Abstract
The murine epidermis contains resident T cells that express a canonical γδ TCR and arise from fetal thymic precursors. These cells are termed dendritic epidermal T cells (DETC) and use a TCR that is restricted to the skin in adult animals. DETC produce low levels of cytokines and growth factors that contribute to epidermal homeostasis. Upon activation, DETC can secrete large amounts of inflammatory molecules which participate in the communication between DETC, neighboring keratinocytes and langerhans cells. Chemokines produced by DETC may recruit inflammatory cells to the epidermis. In addition, cell–cell mediated immune responses also appear important for epidermal–T cell communication. Information is provided which supports a crucial role for DETC in inflammation, wound healing, and tumor surveillance.
Keywords: γδ T cells, DETC, Homeostasis, Wound healing, Inflammation, Skin tumors
Introduction
γδ T cells with invariant or restricted TCR are preferentially located within epithelial tissues that are the points of contact between the body and the outside world. The skin is an important barrier and is under constant assault by pathogens, trauma, and UV irradiation as well as being a common site for malignancy. Thy-1+ dendritic epidermal T cells (DETC) found in murine skin express a canonical γδ TCR composed of Vγ3/Jγ1-Cγ1 and Vδ1/Dδ2/Jδ2-Cδ chains [1–3], and have not been found elsewhere in the adult mouse [4, 5]. Dendritic epidermal T cells monitor epidermal cells and are poised to recognize and respond to undefined self antigens expressed by neighboring keratinocytes after damage or disease. Once keratinocyte distress is detected, the DETC respond by the local secretion of chemokines, cytotoxic effector molecules, growth factors and cytokines that orchestrate skin inflammation, tumor killing and wound healing responses (Fig. 1). Skin-resident γδ T cells and their functions are best characterized in the mouse, and these data are the main focus of this review. However, recent results confirm that there is a population of γδ T cells that is resident in human epidermis along with the more prevalent αβ T cells. Functions of these cells and implications for immune responses by skin-resident T cells in human disease are discussed.
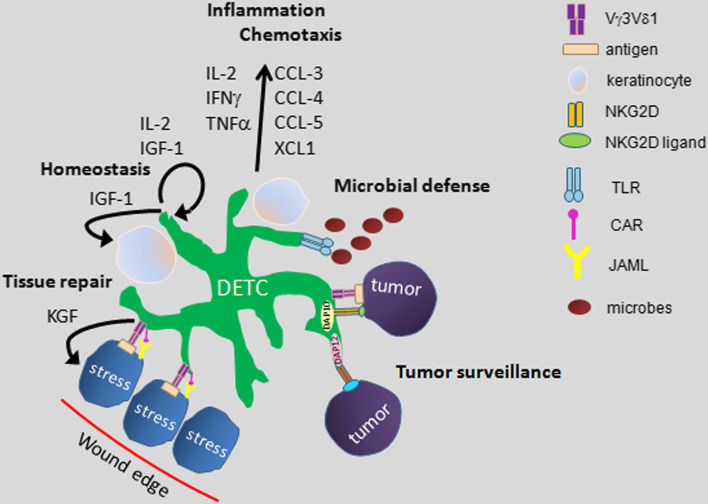
Fig. 1.
Model of functions of skin-resident γδ T Cells. Dendritic epidermal T cells (DETC) are a resident population of Vγ3 Vδ1+ T cells in murine epidermis. Here, they provide a first line of defense against infection, malignancy and injury. DETC contribute to epidermal homeostasis by constitutive production of growth factors and cytokines which promote survival of keratinocytes. Upon infection or other stress to the epidermis, DETC produce a variety of chemokines and cytokines which delicately regulate the cutaneous inflammatory response. When keratinocytes become damaged following injury, this leads to upregulation of an unknown antigen which is subsequently recognized by DETC through the TCR. Additional costimulatory signals are provided to DETC by junctional adhesion molecule-like protein (JAML) recognition of increased Coxsackie and Adenovirus receptor (CAR) expression on wounded keratinocytes. Once activated, DETC locally secrete factors such as keratinocyte growth factors which aid the wound healing response as well as release inflammatory and chemotactic factors to help recruit cells into the site of damage. DETC are also involved in tumor surveillance of cutaneous tumors such as squamous cell carcinoma through TCR and/or NKG2D signals. NKG2D ligands become upregulated on keratinocytes and recognition by DETC leads to anti-tumor effects. The DETC are thus able to provide a multi-pronged defense against disturbance of epidermal homeostasis
Localization of γδ T cells to the epidermis
TCR γ and δ genes are rearranged and expressed in a series of overlapping waves during development with cells bearing specific TCR preferentially or exclusively migrating from the thymus to specific epithelial and lymphoid sites. Although theoretically γδ T cell receptors are capable of great combinatorial diversity, in contrast to αβ T cells, γδ T cells express highly restricted or invariant TCR. Some of the features of the ontogeny, tissue tropism, and antigen receptor diversity of γδ T cells are very different from αβ T cells. DETC precursors are the first T cells to develop in the embryonic thymus. During embryonic development the TCRγ genes rearrange in order of proximity to the J with Vγ3 being expressed first followed by Vγ4 [6]. The TCR expressed by these cells is invariant with no junctional diversity due to the lack of expression of terminal deoxynucleotidyl transferase, gene accessibility, and recombination signal sequence restrictions at this stage of fetal development [7–9].
Vγ3+ thymocytes undergo maturation in the fetal thymus as shown by decreased expression of CD24 [10] and upregulation of CD122 [11]. It is unknown if antigen is expressed in the embryonic thymus or if TCR-mediated signals are required for development of DETC precursors. However, evidence suggests a role for cell-mediated selection events. Interestingly, Skint1 was recently identified as a molecule that is exclusively expressed on epithelial cells in the thymus and epidermis [12]. In mice that lack expression of Skint1, Vγ3 Vδ1 DETC precursor cells are found in the embryonic thymus but retain an immature phenotype and do not undergo maturation/selection steps that are required for thymic egress and migration to the skin [12]. These data suggest that Skint1 provides a key signal for DETC development and further study should demonstrate whether Skint1 interacts directly with the DETC TCR or other molecules on the T cell or alternately regulates expression of additional molecules that promote T cell development.
Cytokines play key roles in development of DETC precursors. DETC are absent or greatly diminished in numbers in mice that lack expression of IL-2Rβ, IL-7, IL-7R, IL-15 and IL-15R [13, 14]. Signals provided by IL-7 to IL-7R+ fetal thymocytes were shown to be essential for TCRγ gene transcription and rearrangement [14]. Critical IL-7R signaling for DETC precursor development occurs through the JAK/STAT pathway since JAK3−/− and STAT5−/− mice have decreased V-J rearrangement and lack both fetal Vγ3+ cells and DETC in adult skin [15, 16]. In contrast, IL-2 and IL-15 facilitate expansion and survival of the DETC precursors [13]. The restrictions in early fetal gene rearrangement, together with specific signals from cytokines and Skint1, provide a window during ontogeny for development of DETC that is missing in the adult.
DETC thymic precursor cells that have undergone appropriate maturation and selection events receive signals that regulate thymic egress and localization to the skin. Although this process is not completely understood, several relevant molecules have recently been identified. E and P selectin ligands are expressed on DETC in adult skin, as well as on DETC precursors in the fetal thymus [17]. Mice deficient in E or P selectin ligands show a dramatic decrease of DETC numbers in the epidermis [17]. Since CCL27 is abundantly expressed in the skin, this raises the possibility that its receptor CCR10 functions as a skin-homing receptor for DETC precursors. Vγ3+ DETC precursors develop normally in the fetal thymus of CCR10-deficient mice but are defective in migration into the skin [18]. However, the few DETC which seed the skin expand locally to reach normal, homeostatic numbers [18]. CCR10−/− DETC display abnormal morphology and are also found in the dermis in contrast to the typical epidermal restricted localization [15]. Mice deficient in the chemokine receptor CCR4 also have normal numbers of Vγ3+ fetal thymic DETC precursors [17]. However, Vγ3+ DETC are greatly diminished and the adult CCR4-deficient epidermis is predominantly populated by T cells expressing alternate γδ TCRs [17]. Further study is needed to determine how adhesion molecules, chemokine receptors and other molecules contribute to DETC homing to the epidermis as well as important issues of DETC survival and epidermal retention.
Role of epidermal γδ T cells in maintenance of epidermal homeostasis
The epidermis is ideally constructed to protect the body from outside insults and therefore utilizes multiple mechanisms to resolve local trauma and preserve tissue homeostasis. The keratinocytes have a rapid rate of turnover as they constantly differentiate and renew the epithelium. Maintenance of homeostasis requires a balance between the keratinocyte proliferation and apoptosis that follows terminal differentiation. Rates of proliferation and apoptosis are modulated in response to stress or trauma to facilitate tissue repair [19]. Epithelial diseases such as psoriasis can occur when there is dysregulation of this balance. Many factors contribute to epidermal homeostasis including the tissue-resident γδ T cells [20, 21].
Mice that lack DETC (TCRδ−/− mice) have increased epidermal apoptosis compared to wild-type mice suggesting a role for DETC in regulation of epidermal homeostasis [20, 21]. Insulin-like growth factor 1 (IGF-1) mediates epidermal development and maintenance through binding the heterotetrameric IGF-1 receptor expressed by keratinocytes [22]. DETC constitutively produce low levels of IGF-1 [21] that can prevent keratinocyte apoptosis by inducing expression of anti-apoptotic Bcl-2 family members [23]. Addition of recombinant IGF-1 or DETC to TCRδ−/− mice abrogates the abnormal apoptosis and restores epidermal homeostasis to these mice [21].
Regulation of homeostasis is critical for proper function of epithelial border tissues including the epidermis. The skin is constantly under assault through exposure to UV irradiation, infection, allergens, chemicals, and injury. As a consequence, homeostasis is interrupted. Following resolution of the trauma, skin homeostasis must be restored to maintain effective barrier and sentinel functions. Resident intraepithelial T cells including DETC may be effective targets for treatment when tissue homeostasis is dysregulated during epithelial diseases.
Role of epidermal T cells in skin inflammation
The close physical contact between DETC and keratinocytes suggests the possibility of a functional interaction. While the role played by DETC during tissue homeostasis has been extensively studied, the role of DETC-produced factors during skin inflammation is less clear. It seems likely that DETC not only respond to epidermal cytokines but also secrete factors which modulate the immunological functions of neighboring and peripheral cells. Upon cognate stimulation, DETC can secrete a variety of cytokines and chemokines which may play important roles in cutaneous inflammation and crosstalk between DETC, keratinocytes and dendritic cells.
Chemokines can selectively recruit immune cells to inflamed tissues including the skin. DETC have been analyzed for their expression of various chemokines such as CCL-3, also known as macrophage inflammatory protein (MIP1α), CCL-4 (MIP-1β), CCL5 (RANTES) and XCL1 (lymphotactin) [24, 25]. Transcript abundance is detected in low amounts under resting conditions but is rapidly upregulated upon activation. Migration of CD8+ αβ T cells in vitro was induced by activated DETC and migration could be blocked most extensively by neutralizing anti-XCL1 antibody with a more moderate effect of neutralizing additional chemokine activity [25]. This suggests that DETC may play important roles in directing migration and recruitment of specialized cells to sites of inflammation or damage. Indeed, migration of αβ T cells and macrophages to the epidermis is delayed in TCRδ−/− mice during the inflammatory phase of wound repair [26].
In addition to chemokines, activated DETC produce several known cytokines including IL-2, IL-3, granulocyte–macrophage colony stimulating factor (GM-CSF), interferon (IFN)γ, and tumor necrosis factor (TNF)α. Comparable to chemokines, these cytokines are detected at low amounts under resting conditions but expression is rapidly upregulated upon stimulation. Interestingly, TNF-β mRNA is abundant under resting conditions but is suppressed upon DETC activation. Other cytokines such as TGFβ1 are less affected by cell activation and are abundantly expressed in both resting and activated cells. Transcript abundance for other cytokines including IL-1α, IL-1β, IL-6 and IL-7, IL-10 and IL-13 are only marginally detectable [25], and intracellular cytokine staining failed to detect IL-4, IL-10, and IL-5 [25]. Various cytokines have been detected in γδ T cells, including DETC, ex vivo following infection, barrier disruption, tumor formation and other insults [27–30]. This suggests that findings from in vitro studies are relevant for in vivo situations. However, additional studies are needed to better understand distinctive subpopulations of γδ T cell-specific cytokine production in vivo.
DETC appear to be a rich source for a variety of cytokines and chemokines, which are mainly pro-inflammatory but do not exclusively fit into a clear T helper (Th) cell-based scheme of restricted cytokine production as observed for Th0,TH1, Th2, Th17, and T regulatory cells. Thus, further analyses will be helpful to gain more insight into the regulation, limitation and plasticity of DETC signature cytokines. The ability to produce many inflammatory cytokines suggests that DETC may be critically involved in cutaneous inflammatory responses.
Role of epidermal T cells in inflammatory skin diseases
In vivo, skin inflammation is caused by numerous factors, including infections, allergy, irritants, autoimmune reactions, UV and drug-induced inflammation among others. DETC may play relevant roles in these inflammatory conditions as has been suggested by results from several groups.
Localization of large numbers of γδ T cells in epithelial layers including the skin suggests that these cells form a first line of defense against invading pathogens. Indeed, mice lacking DETC (TCRδ−/−) were found to develop much larger skin lesions with higher bacterial counts and impaired neutrophil recruitment upon cutaneous infection with Staphylococcus aureus [31, 32]. DETC may also exert a protective role against Gram-negative bacteria since DETC respond to in vitro stimulation by Gram-negative bacteria by enhanced secretion of GM-CSF [33]. Importantly, bacterial stimulation apparently does not require the presence of additional cells [33], which suggests that DETC, like other γδ T cells, express innate pattern recognition receptors such as Toll-like receptors (TLR), which enable them to directly recognize microbial patterns [34]. Indeed, DETC have been reported to upregulate expression of TLR4 during cutaneous inflammation [35]. Future analyses of the abundance of TLRs and other innate pattern recognition receptors on DETC will aid in the understanding of how DETC sense microbial infections.
Much work has been performed to elucidate the role of γδ T cells in contact hypersensitivity models. Delayed-type contact hypersensitivity (CS) responses are an exemplified model of T cell-mediated immunity. Cutaneous CS consists of the afferent or initial sensitizing phase followed by the efferent or elicitation phase. The latter phase occurs when epidermal cells encounter antigens to which they have previously been exposed and is characterized in mice by localized swelling and in humans by eczema of the skin. Early publications reported that γδ T cells assist αβ T cells in adoptive transfer of contact hypersensitivity [36]. The requirement for γδ T cells appears unnecessary for the initiation of CS but is important for the systemic transfer of CS by αβ T cells. More recent studies have shown that γδ T cells are in fact required to elicit CS responses and act in an antigen-nonspecific and non-MHC-restricted manner to promote the antigen/MHC-specific αβ T cell response leading to CS [36]. However, these experiments used lymph node and splenic T cells obtained after sensitization for transferring CS and did not distinguish between peripheral and epidermis-specific Vγ3 Vδ1 cells. Additional studies from the same group further reveal that surprisingly augmented CS responses are observed following in vivo treatment with anti-Vγ3 and anti-Vδ4 antibodies. Since γδ T cell subsets were not depleted from the peripheral blood, lymphoid organs or epidermis, the authors conclude that the antibody treatment induced activation rather than depletion of γδ T cells subsets which then augmented CS responses [37–39]. These results are further supported by evidence that indeed Vγ3+ DETC are the required subset of γδ T cells to mediate αβ T cell-mediated CS responses [40, 41]. Following exposure to contact sensitizer on the skin, the absolute number of γδ T cells increases in the regional lymph nodes and accumulate at the antigen challenge site, with the vast majority being Vγ3+. These Vγ3+ cells proliferate in response to IL-7, but only poorly to IL-2 and IL-4. Furthermore, injection of mice with mAb to IL-7 inhibits accumulation of Vγ3+ cells both in the lymph nodes after skin sensitization and at the antigen-challenge site [41]. These results support the conclusion that DETC are the original source of the γδ cells found in the lymph node after skin sensitization and at the site of challenge, supporting a key role for DETC in CS responses.
Other studies on skin inflammation by Girardi et al. [42] have provided further insight into the role of skin-resident Vγ3+ T cells. TCRδ−/− mice on some genetic backgrounds (FVB and NOD) spontaneously develop localized dermatitis, whereas other strains do not (C57BL/6). The pathology of the observed dermatitis in susceptible strains requires the presence of αβ T cells since mice lacking both αβ and γδ T cells are asymptomatic. FVB and NOD TCRδ−/− strains appear to be more susceptible to allergic and irritant contact dermatitis reactions than their γδ T cell-sufficient controls, whereas no significant differences were observed in γδ T cell-sufficient or -deficient C57BL/6 mice. Adoptive transfer experiments revealed that in fact the Vγ3+ DETC are necessary and sufficient to downregulate spontaneous and irritant contact dermatitis in FVB TCRδ−/−. However, the underlying mechanisms for these results are largely unknown. Do DETC produce regulatory cytokines which dampen αβ T cells responses? Are cell–cell interactions between DETC, DC and T cells required to mediate their regulatory function? In addition, data are not provided showing whether Vγ3+ cells are also necessary and sufficient to downregulate allergic contact dermatitis in susceptible strains. Caution should be present when interpreting early publications in which FVB mice were provided by Taconic (FVBtac). A mutation in the Skint1 gene in these mice results in impaired thymic maturation selectively of Vγ3 Vδ1 DETC progenitors [12]. As a result, DETC lack the Vγ3 Vδ1 TCR, but instead express a heterogeneous mixture of γδ T cells [43].
By contrast to the conventional CS response which is induced by immunization via skin painting with sensitizing haptens, mice respond to high intravenous doses of soluble hapten by developing tolerance. Several studies have demonstrated that high dose tolerance may be associated with the development of regulatory T cells that limit the response of CS effector cells [44, 45]. Intriguingly, it could be shown that γδ T cells can mediate downregulation of both αβ and γδ CS effectors in vivo and IFN-γ production by these CS effectors in vitro [46]. These results, together with the findings that γδ T cells have the capacity to negatively regulate αβ T cell-driven allergic IgE responses [47], expose novel roles of γδ T cells. However, further studies analyzing Vγ3+ DETC in this tolerance model are useful and will aid the understanding of the role of DETC in regulating inflammatory and allergic responses.
The presumed role of γδ T cells as a counter-regulator of αβ T cell responses has also become attractive in the setting of autoimmune-induced skin inflammation. Cutaneous graft-versus-host (GVHD) disease, in which epidermal structures are selectively destroyed, can be induced by local injections of autoreactive αβ T cells. This autoimmune response is self-limiting, and epidermis that spontaneously recovers from the destruction becomes resistant to re-challenge of GVHD [48]. The local resistance correlates well with the expansion of DETC, which supports a role for DETC against autoimmune attacks [49]. In contrast, TCRδ−/− mice are not resistant to the re-challenge of GVHD, further supporting the indispensable role for DETC in this scenario [49].
Together, results from several systems indicate that DETC can produce a variety of cytokines and chemokines which are soluble mediators of intercellular communication and may substantially regulate inflammatory responses. The functional roles of DETC in in vivo CS reactions are quite well characterized; however, the signaling pathways, cytokine and chemokine responses involved in mediating CS elicitation or downregulation remain less clear.
Role of epidermal T cells in skin tumor immunity
In addition to protecting epithelial tissues from disruption and excessive inflammatory responses, DETC can exert anti-tumor function. DETC share characteristics of typical innate immune effector cells such as exhibiting rapid functional responses, recognition of innate ligands, and expression of NKG2D, but they also express a rearranged T cell receptor, a hallmark for adaptive immune cells; all these features may provide tools for exerting anti-tumor function. In fact, DETC can lyse skin-derived tumors as well as the lymphoma cell line YAC-1 targets effectively, and this occurs in a non-MHC-restricted manner [50]. Moreover, confluent monolayers of melanoma or the cutaneous squamous cell carcinoma cell line Pam 212 are disrupted completely by addition of DETC. The cytolytic activity of DETC appears to be specific for tumor cells, since normal mouse keratinocyte monolayers remain intact under the same conditions [50].
Further evidence for the role of DETC in tumor immune surveillance is provided by work from Girardi et al. [51]. Mice lacking γδ T cells are highly susceptible to cutaneous malignancies induced by xenograft transplants or chemical carcinogenesis with DMBA/TPA [51]. While DETC confer protection from DMBA/TPA-induced papillomas and carcinomas, αβ T cells seem to promote tumor progression in this model of carcinogenesis [51]. In contrast, in transplantable tumor models using the PDV keratinocyte tumor cell line, both γδ and αβ T cells appear to be important for the number of developing tumors [51]. One mechanism by which γδ T cells regulate tumor development in the DMBA/TPA model is through NKG2D recognition of the stress ligand retinoic acid early transcript 1 (Rae1) protein, which is induced in the skin by DMBA/TPA treatment. NKG2D-expressing skin γδ T cells can kill Rae1-expressing targets in vitro [51], but in transgenic mice expressing Rae1 in the skin, NKG2D expression is downregulated on lymphocytes, and consequently these mice are more susceptible to papilloma induction than are wild-type mice [52]. These results suggest that long-lasting activation of skin-resident γδ T cells may render them anergic.
Murine NKG2D recognizes not only Rae1 but also several other ligands, including murine UL-16 binding protein-like transcript-1 (Mult1) and histocompatibility 60 (H60a–H60c), reviewed in [53]. A controversial issue with respect to NKG2D function is whether it provides a stimulatory or co-stimulatory signal to responding cells, including DETC. Recent studies have provided insight into the role of NKG2D. NKG2D–H60c interaction alone is not sufficient to activate DETC, but instead provides a co-stimulatory signal that is important for activating DETC in response to stimulation with keratinocytes [54]. However, this observation is in clear contrast to an earlier report suggesting direct activation of DETC through NKG2D [55]. A possible explanation for this reported discrepancy is that NKG2D can function in several contexts. NKG2D usually associates with the adaptor molecule DAP10 which does not contain immunoreceptor tyrosine-based activation motifs (ITAM) and is therefore believed to mediate co-stimulatory signals and not direct activation [56, 57]. However, DAP10 can, in some cells such as natural killer cells, provide a sufficient signal to induce cytotoxicity and possibly even cytokine production [58, 59]. Under certain circumstances, NKG2D may instead associate with the ITAM-containing DAP12 signaling subunit [56, 57], potentially resulting in the ability to directly activate cells. Intriguingly, NKG2D engagement in the absence of TCR engagement has been reported to be sufficient for activating cytotoxicity and cytokine release by DETC, through expression of NKG2D-associated DAP12 [55].
Interestingly, γδ T cells, including DETC, can produce cytolytic effector molecules such as perforin and granzymes which are required to directly kill tumor cells [60, 61]. In addition, skin γδ T cells produce a variety of cytokines, such as IFN-γ and IL-2 [20] which may contribute to the anti-tumor response [62–64]. The role of cytokine production by DETC in their anti-tumor function has not been well studied, and may provide interesting parallels and insights into tumor-associated inflammation which may provide either a tumor-promoting or -inhibiting signal.
Role of epidermal γδ T cells in wound healing
Wound healing is a complex process requiring constant communication between cells in the form of cytokine release, cell-to-cell contacts and cell-to-matrix interactions. The healing process can be divided into several overlapping steps. These include matrix deposition initially in the form of a blood clot and subsequently as granulation tissue, the induction of epithelial cell proliferation and differentiation, the recruitment of inflammatory cells, and the formation of scar tissue [65–67]. In pathologic conditions including spinal cord injuries, infection, malnutrition, diabetes, and other chronic diseases, the ordered and efficient processes are disrupted and chronic, non-healing wounds can be the result. As the population ages, increased numbers of patients with chronic wounds are expected resulting in escalating costs to the health care system [68, 69]. Over 4 million patients in the US are afflicted with chronic venous leg ulcers, pressure sores, ischemic ulcers, and diabetic foot ulcers [65, 68, 69]. Chronic wounds contribute to morbidity and mortality of these patients [70]. Patients with a severe thermal injury are at a significant risk for sepsis due to extensive skin loss and delayed healing. Morbidity and mortality is directly correlated to time until re-epithelialization [71]. It is clear that a better understanding of the cells and mechanisms that contribute to an effective wound healing process is necessary so that new strategies for treatment of wounds can be devised.
Since DETC are ideally localized to monitor the epidermis for damage, it seemed likely that they could contribute to tissue repair. Activated DETC inducibly secrete keratinocyte growth factors (KGF-1/FGF-7 and KGF-2/FGF-10), potent epithelial cell mitogens [72–74]. KGF-1 and KGF-2 are members of the rapidly growing fibroblast growth factor (FGF) family. KGF-1 acts specifically on epithelial cells, including keratinocytes, to stimulate proliferation and migration of these cells as well as in wound healing [73–75]. In addition, activated DETC produce chemokines that may affect migration of cells into the epidermis following trauma [25]. These results suggest that DETC may participate not only in maintenance of epithelial homeostasis but also in tissue repair.
In the absence of the canonical DETC, there are defects in keratinocyte proliferation, tissue re-epithelialization, inflammation, and wound closure following wounding. DETC are activated at wound sites and produce cytokines including the epithelial growth factors KGF-1 and KGF-2 [76]. A keratinocyte-responsive γδ TCR is necessary for activation of the DETC by damaged keratinocytes during wound healing and is also required for the maintenance of T cells in the epidermis [77]. In addition, DETC are necessary for the recruitment of inflammatory cells into the wound site. In a novel mechanism, DETC-produced KGFs stimulate production of hyaluronan by epidermal cells which then controls migration of macrophages into wounds [26]. These results indicate that DETC recognize antigen(s) expressed by stressed or damaged keratinocytes [78] and that recognition of this unknown antigen regulates DETC functional responses.
However, recognition of TCR antigens alone appears to be insufficient for DETC to assist in wound healing. A new cell surface pair of receptor-ligand molecules was recently identified that specifically provides costimulatory signals to epithelial-resident γδ T cells, including DETC [79, 80]. The junctional adhesion molecule-like protein (JAML) is upregulated on activated DETC. Binding of JAML to its ligand on keratinocytes, the Coxsackie and Adenovirus receptor (CAR), provides costimulation leading to cellular proliferation and cytokine and growth factor production. Typically, CAR expression is upregulated on keratinocytes located around wounds in normal mice and this increased expression is sustained for several days. Junctional adhesion molecule-like protein is similarly upregulated on DETC in wounded tissues suggesting a role for costimulation in wound healing responses of DETC. Inhibition of JAML–CAR-mediated costimulation leads to diminished γδ T cell activation and delayed wound closure similar to that seen in the absence of γδ T cells [79].
Since DETC play a positive role in wound healing, this raises the possibility that human epidermal-resident T cells may have similar roles in healing wounds and that their functions may be absent or aberrant in patients with chronic wounds. Human epidermis differs from mouse epidermis in that the major population of epidermal T cells bears an αβ TCR with 1–20% of the cells expressing the Vδ1 TCR. Vδ1 is also expressed by human intestinal γδ T cells [81] while peripheral blood and dermal γδ T cells express the Vδ2 TCR [82]. In addition, human skin is significantly thicker than mouse skin and has reduced hair density. These differences must be considered in the translation of findings from work in mouse skin to patient skin. Therefore, it was important to determine whether human epidermal T cells contribute to wound healing and if healing responses are specific to the epidermal γδ T cells or also shared by epidermal-resident αβ T cells. Initial studies showed that both αβ and γδ T cells isolated from normal human epidermis could produce growth factors, including IGF-1 following in vitro activation [83]. Since IGF-1 has been shown to contribute to healing of human wounds, this supported the possibility that both epidermal resident T cell populations could potentially contribute to wound healing. This was tested directly by comparing epidermal T cells isolated from acute healing wounds 3–4 days post-injury to T cells from chronic wounds with impaired healing at greater than 2 months following initial injury. In patients with acute, healing wounds, both αβ and γδ epidermal T cells are activated and produce growth factors that can contribute to healing [83]. In contrast, epidermal T cells from patients with chronic, non-healing wounds are not activated nor secreting growth factors. These T cells are further unable to produce growth factors in response to ex vivo stimulation suggesting an impaired or anergic status of epidermal T cells in chronic wound tissue. These results indicate that epidermal T cells in both mouse and man contribute to effective wound healing and that T cell responses may be defective in patients with chronic wounds.
New strategies for modulation of the activation of epidermal T cells in vivo or in vitro for immunotherapy could have major wound healing benefits. It will be interesting to determine the status of expression of molecules such as JAML and CAR in patients with chronic wounds. Costimulatory molecules have been proposed as targets for immunotherapy, raising the possibility of future therapeutic roles for the epithelial γδ T cell costimulatory pair, JAML and CAR, in wound healing and inflammatory diseases. A greater understanding of the biology of skin γδ T cells in wound healing may have an impact on development of future strategies for significant improvements in the quality of tissue repair.
Conclusions
In summary, numerous studies of DETC function indicate that the cutaneous immune system is essential for protection of epidermal integrity, response to environmental stimuli, regulation of inflammation, tumor surveillance, and wound healing. These protective roles of DETC highlight this population as an integral part of the skin immune system and emphasize the importance of the identification of DETC TCR ligands. The relevance of these murine studies for human skin disease are supported by the recent reports of similar functions for epidermal-resident T cells in human wound healing. The consequences of the constant exposure of the skin to insults, infection and injury are mitigated by the contributions of the DETC to maintenance of tissue homeostasis. Future studies should clarify the importance of particular molecules and mechanisms utilized for development and function of skin-resident γδ T cells as well as identify therapeutic targets for manipulation of these cells to combat epithelial diseases.
Acknowledgments
The authors are supported by grants from the National Institutes of Health (AI007244, AI64811, AI36964, and GM80301), L’Oreal, Deutsche Dermatologische Gesellschaft and the Arbeitsgemeinschaft Dermatologische Forschung.
Abbreviations
- DETC
Dendritic epidermal T cells
- JAML
Junctional adhesion molecule-like protein
- CAR
Coxsackie and adenovirus receptor
- CS
Delayed-type contact hypersensitivity
- GVHD
Graft versus host disease
- TLR
Toll-like receptor
- Rae-1
Retinoic acid early transcript 1
- Mult1
UL-16 binding protein-like transcript-1
- ITAM
Immunoreceptor tyrosine-based activation motif
References
- 1.Asarnow DM, Goodman T, LeFrancois L, Allison JP. Distinct antigen receptor repertoires of two classes of murine epithelium-associated T cells. Nature. 1989;341:60–62. doi: 10.1038/341060a0. [DOI] [PubMed] [Google Scholar]
- 2.Havran WL, Grell S, Duwe G, Kimura J, Wilson A, Kruisbeek AM, O’Brien RL, Born W, Tigelaar RE, Allison JP. Limited diversity of T-cell receptor γ-chain expression of murine Thy-1+ dendritic epidermal cells revealed by Vγ3-specific monoclonal antibody. Proc Natl Acad Sci USA. 1989;86:4185–4189. doi: 10.1073/pnas.86.11.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asarnow DM, Kuziel WA, Bonyhadi M, Tigelaar RE, Tucker PW, Allison JP. Limited diversity of γδ antigen receptor genes of Thy-1+ dendritic epidermal cells. Cell. 1988;55:837–847. doi: 10.1016/0092-8674(88)90139-0. [DOI] [PubMed] [Google Scholar]
- 4.Havran WL, Allison JP. Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature. 1988;335:443–445. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- 5.Itohara S, Farr AG, Lafaille JJ, Bonneville M, Takagaki Y, Haas W, Tonegawa S. Homing of a γδ thymocyte subset with homogeneous T-cell receptors to mucosal epithelia. Nature. 1990;343:754–757. doi: 10.1038/343754a0. [DOI] [PubMed] [Google Scholar]
- 6.Xiong N, Baker JE, Kang C, Raulet DH. The genomic arrangement of T cell receptor variable genes is a determinant of the developmental rearrangement pattern. Proc Natl Acad Sci USA. 2004;101:260–265. doi: 10.1073/pnas.0303738101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uche UN, Huber CR, Raulet DH, Xiong N. Recombination signal sequence-associated restriction on TCRδ gene rearrangement affects the development of tissue-specific γδ T cells. J Immunol. 2009;183:4931–4939. doi: 10.4049/jimmunol.0901859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiong N, Raulet DH. Development and selection of γδ T cells. Immunol Rev. 2007;215:15–31. doi: 10.1111/j.1600-065X.2006.00478.x. [DOI] [PubMed] [Google Scholar]
- 9.Xiong N, Zhang L, Kang C, Raulet DH. Gene placement and competition control T cell receptor γ variable region gene rearrangement. J Exp Med. 2008;205:929–938. doi: 10.1084/jem.20071275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leclercq G, Plum J, Nandi D, De Smedt M, Allison JP. Intrathymic differentiation of Vγ3 T cells. J Exp Med. 1993;178:309–315. doi: 10.1084/jem.178.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiong N, Kang C, Raulet DH. Positive selection of dendritic epidermal γδ T cell precursors in the fetal thymus determines expression of skin-homing receptors. Immunity. 2004;21:121–131. doi: 10.1016/j.immuni.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Boyden LM, Lewis JM, Barbee SD, Bas A, Girardi M, Hayday AC, Tigelaar RE, Lifton RP. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal γδ T cells. Nat Genet. 2008;40:656–662. doi: 10.1038/ng.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharp LL, Jameson JM, Witherden DA, Komori HK, Havran WL. Dendritic epidermal T-cell activation. Crit Rev Immunol. 2005;25:1–18. doi: 10.1615/CritRevImmunol.v25.i1.10. [DOI] [PubMed] [Google Scholar]
- 14.Ye SK, Maki K, Lee HC, Ito A, Kawai K, Suzuki H, Mak TW, Chien Y, Honjo T, Ikuta K. Differential roles of cytokine receptors in the development of epidermal γδ T cells. J Immunol. 2001;167:1929–1934. doi: 10.4049/jimmunol.167.4.1929. [DOI] [PubMed] [Google Scholar]
- 15.Ye SK, Agata Y, Lee HC, Kurooka H, Kitamura T, Shimizu A, Honjo T, Ikuta K. The IL-7 receptor controls the accessibility of the TCRγ locus by Stat5 and histone acetylation. Immunity. 2001;15:813–823. doi: 10.1016/S1074-7613(01)00230-8. [DOI] [PubMed] [Google Scholar]
- 16.Kang J, DiBenedetto B, Narayan K, Zhao H, Der SD, Chambers CA. STAT5 is required for thymopoiesis in a development stage-specific manner. J Immunol. 2004;173:2307–2314. doi: 10.4049/jimmunol.173.4.2307. [DOI] [PubMed] [Google Scholar]
- 17.Jiang X, Campbell JJ, Kupper TS. Embryonic trafficking of γδ T cells to skin is dependent on E/P selectin ligands and CCR4. Proc Natl Acad Sci USA. 2010;107:7443–7448. doi: 10.1073/pnas.0912943107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin Y, Xia M, Sun A, Saylor CM, Xiong N. CCR10 is important for the development of skin-specific γδ T cells by regulating their migration and location. J Immunol. 2010;185:5723–5731. doi: 10.4049/jimmunol.1001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee P, Lee DJ, Chan C, Chen SW, Ch’en I, Jamora C. Dynamic expression of epidermal caspase 8 simulates a wound healing response. Nature. 2009;458:519–523. doi: 10.1038/nature07687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jameson JM, Sharp LL, Witherden DA, Havran WL. Regulation of skin cell homeostasis by γδ T cells. Front Biosci. 2004;9:2640–2651. doi: 10.2741/1423. [DOI] [PubMed] [Google Scholar]
- 21.Sharp LL, Jameson JM, Cauvi G, Havran WL. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat Immunol. 2005;6:73–79. doi: 10.1038/ni1152. [DOI] [PubMed] [Google Scholar]
- 22.Edmondson SR, Thumiger SP, Werther GA, Wraight CJ. Epidermal homeostasis: the role of the growth hormone and insulin-like growth factor systems. Endocr Rev. 2003;24:737–764. doi: 10.1210/er.2002-0021. [DOI] [PubMed] [Google Scholar]
- 23.Su HY, Cheng WT, Chen SC, Lin CT, Lien YY, Liu HJ, Gilmour RS. Mouse keratinocytes express c98, a novel gene homologous to bcl-2, that is stimulated by insulin-like growth factor 1 and prevents dexamethasone-induced apoptosis. Biochim Biophys Acta. 2004;1676:127–137. doi: 10.1016/j.bbaexp.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Matsue H, Cruz PD, Jr, Bergstresser PR, Takashima A. Profiles of cytokine mRNA expressed by dendritic epidermal T cells in mice. J Invest Dermatol. 1993;101:537–542. doi: 10.1111/1523-1747.ep12365917. [DOI] [PubMed] [Google Scholar]
- 25.Boismenu R, Feng L, Xia YY, Chang JC, Havran WL. Chemokine expression by intraepithelial γδ T cells. Implications for the recruitment of inflammatory cells to damaged epithelia. J Immunol. 1996;157:985–992. [PubMed] [Google Scholar]
- 26.Jameson JM, Cauvi G, Sharp LL, Witherden DA, Havran WL. γδ T cell-induced hyaluronan production by epithelial cells regulates inflammation. J Exp Med. 2005;201:1269–1279. doi: 10.1084/jem.20042057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor KR, Mills RE, Costanzo AE, Jameson JM. γδ T cells are reduced and rendered unresponsive by hyperglycemia and chronic TNFα in mouse models of obesity and metabolic disease. PLoS One. 2010;5(7):e11422. doi: 10.1371/journal.pone.0011422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang T, Scully E, Yin Z, Kim JH, Wang S, Yan J, Mamula M, Anderson JF, Craft J, Fikrig E. IFN-γ-producing γδ T cells help control murine West Nile virus infection. J Immunol. 2003;171:2524–2531. doi: 10.4049/jimmunol.171.5.2524. [DOI] [PubMed] [Google Scholar]
- 29.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vδ1+ γδ T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J Immunol. 2007;178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 30.Gao Y, Yang W, Pan M, Scully E, Girardi M, Augenlicht LH, Craft J, Yin Z. γδ T cells provide an early source of interferon γ in tumor immunity. J Exp Med. 2003;198:433–442. doi: 10.1084/jem.20030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, Magorien JE, Blauvelt A, Kolls JK, Cheung AL, Cheng G, Modlin RL, Miller LS. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest. 2010;120(5):1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molne L, Corthay A, Holmdahl R, Tarkowski A. Role of γδ T cell receptor-expressing lymphocytes in cutaneous infection caused by Staphylococcus aureus . Clin Exp Immunol. 2003;132:209–215. doi: 10.1046/j.1365-2249.2003.02151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leclercq G, Plum J. Stimulation of TCR Vγ3 cells by gram-negative bacteria. J Immunol. 1995;154:5313–5319. [PubMed] [Google Scholar]
- 34.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 35.Shimura H, Nitahara A, Ito A, Tomiyama K, Ito M, Kawai K. Up-regulation of cell surface Toll-like receptor 4-MD2 expression on dendritic epidermal T cells after the emigration from epidermis during cutaneous inflammation. J Dermatol Sci. 2005;37:101–110. doi: 10.1016/j.jdermsci.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Ptak W, Askenase PW. γδ T cells assist αβ T cells in adoptive transfer of contact sensitivity. J Immunol. 1992;149:3503–3508. [PubMed] [Google Scholar]
- 37.Szczepanik M, Lewis J, Geba GP, Ptak W, Askenase PW. Positive regulatory γδ T cells in contact sensitivity: augmented responses by in vivo treatment with anti-γδ monoclonal antibody, or anti-Vγ5 or Vδ4. Immunol Invest. 1998;27:1–15. doi: 10.3109/08820139809070886. [DOI] [PubMed] [Google Scholar]
- 38.Szczepanik M, Nowak B, Askenase PW, Ptak W. Cross-talk between γδ T lymphocytes and immune cells in humoral response. Immunology. 1998;95:612–617. doi: 10.1046/j.1365-2567.1998.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ushio H, Tsuji RF, Szczepanik M, Kawamoto K, Matsuda H, Askenase PW. IL-12 reverses established antigen-specific tolerance of contact sensitivity by affecting costimulatory molecules B7–1 (CD80) and B7–2 (CD86) J Immunol. 1998;160:2080–2088. [PubMed] [Google Scholar]
- 40.Dieli F, Asherson GL, Sireci G, Dominici R, Gervasi F, Vendetti S, Colizzi V, Salerno A. γδ cells involved in contact sensitivity preferentially rearrange the Vγ3 region and require interleukin-7. Eur J Immunol. 1997;27:206–214. doi: 10.1002/eji.1830270131. [DOI] [PubMed] [Google Scholar]
- 41.Dieli F, Ptak W, Sireci G, Romano GC, Potestio M, Salerno A, Asherson GL. Cross-talk between Vβ8+ and γδ+ T lymphocytes in contact sensitivity. Immunology. 1998;93:469–477. doi: 10.1046/j.1365-2567.1998.00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Girardi M, Lewis J, Glusac E, Filler RB, Geng L, Hayday AC, Tigelaar RE. Resident skin-specific γδ T cells provide local, nonredundant regulation of cutaneous inflammation. J Exp Med. 2002;195:855–867. doi: 10.1084/jem.20012000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewis JM, Girardi M, Roberts SJ, Barbee SD, Hayday AC, Tigelaar RE. Selection of the cutaneous intraepithelial γδ+ T cell repertoire by a thymic stromal determinant. Nat Immunol. 2006;7:843–850. doi: 10.1038/ni1363. [DOI] [PubMed] [Google Scholar]
- 44.Marcinkiewicz J, Bereta M, Malinowski J, Ptak W. The induction of oxazolone-specific T suppressor afferent cells in mice by hapten-modified isologous IgG. Eur J Immunol. 1984;14:759–762. doi: 10.1002/eji.1830140818. [DOI] [PubMed] [Google Scholar]
- 45.Rosenstein RW, Murray JH, Cone RE, Ptak W, Iverson GM, Gershon RK. Isolation and partial characterization of an antigen-specific T-cell factor associated with the suppression of delayed type hypersensitivity. Proc Natl Acad Sci USA. 1981;78:5821–5825. doi: 10.1073/pnas.78.9.5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szczepanik M, Anderson LR, Ushio H, Ptak W, Owen MJ, Hayday AC, Askenase PW. γδ T cells from tolerized αβ T cell receptor (TCR)-deficient mice inhibit contact sensitivity-effector T cells in vivo, and their interferon-γ production in vitro. J Exp Med. 1996;184:2129–2139. doi: 10.1084/jem.184.6.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McMenamin C, Pimm C, McKersey M, Holt PG. Regulation of IgE responses to inhaled antigen in mice by antigen-specific γδ T cells. Science. 1994;265:1869–1871. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 48.Shiohara T, Moriya N, Gotoh C, Hayakawa J, Nagashima M, Saizawa K, Ishikawa H. Loss of epidermal integrity by T cell-mediated attack induces long-term local resistance to subsequent attack. I. Induction of resistance correlates with increases in Thy-1+ epidermal cell numbers. J Exp Med. 1990;171:1027–1041. doi: 10.1084/jem.171.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shiohara T, Moriya N, Hayakawa J, Itohara S, Ishikawa H. Resistance to cutaneous graft-vs.-host disease is not induced in T cell receptor δ gene-mutant mice. J Exp Med. 1996;183:1483–1489. doi: 10.1084/jem.183.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaminski MJ, Cruz PD, Jr, Bergstresser PR, Takashima A. Killing of skin-derived tumor cells by mouse dendritic epidermal T-cells. Cancer Res. 1993;53:4014–4019. [PubMed] [Google Scholar]
- 51.Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, Hobby P, Sutton B, Tigelaar RE, Hayday AC. Regulation of cutaneous malignancy by γδ T cells. Science. 2001;294:605–609. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 52.Oppenheim DE, Roberts SJ, Clarke SL, Filler R, Lewis JM, Tigelaar RE, Girardi M, Hayday AC. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol. 2005;6:928–937. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- 53.Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunol Rev. 2010;235:267–285. doi: 10.1111/j.0105-2896.2010.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whang MI, Guerra N, Raulet DH. Costimulation of dendritic epidermal γδ T cells by a new NKG2D ligand expressed specifically in the skin. J Immunol. 2009;182:4557–4564. doi: 10.4049/jimmunol.0802439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nitahara A, Shimura H, Ito A, Tomiyama K, Ito M, Kawai K. NKG2D ligation without T cell receptor engagement triggers both cytotoxicity and cytokine production in dendritic epidermal T cells. J Invest Dermatol. 2006;126:1052–1058. doi: 10.1038/sj.jid.5700112. [DOI] [PubMed] [Google Scholar]
- 56.Diefenbach A, Tomasello E, Lucas M, Jamieson AM, Hsia JK, Vivier E, Raulet DH. Selective associations with signaling proteins determine stimulatory versus costimulatory activity of NKG2D. Nat Immunol. 2002;3:1142–1149. doi: 10.1038/ni858. [DOI] [PubMed] [Google Scholar]
- 57.Gilfillan S, Ho EL, Cella M, Yokoyama WM, Colonna M. NKG2D recruits two distinct adapters to trigger NK cell activation and costimulation. Nat Immunol. 2002;3:1150–1155. doi: 10.1038/ni857. [DOI] [PubMed] [Google Scholar]
- 58.Rosen DB, Araki M, Hamerman JA, Chen T, Yamamura T, Lanier LL. A structural basis for the association of DAP12 with mouse, but not human, NKG2D. J Immunol. 2004;173:2470–2478. doi: 10.4049/jimmunol.173.4.2470. [DOI] [PubMed] [Google Scholar]
- 59.Zompi S, Hamerman JA, Ogasawara K, Schweighoffer E, Tybulewicz VL, Di Santo JP, Lanier LL, Colucci F. NKG2D triggers cytotoxicity in mouse NK cells lacking DAP12 or Syk family kinases. Nat Immunol. 2003;4:565–572. doi: 10.1038/ni930. [DOI] [PubMed] [Google Scholar]
- 60.Shojaei H, Oberg HH, Juricke M, Marischen L, Kunz M, Mundhenke C, Gieseler F, Kabelitz D, Wesch D. Toll-like receptors 3 and 7 agonists enhance tumor cell lysis by human γδ T cells. Cancer Res. 2009;69:8710–8717. doi: 10.1158/0008-5472.CAN-09-1602. [DOI] [PubMed] [Google Scholar]
- 61.Krahenbuhl O, Gattesco S, Tschopp J. Murine Thy-1+ dendritic epidermal T cell lines express granule-associated perforin and a family of granzyme molecules. Immunobiology. 1992;184:392–401. doi: 10.1016/S0171-2985(11)80596-6. [DOI] [PubMed] [Google Scholar]
- 62.Atkins MB. Cytokine-based therapy and biochemotherapy for advanced melanoma. Clin Cancer Res. 2006;12:2353s–2358s. doi: 10.1158/1078-0432.CCR-05-2503. [DOI] [PubMed] [Google Scholar]
- 63.Bonmort M, Ullrich E, Mignot G, Jacobs B, Chaput N, Zitvogel L. Interferon-γ is produced by another player of innate immune responses: the interferon-producing killer dendritic cell (IKDC) Biochimie. 2007;89:872–877. doi: 10.1016/j.biochi.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 64.Murugaiyan G, Saha B. Protumor vs. antitumor functions of IL-17. J Immunol. 2009;183:4169–4175. doi: 10.4049/jimmunol.0901017. [DOI] [PubMed] [Google Scholar]
- 65.Baum CL, Arpey CJ. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg. 2005;31:674–86. doi: 10.1111/j.1524-4725.2005.31612. [DOI] [PubMed] [Google Scholar]
- 66.Gailit J, Clark RA. Wound repair in the context of extracellular matrix. Curr Opin Cell Biol. 1994;6:717–725. doi: 10.1016/0955-0674(94)90099-X. [DOI] [PubMed] [Google Scholar]
- 67.Strecker-McGraw MK, Jones TR, Baer DG. Soft tissue wounds and principles of healing. Emerg Med Clin North Am. 2007;25:1–22. doi: 10.1016/j.emc.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 68.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 69.Izadi K, Ganchi P. Chronic wounds. Clin Plast Surg. 2005;32:209–222. doi: 10.1016/j.cps.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 70.Phillips TJ. Chronic cutaneous ulcers: etiology and epidemiology. J Invest Dermatol. 1994;102:38S–41S. doi: 10.1111/1523-1747.ep12388556. [DOI] [PubMed] [Google Scholar]
- 71.Wolfe RA, Roi LD, Flora JD, Feller I, Cornell RG. Mortality differences and speed of wound closure among specialized burn care facilities. JAMA. 1983;250:763–766. doi: 10.1001/jama.250.6.763. [DOI] [PubMed] [Google Scholar]
- 72.Rubin JS, Osada H, Finch PW, Taylor WG, Rudikoff S, Aaronson SA. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Natl Acad Sci USA. 1989;86:802–806. doi: 10.1073/pnas.86.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Werner S, Peters KG, Longaker MT, Fuller-Pace F, Banda MJ, Williams LT. Large induction of keratinocyte growth factor expression in the dermis during wound healing. Proc Natl Acad Sci USA. 1992;89:6896–6900. doi: 10.1073/pnas.89.15.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Finch PW, Rubin JS, Miki T, Ron D, Aaronson SA. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science. 1989;245:752–755. doi: 10.1126/science.2475908. [DOI] [PubMed] [Google Scholar]
- 75.Staiano-Coico L, Krueger JG, Rubin JS, D’Limi S, Vallat VP, Valentino L, Fahey T, 3rd, Hawes A, Kingston G, Madden MR, et al. Human keratinocyte growth factor effects in a porcine model of epidermal wound healing. J Exp Med. 1993;178:865–878. doi: 10.1084/jem.178.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, Havran WL. A role for skin γδ T cells in wound repair. Science. 2002;296:747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 77.Jameson JM, Cauvi G, Witherden DA, Havran WL. A keratinocyte-responsive γδ TCR is necessary for dendritic epidermal T cell activation by damaged keratinocytes and maintenance in the epidermis. J Immunol. 2004;172:3573–3579. doi: 10.4049/jimmunol.172.6.3573. [DOI] [PubMed] [Google Scholar]
- 78.Havran WL, Chien YH, Allison JP. Recognition of self antigens by skin-derived T cells with invariant γδ antigen receptors. Science. 1991;252:1430–1432. doi: 10.1126/science.1828619. [DOI] [PubMed] [Google Scholar]
- 79.Witherden DA, Verdino P, Rieder SE, Garijo O, Mills RE, Teyton L, Fischer WH, Wilson IA, Havran WL. The junctional adhesion molecule JAML is a costimulatory receptor for epithelial γδ T cell activation. Science. 2010;329:1205–1210. doi: 10.1126/science.1192698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Verdino P, Witherden DA, Havran WL, Wilson IA. The molecular interaction of CAR and JAML recruits the central cell signal transducer PI3 K. Science. 2010;329(5996):1210–1214. doi: 10.1126/science.1187996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morita CT, Parker CM, Brenner MB, Band H. TCR usage and functional capabilities of human γδ T cells at birth. J Immunol. 1994;153:3979–3988. [PubMed] [Google Scholar]
- 82.Trejdosiewicz LK, Smart CJ, Oakes DJ, Howdle PD, Malizia G, Campana D, Boylston AW. Expression of T-cell receptors TcR1 (γδ) and TcR2 (αβ) in the human intestinal mucosa. Immunology. 1989;68:7–12. [PMC free article] [PubMed] [Google Scholar]
- 83.Toulon A, Breton L, Taylor KR, Tenenhaus M, Bhavsar D, Lanigan C, Rudolph R, Jameson J, Havran WL. A role for human skin-resident T cells in wound healing. J Exp Med. 2009;206:743–750. doi: 10.1084/jem.20081787. [DOI] [PMC free article] [PubMed] [Google Scholar]