Abstract
Purpose
Racial/ethnic variation in fracture risk is well-documented, but the mechanisms by which such heterogeneity arises are poorly understood. We analyzed data from black, Hispanic and white men enrolled in the Boston Area Community Health/Bone (BACH/Bone) Survey to determine the contributions of risk factors to racial/ethnic differences in bone mineral content (BMC) and density (BMD).
Methods
In a population-based study, BMC, BMD and body composition were ascertained by DXA. Socioeconomic status, health history and dietary intake were obtained via interview. Hormones and markers of bone turnover were obtained from non-fasting blood samples. Multivariate analyses measured percentage reductions in estimated racial/ethnic differences in BMC/BMD accompanying the successive removal of covariates from linear regression models.
Results
Black men demonstrated greater BMC than their Hispanic and white counterparts. At the femoral neck, adjustment for covariables was sufficient to reduce these differences by 46% and 35%, respectively. While absolute differences in BMC were smaller at the distal radius than femoral neck, the proportionate reductions in racial/ethnic differences after covariable adjustment were comparable or greater. Multivariate models provided evidence that lean and fat mass, serum 25(OH)D, osteocalcin, estradiol, and aspects of socioeconomic status influence the magnitude of racial/ethnic differences in BMC, with lean and fat mass providing the strongest effects. Results for BMD were similar but typically of lesser magnitude and statistical significance.
Conclusions
These cross-sectional analyses demonstrate that much of the racial/ethnic heterogeneity in measures of bone mass and density can be accounted for through variation in body composition, diet, and sociodemographic factors.
Keywords: bone densitometry, epidemiology, men, osteoporosis, population study, race/ethnicity
Introduction
Despite recent declines in the age-specific prevalence of osteoporosis, the aging of the U.S. population [1], persistent disparities in socioeconomic status and access to health care, and the explosive projected growth of the disease burden borne by minority populations [2] underscore the importance of understanding racial and ethnic differences in bone fragility. The existence of racial/ethnic heterogeneity in bone mineral density (BMD) and the risk of osteoporotic fracture is well-established, particularly as it concerns black and white American men and women [3–9]. Relative to their white counterparts, black Americans are at decreased risk of fracture, exhibiting elevated BMD and lower rates of osteopenia and osteoporosis. As protection against fracture, these would appear to overwhelm black Americans’ seemingly disproportionate exposure to detrimental factors and increased risk of syndromes of aging such as frailty [10]. Although some studies have indicated similarly decreased risk of fracture among certain Hispanic subpopulations, the evidence concerning the overall risk posed to Hispanic Americans is mixed [11–15], and existing large studies are for the most part restricted to Mexican-American men and women.
Using data from the Boston Area Community Health/Bone (BACH/Bone) Survey, we have previously demonstrated the existence of substantial racial/ethnic variation in bone mineral content (BMC), bone mineral density (BMD), serum markers of bone turnover, and proximal femur geometry among men living in greater Boston, MA, located in the northeast United States [9, 16, 17]. While we have observed that aspects of body are influential in determining BMC as well as geometric indices of hip strength and their attendant race/ethnic variation [18–20], the relative importance of these parameters compared with other variables - such as dietary intake and socioeconomic status - remains unknown.
To shed further light on the independent importance of these factors in accounting for racial/ethnic heterogeneity in potential indicators of fracture risk, we conducted an analysis of the BACH/Bone subjects’ femoral neck and distal radius BMC and BMD in the context of multiple covariate groups, considered both singly and in concert. The goal of the analysis was to determine the independent contributions of socioeconomic influences, health and chronic illness, nutrition and health behaviors, bone formation and resorption, serum androgen and estrogen, and body composition to age-adjusted racial/ethnic differences in BMC and BMD.
Methods
Design
The BACH/Bone Survey is a cross-sectional investigation of bone health in randomly-sampled, community-dwelling men of age 30–79 y, with data obtained on a male subset of subjects previously enrolled in the parent Boston Area Community Health (BACH) survey. Full details of the BACH and BACH/Bone design and data collection have been previously published [9, 21]. In brief, BACH data collection consisted of early-morning in-home interviews and blood draws. For BACH/Bone subjects, these measurements were supplemented by an additional blood draw, and by dual energy X-ray absorptiometry (DXA) performed at the Boston University School of Medicine (BUSM).
The BACH design implemented oversampling of self-identified black and Hispanic subjects, so that the resulting study sample was approximately evenly divided by race/ethnicity. BACH/Bone consisted of a subset of male BACH subjects (N=1,219; 367 black men, 401 Hispanic men, and 451 white men). Enrollment criteria stipulated that subjects weigh no more than 300 lbs. and be able to lift themselves onto the DXA scan table. DXA data are available on 1,209 of 1,219 subjects.
Written informed consent was obtained from each subject (independently for both BACH and BACH/Bone), and study subjects received $100 and $75 remuneration for participation in BACH and BACH/Bone, respectively. The BACH protocol was approved by the Institutional Review Board (IRB) of New England Research Institutes (NERI), and the BACH/Bone protocol was approved by IRBs at BUSM and NERI.
For this analysis, potential factors accounting for racial/ethnic differences in BMC or BMD were chosen based on their availability in the BACH/Bone study and their observed associations with bone mass and density in the literature. Preference was given to body composition components obtained by DXA over more general measures of body mass and size such as weight or waist circumference.
Measurement
BMC and BMD at the femoral neck and 1/3 distal radius, as well as total body (excluding head) fat mass (FM) and nonfat mass, were measured using a QDR 4500W densitometer (Hologic, Inc., Waltham, MA). The DXA system was monitored for drift, and measurements were performed by technicians trained by Hologic and certified by the International Society for Clinical Densitometry. The coefficients of variation for BMD at sites considered in this report are less than 1.5% [22].
Total lean mass (LM) was obtained by subtracting total body BMC from total nonfat mass. Subjects’ height was measured using a stadiometer. Race/ethnicity was determined via self-identification according to federal guidelines [23]. Age, education and income, self-assessed general health and history of diagnosed chronic illness, smoking status, daily alcohol consumption, time spent outdoors each day, vitamin supplement use, and country of birth, were obtained through self-report. Use of medications was obtained using a manual inventory of subjects’ medication containers, from which the total number of medications used was extracted as an index of cumulative drug exposure. Dietary intake was measured using the validated Block Food Frequency Questionnaire [24].
Circulating hormone and turnover marker concentrations were obtained in separate venous blood draws in the BACH and BACH/Bone Survey visits, respectively. Testosterone and SHBG were measured at the Children’s Hospital Medical Center Research Laboratories (Boston, MA) by competitive electrochemiluminescence immunoassays on the 2010 Elecsys system (Roche Diagnostics, Indianapolis, IN). The lower limits of detection for testosterone and sex hormone-binding globulin were 2 ng/dL (0.07 nmol/L) and 3 nmol/L, respectively. The inter-assay coefficients of variation (CV) for testosterone at concentrations of 24–700 ng/dL (0.83–24.31 nmol/L) were 7.4-1.7% and 2.4–2.7% for sex hormone-binding globulin at concentrations between 25–95 nmol/L. Estradiol was measured by the Mayo Clinic Core Laboratory (Rochester, MN) with liquid chromatography-tandem mass spectrometry. The lower limit of detection was 12.5 pg/mL (46 pmol/L). For reliable measurement of estradiol levels in the low range, values less than 12.5 pg/mL (46 pmol/L) were calculated by manual integration of chromatograms. The inter-assay CVs for estradiol concentrations 25–373 pg/mL (92–1,369 pmol/L) ranged between 16.6%-9.3%. Free testosterone and estradiol concentrations were calculated from total testosterone or estradiol and sex hormone-binding globulin concentrations using mass action equations [25, 26].
Serum measures obtained in BACH/Bone were performed at The Core Laboratory, BUSM. Turnover markers included serum intact osteocalcin (OC), a marker of bone formation, and serum C-terminal telopeptides of Type-1 collagen (CTx), a marker of bone resorption. OC was measured in duplicate with the Nichols Advantage System (Nichols Institute Diagnostics, San Clemente, CA). CTx was measured with Serum CrossLaps ELISA (Nordic Bioscience Diagnostics, Herlev, Denmark). Inter-assay CVs for OC and CTx are less than 10% and 8.1%, respectively. Serum 25-hydroxyvitamin D [25(OH)D] [25(OH)D2 + 25(OH)D3] concentration was measured in duplicate (the average of the two are presented) at the Core Laboratory, BUSM, using a competitive binding protein (CPB) assay without prior chromatography [27]. Inter-assay coefficients of variation are 10–15%. The reference range is 20–100 ng/mL (50–250 nmol/L). The lower limit of detection (LLD) for the serum 25(OH)D assay was 5 ng/mL (12.5 nmol/L); n = 21 men (16 black and 5 Hispanic men) with values less than the LLD were coded to 5 ng/mL.
Statistical Analysis
Racial/ethnic variation in bone parameters was assumed to be expressed via mean differences between subjects of differing race/ethnicity. Data analyses described here therefore focused specifically on measuring the relative contributions of multiple covariate groupings to these differences. Analyses involved a multi-step process. In Step 1, covariates were tested for unadjusted association with race/ethnicity via ANOVA and Wald tests using all available data, with a generous threshold of p < 0.20 employed as a marker of preliminary evidence in favor of association. Variables not meeting this preliminary threshold were removed, while those meeting the threshold were organized into subgroups corresponding to potential domains of influence over racial/ethnic heterogeneity in BMC/BMD. A separate “base” group of explanatory factors included age and height in addition to race/ethnicity. In Step 2, linear regression models containing all factors retained from Step 1 (in addition to the base group of age and height) were constructed for each covariate subgroup and outcome independently. Beginning with a “full” model incorporating all covariates, each covariate in that subgroup was removed individually in a backwards-stepwise fashion. If in removing that covariate the estimated difference between black vs. white, Hispanic vs. white, or Hispanic vs. black subgroups was changed by a factor of 10% or more, the variable was returned to the subgroup model. If, however, none of those estimated differences changed by at least 10%, the relevant covariate was removed from consideration. Using this “change-in-estimates” approach [28] each of the covariate groups was reduced to a more parsimonious subset. In order to facilitate a fair “apples-to-apples” comparison of the influence of individual covariables on racial/ethnic differences, these estimates were restricted to complete case analysis of subjects with available data on all (see derivation of analytic samples described in Results). In Step 3, the remaining covariates were considered in a combined multivariate model for each outcome. The same backwards-stepwise change-in-estimates approach was used to reach the most parsimonious subset of covariates for all four outcomes. Finally, all covariates remaining in any of the four final models were used in a final multivariate model for each outcome.
All statistical analyses were probability weighted to accommodate the complex sampling design of BACH/Bone, and are therefore referenced to the greater Boston population [21]. Analyses were conducted using SUDAAN version 9.0.1 (RTI International, Research Triangle Park, NC, USA). Estimates of covariate effects and reduction in apparent racial/ethnic differences were obtained from multiple linear regression models with BMC and BMD at the femoral neck and 1/3 distal radius as outcome variables.
For reporting purposes, estimates were considered statistically significant if corresponding null hypotheses could be rejected at the 0.05 level.
Results
Sample characteristics are presented in Table 1. Substantial racial/ethnic variation in numerous factors was observed. While the vast majority of black or white subjects were born in the United States, for instance, over three quarters of Hispanic subjects were not. White subjects were slightly taller and displayed greater total fat mass and lesser total lean mass than their black counterparts, while Hispanic subjects generally had the shortest stature, and were slightly younger than other subjects. Greater proportions of black and Hispanic subjects than white subjects reported a diagnosis of either Type 1 or Type 2 diabetes.
Table 1.
Covariate summaries, by race/ethnicity in the base analysis sample (N=1,165); Mean ± SD, or N (%).
| Submodel | Covariates | Black men (N =352) | Hispanic men (N =387) | White men (N =426) | p-value |
|---|---|---|---|---|---|
| Base | Age, y | 47.9 ± 12.4 | 44.4 ± 11.0 | 47.8 ± 13.2 | 0.002 |
| Height, cm | 174.8 ± 7.3 | 169.6 ± 6.2 | 177.3 ± 6.9 | <0.001 | |
| Socioeconomic Influences | Education, y | 13.4 ± 2.9 | 12.0 ± 5.0 | 16.4 ± 3.6 | <0.001 |
| Household income | <0.001 | ||||
| < $10k | 98 (24.4%) | 129 (23.7%) | 48 (9.0%) | ||
| $10k – 29,9k | 97 (25.0%) | 122 (28.7%) | 95 (19.6%) | ||
| $30k – 69,9k | 103 (33.8%) | 79 (33.5%) | 132 (33.0%) | ||
| ≥ $70k | 43 (16.8%) | 19 (14.1%) | 136 (38.4%) | ||
| Married/living with partner | 147 (41.7%) | 259 (66.2%) | 219 (51.5%) | <0.001 | |
| Hours outside/d | 0.05 | ||||
| < 1 | 24 (7.6%) | 35 (7.8%) | 21 (6.2%) | ||
| 1–2 | 44 (10.8%) | 79 (20.2%) | 59 (14.8%) | ||
| 2–4 | 69 (22.5%) | 75 (21.1%) | 84 (20.1%) | ||
| 4–7 | 62 (18.8%) | 55 (11.6%) | 74 (22.4%) | ||
| > 7 | 153 (40.4%) | 143 (39.4%) | 188 (36.6%) | ||
| SES | 53.8 ± 8.3 | 50.7 ± 12.0 | 62.0 ± 8.8 | <0.001 | |
| Born inside U.S. | 285 (79.4%) | 33 (16.0%) | 392 (92.5%) | <0.001 | |
| Health | Diabetes | 62 (14.4%) | 58 (10.9%) | 29 (3.9%) | <0.001 |
| Cancer | 24 (4.7%) | 8 (1.8%) | 42 (8.9%) | 0.001 | |
| Self-rated health | <0.001 | ||||
| Excellent | 49 (16.2%) | 46 (16.7%) | 94 (23.1%) | ||
| Very good | 92 (29.1%) | 54 (22.3%) | 165 (42.0%) | ||
| Good | 137 (38.5%) | 146 (34.5%) | 118 (25.0%) | ||
| Fair/Poor | 74 (16.2%) | 141 (26.4%) | 49 (10.0%) | ||
| Comorbid conditions | 0.008 | ||||
| 0 | 92 (28.0%) | 120 (36.2%) | 91 (23.1%) | ||
| 1–2 | 126 (35.9%) | 157 (40.1%) | 198 (52.4%) | ||
| 3+ | 134 (36.1%) | 110 (23.7%) | 137 (24.5%) | ||
| Major comorbid conditions | 0.002 | ||||
| 0 | 145 (46.9%) | 167 (47.1%) | 192 (51.0%) | ||
| 1 | 110 (27.1%) | 128 (33.2%) | 148 (34.6%) | ||
| 2+ | 97 (26.0%) | 92 (19.7%) | 86 (14.3%) | ||
| Number of Medications | 0.003 | ||||
| 0 | 71 (25.3%) | 82 (22.8%) | 51 (14.6%) | ||
| 1 | 54 (14.6%) | 93 (32.1%) | 76 (18.4%) | ||
| 2 | 49 (14.4%) | 44 (13.7%) | 81 (18.9%) | ||
| 3–4 | 61 (17.6%) | 69 (15.6%) | 102 (24.4%) | ||
| ≥ 5 | 117(28.0%) | 99 (15.8%) | 116 (23.5%) | ||
| Nutrition and Health Behaviors | Smoking status | 0.01 | |||
| Never | 112 (39.6%) | 175 (51.1%) | 183 (46.7%) | ||
| Former | 86 (23.2%) | 124 (26.5%) | 139 (30.6%) | ||
| Current | 154 (37.2%) | 85 (22.4%) | 104 (22.7%) | ||
| Alcohol consumption, drinks/d | <0.001 | ||||
| 0 | 118 (33.6%) | 165 (35.3%) | 106 (20.6%) | ||
| < 1 | 113 (32.0%) | 134 (39.3%) | 154 (40.0%) | ||
| 1–3 | 62 (20.5%) | 54 (18.6%) | 129 (31.5%) | ||
| > 3 | 59 (13.9%) | 33 (6.9%) | 37 (7.9%) | ||
| Vitamin supplement use | 108 (32.1%) | 74 (17.8%) | 193 (40.2%) | <0.001 | |
| Caffeine intake, mg/day | 119.9 ± 120.1 | 216.9 ± 187.0 | 231.8 ± 151.2 | <0.001a | |
| Calories, kcal/day | 1945 ± 740 | 1820 ± 663 | 1951 ± 645 | 0.11b | |
| Protein, g/day | 83.3 ± 20.3 | 81.8 ± 20.1 | 82.6 ± 18.5 | 0.72b | |
| Bone Health Markers | Serum CTx, ng/mL | 0.46 ± 0.33 | 0.46 ± 0.37 | 0.51 ± 0.45 | 0.14a |
| Serum 25(OH)D, ng/mL | 25.1 ± 14.7 | 33.1 ± 14.0 | 37.5 ± 14.0 | <0.001 | |
| Serum OC, ng/mL | 4.2 ± 2.9 | 4.4 ± 2.4 | 5.0 ± 3.6 | 0.05c | |
| Hormones | Total testosterone, ng/dL | 472.0 ± 207.1 | 421.4 ± 160.0 | 429.1 ± 168.2 | 0.08 |
| Free testosterone, ng/dL | 9.6 ± 3.9 | 9.1 ± 3.5 | 8.9 ± 3.4 | 0.29 | |
| SHBG, nmol | 35.7 ± 19.7 | 30.7 ± 14.6 | 33.7 ± 16.2 | 0.05 | |
| Estradiol, pg/mL | 26.0 ± 10.2 | 23.2 ± 8.8 | 23.1 ± 9.0 | 0.05 | |
| Free estradiol, pg/mL | 0.72 ± 0.26 | 0.67 ± 0.26 | 0.66 ± 0.26 | 0.18 | |
| Body Composition | Body mass index, g/m2 | 28.7 ± 5.1 | 28.3 ± 4.7 | 28.3 ± 4.4 | 0.67 |
| Lean mass, kg | 56.4 ± 8.9 | 51.8 ± 7.3 | 55.4 ± 7.1 | <0.001 | |
| Fat mass, kg | 20.6 ± 9.0 | 19.8 ± 7.3 | 23.0 ± 8.5 | <0.001 | |
SES: Socioeconomic status
variable was log transformed for statistical testing
variable was broken into quartiles for statistical testing
variable was square-root transformed for statistical testing
Unadjusted association of covariates with race/ethnicity
Variables that did not evidence marginal association with race/ethnicity (p > .20) are shaded in gray in Table 1, and were excluded in subsequent analyses. As described above, the remaining covariates were divided into groups and analyzed in sub-models. Reduction of groups to those included within the final models then proceeded as described above.
Age and height-adjusted racial/ethnic differences in BMC/BMD
As noted above, estimation of the proportion of racial/ethnic variation explained by each covariate was performed using the subset of subjects with data available on all candidate covariables. Table 2 presents the cumulative effect of missing records on data available for estimation in final multivariate regression models. Comparisons of subjects included in analytic samples (described in Table 2) with BACH/Bone subjects not included in the analytic samples are presented in Table 3, indicating general comparability between those included and not included in the analytic samples.
Table 2.
Derivation of analytic sample. Numbers of subjects not included in final estimation due to exclusions or to missing data are listed sequentially.
| Total BACH/Bone Sample | 1,219 |
| Taking exclusionary medications* | −22 |
| Missing DXA | −32 |
| Base Analytic Sample | 1,165 |
| Missing lean mass/fat mass | −34 |
| Missing country of birth | −1 |
| Missing estradiol | −313 |
| Missing osteocalcin | −29 |
| Missing 25(OH)D | −7 |
| Missing caffeine intake | −126 |
| Missing calorie intake | −89 |
| Missing household income | −23 |
| Analytic Sample Size | 543 |
Exclusionary medications: GnRH agonists and antagonists, androgens, estrogens, progestins, 5-α-reductase inhibitors, drospirenone, ketoconazole, danazol, and clomiphine
Table 3.
Comparison of subjects in the analytic sample to subjects excluded from the analytic sample.
| Included in/Excluded from Analytic Sample Percent or Mean ± SD (N = 543/676) | |
|---|---|
| Race/ethnicity | |
| Black | 20.9%/29.5% |
| Hispanic | 11.7%/14.4% |
| White | 67.4%/56.1% |
| Age, y | 47.4 ± 12.6/48.1 ± 12.9 |
| Height, cm | 176 ± 7/176 ± 8 |
| Household income | |
| < $10k | 16.7%/12.8% |
| $10k–29,9k | 25.5%/19.5% |
| $30k–69,9k | 29.0%/36.7% |
| >=$70k | 28.9%/31.1% |
| SES | 58.2 ± 10.5/58.8 ± 10.0 |
| Born inside U.S. (%Yes) | 83.2%/75.8% |
| Smoking status | |
| Never | 41.7%/47.8% |
| Former | 29.4%/29.4% |
| Current | 28.9%/22.8% |
| Caffeine, mg/day | 216 ± 157/185 ± 154 |
| Calories, kcal/day | 1949 ± 648/1919 ± 698 |
| Estradiol, pg/mL | 23.9 ± 8.7/23.4 ± 9.9 |
| Osteocalcin, ng/mL | 4.88 ± 3.78/4.57 ± 2.64 |
| 25(OH)D, ng/mL | 33.4 ± 14.9/34.5 ± 15.7 |
| Lean mass, kg | 54.8 ± 7.4/55.6 ± 8.0 |
| Fat mass, kg | 22.4 ± 8.5/21.6 ± 8.6 |
SES: Socioeconomic status
Age and height adjusted (‘base’) racial/ethnic differences are presented at the top of Table 4. Results indicate statistically significant age and height-adjusted differences between black and white, and black and Hispanic, subsamples in all bone outcomes, but that Hispanic vs. white differences are statistically insignificant except in the case of BMD at the femoral neck.
Table 4.
Base and final model estimates.
| Model | Subgroup | Covariates | Regression Coefficients |
|||
|---|---|---|---|---|---|---|
| Femoral Neck | 1/3 Distal Radius | |||||
| BMC (g) | BMD (g/cm2) | BMC (g) | BMD (g/cm2) | |||
| Base | Race/Ethnicity | |||||
| Black vs. White | 0.538 | 0.137 | 0.124 | 0.037 | ||
| Black vs. Hispanic | 0.407 | 0.087 | 0.297 | 0.037 | ||
| Hispanic vs. White | 0.131 | 0.050 | −0.173 | −0.000 | ||
| Age, 10y | −0.097 | −0.021 | −0.037 | −0.003 | ||
| Height, cm | 0.036 | 0.003 | 0.006 | 0.002 | ||
| Final | Race/Ethnicity | |||||
| Black vs. White | 0.291 | 0.104 | 0.021 | 0.022 | ||
| Black vs. Hispanic | 0.265 | 0.075 | 0.171 | 0.021 | ||
| Hispanic vs. White | 0.026 | 0.028 | −0.150 | 0.001 | ||
| Age, 10y | −0.079 | −0.021 | −0.017 | −0.001 | ||
| Height, cm | 0.012 | −0.000 | −0.001 | 0.000 | ||
| Socioeconomic Influences | Household income | |||||
| < $10k | −0.165 | −0.021 | −0.005 | −0.003 | ||
| $10k – 29,9k | 0.051 | 0.003 | −0.021 | 0.008 | ||
| $30k – 69,9k | −0.026 | −0.018 | −0.084 | −0.009 | ||
| ≥ $70k | ref | ref | ref | ref | ||
| Born inside U.S. | −0.120 | −0.031 | 0.110 | 0.004 | ||
| Nutrition and Health Behaviors | Smoking status | |||||
| Never | −0.025 | 0.002 | 0.052 | −0.009 | ||
| Former | −0.029 | 0.012 | 0.052 | −0.008 | ||
| Current | ref | ref | ref | ref | ||
| Caffeine intake, mg/daya | −0.128 | −0.021 | −0.013 | 0.001 | ||
| Daily caloric consumption, quintiles | ||||||
| 1 | 0.082 | 0.011 | −0.054 | 0.011 | ||
| 2 | −0.004 | 0.002 | −0.026 | 0.019 | ||
| 3 | −0.161 | −0.019 | −0.105 | 0.018 | ||
| 4 | −0.021 | −0.001 | −0.130 | 0.016 | ||
| 5 | ref | ref | ref | ref | ||
| Bone Health Markers | Serum 25(OH)D, ng/mL | 0.003 | 0.001 | −0.002 | 0.000 | |
| Serum OC, ng/mLb | −0.299 | −0.035 | −0.230 | 0.004 | ||
| Hormones | Estradiol, pg/mL | 0.010 | 0.002 | 0.001 | −0.000 | |
| Body Composition | Lean mass, kg | 0.057 | 0.008 | 0.020 | 0.004 | |
| Fat mass, kg | −0.013 | −0.000 | −0.015 | −0.001 | ||
Ref: referent; BMC: bone mineral content; BMD: bone mineral density
Bolded estimates were statistically significantly associated with outcomes at the 0.05 level.
Variable was log transformed
Variable was square-root transformed
Reduction of racial/ethnic differences by control for external covariates
Final model estimates are presented below the base models in Table 4. The substantial reduction in the number of covariates listed (in comparison to Table 1) is indicative of the number of covariates that were unable to account for a meaningful proportion of mean racial/ethnic differences in any outcome. For instance, none of the variables listed in the health/comorbidity subgroup in Table 1 altered the estimates of unadjusted racial/ethnic differences by more than 10% when entered sequentially into models for each outcome, and hence they were eliminated during the model-reduction method described above.
The cumulative influence of all covariates on estimated racial/ethnic heterogeneity in BMC and BMD is illustrated in comparisons of the regression estimates corresponding to race and ethnicity between the base and final models. These indicate that while the mean BMC differences between black and white subsamples remain statistically significant after adjustment for all relevant covariates, they are substantially reduced. For instance, black versus white differences in mean femoral neck BMC and BMD were reduced by approximately 46% (from 0.54 g to 0.29 g) and 29% (from 0.14 g/cm2 to 0.10 g/cm2), respectively, in the final vis-à-vis the ‘base’ models. The base differences between black and Hispanic men in BMC were similarly reduced at both femoral neck and distal radius.
Independent changes in estimated racial/ethnic differences associated with individual covariates
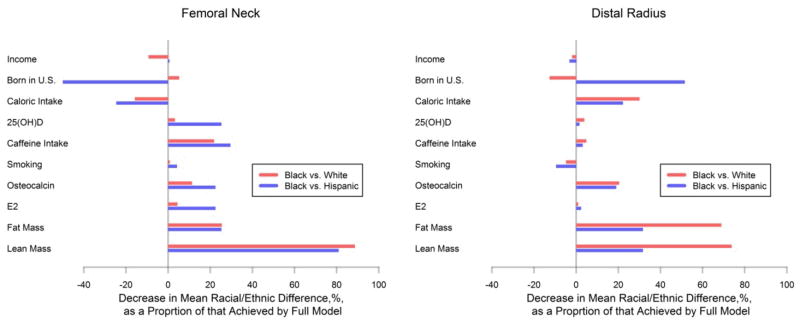
Figure 1 presents change-in-estimates results for final model components in models of femoral neck and distal radius BMC. Each bar shown represents the independent contribution of each covariate to the overall, full model reduction in racial/ethnic differences described above and displayed in Table 4 (comparisons of white and Hispanic subjects are not shown as these differences are much less significant than the others; see Table 4). These results demonstrate the powerful ability of body composition parameters (lean and fat mass) to independently account for a great deal of racial/ethnic variation in BMC, with less substantial but consistent influences of caffeine intake and osteocalcin. Comparisons of left panel to right panel indicate that the influence of lean mass is greater than that of fat mass at the femoral neck, but that the two are essentially identically important at the distal radius.
Figure 1. Change in model-based estimates of mean racial/ethnic differences in BMC resulting from excluding individual factors from multivariate models.
For each panel, the length of the horizontal bars measures the ratio of (a) the difference between the full model and the full model excluding the relevant covariate (listed vertically on left) to (b) the difference between the full model and a model including only age and height as covariates. Thus each bar depicts the independent contribution of each factor to the overall reduction in racial/ethnic differences achieved by the total model, excluding the changes due to any other factors. Results underscore the dominant independent role of body mass; at both the femoral neck and distal radius, failure to consider lean mass alone would result in increases of over 75% in the adjusted mean difference in BMC between black and white subsamples. Results lying to the left of the vertical gray lines indicate that including the relevant covariate in the full model serves to increase the estimated racial/ethnic differences.
Discussion
In these cross-sectional analyses, we demonstrate that control for six categories of covariates was sufficient to account for the majority of racial/ethnic variation in age and height-adjusted BMC, and a substantial proportion of the corresponding differences in BMD. In particular, the largest differences (between black and white subjects) in BMC were reduced by 60–70% through statistical adjustment in a multivariate regression model. We observed, however, that of the numerous covariates that have potential importance in explaining racial/ethnic differences in BMC/BMD, many exhibited no ability to account for those differences in sub-models controlling for the other factors included within the same covariate group.
The dominant influence of fat mass and lean mass on BMC is consistent with data from many studies, and with our published analyses of BMC and proximal femur geometry in this population [18, 19]. As we have previously observed in data from this cohort, increased FM is associated with decreased BMC once LM is held constant (this is indicated by the negative coefficients associated with FM in Table 4). In the models presented here, however, both LM and FM appeared to contribute substantially to estimated racial/ethnic differences in BMC/BMD, implying that both FM and LM may be important in accounting for the difference between (for instance) black and white subjects’ risk of osteoporotic fracture. Thus, while racial/ethnic differences in total body LM and FM do not, individually, appear to be very large (Table 1), the presence of both elevated fat mass and reduced LM in white subjects may be critical to racial/ethnic differences in BMC, despite the role of obesity in fracture risk itself remaining unclear [29]. That lean mass would display a stronger association with racial/ethnic differences at a load-bearing (femoral neck) vs. non-load-bearing (distal radius) site, as indicated by Figure 1, accords with intuition.
In general, the covariable factors considered were more successful in accounting for racial/ethnic differences in BMC than in BMD, and we have previously observed [18] that BMC is more tightly associated with the other body composition components (fat and lean body mass) than is BMD. It is possible that racial/ethnic variation in bone size (of which BMD is a function) in part accounts for this discrepancy.
While the models described here suggest that body composition exercises the strongest proximal influence over racial/ethnic heterogeneity in BMC and BMD, lack of longitudinal data makes it difficult to diagnose the indirect effects that may be embedded in the apparent association between BMC/BMD and the other body composition compartments. It should again be emphasized that the analyses described here were primarily intended to account for racial/ethnic variation in BMC/BMD, and covariates presented in the final model were included because they demonstrated the potential to do that for at least one of the outcome variables, the statistical significance (or lack thereof) of their overall association with BMC/BMD notwithstanding.
In these analyses we observed certain differences in results according to whether observations were obtained at the distal radius or the femoral neck, in that the magnitude of reductions in racial/ethnic differences in BMC displayed limited evidence of variation by site (Figure 1), for instance in the lean and fat mass contributions as described above. These sites differ in the relative proportion of trabecular or cancellous bone; at the femoral neck site, approximately 25% of the bone is trabecular, whereas in the distal radius, up to 10% is trabecular [30]. Because trabecular bone is thought to be more metabolically active than compact bone, it is conceivable that BMC/BMD in the femoral neck would demonstrate stronger associations with covariables lying along metabolic pathways leading to osteogenesis and turnover than would BMC/BMD at the radius. Our cross-sectional results seem to support this interpretation in a limited sense, as vitamin D and caffeine intake appear to generate greater independent contributions to racial/ethnic differences in BMC at the femoral neck than the distal radius (Figure 1). This is consistent with previous research, where, for instance, supplemental vitamin D was associated with relatively large effects on the more trabecular-rich lumbar spine [31]. However, it should yet again be noted that because this analysis targets reduction in racial/ethnic differences (rather than pure strength of association with BMC or BMD), it should be interpreted in that light; a hypothetical covariate of critical importance to BMC or BMD but that displays no racial/ethnic variation would be removed from consideration in these analyses, by design.
Certain limitations of these analyses should be noted. Among these is the unavoidable reality of the cross-sectional study design, preventing the examination of change with time in both racial/ethnic differences in bone mass and density, and the observation of contributions of alterations in potential explanatory factors to those changes. An additional limitation is the inability of this study of adult men to account for developmental variation in skeletal health. To the degree that later life manifestations of racial/ethnic variations in BMC/BMD reflect variation in exposures earlier in life, they cannot be addressed by the analyses described here. Finally, the inclusion of numerous covariate factors on which it is possible for subjects to have missing records insures that some subjects are not considered in certain analyses. Table 3 indicates, however, that the subjects excluded from the analysis samples are for the most part quite similar to those included in the analysis samples.
These results strongly suggest that much of the racial/ethnic variation in adult male fracture risk is mediated by the joint influences of numerous factors, with body composition, bone formation, and caffeine intake acting as powerful proximal indicators of racial/ethnic differences in BMC and BMD. Given the aging of the U.S. population and the accelerating epidemics of obesity and diabetes, the role of body composition in fall and fracture risk demands increased attention.
Acknowledgments
The authors appreciate the statistical programming support of Gavin S. Miyasato, S.M.
Grant support: The project described was supported by Award Number R01AG020727 from the National Institute on Aging. The parent study (BACH) was supported by grant U01DK056842 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, the National Institute of Diabetes and Digestive and Kidney Diseases, or the National Institutes of Health.
Footnotes
Disclosures: The authors have nothing to disclose.
References
- 1.Looker AC, Melton LJ, 3rd, Harris TB, Borrud LG, Shepherd JA. Prevalence and trends in low femur bone density among older US adults: NHANES 2005–2006 compared with NHANES III. J Bone Miner Res. 2009;25:64–71. doi: 10.1359/jbmr.090706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 3.Barrett-Connor E, Siris ES, Wehren LE, Miller PD, Abbott TA, Berger ML, Santora AC, Sherwood LM. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res. 2005;20:185–194. doi: 10.1359/JBMR.041007. [DOI] [PubMed] [Google Scholar]
- 4.George A, Tracy JK, Meyer WA, Flores RH, Wilson PD, Hochberg MC. Racial differences in bone mineral density in older men. J Bone Miner Res. 2003;18:2238–2244. doi: 10.1359/jbmr.2003.18.12.2238. [DOI] [PubMed] [Google Scholar]
- 5.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC, Jr, Lindsay R. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468–489. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 6.Marshall LM, Zmuda JM, Chan BK, Barrett-Connor E, Cauley JA, Ensrud KE, Lang TF, Orwoll ES. Race and ethnic variation in proximal femur structure and BMD among older men. J Bone Miner Res. 2008;23:121–130. doi: 10.1359/JBMR.070908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tracy JK, Meyer WA, Flores RH, Wilson PD, Hochberg MC. Racial differences in rate of decline in bone mass in older men: the Baltimore men’s osteoporosis study. J Bone Miner Res. 2005;20:1228–1234. doi: 10.1359/JBMR.050310. [DOI] [PubMed] [Google Scholar]
- 8.Tracy JK, Meyer WA, Grigoryan M, Fan B, Flores RH, Genant HK, Resnik C, Hochberg MC. Racial differences in the prevalence of vertebral fractures in older men: the Baltimore Men’s Osteoporosis Study. Osteoporos Int. 2006;17:99–104. doi: 10.1007/s00198-005-1919-z. [DOI] [PubMed] [Google Scholar]
- 9.Araujo AB, Travison TG, Harris SS, Holick MF, Turner AK, McKinlay JB. Race/ethnic differences in bone mineral density in men. Osteoporos Int. 2007;18:943–953. doi: 10.1007/s00198-006-0321-9. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch C, Anderson ML, Newman A, Kop W, Jackson S, Gottdiener J, Tracy R, Fried LP. The association of race with frailty: the cardiovascular health study. Ann Epidemiol. 2006;16:545–553. doi: 10.1016/j.annepidem.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Bauer RL. Ethnic differences in hip fracture: a reduced incidence in Mexican Americans. Am J Epidemiol. 1988;127:145–149. doi: 10.1093/oxfordjournals.aje.a114774. [DOI] [PubMed] [Google Scholar]
- 12.Espino DV, Palmer RF, Miles TP, Mouton CP, Wood RC, Bayne NS, Markides KP. Prevalence, incidence, and risk factors associated with hip fractures in community-dwelling older Mexican Americans: results of the Hispanic EPESE study. Establish Population for the Epidemiologic Study for the Elderly. J Am Geriatr Soc. 2000;48:1252–1260. doi: 10.1111/j.1532-5415.2000.tb02598.x. [DOI] [PubMed] [Google Scholar]
- 13.Espino DV, Silva Ross J, Oakes SL, Becho J, Wood RC. Characteristics of hip fractures among hospitalized elder Mexican American Black and White Medicare beneficiaries in the Southwestern United States. Aging Clin Exp Res. 2008;20:344–348. doi: 10.1007/BF03324866. [DOI] [PubMed] [Google Scholar]
- 14.Silverman SL, Madison RE. Decreased incidence of hip fracture in Hispanics, Asians, and blacks: California Hospital Discharge Data. Am J Public Health. 1988;78:1482–1483. doi: 10.2105/ajph.78.11.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lauderdale DS, Jacobsen SJ, Furner SE, Levy PS, Brody JA, Goldberg J. Hip fracture incidence among elderly Hispanics. Am J Public Health. 1998;88:1245–1247. doi: 10.2105/ajph.88.8.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Travison TG, Beck TJ, Esche GR, Araujo AB, McKinlay JB. Age trends in proximal femur geometry in men: variation by race and ethnicity. Osteoporos Int. 2008;19:277–287. doi: 10.1007/s00198-007-0497-7. [DOI] [PubMed] [Google Scholar]
- 17.Leder BZ, Araujo AB, Travison TG, McKinlay JB. Racial and ethnic differences in bone turnover markers in men. J Clin Endocrinol Metab. 2007;92:3453–3457. doi: 10.1210/jc.2006-2695. [DOI] [PubMed] [Google Scholar]
- 18.Travison TG, Araujo AB, Esche GR, Beck TJ, McKinlay JB. Lean mass and not fat mass is associated with male proximal femur strength. J Bone Miner Res. 2008;23:189–198. doi: 10.1359/JBMR.071016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Travison TG, Araujo AB, Esche GR, McKinlay JB. The relationship between body composition and bone mineral content: threshold effects in a racially and ethnically diverse group of men. Osteoporos Int. 2008;19:29–38. doi: 10.1007/s00198-007-0431-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiu GR, Araujo AB, Travison TG, Hall SA, McKinlay JB. Relative contributions of multiple determinants to bone mineral density in men. Osteoporos Int. 2009;20:2035–2047. doi: 10.1007/s00198-009-0895-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKinlay JB, Link CL. Measuring the urologic iceberg: design and implementation of the Boston Area Community Health (BACH) Survey. Eur Urol. 2007;52:389–396. doi: 10.1016/j.eururo.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baran D. Manufacturer’s specifications. Hologic Inc; Bedford, MA: 1995. Precision in fan beam densitometry: A multi-site validation. [Google Scholar]
- 23.Wallman KK, Evinger S, Schechter S. Measuring our nation’s diversity: developing a common language for data on race/ethnicity. Am J Public Health. 2000;90:1704–1708. doi: 10.2105/ajph.90.11.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 25.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 26.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 27.Chen TC, Turner AK, Holick MF. Methods for the determination of the circulating concentration of 25-hydroxyvitamin D. J Nutr Biochem. 1990;1:315–319. doi: 10.1016/0955-2863(90)90067-u. [DOI] [PubMed] [Google Scholar]
- 28.Rothman KJ, Greenland S, editors. Modern Epidemiology. Lippincott-Raven; Philadelphia: 1998. [Google Scholar]
- 29.Beck TJ, Petit MA, Wu G, LeBoff MS, Cauley JA, Chen Z. Does obesity really make the femur stronger? BMD, geometry, and fracture incidence in the women’s health initiative-observational study. J Bone Miner Res. 2009;24:1369–1379. doi: 10.1359/JBMR.090307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mundy GR, Chen D, Oyajobi BO. Chapter 7. Bone Remodeling. In: Favus MA, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. American Society for Bone and Mineral Research; Washington, DC: 2003. pp. 46–57. [Google Scholar]
- 31.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337:670–676. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]