Abstract
Using functional tissue engineering principles, our laboratory has produced tendon repair tissue which matches the normal patellar tendon force-displacement curve up to 32% of failure. This repair tissue will need to withstand more strenuous activities, which can reach or even exceed 40% of failure force. To improve the linear stiffness of our tissue engineered constructs (TECs) and tissue engineered repairs, our lab is incorporating the glycosaminoglycan chondroitin-6-sulfate (C6S) into a type I collagen scaffold. In this study, we examined the effect of C6S incorporation and mechanical stimulation cycle number on linear stiffness and mRNA expression (collagen types I and III, decorin and fibronectin) for mesenchymal stem cell (MSC)- collagen sponge TECs. The TECs were fabricated by inoculating MSCs at a density of 0.14x106 cells/construct onto pre-cut scaffolds. Primarily type I collagen scaffold materials, with or without C6S, were cultured using mechanical stimulation with three different cycle numbers (0, 100, or 3000 cycles/day). After two weeks in culture, TECs were evaluated for linear stiffness and mRNA expression. C6S incorporation and cycle number each played an important role in gene expression, but only the interaction of C6S incorporation and cycle number produced a benefit for TEC linear stiffness.
Keywords: Tissue engineering, collagen-GAG, mechanical stimulation, patellar tendon
Introduction
Tendon and ligament injuries remain the most common and significant musculoskeletal injuries. Each year, over 16 million patients in the US present with soft connective tissue injuries to tendon, ligament, and capsular structures.1 The knee accounts for roughly 23% of activity-related injuries.3 Left untreated, many of these injuries result in significant dysfunction and disability for the patient.2–4
Repair outcome after tendon and ligament injury varies depending on the type of treatment and the extent of injury. Direct repair is limited by the intrinsic healing capacity of the tissue and the extent of tissue disruption.5 With poor healing and extensive damage, surgeons may use a graft to replace the tissue. Autografts are limited by availability and impaired recovery due to harvest site morbidity and pain.4 Allografts are also used, but suffer from high cost, limited availability, potential disease transmission, and immune rejection.4 Overall, grafts can lose strength over time and fail to fully incorporate into bone.3, 4
Frequent injuries and the challenges of traditional repair have led some researchers to consider novel treatments such as tissue engineered constructs. Tissue engineered constructs (TECs) are designed to aid in natural tissue regeneration or replacement and eventually degrade. TECs are commonly composed of a biodegradable polymeric scaffold and cells. Synthetic polymeric scaffolds can be processed into unique 3D geometries, possess relatively good mechanical strength, and have a controllable degradation rate. However, without surface modification, these scaffolds do not support extensive cellular adhesion, infusion and/or proliferation.6–8 By contrast, natural polymeric scaffolds, such as type I collagen, are highly biocompatible and can support cell adhesion and proliferation. Unfortunately, these scaffolds exhibit lower mechanical strength than synthetic polymeric scaffolds.8 Physical cross-linking can increase collagen stiffness, but can also make the scaffold brittle and disturb surface markers for cell adhesion and migration, thus limiting tissue ingrowth and remodeling.9
Using the principles of functional tissue engineering,10, 11 our laboratory is designing more effective collagen-based TECs for tendon repair.2, 12–15 We first established the functional range of normal tendon loading for activities of daily living (ADLs) by recording in vivo forces in numerous tendons in the rabbit16–18 and goat models.19 Peak in vivo forces in the goat patellar tendon (PT) reached 32% of normal PT failure force.19 While our current tissue engineered repairs, which use mechanically preconditioned mesenchymal stem cell (MSC)-collagen sponge TECs, can sustain this load,2 tendons can function at up to 40% of failure during more strenuous activities.10 To accommodate these activities, our goal is to produce repair tissue that matches the normal PT failure curve up to 40% of normal failure loads.
One strategy to improve TEC linear stiffness and tissue engineered repair is to incorporate the glycosaminoglycan (GAG) chondroitin-6-sulfate (C6S) into the collagen scaffold. Adding C6S into our collagen scaffold can potentially: 1) improve sponge biomechanics20, 21 and 2) create a more homogenous scaffold by “linking” discontinuous fibrils.22, 23 While not the predominant GAG in tensile-load bearing tendons22, C6S does bind to decorin24, which is essential for proper collagen fibrillogenesis in tendon.25, 26 C6S incorporation thus has the potential to positively regulate type I collagen fibrillogenesis.24
Our study objectives were to determine the individual and interactive effects of incorporating C6S and mechanical stimulation on in vitro linear stiffness and mRNA expression (collagen type I, collagen type III, decorin and fibronectin) of MSC-collagen sponge TECs. We hypothesized that: 1) Under static culture conditions, incorporating C6S will increase linear stiffness and gene expression; 2) Mechanical stimulation of TECs without C6S will improve linear stiffness and gene expression; and 3) Combining C6S and mechanical stimulation will synergistically increase both TEC linear stiffness and gene expression levels.
Materials and Methods
Experimental Design
A collagen sponge scaffold fabricated with and without C6S (COL-C6S and COL, respectively; Engineered Skin Lab, Shriners Hospitals for Children, Cincinnati, OH) was evaluated. COL and COL-C6S scaffolds were analyzed for average pore diameter (4 per sample, n=5 per group) using scanning electron microscopy (SEM) and for relative crosslink density (n = 3 per scaffold) using differential scanning calorimetry (DSC). MSC-collagen sponge TECs were created using banked cell lines harvested from ten (n=10) skeletally mature, female New Zealand White rabbits. MSCs were sub-cultured to passage two (P2) using previously described techniques.12,13 For each scaffold type, COL and COL-C6S, three treatment levels of mechanical stimulation were tested: static culture, mechanically stimulated with 100 cycles/day and mechanically stimulated with 3000 cycles/day. TECs were evaluated for biomechanics and mRNA expression (collagen types I and III, decorin, and fibronectin). For each test condition, ten TECs (one per cell line) were dedicated to each response measure, biomechanics and gene expression (Table I).
Table I. Experimental Design.
Ten constructs (one from each cell line) were dedicated to each response measure.
| Scaffold | Mechanical Stimulation | Response Measure |
|
|---|---|---|---|
| Biomechanics | Biochemistry | ||
| COL | Static | n = 9 | n = 10 |
| 100 cycles/day | n = 10 | n = 10 | |
| 3000 cycles/day | n = 10 | n = 10 | |
| COL-C6S | Static | n = 9 | n = 10 |
| 100 cycles/day | n = 10 | n = 10 | |
| 3000 cycles/day | n = 10 | n = 10 | |
Collagen Scaffold Fabrication
Collagen sponge scaffolds, with and without chondroitin-6-sulfate, were fabricated at the Engineered Skin Lab.28 Briefly, comminuted bovine collagen (Kensey-Nash) was solubilized in acetic acid (0.55% wt./vol.) and homogenized by rapid mixing at 5200 rpm. C6S (0.05% wt./vol.) was co-precipitated with the collagen solution through the slow addition of a C6S-acetic acid solution during homogenization to ensure even dispersion of the C6S.28 The collagen-C6S mixture was injected into custom designed casting frames and frozen by submersion in a 95% EtOH bath. The frozen sheet of collagen-C6S was then lyophilized and dehydrothermally cross-linked at 140°C for 24 hours. Collagen sponges (COL; 0.6%wt./vol.) were fabricated, as described, without the addition of C6S.
Scanning Electron Microscopy (SEM)
Collagen scaffold morphology was examined by scanning electron microscopy (FEI Sirion), and average pore diameter was determined using ImageJ software. Samples were collected from dry collagen sponges, sputter coated with gold-palladium and imaged in secondary electron mode (5 kV). From the images (4 per sample, 5 samples per group), the diameter of at least 25 pores from each scaffold type was measured.
Differential Scanning Calorimetry (DSC)
As a relative measure of crosslink density, peak denaturation temperature (Tg) of COL and COL-C6S scaffolds was quantified (n = 3 per scaffold). Samples (5–10 mg in dry wt) were sealed in an aluminum cup. An empty cup served as a reference. DSC was performed from 25 to 150°C at a ramp rate of 10°C/min (TA Instruments DSC 2920). Scaffolds formed with acid-soluble collagen served as a negative control.
Tissue Engineered Construct Preparation
COL and COL-C6S constructs were prepared as previously described.2 COL and COL-C6S sponges were cut to fit in wells of a silicone dish. Two 4mm holes were created to allow the scaffold to be secured over the posts in each well. Scaffolds were soaked in 70% ethanol for 24 hours, rinsed with PBS (Gibco) and MSC growth media, and placed in the silicone dishes. MSC were inoculated (0.14x106 cells) on the scaffold in 0.4ml of MSC growth media. All TECs were incubated for two weeks and fed three times weekly (ADV-DMEM, 5% L-ascorbic acid 2-phosphate, 5% FBS, 1% GlutaMAX ™, 1% AB/AM).
Mechanical Stimulation
After two days of static culture, TECs allocated for mechanical stimulation were placed into our pneumatic system.2, 13–15, 27 Static culture TECs remained in the incubator. TECs were stimulated to a peak strain of 2.4%, at 1Hz, for eight hours a day with either 100 or 3000 cycles/day.16–19 After two weeks in culture, static and stimulated (2 days of acclimation, 12 days of stimulation) TECs were prepared for biomechanical testing (n=10) or gene expression analysis (n=10). Biomechanical testing samples were stored at −80°C; gene expression analysis samples were treated with RNAlater (QIAGEN Inc; Valencia, CA) for 6 hours, snap frozen in liquid nitrogen and placed in a −80°C freezer to prevent RNA degradation.
Biomechanical Evaluation of Constructs
TECs were removed from the freezer, allowed to thaw to room temperature, and re-hydrated in PBS prior to testing. The width and thickness of each construct were measured using calipers. TEC ends were secured into testing grips and loaded into the testing system (TestResources, Inc.; Shakopee, MN). Constructs were failed in tension at a strain rate of 10%/sec. Force-elongation plots were used to determine linear stiffness.
Biochemical Evaluation of Constructs
RNA was extracted using RNeasy mini kit (QIAGEN Inc.).13 First-strand cDNA was generated using a conventional reverse transcriptase reaction (MuLV reverse transcriptase, Applied Biosystems; Foster City, CA). Using rabbit specific primers (collagen type I, collagen type III, decorin, fibronectin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH)29, 30) reverse-transcribed RNA was amplified using conventional polymerase chain reaction (PCR). Products were verified using electrophoresis and SYBR safe DNA gel stain (Invitrogen Molecular Probes; Eugene, OR). Messenger RNA levels were then quantified in duplicate using quantitative real-time PCR (qRT-PCR) and normalized to GAPDH expression. GAPDH was used as the housekeeping gene because previous studies by our laboratory have shown GAPDH levels are not affected by mechanical stimulation.13 Primer sequences used for gene expression analysis were published previously.13
Statistical Analysis
Differences in linear stiffness and gene expression were compared using a one-way ANOVA with C6S incorporation and cycle number as fixed factors. Data were normal and heteroscedastic. Tamhane’s analysis was used for post-hoc testing. The significance level was set at p < 0.05. Note: qRT-PCR found no RNA for COL-C6S constructs at 100 cycles/day and no collagen type III mRNA in static COL and COL-C6S constructs. These groups were excluded from the analysis.
Results
TEC width and thickness averaged 8.2±0.6mm and 1.8±0.4mm, respectively (mean±SD). These dimensions were not affected by C6S incorporation or mechanical stimulation.
C6S incorporation and mechanical stimulation cycle number produced both independent and interactive effects on stiffness and gene expression (Table II). Independently, each factor significantly altered mRNA expression of collagen type I (p < 0.001), collagen type III (p < 0.001), decorin (p ≤ .005) and fibronectin (p < 0.001). Interactively, the two factors altered TEC linear stiffness (p = 0.006) and mRNA expression of collagen type I (p < 0.001), decorin (p = 0.001) and fibronectin (p < 0.001). GAPDH expression levels were consistent under static culture conditions (0.001±0.0003 and 0.0009±0.0002, COL and COL-C6S, respectively; mean±SEM), with 100 cycles/day (0.0011±0.0003 and 0.0012±0.0003) and 3000 cycles/day of mechanical stimulation (0.001±0.0003 and 0.003±0.0003).
Table II.
Biomechanics and Gene Expression Data for MSC-Collagen Sponge TECs (Mean ± SEM) Cultured for Two Weeks Statically and with Mechanical Stimulation.
| Scaffold Material |
Cycle Number (Cycles/Day) |
Stiffness (N/mm) | Collagen Type I |
Collagen Type III |
Decorin | Fibronectin |
|---|---|---|---|---|---|---|
| COL | As Fabricated | 0.027 ± 0.0045 | - | - | - | - |
| 0 | 0.053 ± 0.006 | 0.022 ± 0.004 | No Expression | 0.026 ± 0.006 | 0.276 ± 0.064 | |
| 100 | 0.050 ± 0.004 | 0.004 ± 0.003 | 0.024 ± 0.010 | 0.007 ± 0.002 | 4.40e-4 ± 9.33e-5 | |
| 3000 | 0.041 ± 0.005 | 0.076 ± 0.005 | 5.09 ± 0.735 | 0.052 ± 0.012 | 0.030 ± 0.005 | |
| COL-C6S | As Fabricated | 0.025 ± 0.003 | - | - | - | - |
| 0 | 0.057 ± 0.009 | 0.070 ± 0.022 | No Expression | 0.710 ± 0.218 | 1.94 ± 0.225 | |
| 100 | 0.080 ± 0.006 | No RNA | No RNA | No RNA | No RNA | |
| 3000 | 0.032 ± 0.003 | 0.600 ± 0.086 | 0.022 ± 0.003 | 0.005 ± 0.001 | 0.004 ± 0.001 |
Effect of C6S Incorporation on Inherent Scaffold Structure and Biomechanics
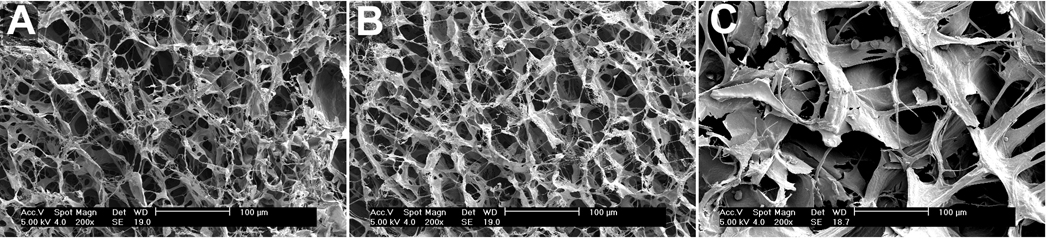
Adding C6S to the collagen sponge left the scaffold structure unchanged (Fig. 1A and B) with no significant differences in pore diameter (55.4±2.4um and 53.1±8.9um, COL and COL-C6S, respectively; mean±SD). C6S incorporation did not significantly improve relative crosslink density (83.0±1.4°C and 80.2±1.4°C, COL and COL-C6S, respectively; mean±SD). In addition, no significant differences were found in the as-fabricated stiffness of COL and COL-C6S sponges (0.027±0.0045N/mm and 0.025±0.003N/mm, respectively; mean±SEM).
Figure 1. Scanning Electron Microscopy of Scaffold Materials.
Incorporation of C6S had no effect on the structure or pore size of COL and COL-C6S scaffolds (A and B, respectively). However, CD-COL had larger pores with a wider pore size distribution (C).
Effect of C6S Incorporation on TEC Linear Stiffness and Gene Expression
Static culture
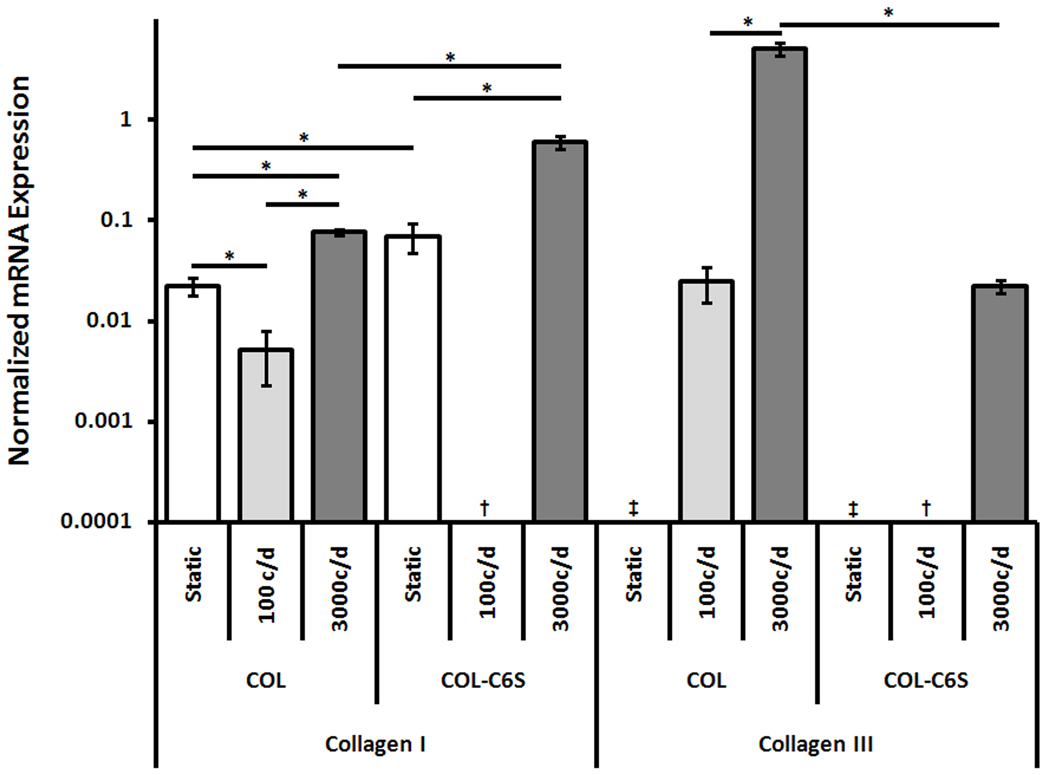
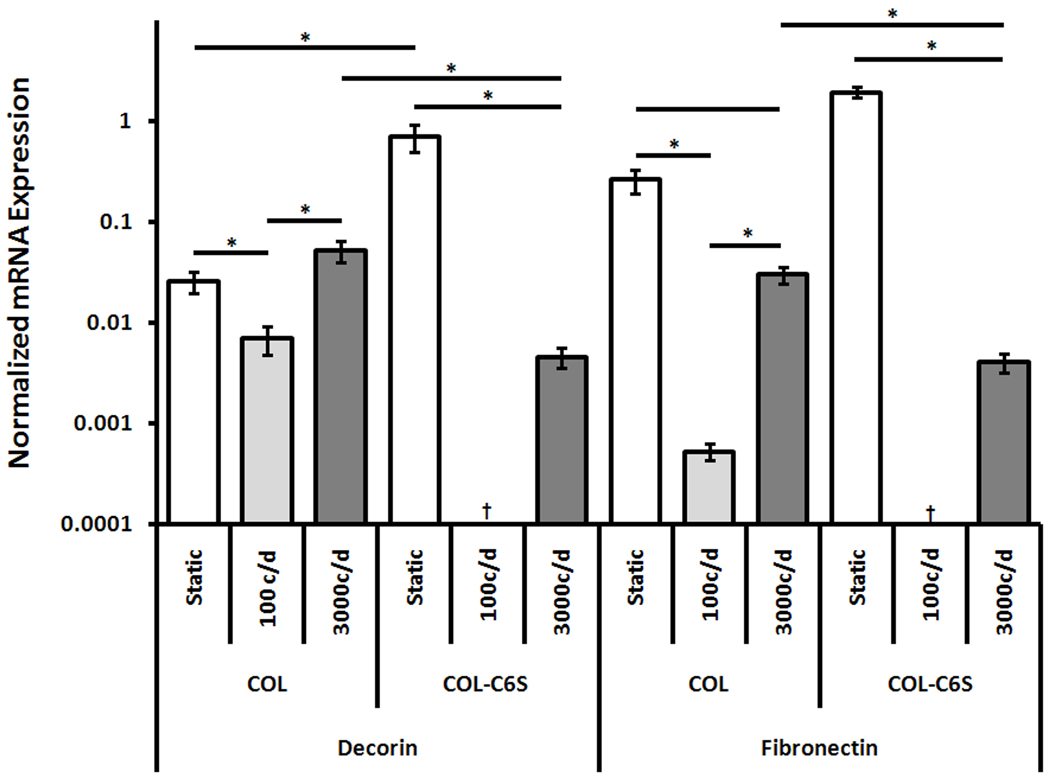
C6S incorporation did not alter TEC linear stiffness but did significantly affect nearly all mRNA expression levels (Fig. 3, 4). The addition of C6S significantly increased collagen type I (p = 0.049), decorin (p = 0.006) and fibronectin (p < 0.001) expression. Neither COL nor COL-C6S constructs expressed collagen type III.
Figure 3. mRNA Expression of Collagen Types I and III Normalized by GAPDH (Mean±SEM).
The addition of C6S increased collagen type I expression when TECs were cultured statically and stimulated with 3000 cycles/day. Mechanical stimulation with 3000 cycles/day increased collagen type I expression. c/d, cycles/day; ‡, No Expression; †, No RNA. * p < 0.05.
Figure 4. mRNA Expression of Decorin and Fibronectin Normalized by GAPDH (Mean±SEM).
The addition of C6S increased decorin and fibronectin expression when TECs were cultured statically but reduced expression when TECs were stimulated with 3000 cycles/day. c/d, cycles/day; ‡, No Expression; †, No RNA. * p < 0.05.
100 cycles/day
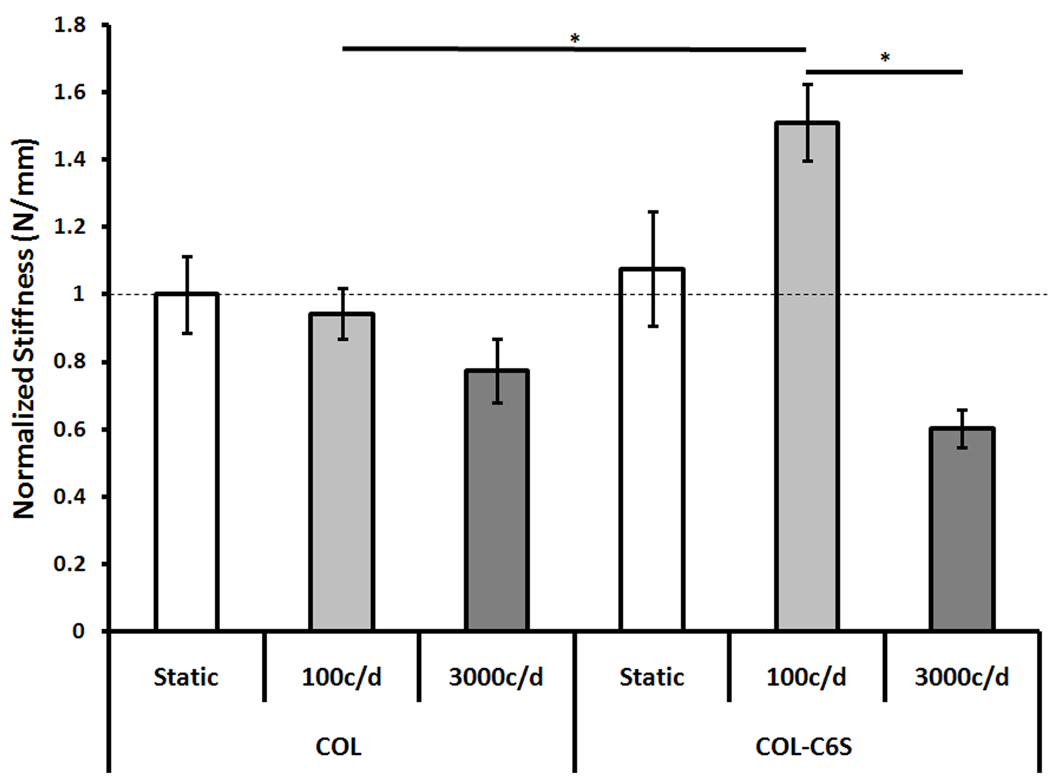
C6S incorporation produced a significant increase in TEC linear stiffness (p = 0.016; Fig. 2). However, adding C6S resulted in no detectable RNA expression.
Figure 2. Linear Stiffness Normalized by Static Control (Mean±SEM).
The addition of C6S increased TEC stiffness when constructs were stimulated with 100 cycles/day. However, mechanical stimulation did not significantly improve linear stiffness above static controls. c/d, cycles/day. * p < 0.05.
3000 cycles/day
C6S incorporation had no effect on TEC linear stiffness but did significantly alter gene expression patterns (Fig. 3, 4). Adding C6S significantly increased collagen type I expression (p = 0.002) but significantly decreased expression levels of collagen type III (p < 0.001), decorin (p = 0.041), and fibronectin (p = 0.009).
Effect of Cycle Number on TEC Linear Stiffness and Gene Expression
COL constructs
Cycle number had no effect on linear stiffness but did significantly affect mRNA expression of collagen types I and III, decorin, and fibronectin (Fig. 3, 4). Collagen type I expression was highest for constructs stimulated with 3000 cycles/day (p < 0.001), followed by statically cultured constructs and those stimulated with 100 cycles/day (p = 0.016). Collagen type III expression was also highest for constructs stimulated with 3000 cycles/day (p < 0.001). Decorin expression in constructs cultured statically and stimulated with 3000 cycles/day were both higher than those stimulated with 100 cycles/day (p ≤ 0.046) but were not different than each other. Fibronectin expression was highest for constructs cultured statically (p ≤ 0.012) and constructs stimulated with 3000 cycles/day had higher expression than those stimulated with 100 cycles/day (p = 0.001).
COL-C6S constructs
Increasing cycle number from 100 to 3000 cycles/day significantly decreased TEC linear stiffness (p < 0.001). However, mechanical stimulation with 100 or 3000 cycles/day did not improve construct stiffness above those cultured statically (Fig. 2). Introducing mechanical stimulation also affected COL-C6S mRNA expression levels (Fig. 3, 4). Stimulating COL-C6S constructs with 3000 cycles/day increased expression of collagen type I (p = 0.001) but decreased expression of decorin (p = 0.005) and fibronectin (p < 0.001) when compared to static controls. Collagen type III was only expressed by constructs stimulated with 3000 cycles/day.
Interactive Effects of C6S Incorporation and Cycle Number on TEC Linear Stiffness and Gene Expression
C6S incorporation and cycle number interacted to significantly affect both construct linear stiffness and mRNA expression levels of collagen type I and fibronectin. The combination of C6S incorporation and stimulation with 100 cycles/day significantly increased TEC stiffness above COL constructs stimulated with both 100 and 3000 cycles/day (p = 0.016 and 0.002, respectively). Adding C6S in conjunction with 3000 cycles/day of stimulation significantly increased mRNA expression of collagen type I above all treatment groups involving COL constructs (p ≤ 0.002). Under static culture conditions, the addition of C6S produced the highest fibronectin expression (p < 0.001). Additionally, in combination with 3000 cycles/day of stimulation, the addition of C6S significantly increased fibronectin expression above COL constructs stimulated with 100 cycles/day (p = 0.029) but decreased expression when compared to static COL constructs (p = 0.022).
Discussion
This study was designed to examine how incorporating chondroitin-6-sulfate (C6S) into the scaffold and mechanical stimulation into the culture period affect linear stiffness and mRNA expression levels of MSC-collagen sponge tissue engineered constructs (TECs). Each factor uniquely affected the biochemical and biomechanical responses of the TEC. Incorporating C6S increased nearly all of the mRNA levels under static culture and increased linear stiffness when TECs were exposed to 100 cycles/day of mechanical stimulation. The benefits of incorporating C6S and mechanical stimulation may be attributed to altered cell-matrix and matrix-matrix interactions.
Gene expression levels produced by COL-C6S constructs may be correlated with the nutrient levels available to the cells. Increased mRNA levels of COL-C6S constructs cultured statically could be due to a higher concentration of nutrients reaching the cells. The negatively charged C6S incorporated into the collagen sponge should make the scaffold swell and increase the water content. If media is pulled into the scaffold, the cells may be provided with a greater influx of nutrients, allowing them to be more metabolically active. However, when mechanical stimulation with 3000 cycles/day was introduced, mRNA expression for nearly all genes decreased. This may be attributed to the fact that when TECs are strained during stimulation, the TEC thickness decreases during each stimulation cycle due to Poisson’s effect. The media could be forced out of the scaffold and limit the cells’ ability to use the nutrients. Therefore, the benefit of incorporating C6S appears to be attenuated in the presence of mechanical stimulation.
Cellular adaptation to mechanical loading is not only affected by the loading frequency, or cycle number, but also by cell-matrix interactions.36 The reduced mRNA levels or lack of RNA for COL and COL-C6S constructs, respectively, when exposed to 100 cycles/day may be due to either reduced cell viability or reduced cell activity. In an attempt to reduce strain or because they were being stress shielded by the stiff construct matrix, the cells may have released integrins, and potentially detached.36 On the other hand, when cells are engaged in persistent sub-threshold interactions, they can become insensitive to activation37 and potentially quiescent. In contrast, stimulation with 3000 cycles/day may have surpassed this sub-threshold level of stimulation and contributed to increased mRNA expression of fibrillar genes. However, if matrix deposition occurred, it was likely balanced by some level of matrix degradation, as evidenced by unaltered linear stiffness with 3000 cycles/day of stimulation.
The increase in linear stiffness produced by C6S incorporation when TECs were exposed to 100 cycles/day of stimulation may be attributed to altered cell-matrix interactions. If type I collagen and C6S deform differently in response to uniaxial tension, this would alter cell-matrix interactions and potentially cellular adaptation to mechanical stimulation. Additionally, C6S molecules within the scaffold have the potential to form interfibrillar bonds that act as a link between discontinuous collagen fibrils.22, 23 Collagen-C6S interactions could aid in distributing the mechanical signal throughout the construct, consequently producing a more homogenous, rather than localized, response to the stimulus. These matrix-matrix interactions may contribute to the cell-mediated effects discussed above.
Our current results disagree with several of the findings from a previous study in our laboratory which demonstrated that mechanical stimulation (2.4% strain, 8 hours/day for 12 days) of MSC-collagen sponge TECs improved linear stiffness and mRNA expression of collagen types I and III.2, 13–15, 27 Using a commercially-derived collagen sponge (CD-COL; Kensey-Nash, Exton, Pa), the TECs showed orders of magnitude higher mRNA expression levels than the current TECs created using COL and COL-C6S sponges. To help understand these differences, we compared the structure of CD-COL sponges with those of COL and COL-C6S sponges. Scanning electron microscopy revealed that, despite using the same source of raw material, the structure of the CD-COL sponge was qualitatively and quantitatively different than that of COL and COL-C6S (Fig. 1). CD-COL sponges were comprised of thick reticulations of collagen with larger pores and a wide pore size distribution while both the COL and COL-C6S sponges contained thin collagen reticulations with smaller and more uniform pores (Fig.1). Additionally, the average pore diameter within the CD-COL sponge (142.5±16.1um; mean±SD) was approximately three times that of the COL and COL-C6S groups (55.4±2.4um and 53.1±8.9um, respectively). However, peak denaturation temperature, a measure of relative crosslink density, was not significantly altered by processing the CD-COL (80.4±4.5°C; mean±SD), COL (83.0±1.4°C) and COL-C6S sponges (80.2±1.4°C). These results suggest that other factors, like pore size and pore size distribution, may be important to control in scaffold materials. A study is currently underway to identify the optimal pore size for rabbit MSCs on a collagen-C6S sponge to ensure sufficient linear stiffness and enhanced expression of relevant genes important in tendon repair.
The influence of MCS-collagen sponge TEC mRNA expression levels at the time of surgery on repair tissue biomechanics remains unclear. Collagen types I and III are both important in tendon healing with the ratio of collagen type III to collagen type I increasing early in tendon healing before eventually decreasing during the remodeling phase.32 Decorin, the predominant proteoglycan in tensile load-bearing tendon, mediates type I collagen fibrillogenesis and matrix assembly25, 26 and participates in fibril-to-fibril force transfer.23 Fibronectin plays a key role in ECM-cell interactions such as adhesion, migration, growth and differentiation33 and also serves to mediate post-translation collagen fibril modifications and assembly.34 Despite understanding the function of these factors, we still do not know the magnitude and timing of expression needed to dramatically improve repair biomechanics in our PT defect model. A future paired in vitro-in vivo study should help us to better understand how in vitro mRNA expression relates to repair biomechanics in vivo.
There are several limitations to consider. 1) Since TEC aspect ratio was approximately 2:1, end effects could have influenced the mechanical properties. However, low aspect ratio has been shown to have a greater impact on failure properties than the sub-failure linear stiffness which we monitored.35 2) Stimulation-based increases in TEC stiffness have been attributed to increased collagen fibril alignment and ECM deposition by MSCs. However, TEC architecture and cellular contribution to TEC stiffness were not examined in this study. Future studies will implement methods such as SEM, TEM or FTIR to evaluate not only TEC architecture (pore size, pore size distribution, etc.) but also potential inter-fibrillar bonds between collagen and C6S. We will also compare acellular and cellular TEC stiffness at various time points in the culture period to understand how cells affect TEC biomechanics. 3) Gene expression data was only collected after two weeks in culture. Consequently, we do not yet understand the temporal changes in mRNA expression. We plan to add time points for gene expression in future studies to help understand the development of TECs in culture. 4) Type I collagen production was not evaluated. Since the sponge scaffold is collagen-based, it is difficult to differentiate newly synthesized collagen from matrix collagen. Although immunohistochemical methods are available to stain for type I pro-collagen, the concern is that the collagen may not be integrated into the matrix. Future studies may incorporate radio-labeling to quantify the collagen produced by the MSCs. 5) Cellular viability (living vs. dead) and activity (proliferating vs. quiescent) were not assessed in our constructs. Understanding the viability and activity of our constructs would help clarify whether the lower mRNA expression produced by COL and COL-C6S constructs was due to a lack of viable cells or reduced cellular activity.
In conclusion, this study demonstrates that incorporating C6S and applying 100 cycles/day of mechanical stimulation increases the linear stiffness of MSC-collagen sponge TECs. While C6S incorporation and cycle number each play an important role in gene expression of COL and COL-C6S TECs, their impact is not mutually exclusive. Instead, their interaction was found to produce a benefit for TEC linear stiffness. However, these in vitro results need to be paired with an in vivo study before we can conclude whether these treatments will have a significant impact on tendon healing.
Acknowledgements
This research was partially supported by a grant from the National Institutes of Health (AR 46574) given to the University of Cincinnati. The authors also wish to thank Abhishek Jain for assistance with biomechanical testing.
References
- 1.Praemer A, Furner S, Rice D. Musculoskeletal conditions in the united states. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1999. [Google Scholar]
- 2.Juncosa-Melvin N, Shearn JT, Boivin GP, et al. Effects of mechanical stimulation on the biomechanics and histology of stem cell-collagen sponge constructs for rabbit patellar tendon repair. Tissue Eng. 2006;12:2291–2300. doi: 10.1089/ten.2006.12.2291. [DOI] [PubMed] [Google Scholar]
- 3.McGuire DA, Hendricks SD. Allografts in sports medicine. Oper Tech Sports Med. 2007;15:46–52. [Google Scholar]
- 4.Baer GS, Harner CD. Clinical outcomes of allograft versus autograft in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26:661–681. doi: 10.1016/j.csm.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Woo SL, Vogrin TM, Abramowitch SD. Healing and repair of ligament injuries in the knee. J Am Acad Orthop Surg. 2000;8:364–372. doi: 10.5435/00124635-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Qin T, Yang Z, Wu Z, et al. Adhesion strength of human tenocytes to extracellular matrix component-modified poly(DL-lactide-co-glycolide) substrates. Biomaterials. 2005;26:6635–6642. doi: 10.1016/j.biomaterials.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 7.Sahoo S, Ouyang H, James C, et al. Characterization of a novel polymeric scaffold for potential application in tendon/ligament tissue engineering. Tissue Eng. 2006;12:91–99. doi: 10.1089/ten.2006.12.91. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Ramanath HS, Wang D. Tendon tissue engineering using scaffold enhancing strategies. Trends Biotechnol. 2008;26:201–209. doi: 10.1016/j.tibtech.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Cornwell KG, Lei P, Andreadis ST, Pins GD. Crosslinking of discrete self-assembled collagen threads. J Biomed Mater Res A. 2007;80:362–371. doi: 10.1002/jbm.a.30893. [DOI] [PubMed] [Google Scholar]
- 10.Guilak F, Butler DL, Mooney D, Goldstein SA. Functional tissue engineering. New York: Springer-Verlag; 2003. 480 pp. [Google Scholar]
- 11.Butler DL, Juncosa-Melvin N, Boivin GP, et al. Functional tissue engineering for tendon repair. J Orthop Res. 2008;26:1–9. doi: 10.1002/jor.20456. [DOI] [PubMed] [Google Scholar]
- 12.Awad HA, Boivin GP, Dressler MR, et al. Repair of patellar tendon injuries using a cell-collagen composite. J Orthop Res. 2003;21:420–431. doi: 10.1016/S0736-0266(02)00163-8. [DOI] [PubMed] [Google Scholar]
- 13.Juncosa-Melvin N, Matlin KS, Holdcraft RW, et al. Mechanical stimulation increases collagen type I and collagen type III gene expression of stem cell-collagen sponge constructs for patellar tendon repair. Tissue Eng. 2007;13:1219–1226. doi: 10.1089/ten.2006.0339. [DOI] [PubMed] [Google Scholar]
- 14.Shearn JT, Juncosa-Melvin N, Boivin GP, et al. Mechanical stimulation of tendon tissue engineered constructs. J Biomech Eng. 2007;129:848–854. doi: 10.1115/1.2800769. [DOI] [PubMed] [Google Scholar]
- 15.Nirmalanandhan VS, Dressler MR, Shearn JT, et al. Mechanical stimulation of tissue engineered tendon constructs: Effect of scaffold materials. J Biomech Eng. 2007;129:919–923. doi: 10.1115/1.2800828. [DOI] [PubMed] [Google Scholar]
- 16.Malaviya P, Butler DL, Korvick DL, Proch FS. In vivo tendon forces correlate with activity level and remain bounded. J Biomech. 1998;31:1043–1049. doi: 10.1016/s0021-9290(98)00123-7. [DOI] [PubMed] [Google Scholar]
- 17.Juncosa N, West JR, Galloway MT, et al. In vivo forces used to develop design parameters for tissue engineered implants for rabbit patellar tendon repair. J Biomech. 2003;36:483–488. doi: 10.1016/s0021-9290(02)00459-1. [DOI] [PubMed] [Google Scholar]
- 18.West JR, Juncosa N, Galloway MT, et al. Characterization of in vivo Achilles tendon forces in rabbits during treadmill locomotion at varying speeds and inclinations. J Biomech. 2004;37:1647–1653. doi: 10.1016/j.jbiomech.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 19.Korvick DL, Cummings JF, Grood ES, et al. The use of an implantable force transducer to measure patellar tendon forces in goats. J Biomech. 1996;29:557–561. doi: 10.1016/0021-9290(95)00036-4. [DOI] [PubMed] [Google Scholar]
- 20.Yannas IV, Burke JF. Design of an artificial skin I. J Biomed Mater Res. 1980;14:65–81. doi: 10.1002/jbm.820140108. [DOI] [PubMed] [Google Scholar]
- 21.Yannas IV, Burke JF, Gordon PL. Design of an artificial skin II. J Biomed Mater Res. 1980;14:107–131. doi: 10.1002/jbm.820140203. [DOI] [PubMed] [Google Scholar]
- 22.Scott JE, Orford CR, Hughes EW. Proteoglycan-collagen arrangements in developing rat tail tendon. Biochem J. 1981;195:573–581. doi: 10.1042/bj1950573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Redaelli A, Vesentini S, Soncini M, et al. Possible role of decorin glycosaminoglycans in fibril to fibril force transfer in relative mature tendons. J Biomech. 2003;36:1555–1569. doi: 10.1016/s0021-9290(03)00133-7. [DOI] [PubMed] [Google Scholar]
- 24.Yoon JH, Halper J. Tendon proteoglycans. J Musculoskelet Neuronal Interact. 2005;5:22–34. [PubMed] [Google Scholar]
- 25.Zhang G, Ezura Y, Chervoneva I, et al. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem. 2006;98:1436–1449. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
- 26.Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj J. 2002;19:249–255. doi: 10.1023/A:1025383913444. [DOI] [PubMed] [Google Scholar]
- 27.Nirmalanandhan VS, Rao M, Shearn JT, et al. Effect of scaffold material, construct length and mechanical stimulation on the in vitro stiffness of the engineered tendon construct. J Biomech. 2008;41:822–828. doi: 10.1016/j.jbiomech.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Boyce ST, Christianson DJ, Hansbrough JF. Structure of a collagen-GAG dermal skin substitute optimized for cultured human epidermal keratinocytes. J Biomed Mater Res. 1988;22:939–957. doi: 10.1002/jbm.820221008. [DOI] [PubMed] [Google Scholar]
- 29.Guehring T, Omlor GW, Lorenz H, et al. Stimulation of gene expression and loss of anular architecture caused by experimental disc degeneration. Spine. 2005;30:2510–2515. doi: 10.1097/01.brs.0000186591.17114.e9. [DOI] [PubMed] [Google Scholar]
- 30.Boykiw R, Sciore P, Reno C, et al. Altered levels of extracellular matrix molecule mRNA in healing rabbit ligaments. Matrix Biology. 1998;17:371–378. doi: 10.1016/s0945-053x(98)90089-0. [DOI] [PubMed] [Google Scholar]
- 31.Velling T, Risteli J, Wennerberg K, et al. Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins α11β1 and α2β1. J Biol Chem. 2002;277:37377–37381. doi: 10.1074/jbc.M206286200. [DOI] [PubMed] [Google Scholar]
- 32.Frank C, Schachar N, Dittrich D. Natural history of healing in the repaired medial collateral ligament. J Orthop Res. 1983;1:179–188. doi: 10.1002/jor.1100010209. [DOI] [PubMed] [Google Scholar]
- 33.Yamada KM. Fibronectins. Curr Opin Cell Biol. 1989;1:956–963. doi: 10.1016/0955-0674(89)90065-3. [DOI] [PubMed] [Google Scholar]
- 34.Banes AJ, Link GW, Bevin AG, et al. Tendon synovial cells secrete fibronectin in vivo and in vitro. J Orthop Res. 1988;6:73–82. doi: 10.1002/jor.1100060110. [DOI] [PubMed] [Google Scholar]
- 35.Cook RD. Advanced mechanics of materials. Prentice Hall. 1999:1–2. [Google Scholar]
- 36.Banes AJ, Tsuzaki M, Yamamoto J, et al. Mechanoreception at the cellular level. Biochem Cell Biol. 1995;73:349–365. doi: 10.1139/o95-043. [DOI] [PubMed] [Google Scholar]
- 37.Grossman Z, Paul WE. Adaptive cellular interactions in the immune system. Proc Natl Acad Sci. 1992;89:10365–10369. doi: 10.1073/pnas.89.21.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]