Abstract
The gelatinases, matrix metalloproteinase (MMP)-9 and -2, are produced as latent, inactive enzymes that can be proteolytically activated by a number of proteases. In many normal and pathological conditions, where the expression of MMPs is deregulated, changes in the expression of other proteases have also been reported. Human kallikrein-related peptidase 7 (KLK7), a chymotryptic-like serine protease, is overexpressed in many different types of neoplastic conditions, which have also been shown to express high levels of both MMP-9 and -2. Since the activation of MMPs by KLK7 has never been examined, we sought to determine whether KLK7 can activate these MMPs. To test this hypothesis KLK7 was incubated with the recombinant MMPs and the products of the reaction were analyzed for their activity. Incubation of proMMP-9 with KLK7 resulted in the production of a novel truncated, active MMP-9 lacking the C-terminal hemopexin domains. In contrast, KLK7 degraded, but did not activate, proMMP-2. The novel activation of proMMP-9 by KLK7 was further confirmed using conditioned medium prepared from an MMP-9-expressing cell line, MDA-MMP-9. Our results clearly establish that KLK7 activates proMMP-9 to produce a novel truncated, active MMP-9 product not generated by other proteases. These findings suggest that KLK7 may play an important role in the activation of MMP-9 in tumors that express high levels of both these proteases and the resulting truncated MMP may possess altered substrate specificities compared with full-length MMP-9 activated by other proteases.
Keywords: gelatinase, pancreatic cancer, proteolytic cascade, zymogen activation
1. Introduction
Matrix metalloproteinases (MMPs) comprise a family of zinc-dependent, neutral endopeptidases that participate in the degradation of the extracellular matrix and remodeling in both normal and pathological conditions, including cancer. During metastasis, tumor cells must detach from the primary tumor, migrate, and invade surrounding tissues – processes that are actively facilitated by MMPs. Members of the MMP family have been studied in detail for their ability to influence the progression of different types of cancers [1–3]; and various MMPs have been shown to regulate tumor progression by proteolysis of extracellular matrix proteins [4], remodeling the tumor microenvironment [5], processing cell-cell adhesion molecules [6], selecting tumor cells resistant to apoptosis [7], and promoting tumor angiogenesis [8]. In particular, degradation of the basement membrane, comprised predominately of type IV collagen, is carried out by the gelatinases (MMP-2 and MMP-9)
MMPs are secreted as latent, inactive proenzymes that can be converted into active enzymes by proteolytic removal of the propeptide domain containing a highly conserved cysteine residue that forms an intramolecular complex with the active site zinc atom [9–10]. Many extracellular proteases have been demonstrated to directly activate MMPs including other MMPs (e.g., MMP-3 activation of the MMP-9 [11]) and non-MMP proteins (e.g., plasmin activation of MMP-3 [12]). High levels of metalloproteinases, especially MMP-2 and/or MMP-9 have been demonstrated in many cancers, including pancreatic [13–20], breast [21], cervical [22], and ovarian cancer [23]. Similarly, the human kallikrein-related peptidases constitute an important group of serine proteases that are up-regulated in various cancers (reviewed in [24–25]). In particular, kallikrein-like peptidase 7 (KLK7) has also been reported to be overexpressed in human pancreatic [26], breast [27], cervical [28], and ovarian cancer [29–31]. Dysregulated KLK expression in neoplastic tissues may play a role in tumor growth, invasion, metastasis, and angiogenesis (reviewed in [24, 32–33]). The co-expression of KLK7 and MMP-2 and -9 prompted us to investigate whether KLK7 could activate these MMPs. Results from our experiments clearly show that KLK7 can activate purified proMMP-9, but not proMMP-2, in vitro to produce a novel active MMP-9 gelatinolytic fragment that lacks the C-terminal hemopexin domains, which is in contrast to the 83-kDa product produced by other proteases. Kallikrein-related peptidase 7 was also able to activate proMMP-9 secreted in conditioned medium by MDA-MMP-9 cells in a similar fashion. These results highlight a novel role for KLK7 in the activation of MMP-9 in tissues where both these proteases are highly expressed and should temper the assignment of gelatinolytic activity based solely on gel mobility in mixtures of MMPs. Importantly such activation of MMP-9 by KLK7 in pathological conditions, like cancer, could result in truncated MMP-9 that may exhibit altered substrate specificity and not be targeted by current therapies.
2. Materials and methods
2.1 Cell culture and conditioned media
MDA-MMP-9 cells (kindly provided by Dr. James P. Quigley) and the parental breast cancer cell line MDA-MB-231 were seeded in 10-cm dishes and grown to 70% confluence in Dulbecco’s Modified Eagle’s Medium (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (Atlanta Biologicals, Norcross, GA) at 37°C in a 5% CO2/air environment. Geneticin (600 µg/ml) was included in the culture medium for the MDA-MMP-9 cells. For preparation of conditioned media, growth media was removed, cells were washed twice in phosphate-buffered saline, and incubated in serum-free medium (SFM) for 48 hours. After incubation, the conditioned media was removed, centrifuged at 1500 rpm at 4°C to remove any cell debris, aliquoted, and stored at −20°C.
2.2 Activation of proKLK7
Recombinant, proKLK7 (100 µg/mL) (R&D Systems, Minneapolis, MN) was proteolytically activated using thermolysin as described previously [34]. To activate with plasmin, equal molar amounts of proKLK7 and plasmin (EMD Chemicals) were incubated at 37°C for 4 hours in 50 mM Tris–HCl, pH 7.2, 0.15 M NaCl. Plasmin activity was terminated by addition of D-Val-Phe-Lys chloromethyl ketone (VFK-CK, EMD Chemicals), a selective irreversible plasmin inhibitor.
2.3 Gelatin zymography
For gelatin zymography, mixtures after incubation were mixed with non-reducing SDS-PAGE sample buffer and incubated at RT for 10 min. The samples were then resolved in 15 or 20% acrylamide SDS-PAGE gels containing 10 mg/ml of porcine gelatin. After electrophoresis, the gels were washed for 1 h in renaturing buffer (2.5% Triton X-100) and incubated overnight in developing buffer (50 mM Tris, pH 7.5, 200 mM NaCl, and 5 mM CaCl2) at 37°C with constant shaking. The gels were then stained with 0.1% Coomassie Brilliant Blue R-250 (Bio-Rad Laboratories, Richmond, CA) in 50% methanol and 10% acetic acid overnight. The bands were visualized after repeated washes with a 50% methanol, 10% acetic acid solution.
2.4 Activation of proMMP-9 and proMMP-2 by KLK7
ProMMP-9 and proMMP-2 (1 pmol) (Chemicon, Temecula, CA) were incubated with 0.2 pmol of thermolysin-activated KLK7 at 37°C in KLK7 activity buffer (50 mM Tris-HCl, pH 8.5, 0.15 M NaCl) containing 50 mM EDTA (to inhibit thermolysin). At various time intervals samples were removed and resolved on 4–12% Bis-Tris polyacrylamide gels (Invitrogen) for western blots or 20% polyacrylamide gels containing 0.1% gelatin to monitor gelatinolytic activity. As controls, proMMP-9 or proMMP-2 alone or with 1 ng of thermolysin, were incubated under similar conditions separately for 4 hours. As a further control, thermolysin-activated KLK7 was incubated in the presence of 50 mM EDTA for 4 hours.
2.5 Activation of proMMP-9 by KLK7 in conditioned medium of MDA-MMP-9 cells
Conditioned media (20 µl) from MDA-MMP-9 cells was incubated with 100 ng of thermolysin-activated KLK7 in the presence of 50 mM EDTA at 37°C for 0, 30, 60, 120, 240 minutes. At each time point, an aliquot was removed for gelatin zymography. As controls, 20 µl of MDA-MMP-9 conditioned media was incubated alone or with 10 ng of thermolysin in presence of 50 mM EDTA at 37°C for 4 hours. As a positive control, 1 pmol of recombinant proMMP-9 was incubated with 0.2 pmol of thermolysin-activated KLK7 in 20 µl of SFM containing 50 mM EDTA for 4 hours at 37°C.
2.6 Activation of recombinant proMMP-9 by KLK7 in MDA-MB-231 conditioned media
ProMMP-9 (1 pmol) was incubated with 0.2 pmol of thermolysin-activated KLK7 in 20 µl of MDA-MB-231 conditioned media in the presence of 50 mM EDTA at 37°C. As controls, 20 µl of conditioned media was incubated alone, with 100 ng proMMP-9, or with 100 ng proMMP-9 and 1 ng thermolysin at 37°C in the presence of 50 mM EDTA. As a positive control, proMMP-9 was incubated with thermolysin-activated KLK7 in 20 µl of SFM containing 50 mM EDTA at 37°C. After four hours, the samples were analyzed by gelatin zymography, visualized by Coomassie blue staining, and the sizes of the novel gelatinolytic fragments were determined using an AlphaEase image documentation system and analysis software (Alpha Innotech, San Leandro, CA).
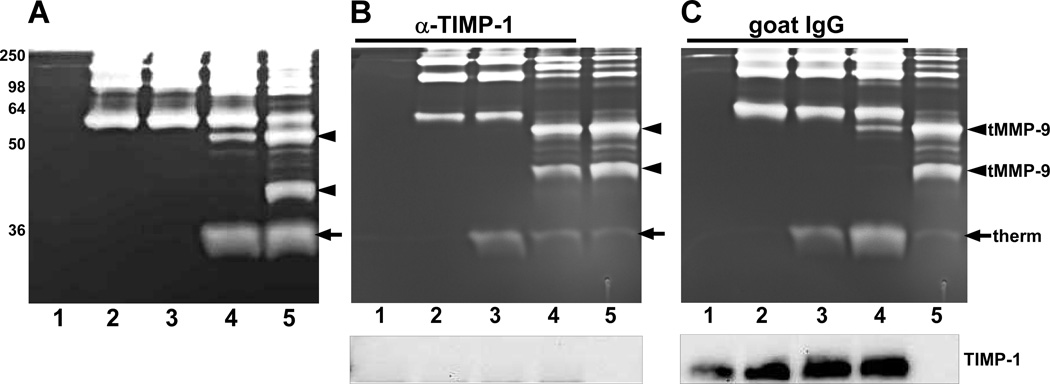
2.7 Activation of proMMP-9 by KLK7 in TIMP-1-immunodepleted conditioned medium of MDA-MB-231 cells
MDA-MB-231 conditioned medium (500 µl) was incubated with 2 µg of anti-TIMP-1 antibody (R&D Systems) on a rotating mixer overnight at 4°C. As a control, 500 µl of conditioned medium was incubated with 2 µg of normal goat IgG antibody (R&D Systems) under similar conditions. Immunocomplexes were removed by addition of a 50% slurry of protein G Sepharose 4 Fast Flow (GE Healthcare, Piscataway, NJ), mixing for 2 hours at 4°C, followed by centrifugation. ProMMP-9 (1 pmol) was incubated with 0.2 pmol thermolysin-activated KLK7 in 20 µl of TIMP-1-immunodepleted MDA-MB-231 conditioned medium or control IgG-treated conditioned medium in the presence of 50 mM EDTA for 4 hours at 37°C. As controls, 20 µl of TIMP-1 or control immunodepleted conditioned medium was incubated alone, with 1 pmol of proMMP-9, or with 1 pmol of proMMP-9 and 1 ng thermolysin in presence of 50 mM EDTA for 4 hours at 37°C. As a positive control, 1 pmol of proMMP-9 was incubated with 0.2 pmol thermolysin-activated KLK7 in 20 µl of SFM containing 50 mM EDTA for 4 hours at 37°C. At the end of incubation, one aliquot was subjected to gelatin zymography and a second aliquot was analyzed by western blot for the presence of TIMP-1.
2.8 Western blot analysis of KLK-7-cleaved proMMP-9
For detection of proMMP-9 products cleaved by KLK7 using western blot analysis, PVDF membranes were incubated with MMP-9 antibodies generated using full-length proMMP-9 (AF911, R&D Systems), the catalytic domain of MMP-9 (AB19016, Millipore), or peptide immunogens located in the C-terminus of MMP-9 (M5177, Sigma-Aldrich; 56-2A4, Millipore).
2.9 N-terminal sequence analysis of KLK-7-cleaved MMP-9
To determine the KLK7 cleavage site of MMP-9, 50 pmol of proMMP-9 was incubated with 10 pmol of thermolysin-activated KLK7 for 4 hours at 37°C. The reaction was terminated by heating at 95°C for 5 minutes following the addition of SDS-PAGE sample buffer. The products were resolved on a 14% acrylamide/SDS gel, transferred to PVDF membrane, and visualized with 0.2% Ponceau-S stain in 1% acetic acid. The membrane with truncated ~48-kDa band was excised, washed in methanol followed by distilled water, and submitted to the Harvard Microchemistry Facility for protein sequence analysis.
3. Results
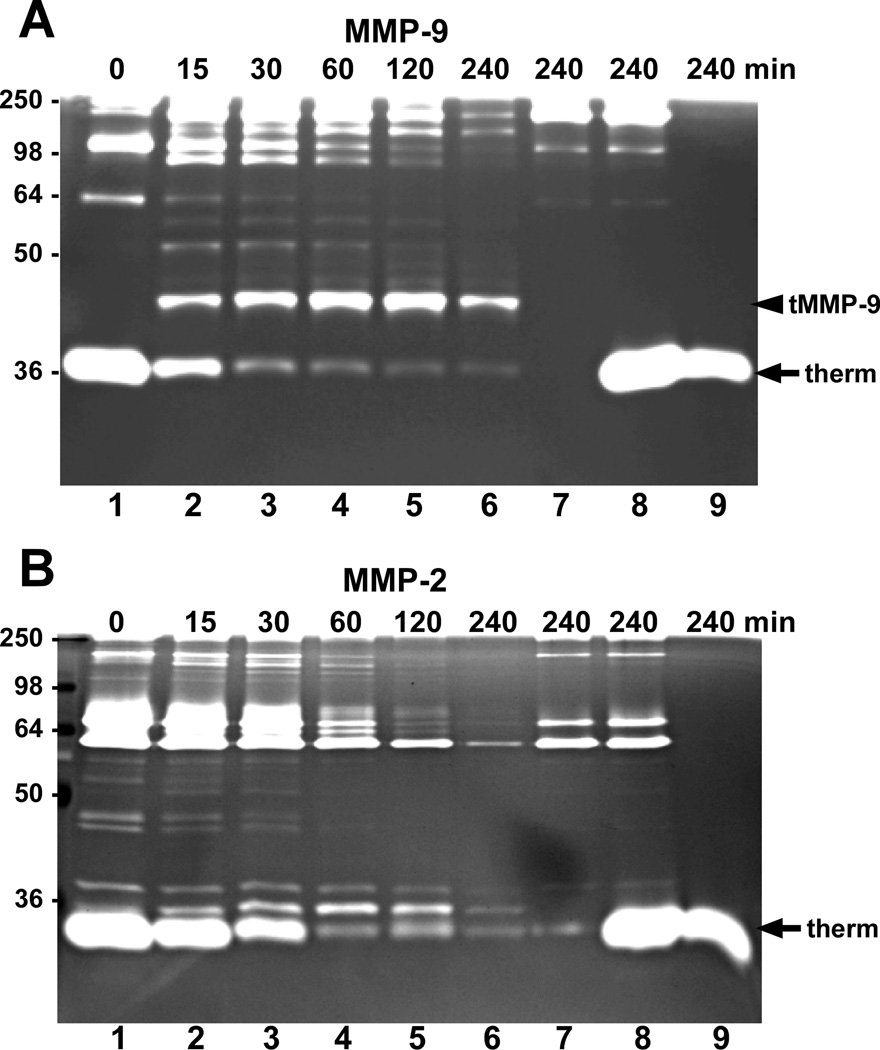
Since MMPs -2 and -9 and KLK7 have been observed to be overexpressed in various cancers, we examined the ability of KLK7 to activate these MMPs. Upon incubation, recombinant proMMP-9 was cleaved by KLK7 in a time-dependent manner giving rise to a distinct truncated, proteolytically active product as visualized by gelatin zymography (Fig.1A). By 4 hours, the full-length 92-kDa proMMP-9 was almost entirely cleaved by KLK7 resulting in a low molecular weight gelatinase activity (Fig.1A, arrowhead labeled “tMMP-9”). To confirm that the gelatinolytic activity resulted from MMP-9 activated by KLK7 and not by the thermolysin used to activate KLK7, proMMP-9 was incubated without and with thermolysin in the presence of EDTA (Fig. 1A, lanes 7 and 8, respectively). Although thermolysin exhibited significant gelatinase activity (Fig. 1A, arrow labeled “therm”), it did not produce the truncated, active MMP-9 band observed in the presence of KLK7. Similarly, gelatin zymography of thermolysin-activated KLK7 only elicited gelatinolytic activity from the thermolysin and not KLK7 (Fig. 1A, lane 9). To inhibit thermolysin activity, EDTA was included in the reactions during the incubation period; however, thermolysin activity was restored following electrophoresis as indicated by its robust gelatinolytic activity in the control reactions. To rule out the possibility that EDTA in the reactions could have influenced the conformation of MMP-9 leading to this unique activation with KLK7, we tested MMP-9 activation using plasmin-activated KLK7. Gelatin zymography of the reaction products revealed that a similar truncated, gelatinolytically-active MMP-9 fragment was produced with either plasmin-activated KLK7 or thermolysin-activated KLK7 (data not shown); thus, eliminating the notion that EDTA influences the activation of proMMP-9 by KLK7. In contrast to the activation of MMP-9 by KLK7, incubation of proMMP-2 with KLK7 resulted in a time-dependent degradation of MMP-2 gelatinase activity without yielding any distinct major gelatinolytic fragments (Fig. 1B). Thus, among these MMPs, activation by KLK7 appears to be specific for MMP-9.
Fig. 1.
ProMMP-9 but not proMMP-2 is activated by KLK7. (A) Recombinant proMMP-9 (1 pmol) or (B) proMMP-2 was incubated with 0.2 pmol of thermolysin-activated KLK7 at 37°C for the indicated times (lanes 1–6) in activity buffer containing 50 mM EDTA and the products were separated by SDS-PAGE and visualized by gelatin zymography. As controls, 1 pmol of the corresponding proMMP was incubated without (lane 7) or with thermolysin (lane 8) in the presence of EDTA (to inhibit thermolysin activity) for 4 hours, or 0.2 pmol of thermolysin-activated KLK7 was incubated for 4 hours (lane 9) prior to gel analysis. The predominant proteolytically active truncated MMP-9 (tMMP-9) fragment produced by KLK7 is denoted with arrowheads and gelatinolytic activity corresponding to thermolysin (therm) is indicated by the arrows. Sizes of the protein markers (kDa) are indicated on the left.
To determine the site of cleavage by KLK7 to produce the truncated MMP-9, proMMP-9 was incubated with KLK7 and the reaction products were loaded in two lanes of a 14% SDS-PAGE gel. Following electrophoresis, the two lanes were divided and one was stained with Coomassie blue to visualize the reaction products (Fig. 2) while the other was transferred to PVDF membrane and stained with Ponceau S. The major band observed, corresponding to the truncated MMP-9 band detected by Coomassie blue (Fig. 2, arrowhead labeled “tMMP-9”), was excised and submitted for N-terminal sequence analysis. Five cycles of Edman degradation performed on the KLK7-cleaved MMP-9 yielded the sequence(s) T/[G]-P-R-P-E (amino acid in brackets reported with reasonable confidence). The N-terminal sequence G-P-R-P-E was verified in a second preparation, however, no sequence data were obtained for the 51-kDa catalytic domain in either preparation, presumably because its N-terminus is blocked. Inspection of the proMMP-9 sequence revealed the sequence G-P-R-P-E (residues 444–448, Fig. 3A) located between the consensus HEFGHALGLDH catalytic domain motif and hemopexin-like domains (HP) in the C-terminus. This sequence is preceded by a tyrosine residue, the most favored P1 residue of KLK7 as determined by substrate specificity profiling [35], supporting the notion that this represents a KLK7 cleavage site.
Fig. 2.
N-terminal sequence analysis. Recombinant proMMP-9 was incubated with activated KLK7 and the reaction products were fractionated on a 14% Tris-glycine gel. Following electrophoresis the gel was divided and the reaction products in one portion were visualized by Coomassie blue stain. Sizes of the protein markers (kDa) are indicated on the left. The other gel portion was transferred to PVDF membrane, stained with Ponceau S and the membrane containing the major truncated MMP-9 (tMMP-9) product (corresponding to the band visualized by Coomassie blue) was excised and submitted for N-terminal sequence analysis yielding the sequence GPRPE.
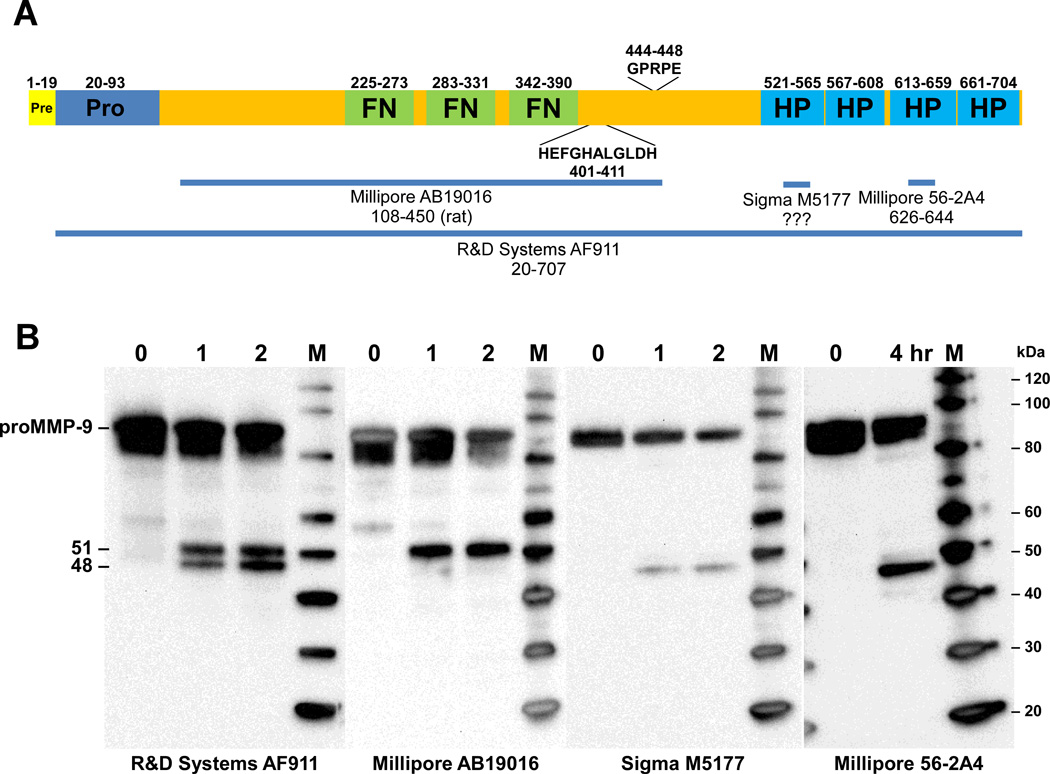
Fig. 3.
Western blot analysis of proMMP-9 cleavage products. (A) Schematic diagram of preproMMP-9 depicting the locations of the fibronectin (FN), zinc-binding catalytic (HEFGHALGLDH), and hemopexin (HP) domains. The MMP-9 immunogens used to generate the MMP-9 antibodies used for western analysis are indicated by the horizontal lines (B) Recombinant proMMP-9 was incubated with activated KLK7 for the indicated times and the reaction products were loaded in multiple lanes of 4–12% Bis-Tris gels. Following electrophoresis, the products were transferred to PVDF membranes and detected with the indicated MMP-9 antibodies. MagicMarkXP (Invitrogen) protein standards (M) were used to determine the size of the MMP-9 products. Sizes of the protein markers (kDa) are indicated on the right.
It is also noteworthy that catalytically active recombinant forms of MMP-9 have been produced that were truncated following the protease domain at almost the same site (GPRPE) cleaved by KLK7 [36–37]. Intriguingly, the mobility of the catalytically active portion of MMP-9 produced by KLK7 identified by gelatin zymography is very similar to that observed for truncated murine MMP-9 [36].
To further characterize the MMP-9 cleavage products produced by KLK7, proMMP-9 was incubated with KLK7 and the reaction products were loaded in multiple lanes of 4–12% Bis-Tris gels. Following transfer to PVDF membranes the cleavage of proMMP-9 by KLK7 was assessed by western blot analysis using various MMP-9 antibodies (Fig. 3B). Using an antibody (AF911, R&D Systems) generated against recombinant proMMP-9, two proteolytic products were detected corresponding to 51 and 48 kDa. Immunoblots incubated with an antibody targeting the catalytic domain of MMP-9 (AB19016, Millipore) revealed that the 51-kDa band represents the novel truncated MMP-9 fragment observed by gelatin zymography (Fig. 1A, arrowhead labeled “tMMP-9”). Consistent with this finding, antibodies produced using peptide immunogens derived from the C-terminus of MMP-9 detected the 48-kDa band, which represents the C-terminal portion of MMP-9 containing the hemopexin domains. This C-terminal fragment would be produced by cleavage between Tyr443 and Gly444 yielding the N-terminal sequence GPRPE that was identified by Edman sequencing. Due to their similar size, the 51- and 48-kDa MMP-9 fragments were only resolved using 4–12% gradient Bis-Tris gels (Fig. 3B) and not when the reaction products were fractionation using a 14% Tris-glycine gel (Fig. 2).
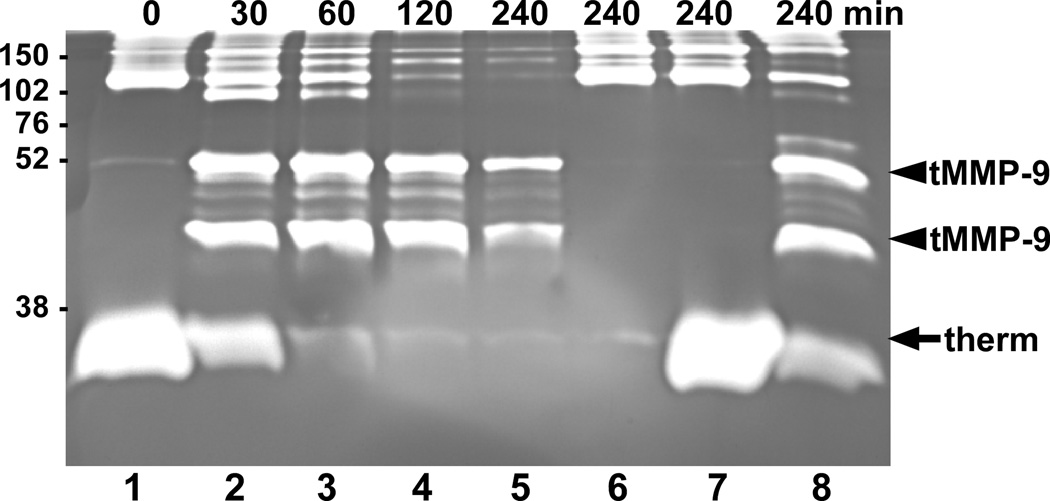
To determine whether KLK7 can activate proMMP-9 in the context of other secreted cellular products, conditioned medium was prepared from MDA-MMP-9 cells, which are MDA-MB-231 cells, derived from a breast carcinoma, transfected to express high levels of MMP-9 [11], but do not express KLK7 [38]. Upon incubation with KLK7, proMMP-9 in the conditioned medium was efficiently cleaved by KLK7 to yield a 51-kDa truncated gelatinolytic fragment (Fig. 4, arrowhead), which is similar to that observed with purified, recombinant MMP-9 (Fig. 1A). Interestingly, an additional gelatinolytically-active MMP-9 band was also seen in experiments using the MDA-MMP-9 conditioned medium. Both fragments were also produced in a control reaction when recombinant proMMP-9 was incubated with KLK7 in SFM rather than KLK7 activity buffer (Fig. 4, lane 8, arrowheads), suggesting that an additional site within MMP-9 becomes susceptible to cleavage by KLK7 in these buffer conditions. Neither the conditioned medium alone nor conditioned medium with thermolysin produced these active MMP-9 products (Fig. 4, lanes 6 and 7, respectively).
Fig. 4.
KLK7 activates proMMP-9 secreted by MDA-MMP-9 cells. Conditioned medium from MDA-MMP-9 cells was incubated at 37°C with 100 ng of thermolysin-activated KLK7 in the presence of 50 mM EDTA for the indicated times (lanes 1–5). As controls, conditioned medium was incubated without (lane 6) or with thermolysin (lane 7) under similar conditions for 4 hours. As a positive control, 1 pmol of proMMP-9 was incubated with 0.2 pmol thermolysin-activated KLK7 (lane 8) in SFM containing EDTA for 4 hrs. At the end of the incubation period the products were analyzed by gelatin zymography. The truncated MMP-9 (tMMP-9) gelatinolytic products produced by KLK7 are denoted by the arrowheads and thermolysin-directed proteolysis (therm) is indicated by the arrow. Sizes of the protein markers (kDa) are indicated on the left.
To identify soluble factors in conditioned media that may regulate KLK7-mediated activation of MMP-9, MDA-MB-231 cells were employed for further in vitro activation studies. MDA-MD-231 cells, the parental cells transfected to produce high levels of MMP-9, did not exhibit detectable levels of MMP-9 as measured by gelatin zymography (Fig. 5A, lane 1). These cells should possess the same cellular factors regulating activation of MMP-9 as the MDA-MMP-9 cells and thus provide a context for manipulating the exogenous levels of both KLK7 and MMP-9. Interestingly, in the presence of MDA-MB-231 conditioned medium KLK7 did not activate recombinant proMMP-9 in a fashion similar to that observed with SFM (Fig. 5A, compare lanes 4 and 5). Incubation with active KLK7 produced only low levels of a ~51-kDa fragment (Fig. 5A, lane 4). These results indicate that there is a factor in the conditioned medium that regulates KLK7 activation of proMMP-9.
Fig. 5.
TIMP-1 in MDA-MB-231 conditioned medium regulates KLK7 activation of proMMP-9. (A) MDA-MB-231 conditioned medium containing 50 mM EDTA was incubated at 37°C for 4 hours with no additions (lane 1), with 1 pmol proMMP-9 (lane 2), with 1 pmol proMMP-9 and thermolysin (lane 3), or with 1 pmol proMMP-9 and 0.2 pmol thermolysin-activated KLK7 (lane 4). As a positive control, 1 pmol proMMP-9 was incubated with 0.2 pmol thermolysin-activated KLK7 in SFM containing EDTA at 37°C for 4 hours (lane 5). At the end of the incubation period, the samples were analyzed by gelatin zymography. MDA-MB-231 conditioned medium was immunodepleted with a TIMP-1 antibody (B) or control goat IgG (C). The immunodepleted conditioned medium was incubated with the additions indicated in (A) at 37°C for 4 hours prior to analysis by gelatin zymography (upper panels) or western blot for TIMP-1 (lower panels). As a positive control, 1 pmol proMMP-9 was incubated with 0.2 pmol thermolysin-activated KLK7 in SFM containing EDTA at 37°C for 4 hours (lanes 5). The truncated MMP-9 (tMMP-9) gelatinolytic products are denoted by the arrowheads and thermolysin-directed proteolysis (therm) is indicated by the arrow. Sizes of the protein markers (kDa) are indicated on the left.
One of the secreted factors that has been studied extensively in regulating the activation, as well as activity, of MMPs is TIMP-1 (tissue inhibitor of metalloproteinase-1) [39]. To test the role of TIMP-1 in KLK7-mediated activation of proMMP-9, proMMP-9 was incubated with KLK7 in MDA-MB-231 conditioned medium with (Fig. 5B) and without (Fig. 5C) immunodepletion of TIMP-1. Immunodepletion of TIMP-1 from MDA-MB-231 conditioned medium resulted in the formation of both truncated MMP-9 products (Fig. 5B, lane 4, arrowheads), similar to the gelatinolytic bands produced with SFM (Fig. 5B, lane 5). Parallel reactions performed with MDA-MB-231 conditioned medium immunodepleted with normal goat IgG exhibited almost complete attenuation of MMP-9 activation by KLK7 (compare Fig. 5B, lane 4 and Fig. 5C, lane 4). The removal of TIMP-1 from the conditioned medium (Fig. 5B, lower panel) and its retention with control immunoglobulins (Fig. 5C, lower panel) was verified by western blot. These results clearly show that TIMP-1 can regulate KLK7-mediated cleavage of MMP-9.
4. Discussion
Proteases, including matrix metalloproteinases, play a key role in the progression of many disease conditions including cancer. The members of this family – including the collagenases, stromelysins, gelatinases, and membrane-type metalloproteases – are zinc-containing, neutral endopeptidases, comprised of multiple domains that are characterized by their ability to degrade various extracellular proteins. Both the expression and the activity of MMPs are regulated and controlled at different levels [40]. Matrix metalloproteinases, like MMP-9, have been found to be expressed by various normal cell types and also by certain cancer cells [41–43]. Previous studies have shown that MMP-9 is a key player in physiological processes in both normal and disease conditions. Higher MMP-9 expression has also been observed in many different types of cancer [21–23] in which both microenvironment- [44–45] and tumor-derived MMP-9 [46] have been shown to influence tumor cell invasion, metastasis [47–48], intravasation [49], and angiogenesis [1, 50].
One of the key mechanisms leading to activation of proteases, in both normal and diseased conditions, is via proteolytic cascades involving the activation of one protease leading to further activation of proteases from same and other protease families. Such proteolytic activation cascades are known to play an important role in processes such as extracellular matrix degradation, which leads to tumor invasion [51] and progression of many different neoplastic conditions [52]. Most of the MMPs are secreted as latent zymogens and are activated by the proteolytic removal of the propeptide domain [10]. Previous studies have shown that many proteases, such as trypsin [53], plasmin [54–55], and chymase [56] as well as other MMPs (e.g., MMP-3 [57], MMP-7 [58]), can activate proMMP-9 in vitro.
Kallikrein-related peptidase 7, a chymotryptic-like serine protease, was initially purified and characterized from human skin extracts and is thought to play a role in desquamation of human skin [59–60], however, increasingly the KLK7 transcript and/or KLK7 protein has been found to be overexpressed in human cancers. Previously, we reported that KLK7 is overexpressed in pancreatic adenocarcinomas [26]. Other laboratories have also demonstrated it is overexpressed in ovarian [29–31, 61], squamous cervical [28], and breast cancers [27]. Interestingly, in many of these cancers there is a parallel in the overexpression of KLK7 and MMPs, such as MMP-9 and MMP-2 [62–64]. Based on these observations, we sought to determine whether KLK7 could activate proMMP-9 and proMMP-2.
Using a series of in vitro experiments we found that KLK7 was able to cleave proMMP-9 to produce a novel truncated fragment with gelatinolytic activity in a time-dependent manner. Activation of the 92-kDa proMMP-9 by other proteases typically yields an 83-kDa active protease. Using a fivefold molar excess of substrate (proMMP-9) to enzyme (KLK7), KLK7-cleaved MMP-9 products exhibited robust gelatinolytic activity that was clearly evident within 15 minutes and the proMMP-9 was completely activated by 4 hours. This rapid activation of proMMP-9 by KLK7 is reminiscent of the activation reported for MMP-3 using similar reaction conditions [57] and appears much more efficient than activation by trypsin [53]. Furthermore, MMP-3 has been demonstrated to generate an active 82-kDa form of MMP-9 that is subsequently converted into a major gelatinase with a mass of 35 kDa [65]. Intriguingly, in a study using a transgenic mouse model of pancreatic islet carcinogenesis, an unidentified 43-kDa gelatinolytic activity was observed during the angiogenic switch triggered by MMP-9 [66], similar in size to the novel gelatinolytic fragments observed in this study. Although no direct conclusions can be made about the involvement of KLK7 in the production of this low-molecular weight gelatinolytic activity, the ability of KLK7 to produce similar gelatinolytic activities from MMP-9 in vitro warrants further investigation into this potential link.
In mixtures of gelatinases, the presence and activity MMP-9 and MMP-2 is often based solely on the size of the proteolytic products observed by gelatin zymography, with higher molecular weight products assigned as MMP-9 and lower molecular weight products designated as MMP-2. In view of the low-molecular weight proteolytically active MMP-9 produced by KLK7, identification of these gelatinases should likely include additional methodologies.
To determine whether the activation of proMMP-9 by KLK7 was specific to this gelatinase, we also tested the ability of KLK7 to activate proMMP-2. In contrast to the vigorous activation of MMP-9, no activation of proMMP-2 was observed upon incubation with KLK7 under similar reaction conditions. These results clearly show KLK7-dependent activation is specific for MMP-9, which is similar to the cleavage profiles observed for other proteolytic MMP activators.
MDA-MMP-9 cells have been used previously in studying activators of MMP-9 [11]. The level of proMMP-9 is much higher than TIMP-1 in these cells and they do not have detectable levels of MMP-2 [11] or KLK7 [38]; thus, this cell line provides a good cell-based system to confirm the activation of proMMP-9 by KLK7. When active KLK7 was incubated with conditioned medium from these cells, a truncated, active fragment was produced similar to that observed using purified components. Interestingly, a second novel gelatinolytic band was also detected. A similar pattern of active MMP-9 products was also observed when purified proMMP-9 was incubated with KLK7 in SFM. The rapid activation of MMP-9 by KLK7 in these experimental settings, suggests that KLK7 may play an important role in the activation of MMP-9 in tissues, where both MMP-9 and KLK7 have been reported to be overexpressed, such as pancreatic cancer.
To identify cellular factors that may regulate KLK7-activation of MMP-9, we employed MDA-MB-231 cells, the parental cell line used to generate the MMP-9-expressing cells. Incubation of proMMP-9 and KLK7 in MDA-MB-231 conditioned medium failed to produce the truncated active MMP-9 band and generated only very low levels of the second truncated fragment, observed with MDA-MMP-9 conditioned medium, suggesting the presence of an inhibitory factor. Since TIMP-1 is known to influence both the activation and activity of MMP-9, the MDA-MB-231 conditioned medium was immunodepleted specifically for TIMP-1 and KLK7-dependent MMP-9 activation was restored. The interaction of TIMP-1 with MMP-9 may prevent its dimerization and activation. Thus, though KLK7 is a novel activator of proMMP-9, the activation is attenuated by TIMP-1 similar to other proteases, suggesting that the balance between proMMP-9 and TIMP-1 cannot be circumvented by KLK7.
Interestingly, the possible link between activation of MMP-9 and a chymotryptic-like protease has been previously reported. In human skin, TNF-α-induced proteolytic activation of MMP-9 was found to be mediated by a chymotryptic-like serine protease and controlled by TIMP-1 [67]. Since KLK7 is a chymotrypsin-like protease predominantly expressed in human skin, it presents an intriguing possibility that KLK7 is responsible for the MMP-9 activation observed in this study.
We further used plasmin-activated KLK7 for testing KLK7-mediated MMP-9 activation to ensure that EDTA included to inhibit thermolysin did not alter the conformation of proMMP-9, thereby exposing sites that are usually not accessible for proteolytic activation of MMP-9 under physiological conditions. Additionally, plasmin activation of KLK7 clearly points out to the possibility of a novel proteolytic activation cascade (plasmin→KLK7→MMP-9) in pathological states where all three protease are expressed.
Though a clear role for the novel truncated MMP-9 we have observed is not known, it is possible that in neoplastic conditions these fragments could have possible pro-tumorigenic effects similar to those ascribed to the 83-kDa form of MMP-9 produced through the actions of other proteases. Most importantly this catalytically active fragment also lacks the C-terminal hemopexin domain, which is thought to mediate protein-protein interactions and to contribute to substrate specificity; thus, this truncated MMP may engage additional substrates not targeted by full-length MMP-9.
In conclusion, the results of this study clearly indicate that unique low-molecular weight active MMP-9 fragments can be produced through the proteolytic action of KLK7 and suggests a novel role of KLK7 during its aberrant expression in human cancers, supporting the notion that it may play a significant part in tumor development and progression. Our present study highlights the need for understanding further the role of other protease families, like KLKs, in activating MMPs and the importance of taking into consideration such proteolytically active fragments while designing new therapies.
Highlights.
-
>
KLK7 and MMP-9 are expressed in pancreatic, breast, cervical, and ovarian cancers.
-
>
KLK7 cleaves proMMP-9 to produce a unique truncated, active gelatinase.
-
>
Active MMP-9 lacking the hemopexin domains may have altered substrate specificities.
Acknowledgments
We greatly appreciate the gift of MDA-MMP-9 (E10) cells from Dr. James Quigley (Scripps Research Institute, La Jolla, CA) and helpful discussion regarding activation of KLK7 with plasmin. We would also like to thank Drs. Tom Kelly (University of Arkansas for Medical Sciences, Little Rock, AR) for critically reading this manuscript and John Neveu (Harvard University) for N-terminal sequence analysis.
This work was supported in part by American Cancer Society grant RSG-02-142-01-CNE (to R.S.H.) and NIH/NIDDK grant DK081690 (to G.K.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 2.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 3.Kleiner DE, Stetler-Stevenson WG. Matrix metalloproteinases and metastasis. Cancer Chemother. Pharmacol. 1999;43 Suppl:S42–S51. doi: 10.1007/s002800051097. [DOI] [PubMed] [Google Scholar]
- 4.Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- 5.Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noe V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, Bruyneel E, Matrisian LM, Mareel M. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J. Cell Sci. 2001;114:111–118. doi: 10.1242/jcs.114.1.111. [DOI] [PubMed] [Google Scholar]
- 7.Fingleton B, Vargo-Gogola T, Crawford HC, Matrisian LM. Matrilysin [MMP-7] expression selects for cells with reduced sensitivity to apoptosis. Neoplasia. 2001;3:459–468. doi: 10.1038/sj.neo.7900190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Handsley MM, Edwards DR. Metalloproteinases and their inhibitors in tumor angiogenesis. Int. J. Cancer. 2005;115:849–860. doi: 10.1002/ijc.20945. [DOI] [PubMed] [Google Scholar]
- 9.Springman EB, Angleton EL, Birkedal-Hansen H, Van Wart HE. Multiple modes of activation of latent human fibroblast collagenase: evidence for the role of a Cys73 active-site zinc complex in latency and a "cysteine switch" mechanism for activation. Proc. Natl. Acad. Sci. U. S. A. 1990;87:364–368. doi: 10.1073/pnas.87.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Wart HE, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc. Natl. Acad. Sci. U. S. A. 1990;87:5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramos-DeSimone N, Hahn-Dantona E, Sipley J, Nagase H, French DL, Quigley JP. Activation of matrix metalloproteinase-9 (MMP-9) via a converging plasmin/stromelysin-1 cascade enhances tumor cell invasion. J. Biol. Chem. 1999;274:13066–13076. doi: 10.1074/jbc.274.19.13066. [DOI] [PubMed] [Google Scholar]
- 12.He C, Wilhelm SM, Pentland AP, Marmer BL, Grant GA, Eisen AZ, Goldberg GI. Tissue Cooperation in a Proteolytic Cascade Activating Human Interstitial Collagenase. Proc. Natl. Acad. Sci. U. S. A. 1989;86:2632–2636. doi: 10.1073/pnas.86.8.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gress TM, Müller-Pillasch F, Lerch MM, Friess H, Büchler M, Adler G. Expression and In-situ Localization of Genes Coding for Extracellular Matrix Proteins and Extracellular Matrix Degrading Proteases in Pancreatic Cancer. Int. J. Cancer. 1995;62:407–413. doi: 10.1002/ijc.2910620409. [DOI] [PubMed] [Google Scholar]
- 14.Bramhall SR, Neoptolemos JP, Stamp GWH, Lemoine NR. Imbalance of Expression of Matrix Metalloproteinases (MMPs) and Tissue Inhibitors of the Matrix Metalloproteinases (TIMPs) in Human Pancreatic Carcinoma. J. Pathology. 1997;182:347–355. doi: 10.1002/(SICI)1096-9896(199707)182:3<347::AID-PATH848>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 15.Koshiba T, Hosotani R, Wada M, Miyamoto Y, Fujimoto K, Lee JU, Doi R, Arii S, Imamura M. Involvement of matrix metalloproteinase-2 activity in invasion and metastasis of pancreatic carcinoma. Cancer. 1998;82:642–650. doi: 10.1002/(sici)1097-0142(19980215)82:4<642::aid-cncr5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 16.Kuniyasu H, Ellis LM, Evans DB, Abbruzzese JL, Fenoglio CJ, Bucana CD, Cleary KR, Tahara E, Fidler IJ. Relative expression of E-cadherin and type IV collagenase genes predicts disease outcome in patients with resectable pancreatic carcinoma. Clin. Cancer Res. 1999;5:25–33. [PubMed] [Google Scholar]
- 17.Gong YL, Xu GM, Huang WD, Chen LB. Expression of matrix metalloproteinases and the tissue inhibitors of metalloproteinases and their local invasiveness and metastasis in Chinese human pancreatic cancer. J. Surg. Oncol. 2000;73:95–99. doi: 10.1002/(sici)1096-9098(200002)73:2<95::aid-jso7>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 18.Ellenrieder V, Alber B, Lacher U, Hendler SF, Menke A, Boeck W, Wagner M, Wilda M, Friess H, Büchler M, Adler G, Gress TM. Role of MT-MMPs and MMP-2 in Pancreatic Cancer Progression. Int. J. Cancer. 2000;85:14–20. doi: 10.1002/(sici)1097-0215(20000101)85:1<14::aid-ijc3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto H, Itoh F, Iku S, Adachi Y, Fukushima H, Sasaki S, Mukaiya M, Hirata K, Imai K. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human pancreatic adenocarcinomas: clinicopathologic and prognostic significance of matrilysin expression. J. Clin. Oncol. 2001;19:1118–1127. doi: 10.1200/JCO.2001.19.4.1118. [DOI] [PubMed] [Google Scholar]
- 20.Harvey SR, Hurd TC, Markus G, Martinick MI, Penetrante RM, Tan D, Venkataraman P, DeSouza N, Sait SN, Driscoll DL, Gibbs JF. Evaluation of urinary plasminogen activator, its receptor, matrix metalloproteinase-9, and von Willebrand factor in pancreatic cancer. Clin. Cancer Res. 2003;9:4935–4943. [PubMed] [Google Scholar]
- 21.Pellikainen JM, Ropponen KM, Kataja VV, Kellokoski JK, Eskelinen MJ, Kosma VM. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special reference to activator protein-2, HER2, and prognosis. Clin. Cancer Res. 2004;10:7621–7628. doi: 10.1158/1078-0432.CCR-04-1061. [DOI] [PubMed] [Google Scholar]
- 22.Asha Nair S, Karunagaran D, Nair MB, Sudhakaran PR. Changes in matrix metalloproteinases and their endogenous inhibitors during tumor progression in the uterine cervix. J. Cancer Res. Clin. Oncol. 2003;129:123–131. doi: 10.1007/s00432-002-0411-9. [DOI] [PubMed] [Google Scholar]
- 23.Schmalfeldt B, Prechtel D, Harting K, Spathe K, Rutke S, Konik E, Fridman R, Berger U, Schmitt M, Kuhn W, Lengyel E. Increased expression of matrix metalloproteinases (MMP)-2, MMP-9, and the urokinase-type plasminogen activator is associated with progression from benign to advanced ovarian cancer. Clin. Cancer Res. 2001;7:2396–2404. [PubMed] [Google Scholar]
- 24.Borgono CA, Michael IP, Diamandis EP. Human tissue kallikreins: physiologic roles and applications in cancer. Mol. Cancer Res. 2004;2:257–280. [PubMed] [Google Scholar]
- 25.Emami N, Diamandis EP. Utility of kallikrein-related peptidases (KLKs) as cancer biomarkers. Clin. Chem. 2008;54:1600–1607. doi: 10.1373/clinchem.2008.105189. [DOI] [PubMed] [Google Scholar]
- 26.Johnson SK, Ramani VC, Hennings L, Haun RS. Kallikrein 7 enhances pancreatic cancer cell invasion by shedding E-cadherin. Cancer. 2007;109:1811–1820. doi: 10.1002/cncr.22606. [DOI] [PubMed] [Google Scholar]
- 27.Talieri M, Diamandis EP, Gourgiotis D, Mathioudaki K, Scorilas A. Expression analysis of the human kallikrein 7 (KLK7) in breast tumors: a new potential biomarker for prognosis of breast carcinoma. Thromb. Haemost. 2004;91:180–186. doi: 10.1160/TH03-05-0261. [DOI] [PubMed] [Google Scholar]
- 28.Santin AD, Cane S, Bellone S, Bignotti E, Palmieri M, De Las Casas LE, Roman JJ, Anfossi S, O'Brien T, Pecorelli S. The serine protease stratum corneum chymotryptic enzyme (kallikrein 7) is highly overexpressed in squamous cervical cancer cells. Gynecol. Oncol. 2004;94:283–288. doi: 10.1016/j.ygyno.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 29.Tanimoto H, Underwood LJ, Shigemasa K, Yan Yan MS, Clarke J, Parmley TH, O'Brien TJ. The stratum corneum chymotryptic enzyme that mediates shedding and desquamation of skin cells is highly overexpressed in ovarian tumor cells. Cancer. 1999;86:2074–2082. [PubMed] [Google Scholar]
- 30.Dong Y, Kaushal A, Brattsand M, Nicklin J, Clements JA. Differential Splicing of KLK5 and KLK7 in Epithelial Ovarian Cancer Produces Novel Variants with Potential as Cancer Biomarkers. Clin. Cancer Res. 2003;9:1710–1720. [PubMed] [Google Scholar]
- 31.Yousef GM, Polymeris ME, Yacoub GM, Scorilas A, Soosaipillai A, Popalis C, Fracchioli S, Katsaros D, Diamandis EP. Parallel overexpression of seven kallikrein genes in ovarian cancer. Cancer Res. 2003;63:2223–2227. [PubMed] [Google Scholar]
- 32.Emami N, Diamandis EP. New insights into the functional mechanisms and clinical applications of the kallikrein-related peptidase family. Mol. Oncol. 2007;1:269–287. doi: 10.1016/j.molonc.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paliouras M, Borgono C, Diamandis EP. Human tissue kallikreins: the cancer biomarker family. Cancer Lett. 2007;249:61–79. doi: 10.1016/j.canlet.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 34.Ramani VC, Haun RS. The extracellular matrix protein fibronectin is a substrate for kallikrein 7. Biochem. Biophys. Res. Commun. 2008;369:1169–1173. doi: 10.1016/j.bbrc.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 35.Debela M, Magdolen V, Schechter N, Valachova M, Lottspeich F, Craik CS, Choe Y, Bode W, Goettig P. Specificity profiling of seven human tissue kallikreins reveals individual subsite preferences. J. Biol. Chem. 2006;281:25678–25688. doi: 10.1074/jbc.M602372200. [DOI] [PubMed] [Google Scholar]
- 36.Rasch MG, Lund IK, Illemann M, Hoyer-Hansen G, Gardsvoll H. Purification and characterization of recombinant full-length and protease domain of murine MMP-9 expressed in Drosophila S2 cells. Protein Expr. Purif. 2010;72:87–94. doi: 10.1016/j.pep.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Sadatmansoori S, MacDougall J, Khademi S, Cooke LS, Guarino L, Meyer EF, Forough R. Construction, expression, and characterization of a baculovirally expressed catalytic domain of human matrix metalloproteinase-9. Protein Expr. Purif. 2001;23:447–452. doi: 10.1006/prep.2001.1542. [DOI] [PubMed] [Google Scholar]
- 38.Paliouras M, Diamandis EP. Coordinated steroid hormone-dependent and independent expression of multiple kallikreins in breast cancer cell lines. Breast Cancer Res. Treat. 2007;102:7–18. doi: 10.1007/s10549-006-9312-y. [DOI] [PubMed] [Google Scholar]
- 39.Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur. J. Cell Biol. 1997;74:111–122. [PubMed] [Google Scholar]
- 40.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heppner KJ, Matrisian LM, Jensen RA, Rodgers WH. Expression of most matrix metalloproteinase family members in breast cancer represents a tumor-induced host response. Am. J. Pathol. 1996;149:273–282. [PMC free article] [PubMed] [Google Scholar]
- 42.Hibbs MS, Hasty KA, Seyer JM, Kang AH, Mainardi CL. Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J. Biol. Chem. 1985;260:2493–2500. [PubMed] [Google Scholar]
- 43.Wilhelm SM, Collier IE, Marmer BL, Eisen AZ, Grant GA, Goldberg GI. SV40-transformed human lung fibroblasts secrete a 92-kDa type IV collagenase which is identical to that secreted by normal human macrophages. J. Biol. Chem. 1989;264:17213–17221. [PubMed] [Google Scholar]
- 44.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Itoh T, Tanioka M, Matsuda H, Nishimoto H, Yoshioka T, Suzuki R, Uehira M. Experimental metastasis is suppressed in MMP-9-deficient mice. Clin. Exp. Metastasis. 1999;17:177–181. doi: 10.1023/a:1006603723759. [DOI] [PubMed] [Google Scholar]
- 46.Himelstein BP, Canete-Soler R, Bernhard EJ, Dilks DW, Muschel RJ. Metalloproteinases in tumor progression: the contribution of MMP-9. Invasion Metastasis. 1994;14:246–258. [PubMed] [Google Scholar]
- 47.Bernhard EJ, Gruber SB, Muschel RJ. Direct evidence linking expression of matrix metalloproteinase 9 (92-kDa gelatinase/collagenase) to the metastatic phenotype in transformed rat embryo cells. Proc. Natl. Acad. Sci. U. S. A. 1994;91:4293–4297. doi: 10.1073/pnas.91.10.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hua J, Muschel RJ. Inhibition of matrix metalloproteinase 9 expression by a ribozyme blocks metastasis in a rat sarcoma model system. Cancer Res. 1996;56:5279–5284. [PubMed] [Google Scholar]
- 49.Kim J, Yu W, Kovalski K, Ossowski L. Requirement for specific proteases in cancer cell intravasation as revealed by a novel semiquantitative PCR-based assay. Cell. 1998;94:353–362. doi: 10.1016/s0092-8674(00)81478-6. [DOI] [PubMed] [Google Scholar]
- 50.John A, Tuszynski G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol. Oncol. Res. 2001;7:14–23. doi: 10.1007/BF03032599. [DOI] [PubMed] [Google Scholar]
- 51.Mignatti P, Rifkin DB. Biology and biochemistry of proteinases in tumor invasion. Physiol. Rev. 1993;73:161–195. doi: 10.1152/physrev.1993.73.1.161. [DOI] [PubMed] [Google Scholar]
- 52.Woodward JK, Holen I, Coleman RE, Buttle DJ. The roles of proteolytic enzymes in the development of tumour-induced bone disease in breast and prostate cancer. Bone. 2007;41:912–927. doi: 10.1016/j.bone.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 53.Ogata Y, Itoh Y, Nagase H. Steps involved in activation of the pro-matrix metalloproteinase 9 (progelatinase B)-tissue inhibitor of metalloproteinases-1 complex by 4-aminophenylmercuric acetate and proteinases. J. Biol. Chem. 1995;270:18506–18511. doi: 10.1074/jbc.270.31.18506. [DOI] [PubMed] [Google Scholar]
- 54.Goldberg GI, Strongin A, Collier IE, Genrich LT, Marmer BL. Interaction of 92-kDa type IV collagenase with the tissue inhibitor of metalloproteinases prevents dimerization, complex formation with interstitial collagenase, and activation of the proenzyme with stromelysin. J. Biol. Chem. 1992;267:4583–4591. [PubMed] [Google Scholar]
- 55.Lijnen HR, Silence J, Lemmens G, Frederix L, Collen D. Regulation of gelatinase activity in mice with targeted inactivation of components of the plasminogen/plasmin system. Thromb. Haemost. 1998;79:1171–1176. [PubMed] [Google Scholar]
- 56.Fang KC, Raymond WW, Blount JL, Caughey GH. Dog mast cell alpha-chymase activates progelatinase B by cleaving the Phe88-Gln89 and Phe91-Glu92 bonds of the catalytic domain. J. Biol. Chem. 1997;272:25628–25635. doi: 10.1074/jbc.272.41.25628. [DOI] [PubMed] [Google Scholar]
- 57.Ogata Y, Enghild JJ, Nagase H. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J. Biol. Chem. 1992;267:3581–3584. [PubMed] [Google Scholar]
- 58.von Bredow DC, Cress AE, Howard EW, Bowden GT, Nagle RB. Activation of gelatinase-tissue-inhibitors-of-metalloproteinase complexes by matrilysin. Biochem. J. 1998;331(Pt 3):965–972. doi: 10.1042/bj3310965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Egelrud T. Purification and preliminary characterization of stratum corneum chymotryptic enzyme: a proteinase that may be involved in desquamation. J. Invest. Dermatol. 1993;101:200–204. doi: 10.1111/1523-1747.ep12363804. [DOI] [PubMed] [Google Scholar]
- 60.Hansson L, Stromqvist M, Backman A, Wallbrandt P, Carlstein A, Egelrud T. Cloning, expression, and characterization of stratum corneum chymotryptic enzyme. A skin-specific human serine proteinase. J. Biol. Chem. 1994;269:19420–19426. [PubMed] [Google Scholar]
- 61.Kyriakopoulou LG, Yousef GM, Scorilas A, Katsaros D, Massobrio M, Fracchioli S, Diamandis EP. Prognostic value of quantitatively assessed KLK7 expression in ovarian cancer. Clin. Biochem. 2003;36:135–143. doi: 10.1016/s0009-9120(02)00446-0. [DOI] [PubMed] [Google Scholar]
- 62.Koshiba T, Hosotani R, Wada M, Fujimoto K, Lee JU, Doi R, Arii S, Imamura M. Detection of matrix metalloproteinase activity in human pancreatic cancer. Surg. Today. 1997;27:302–304. doi: 10.1007/BF00941802. [DOI] [PubMed] [Google Scholar]
- 63.Lebeau A, Nerlich AG, Sauer U, Lichtinghagen R, Lohrs U. Tissue distribution of major matrix metalloproteinases and their transcripts in human breast carcinomas. Anticancer Res. 1999;19:4257–4264. [PubMed] [Google Scholar]
- 64.Sakata K, Shigemasa K, Nagai N, Ohama K. Expression of matrix metalloproteinases (MMP-2, MMP-9, MT1-MMP) and their inhibitors (TIMP-1, TIMP-2) in common epithelial tumors of the ovary. Int. J. Oncol. 2000;17:673–681. [PubMed] [Google Scholar]
- 65.Ries C, Pitsch T, Mentele R, Zahler S, Egea V, Nagase H, Jochum M. Identification of a novel 82 kDa proMMP-9 species associated with the surface of leukaemic cells: (auto-) catalytic activation and resistance to inhibition by TIMP-1. Biochem. J. 2007;405:547–558. doi: 10.1042/BJ20070191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han YP, Nien YD, Garner WL. Tumor necrosis factor-alpha-induced proteolytic activation of pro-matrix metalloproteinase-9 by human skin is controlled by down-regulating tissue inhibitor of metalloproteinase-1 and mediated by tissue-associated chymotrypsin-like proteinase. J. Biol. Chem. 2002;277:27319–27327. doi: 10.1074/jbc.M202842200. [DOI] [PMC free article] [PubMed] [Google Scholar]