Abstract
OBJECTIVES
To evaluate whether a novel GPS-like position-sensing technology will enable accurate co-registration of images between imaging modalities.
BACKGROUND
Co-registration of images obtained by different imaging modalities will allow for comparison and fusion between imaging modalities, and therefore has significant clinical and research implications. We compared US and MR images of carotid endarterectomy (CEA) specimens using a novel position-sensing technology that uses an electromagnetic (EM) transmitter and sensors mounted on a US transducer. We then evaluated in vivo US-US and US-MRI co-registration.
METHODS
Thirteen CEA specimens underwent 3.0 Tesla MRI, after which images were uploaded to a LOGIQ E9 3D (GE Healthcare) US system and registered by identifying 2–3 common points. A similar method was used to evaluate US-MRI co-registration in patients with carotid atherosclerosis. For carotid intima-media thickness (C-IMT) measurements, ten volunteers underwent bilateral carotid US scans co-registered to 3D US maps created on the initial visit, with a repeat scan 2 days later.
RESULTS
For the CEA specimens, there was a mean 20 (standard error [SE] 2.0) frames per MRI slice. The mean frame difference, over 33 registration markers, between MR and US images for Readers 1 and 2 was −2.82 ± 19.32 (mean ± 95% confidence interval [CI]) frames and 2.09 ± 14.68 (mean ± 95% CI) frames, respectively. The US-MRI intraclass correlation coefficients (ICC) for the first and second readers were 0.995 and 0.997, respectively. For patients with carotid atherosclerosis, the mean US frames per MRI slice (9 [SE 2.3]) was within range of that observed with CEA specimens. Inter-visit, intra-reader, and inter-reader reproducibility of C-IMT measurements were consistently high (side-averaged ICC >0.9).
CONCLUSION
Accurate co-registration between US and other modalities is feasible with a GPS-like technology, which has significant clinical and research applicability.
Introduction
All modalities used for imaging atherosclerosis have inherent advantages and disadvantages (1). For example, magnetic resonance imaging (MRI) provides highly detailed, structural, and compositional information but suffers from relatively long study times and poor temporal resolution (2, 3). In contrast, ultrasonography provides real-time imaging with excellent temporal resolution, but image quality can be variable. Combining imaging modalities could theoretically allow their strengths to complement each other and overcome their individual limitations. For example, the portability and immediate availability of real-time data from ultrasonography can be combined with highly detailed, anatomical information from MRI or computed tomography (CT) scans.
A key step for accurate comparisons between modalities involves co-registration, or matching anatomic features between imaging modalities (4). A novel advance in ultrasound (US) technology, namely real-time electromagnetic tracking of the US probe location and orientation, may now allow semi-automated registration between US and other imaging modalities. However, this technology has limited validation (5–7).
Therefore, we pursued the validation of this novel real-time electromagnetic tracking feature now available in a vascular US system, and compared the reliability of its co-registration system with manual registration. We first evaluated ex vivo co-registration between US and MRI using a set of carotid endarterectomy (CEA) surgical specimens and then, in pilot analyses, evaluated in vivo co-registration of US-US and US-MRI.
Methods
All experiments were approved by the institutional review board.
Ex Vivo Co-registration
Samples
Atherosclerotic plaques (n = 13) were obtained after CEA. Specimens were acquired 1–3 hours after surgical resection and preserved in phosphate buffered saline (PBS)/50% glycerol and stored at −20 ºC. This preservation method maintains the ultrastructural properties of the carotid plaque (8).
Sample Preparation
Immediately prior to use, specimens were dialyzed for 24 hours against PBS to remove the glycerol. Samples were then embedded in 3% [weight (grams)/ volume (milliliters)] low-melting agarose gel (FMC Bioproducts, Philadelphia, PA). The agarose gel solution was created by adding distilled water to agarose gel powder and heating the mixture to 60 °C. Prior to pouring the solution over tissue samples, vacuum suction was applied to the solution. We have found that application of vacuum suction to the solution after mixing minimizes the presence of air bubbles, which are hyperechogenic on US and interfere with US imaging. Once tissues were embedded, the solution was allowed to cool to room temperature prior to imaging.
Agarose gel was chosen as a support matrix for several reasons: 1) to minimize damage from tissue manipulation, 2) to preserve spatial arrangement of the morphological features for comparisons between imaging modalities, 3) to minimize chemical interactions with biological tissue which may interfere with MRI and US imaging, and 4) to provide a support medium necessary for US imaging. Plastic pipette tips were embedded near one end of the CEA sample to provide a non-anatomic reference point.
MRI
Magnetic resonance (MR) images were acquired after the CEA samples were embedded in 3% agarose gel in 15 mL culture tubes. These tubes were placed in a custom-built holder designed specifically for acquiring tissue MR images (9). Specimens were imaged using a 6 cm phased array 4-channel carotid coil (Pathway Med Tech, Redmond, WA) on an Excite 3.0 Tesla MRI scanner (General Electric, Wauwatosa, WI). Serial axial proton-density weighted (PDW), T1-weighted (T1W), and T2-weighted (T2W) images were acquired (2-mm slice thickness, matrix 512×512, FOV 100 mm × 100 mm) using a fast-spin echo sequence providing 8–31 slices depending on tissue size with an in-plane resolution of ~0.195 mm. Correction algorithms adjusted for magnetic field strength gradients across the sample image.
Real-time 3D Ultrasound
Following MRI, each of the 13 CEA tissue samples embedded in agarose was extruded intact with the agarose column from its culture tube. The column was then transferred to a plastic box (10.5 × 12.5 × 4.0 cm) and additional 3% molten agarose was added to form an agarose bed prepared as described under the sample preparation section. This procedure was performed to provide adequate surface contact for the ultrasound probe. Samples were imaged with a LOGIQ E9 ultrasound (US) system (General Electric, Wauwatosa, WI) using a “free-hand 3D scanning with electromagnetic field sensors” approach. This approach used a mid-range DC magnetic transmitter to generate a weak magnetic field. Position sensors attached to a 2D vascular US probe sensed this field and recorded the probe position through an in-built circuit system, which functioned similar to a global position system (GPS), thereby allowing the computer to know the position and orientation of the transducer and allowing it to reconstruct 3D volumes (10).
Automated Co-registration
The PDW sequences of the MR images of a given CEA sample were uploaded from a CD-ROM to the US system, and manual marking of 2–3 features was performed between the MR images and a real-time B-mode US scan. The PDW sequence was selected over T1W and T2W, since the PDW sequences were found to have the best image quality (reduced noise, reduced motion artifacts, reduced blurring, and sharp edges). Common features used for initial marking included the plastic marker tip, calcifications, and, if present, the “bifurcation.” After 2–3 common points between the MR images and real-time US scans were marked, the US instrument ran an algorithm to create a transformation matrix, which mapped the 2D probe position to a multiplanar reconstruction of MR images (11). The real-time US scan was now registered to the MR images, such that movement of the US probe resulted in movement through reconstructed MR image planes, displayed next to the US scan.
Validation of Ultrasound Co-registration by Manual Methods
All image data were exported in Digital Imaging and Communications in Medicine (DICOM) format to CD-ROM’s for offline analysis. Image sets were loaded onto a free DICOM viewer. Each image in a set had 3 components: 1) an US image, 2) an US frame number which identified the order of the image in the series, and 3) an MR image co-registered such that several US frames in the sweep would have the same MR image (i.e., more US frames than MR image slices) (Figure 1).
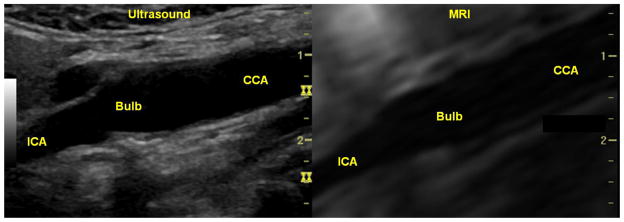
Figure 1. Real-time US scan co-registered with MR images.
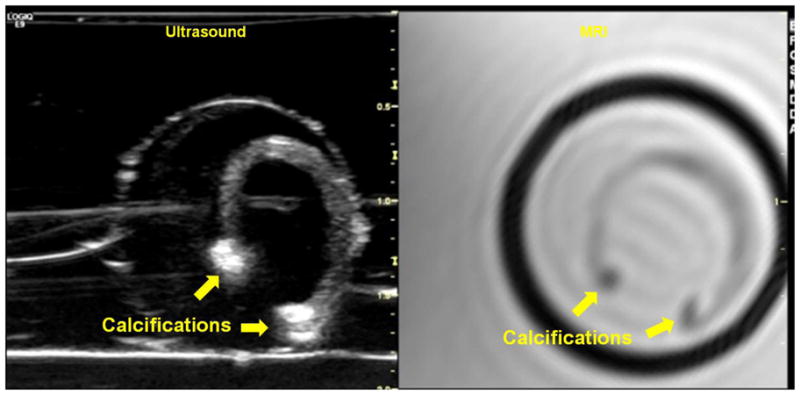
Transverse US scan of a carotid endarterectomy (CEA) tissue specimen (left) co-registered with its corresponding axial MRI slice (right). The specimen appears u-shaped as a result of being sliced longitudinally during surgical resection. The calcifications at both ends of the specimen appear as bright hyperechoic regions on US and dark hypointense regions on the MRI. The circular ring enclosing the tissue specimen on US represents the interface between the surrounding agarose bed and the original agarose cylinder formed when the tissue was initially embedded in a 15 mL cylindrical tube for MR imaging.
Initially, only the US image was blinded out of the 3 components, and 2 readers jointly identified 2–3 features on the MR image per CEA tissue sample. The US frame number for a feature and a description of the feature were noted. The total number of MR images per tissue sample was also noted.
After all features were identified, the readers independently loaded the image sets in a pre-randomized order unique to each reader, and now they were blinded to the US frame number and MR image and unblinded to the US image. Each reader identified the previously described MRI features on the US images. After a feature was satisfactorily identified on a US image, the US frame number was unblinded and recorded as the manually registered frame for that feature.
In Vivo Co-registration
Reproducibility of Carotid Intima-medial Thickness (C-IMT): Ultrasound-Ultrasound co- registration
We then examined application of the co-registration technology for improving the reproducibility of C-IMT measurements. Healthy individuals (n=10) without coronary heart disease, stroke, peripheral vascular disease, hypertension, or current smoking were invited to participate. All volunteers underwent bilateral US scans of the common carotid artery (CCA) for C-IMT measurements on a LOGIQ E9 US machine. Scans were acquired using traditional methodology and using the GPS-like technology. Repeat scans using both methods were performed 2 days later. Neither the traditional method nor the GPS method was ECG gated.
The traditional method involved use of a Meijer’s arc for standardizing the probe angle. Volunteers were positioned supine with the neck slightly hyperextended and rotated ~30° from midline contralateral to the side imaged. The angle on the Meijer’s arc was recorded on the initial visit, and the same angle was used for imaging during the return visit.
For the GPS method, a 3D US sweep of the CCA was performed on the initial visit to create a 3D US map, which was stored. For measurement of C-IMT, both during the initial and return visits, real-time US scans were obtained and co-registered to the 3D map with the GPS-like technology (Figure 2). Semi-automated feature registration using co-registration of corresponding planes followed by point-to-point co-registration (i.e., plane-point method) was performed between the real-time US and the 3D map. The plane containing the carotid bifurcation became the internal registration plane for each subject. The carotid bifurcation served as the registration region.
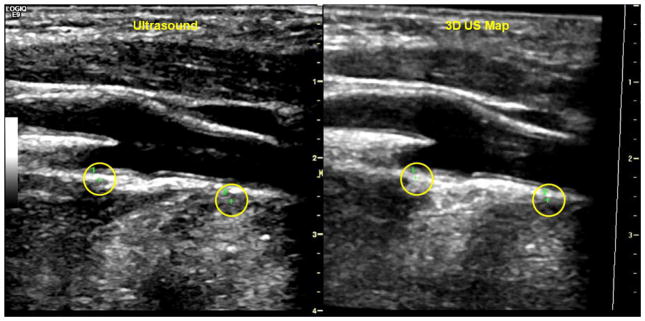
Figure 2. Real-time longitudinal carotid artery scan at the bifurcation registered with a stored 3D US map with green cross markers.
Carotid US scan (left) co-registered to 3D map (right). Green crosses (circled) seen in both the left and right panel indicates that the scan plane was aligned with chosen features on the 3D map. The first cross, labeled with a 1, is located in the far wall of the internal carotid artery just superior to the bifurcation. The second marker, labeled with a 2, is located in the far wall of the common carotid artery about a centimeter inferior to the beginning of the bulb.
Once the plane-point co-registration was completed during the initial visit, point markers (i.e., GPS markers) were placed on the co-registered real-time US scan and saved along with the 3D US map. These markers which appeared as green crosses when the scan plane was aligned with the markers and as red or blue boxes when the scan plane was directed away from the markers were used to identify the scan plane/ image sequence where the C-IMT measurements will be made. After the plane-point co-registration was completed on the return visit, the GPS markers were loaded from memory enabling the operator to return to the location of the scan plane where in C-IMT measurements will be made.
All scans were de-identified and exported in DICOM format for offline processing on a workstation equipped to measure C-IMT (Carotid Analyzer, Medical Imaging Applications LLC, Coralville, IA). The C-IMT measurements took place approximately 2 months after image acquisition. For each side, the mean C-IMT value of the far wall of the distal CCA over a 1 cm length was measured and the combined average of the right and left sides were used for our analysis. Two trained readers blinded to method and to visit order performed the measurements. During the analysis, the readers noted that the quality of the image sequences varied and so they performed a post hoc, blinded, subjective image quality assessment.
US-MRI Co-registration in Patients with Known Carotid Atherosclerosis
To test US-MRI co-registration in a clinical setting, we invited a subset of individuals (n=3, 6 carotid arteries) participating in an ongoing trial (ClinicalTrials.gov NCT00860184) for which Baylor College of Medicine is one center to participate in an ancillary study. The trial enrolls and follows asymptomatic subjects with 50–79% carotid artery stenosis in whom a carotid MRI is performed at baseline. The ancillary study which added 3D ultrasound imaging was in addition approved by the trial principal investigator.
The MRI was first obtained using sequences specified by the trial protocol. The PDW MR image series obtained over an 8 cm length of carotid artery centered on the bifurcation of the carotid artery (2 mm slice thickness, 40 slices total) was then exported in DICOM format and uploaded to a LOGIQ E9 US system. For the US scan, we positioned the subjects similarly to that used for MR imaging to achieve in-plane registration.
The co-registration method between real-time scans and uploaded MR images was similar to that used for ex vivo co-registration between US and MRI. Briefly, real-time US scans were obtained and co-registered to the uploaded MR image using semi-automated feature registration of corresponding planes followed by point-to-point registration (Figures 3 & 4). Validation by manual methods was conducted as described for CEA specimens; we used the disappearance of the flow divider as a common feature to evaluate registration between the imaging modalities.
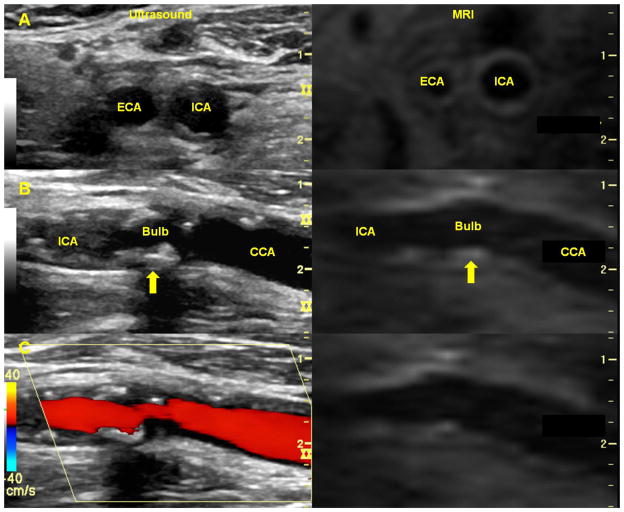
Figure 3. Real-time long-axis carotid US co-registered to a corresponding PDW MRI series in a patient with minimal carotid atherosclerosis in the vessel imaged.
Real-time long-axis US of the right carotid artery (left) co-registered with the corresponding MRI scan (right) in a patient with minimal carotid atherosclerosis in the vessel imaged. In both images, the common carotid artery is seen starting on the right, expanding to the carotid bulb, and tapering down to the internal carotid artery on the left. ICA = internal carotid artery; CCA = common carotid artery.
Figure 4. Real-time carotid co-registered to corresponding PDW MRI series in a patient with carotid atherosclerosis.
In all panels, the real-time carotid US (left) has been co-registered with the corresponding PDW MR series (right). Panel A shows transverse views of the carotid arteries just after the carotid bifurcation with the external carotid artery (ECA) to the left of the internal carotid artery (ICA). The vessel wall boundaries can be seen clearly on the MR image. Long-axis views of the same artery can be seen in Panel B with the ICA on the left and the common carotid artery (CCA) on the right. Multiple regions of calcified plaque appear as bright hyperechoic regions on the real-time US. The plaques may not appear on the corresponding PDW MR images, since calcifications appear as dark hypointense regions on MR images. Corresponding regions of plaque (arrows) have been marked in the carotid bulb. Color Doppler has been overlaid on the real-time US in Panel C.
Statistical Methods
The MRI slices per sample, US frames per sample, and US frames per co-registered MRI slice were described using Stata 11 (Stata, College Station, TX). Absolute agreement between US co-registered frames and manual co-registration were determined by calculating intraclass correlation coefficients (ICC) using a two-way random effect analysis of variance (ICC[2,1] method) model to test strength of absolute agreement (12). ICC’s were also calculated using a multi-level hierarchical model with mixed effects (13), which adjusted for variations within each CEA specimen. Bland-Altman plots were generated to examine for biases inherent to registration methods used and to evaluate 95% limits of agreement between mutual MRI-registered and independent US-registered frames (14).
For the C-IMT measurements performed for US-US co-registration, intraclass correlation coefficients (ICC[2,1] method) were used to determine inter-visit repeatability and inter- and intra-reader reproducibilities. Bland-Altman plots were also generated to examine for biases inherent between methods. For the US-MRI co-registration conducted in patients with known carotid atherosclerosis, the difference in frames for common features observed on US and MRI was noted and described.
Results
Ex Vivo Registration
Among the 13 CEA specimens imaged, 33 features were identified on the MRI series. We found a mean of 19.7 (S.E. 2.1) US scan frames within each registered MRI slice. The number of MRI slices per CEA specimen and US frames per specimen can be found in Table 1.
Table 1.
Parameters of each US scan registered to MRI series (mean ± S.E.)
| Parameter | Value |
|---|---|
| MRI slices per CEA specimen | 13.92 ± 1.05 |
| US frames per CEA specimen | 265.77 ± 28.58 |
| US frames between registered MRI slices | 19.66 ± 2.07 |
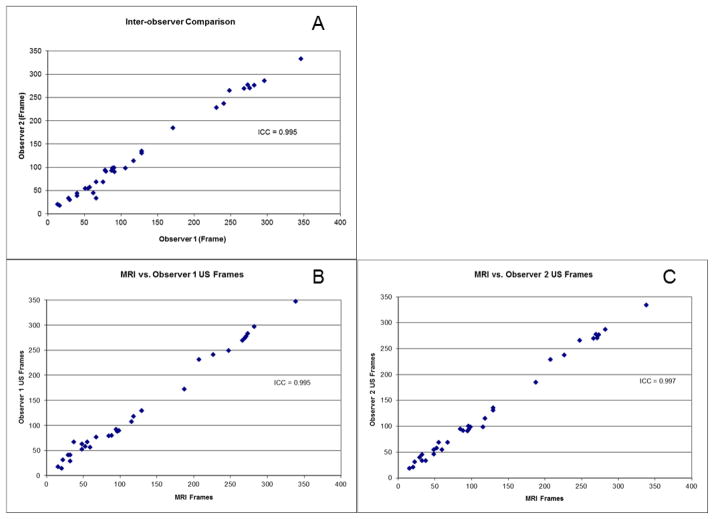
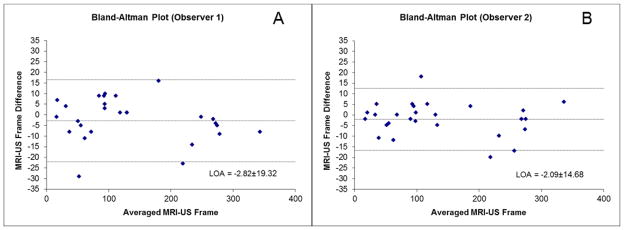
Inter-reader reproducibility using manual registration was excellent, with an intraclass correlation coefficient (ICC[2,1] method), or measure of absolute agreement, of 0.995 (Figure 5A). Correlation of the US system registered US frames with manually registered US frames was also excellent, with a composite ICC(2,1) of 0.995. ICC(2,1) ranged between 0.995 and 0.997 for each reader when manually registered US frame features were compared with the US system registered US frames (Figures 5B & 5C). In fact, after adjusting for variations at the CEA sample level using multi-level modeling, we still found the methods to have excellent agreement with no change in composite ICC.
Figure 5. Inter-observer manual reproducibility (A) and comparison between automated coregistration and co-registration by first (B) and second (C) readers.
Plots of US frames noted by observers on matched US features (A) and comparing manually registered US features by Readers 1 (B) and 2 (C) to the US system registeration.
Bland-Altman plots comparing the US system registered US frame for a MRI slice transition to manually registered US frame showed very low difference (−2.8 frames for Reader 1 and −2.1 frames for Reader 2) and limits of agreement within the mean US frames between MRI slices (−22.1 to 16.5 frames for Reader 1 and −16.8 to 12.6 frames for Reader 2) (Figure 5). Moreover, the 95% limits of agreement for the difference between methods for indexed frame number fell within the upper 95% limits of the mean number of US frames between MRI slices (24.2 indexed US frames between MRI slices). In other words, for a given co-registered MRI slice, US frames indexed by the manual registration method fell within the range of US frames attributed to that MRI slice by the US system registered method. For each observer, only 2 of 33 (6.1%) data points were beyond the limits of agreement and neither of these data points were from the same sample (i.e., points outside the limits of agreement came from different markers for different readers).
In Vivo Ultrasound-Ultrasound Registration for C-IMT measurement
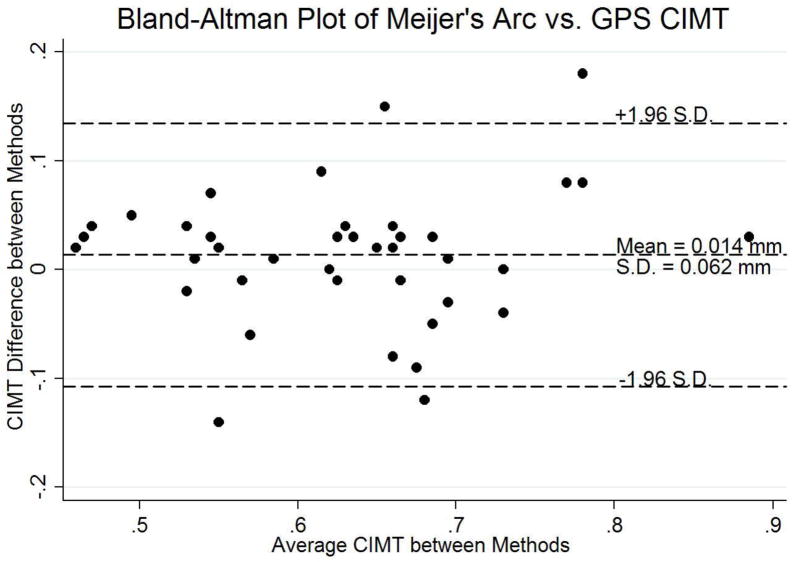
For C-IMT measurements, the mean far wall common C-IMT measured on images acquired with the Meijer’s arc and with the GPS-like technology were 0.61 (SE 0.03) mm and 0.63 (SE 0.03) mm, respectively, for the initial visit, and 0.64 (SE 0.03) mm and 0.64 (SE 0.04) mm, respectively, for the return visit. There was no significant difference in C-IMT measurements between sides and visits for the traditional method (p=0.66 and p=0.10, respectively) and for the GPS method (p=0.43 and p=0.22, respectively).
The mean difference between the two methods was small (0.01 [SD 0.06] mm) when averaged over both sides and visits (p=0.29) (Figure 7). The intra- and inter-reader reproducibilities were high using the GPS-like technology (ICC[2,1] of 0.91 and 0.93, respectively). The inter-visit correlation coefficient for C-IMT measurements on images from the GPS-like technology was consistently greater than those taken from Meijer’s arc (left ICC 0.93 vs. 0.72, right ICC 0.84 vs. 0.80, side-averaged 0.91 vs. 0.84, all respectively) (Table 2). Image qualitity was subjectively noted to be poor for 12 image sets with GPS based imaging as opposed to 1 for the traditional method. However, image quality was adequate for CIMT measurements in all image sets.
Figure 7. Bland-Altman plot (Meijer’s Arc vs. GPS C-IMT).
Bland-Altman plot comparing C-IMT measurements from US scans acquired with the Meijer’s arc vs. those acquired with the GPS-like technology.
Table 2.
Inter-method and inter-visit agreements. Inter-method intraclass correlation coefficients (ICC) type (2,1) between an image acquisition method using the Meijer’s arc and another method using a GPS-like technology are shown on the top half of the table. Inter-visit ICC(2,1) are shown in the bottom half.
| ICC Left | ICC Right | ICC Side-Averaged | |
|---|---|---|---|
| Meijer’s Arc v. GPS | |||
| Visit 1 | 0.94 | 0.78 | 0.90 |
| Visit 2 | 0.66 | 0.86 | 0.84 |
| Visit Average | 0.86 | 0.89 | 0.92 |
| Visit 1 v. Visit 2 | |||
| Meijer’s Arc | 0.72 | 0.80 | 0.84 |
| GPS | 0.93 | 0.84 | 0.91 |
In Vivo Ultrasound-MRI registration
Of the 6 carotid arteries analyzed, one was excluded as we realized during analysis that the wrong vessel was registered (i.e carotid artery was improperly identified on the MRI). Among the analyzed arteries, the absolute frame difference between appearance of a feature on MRI vs. US ranged from 4 to 15 frames per slice transition, with a mean number of 9 (SE 2.3) frames between MR image slices. When the US frame where the MRI slice transition (change) takes place was compared between the GPS-registered US frame and the manually co-registered US frame, there was a low difference (–4.2 frames). The limits of agreement for the mean number of US frames between MR image slices was –13.2 to 4.8 frames. No readings were outside the limits of agreement.
Discussion
Combining imaging modalities has potential value in research and the clinical practice of medicine. Current methods of combining imaging modalities have limitations. They integrate the hardware of two modalities, a setup which requires highly specialized technical expertise, or using post-processing computer algorithms, a process performed post facto (15, 16). A fully integrated system for co-registering real-time US to any imaging modality is now commercially available; however the reliability of the system for vascular use has not been tested.
We first tested the reproducibility of the US system ex vivo and showed excellent agreement (ICC >0.99) between the LOGIQ E9 co-registration and manual registration methods. We also found very low bias between the two methods (<3 frames difference between methods) with the 95% limits of agreement not exceeding the upper 95% estimate of US frames indexed per MRI slice. Therefore, the LOGIQ E9 co-registration method using the GPS-like system reliably co-registers real-time US with MRI of CEA specimens.
Then, we explored the use of the co-registration system for improving carotid intima-media thickness (C-IMT) measurements. We evaluated the use of the US system for creating 3D US maps of the carotid artery, which were then used as references to guide image acquisition in similar planes/ angles during subsequent imaging. We found that inter-visit repeatability was consistently higher using the GPS-like technology than using the traditional Meijer’s arc. Intra-reader repeatability and inter-reader reproducibility was also consistently high for the GPS-like technology. It must be noted that both US methods were not ECG-gated which in turn could have further improved the reproducibility of the CIMT measurements. Finally, image resolution was noted to be qualitatively lower for these scans compared with scans acquired using the Meijer’s arc, probably because image enhancements were disabled with the GPS-like technology. However, image enhancements can be enabled with the GPS-like technology, as seen in the US-MRI co-registration protocol with patients having known carotid atherosclerosis. Therefore we believe that good (regular) quality images can be obtained using this technology. In spite of the image enhancements being disabled, we still found higher inter-visit correlations for C-IMT measurements with scans using the GPS-like technology than with those using the Meijer’s arc. Overall our findings suggest that use of the GPS-like technology can improve the reproducibility of C-IMT measurements, and provide a basis for improving reliability of serial C-IMT comparisons.
Finally, we evaluated US-MRI in vivo co-registration in a small number of patients with known carotid atherosclerosis who were participating in an MRI study. The results in this pilot analysis were consistent with the ex vivo experiment. These results from the pilot in vivo analyses suggest that the reliable co-registration observed ex vivo may translate to in vivo clinical applications as well and warrant further clinical exploration.
Clinical Perspective
This “fusion” technology clearly has significant clinical and research applicability. From a clinical perspective, the technology can be used to map real-time US scans to previously acquired high-resolution MR image sets or images from other imaging modalities such as CT. A physician could therefore have the co-registered high-resolution images from the MRI or CT available for real-time navigation during a procedure to verify the anatomic location of interest and fuse the US information with the same images. Functional information gained from real-time US can be overlaid, or fused, with high-resolution images from different modalities for improving interpretation of the functional information. Another important clinical use could be recalling mapped US images in serial follow-up exams (as seen in our US-US registration experiment) to allow direct comparisons for changes (e.g., in dimensions) over time. With extension of the same technology to cardiac imaging one could think of the many potential scenarios where this technology will have clinical utility.
However, we must recognize that imaging cardiovascular structures which may pulsate, contract or twist will bring with it a set of challenges. For example, what would happen if the heart rate changes between the time of the MR/ CT imaging and the ultrasound? It is possible that using electrocardiography (ECG)-gating and developing computer algorithms that match phases of the cardiac cycle for volumetric comparisons one could circumvent this issue; however further work is needed to understand all the issues with such a scenario. Another potential issue (which is an issue with any current clinical imaging) would be irregular heart rate. Ultimately, the challenges for clinical implementation may depend on the purpose for fusing MR/ CT imaging with real-time US. If one desires high precision from the fusion say for example in a biopsy or a stent graft implantation perfect co-registration will be required. On the other hand if one is primarily interested in the functional significance of an anatomical finding or in harnessing the advantages specific to each imaging modality in evaluating an anatomical structure or its function, these challenges (e.g., pulsatility, etc.) may be less of an issue and the technology in its current form could readily be used to bring images from another imaging modality to ultrasound. For example, an area of interest not clearly seen on routine ultrasonography may be identified on MR/ CT imaging and its functional significance then interrogated after fusion with ultrasonography
Finally additional factors that will be critical in the adaptation of this technology and dictate clinical scenarios in which one will use this technology include the additional time required for the registration/fusion and technologist training in other imaging modalities, i.e. being able to identify orientation and anatomy in other imaging modalities, as evidenced by our mis-identification of one carotid artery during our in vivo ultrasound-MRI co-registration.
Research Perspective
For research, prospective clinical studies using B-mode US imaging are often criticized for poor scan plane reproducibility of anatomic locations, which can become highly significant for making very small measurements (17). For example, common C-IMT requires a measurement precision on the order of ~0.001 mm to detect progression, a precision that is sensitive to scan plane reproducibility (18). The American Society of Echocardiography recognized such an issue when releasing its 2008 C-IMT Consensus Statement and suggested the use of a Meijer’s Arc, an external reference system, to improve its reproducibility (19). However, US scans using the Meijer’s Arc are still subject to scan plane variations (although to a lesser degree). Furthermore, the C-IMT’s of the internal carotid artery and bifurcation suffer greater inter-observer variability than C-IMT’s of the common carotid and may benefit from improved scan plane reproducibility as well (20). Allowing co-registration may further improve the reproducibility of these measurements.
Our C-IMT study comparing this technology with traditional methods using the Meijer’s arc support the use of this technology for fulfilling a role in improving scan plane reproducibility for C-IMT measures and for general prospective studies using ultrasound imaging. Similarly, this technology could have great use in studies that use echocardiographic end points to ensure that measurements are made in similar planes. At the very least, the technology may simply allow for serial follow-up of an area of interest on US and allow for comparisons of real-time US with other imaging modalities.
Limitations
This study and the technology used have several limitations. As with any new medical technology, time must be spent learning the implementation of the GPS-like technology in a clinical setting. The system required initial registration of readily identifiable image features, making it semi-automated. The DC magnetic array should not be moved once the initial registration process has been completed; otherwise the probe position would not be detected correctly. Similarly, once images are registered, the patient cannot be allowed to move either. Also, the co-registration system is currently rigid and non-elastic and cannot account for changes in anatomic position (e.g. flexion, extension, rotation of joints). Thus, patients must be positioned in a manner similar to that on the co-registered image modality (e.g., MRI or CT), which may not be the optimal position for US imaging. Additionally, confirmation of co-registration using manual methods can be complicated by US sweeps following a staggered back-and-forth path instead of following a continuous path in one direction. For the C-IMT study, images acquired with the GPS-like technology had lower resolution compared with those acquired with the Meijer’s arc due to image enhancements being disabled for our protocol.
From a study stand point, the sample sizes for all of our studies were small. Our ICC for measuring C-IMT from scans acquired with the Meijer’s arc was lower than has been previously reported since the LOGIQ E9 vascular US instrument we used did not have electrocardiography (ECG)-gating. We chose to use this system for both methods in order to reduce instrument related variability/ quality for CIMT measurement. However, it must be noted that the LOGIQ E9 vascular US systems now have ECG-gating capability.
Conclusion
A novel position-sensing technology that uses an electromagnetic array enables excellent real-time registration of US scan frames to MRI data and stored 3D US references. This technology may have applications to other imaging modalities and to clinical and research use.
Figure 6. Bland-Altman plot (automated vs. first [A] and second [B] readers).
Bland-Altman plot comparing manually registered US frames of Readers 1 (A) and 2 (B) with the US system registration.
Acknowledgments
Funding: E.Y.Y. is supported by an American Heart Association South Central Affiliate Postdoctoral Fellowship grant and received support from a National Institutes of Health / National Heart, Lung, and Blood Institute (NIH/NHLBI) T32 HL007812 training grant. S.S.V. is supported by a Veterans Affairs Career Development Award. V.N. is supported by an NIH/NHLBI K23 HL096893 grant and received grant support from the Gulf Coast Regional Medical Foundation.
We thank the participants of the preliminary studies for their contributions and Joanna Brooks, B.A., for editorial assistance. Dr. Yang was supported by a National Institutes of Health (NIH) / National Heart, Lung, and Blood Institute (NHLBI) T32 HL007812 training grant and an American Heart Association South Central Affiliate Postdoctoral Fellowship grant at different times during this study. Dr. Nambi is supported by a NIH/NHLBI K23 HL096893 grant and received grant support from the Gulf Coast Regional Medical foundation. Clinical trial NCT00860184 is sponsored by VPDiagnostics, Inc. (Seattle WA) and supported by NIH/NHLBI R44 HL070576.
Footnotes
Relationship with industry: M.J.W., W.Z., and A.L.H. are employees of General Electric Healthcare. V.N. holds research agreements with General Electric Healthcare and TomTec.
The first and senior authors performed all the analyses and drafted the manuscript. They also made the revisions based on feedback received from all other co-authors (including the three authors who are General Electric Healthcare employees) who had an opportunity to review and comment on the manuscript. The first and last authors made the final decisions regarding the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Collins R, Cranny G, Burch J, Aguiar-Ibanez R, Craig D, Wright K, et al. A systematic review of duplex ultrasound, magnetic resonance angiography and computed tomography angiography for the diagnosis and assessment of symptomatic, lower limb peripheral arterial disease. Health Technol Assess. 2007 May;11(20):iii–iv. xi–xiii, 1–184. doi: 10.3310/hta11200. [DOI] [PubMed] [Google Scholar]
- 2.Raghavan P, Mukherjee S, Gaughen J, Phillips CD. Magnetic resonance angiography of the extracranial carotid system. Top Magn Reson Imaging. 2008 Oct;19(5):241–9. doi: 10.1097/RMR.0b013e3181a8df26. [DOI] [PubMed] [Google Scholar]
- 3.Meenan RT, Saha S, Chou R, Swarztrauber K, Krages KP, O’Keefee-Rosetti M, et al. Effectiveness and cost-effectiveness of echocardiography and carotid imaging in the management of stroke. Evid Rep Technol Assess (Summ) 2002 Jul;(49):1–10. [PMC free article] [PubMed] [Google Scholar]
- 4.Hill DL, Batchelor PG, Holden M, Hawkes DJ. Medical image registration. Phys Med Biol. 2001 Mar;46(3):R1–45. doi: 10.1088/0031-9155/46/3/201. [DOI] [PubMed] [Google Scholar]
- 5.Jochen K, Sheng X, Neil G, Peter G, Peter C, Iclal O, et al., editors. Fusion of real-time transrectal ultrasound with preacquired MRI for multimodality prostate imaging. SPIE; 2007. [Google Scholar]
- 6.Xu S, Kruecker J, Turkbey B, Glossop N, Singh AK, Choyke P, et al. Real-time MRI-TRUS fusion for guidance of targeted prostate biopsies. Comput Aided Surg. 2008 Sep;13(5):255–64. doi: 10.1080/10929080802364645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung EM, Schreyer AG, Schacherer D, Menzel C, Farkas S, Loss M, et al. New real-time image fusion technique for characterization of tumor vascularisation and tumor perfusion of liver tumors with contrast-enhanced ultrasound, spiral CT or MRI: first results. Clin Hemorheol Microcirc. 2009;43(1):57–69. doi: 10.3233/CH-2009-1221. [DOI] [PubMed] [Google Scholar]
- 8.Morrisett J, Vick W, Sharma R, Lawrie G, Reardon M, Ezell E, et al. Discrimination of components in atherosclerotic plaques from human carotid endarterectomy specimens by magnetic resonance imaging ex vivo. Magn Reson Imaging. 2003 Jun;21(5):465–74. doi: 10.1016/s0730-725x(02)00643-4. [DOI] [PubMed] [Google Scholar]
- 9.Choudhary S, Higgins C, Chen I, Reardon M, Lawrie G, Vick Gr, et al. Quantitation and localization of matrix metalloproteinases and their inhibitors in human carotid endarterectomy tissues. Arterioscler Thromb Vasc Biol. 2006 Oct;26(10):2351–8. doi: 10.1161/01.ATV.0000239461.87113.0b. [DOI] [PubMed] [Google Scholar]
- 10.Leotta DF, Detmer PR, Martin RW. Performance of a miniature magnetic position sensor for three-dimensional ultrasound imaging. Ultrasound Med Biol. 1997;23(4):597–609. doi: 10.1016/s0301-5629(97)00043-4. [DOI] [PubMed] [Google Scholar]
- 11.Mankovich NJ, Robertson DR, Cheeseman AM. Three-dimensional image display in medicine. J Digit Imaging. 1990 May;3(2):69–80. doi: 10.1007/BF03170565. [DOI] [PubMed] [Google Scholar]
- 12.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979 Mar;86(2):420–8. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 13.Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata. 2. Stata Press; 2008. [Google Scholar]
- 14.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999 Jun;8(2):135–60. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 15.Tang AM, Kacher DF, Lam EY, Brodsky M, Jolesz FA, Yang ES. Multi-modal imaging: simultaneous MRI and ultrasound imaging for carotid arteries visualization. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:2603–6. doi: 10.1109/IEMBS.2007.4352862. [DOI] [PubMed] [Google Scholar]
- 16.Slomka PJ, Baum RP. Multimodality image registration with software: state-of-the-art. Eur J Nucl Med Mol Imaging. 2009 Mar;36( Suppl 1):S44–55. doi: 10.1007/s00259-008-0941-8. [DOI] [PubMed] [Google Scholar]
- 17.Bliddal H, Boesen M, Christensen R, Kubassova O, Torp-Pedersen S. Imaging as a follow-up tool in clinical trials and clinical practice. Best Pract Res Clin Rheumatol. 2008 Dec;22(6):1109–26. doi: 10.1016/j.berh.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Mackinnon AD, Jerrard-Dunne P, Sitzer M, Buehler A, von Kegler S, Markus HS. Rates and determinants of site-specific progression of carotid artery intima-media thickness: the Carotid Atherosclerosis Progression Study. Stroke. 2004 Sep;35(9):2150–4. doi: 10.1161/01.STR.0000136720.21095.f3. [DOI] [PubMed] [Google Scholar]
- 19.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008 Feb;21(2):93–111. doi: 10.1016/j.echo.2007.11.011. quiz 89–90. [DOI] [PubMed] [Google Scholar]
- 20.Kanters SD, Algra A, van Leeuwen MS, Banga JD. Reproducibility of in vivo carotid intima-media thickness measurements: a review. Stroke. 1997 Mar;28(3):665–71. doi: 10.1161/01.str.28.3.665. [DOI] [PubMed] [Google Scholar]