reprod. 2003;18(1):199–206. doi: 10.1093/humrep/deg005. [DOI] [PubMed] [Google Scholar]
- 47.Eagle KA, Guyton RA, Davidoff R, Ewy GA, Fonger J, Gardner TJ, Gott JP, Herrmann HC, Marlow RA, Nugent W, O’Connor GT, Orszulak TA, Rieselbach RE, Winters WL, Yusuf S, Gibbons RJ, Alpert JS, Garson A, Jr., Gregoratos G, Russell RO, Ryan TJ, Smith SC., Jr. ACC/AHA guidelines for coronary artery bypass graft surgery: executive summary and recommendations: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1991 guidelines for coronary artery bypass graft surgery) Circulation. 1999;100(13):1464–1480. doi: 10.1161/01.cir.100.13.1464. [DOI] [PubMed] [Google Scholar]
- 48.Takaro T, Pifarre R, Fish R. Veterans Administration Cooperative Study of medical versus surgical treatment for stable angina--progress report. Section 3. Left main coronary artery disease. Prog Cardiovasc Dis. 1985;28(3):229–234. doi: 10.1016/0033-0620(85)90018-0. [DOI] [PubMed] [Google Scholar]
- 49.Gepner AD, Wyman RA, Korcarz CE, Aeschlimann SE, Stein JH. An abbreviated carotid intima-media thickness scanning protocol to facilitate clinical screening for subclinical atherosclerosis. J Am Soc Echocardiogr. 2007;20(11):1269–1275. doi: 10.1016/j.echo.2007.03.009. [DOI] [PubMed] [Google Scholar]