Abstract
Iejimalide B, a structurally unique 24-membered polyene macrolide having a previously underutilized mode of anticancer activity, was synthesized according to a strategy employing Julia-Kocienski olefinations, a palladium-catalyzed Heck reaction, a palladium-catalyzed Marshall propargylation, a Keck-type esterification, and a palladium-catalyzed macrolide-forming, intramolecular Stille coupling of a highly complex cyclization substrate. The overall synthesis is efficient (19.5% overall yield for 15 linear steps) and allows for more practical scaled-up synthesis than previously reported strategies that differed in the order of assembly of key subunits and in the method of macrocyclization. The present synthesis paves the way for efficient preparation of analogues for drug development efforts.
Introduction
Natural products have long served as invaluable resources in the search for structurally novel compounds as leads for the development of drugs having many therapeutic applications, including antibiotics and anticancer agents. More than two decades ago, a bioassay guided screen of marine organism metabolites led to the discovery of the iejimalides,1 a class of natural macrolides originally isolated from the tunicate species Eudistoma cf. rigida native to the coral reefs of Ie Island (Iejima) near Okinawa, Japan. As disclosed by Kobayashi and coworkers,1a,b the unique structural architecture of the iejimalides consists of a novel 24-membered polyene macrolide core structure containing five chiral centers, and four dienes. Of these dienes, three have the E,E-configuration, one has the E,Z-configuration, and one is a skipped triene. Three of the dienes reside in the macrocyclic ring whereas the fourth is seen in an interesting side chain terminated in an N-formyl l-serine residue (Figure 1). 1c This structural assignment resulted from a correction of earlier studies.1a,b
Figure 1.
Structures of iejimalide A through D
Bioassays2 showed that the iejimalides possess potent growth inhibitory activity against a wide range of human tumor cell lines, with iejimalide B being especially potent. Recently reported data from NCI,3 obtained for samples provided by our laboratories, showed that iejimalide B is cytostatic (GI50) at <5 nM against 40 of the 60 cell lines tested in the NCI cancer screen. A recent study showed that the iejimalides may have an underexplored mode of anticancer activity, which may lead to new cancer therapy modalities4. Treatment of cancer cells in culture leads to inhibition of vacuolar ATPase (V-ATPase) and downstream effects including promotion of reactive oxygen species and an apoptotic cascade in some cell lines. The V-ATPase is an intracellular membrane-bound multicomponent protein complex, which maintains the proton gradient of intracellular compartments, organelles, and extracellular microenvironments, which have critical roles in many biological processes.5,6 Also, certain pathologies are associated with the functional status of V-ATPase including association with invasiveness and metastasis of some cancers.7 Iejimalides are among a privileged class of natural products that show inhibition of V-ATPase.2a,8, Recent proteomic evidence supports a similar cellular mode of action for the iejimalides when compared with established natural product V-ATPase inhibitors.9 Despite the increasing significance of the cellular regulatory features associated with V-ATPase,10,11 little is known about other specific cellular target(s) of the iejimalides and the pathways leading to growth arrest and apoptosis.4a,b
A major limitation regarding studies of the biological action and therapeutic development of iejimalides is that the original natural source provides only miniscule amounts of the iejimalides (0.0003–0.0006% of the tunicate wet weight). The Kobayashi laboratory performed re-extraction from a Cystodytes sp. to accumulate enough material to assign the absolute configuration of all the stereocenters (4R,9S,17S,22S,23S,32S) and to revise the configuration of the C13-C14 double bond from E to Z.1c,d Our laboratories conducted five collections of E. rigida over a period of a few years to obtain milligram quantities of the iejimalides for initial studies of biological activity.2b,3,4
The potent anti-tumor activity of the iejimalides through an important cellular mechanism, their challenging, unique structures, natural scarcity, and unique biological origins have aroused diverse interests in these compounds,12,13,14 which deserve more comprehensive studies and evaluation for anticancer drug development. To this end, an objective has been to provide sufficient amounts of these fascinating compounds for further biological evaluation through high-efficiency chemical synthesis. A rather elegant ring closing alkene metathesis reaction of a highly functionalized, polyunsaturated macrocyclization substrate was the most prominent feature of the Fürstner approach.12d,e In contrast, in an initial synthesis of iejimalide B reported by our laboratory,12f the key macrocyclization was accomplished by a Shiina lactonization as one of the earliest reports of the use of this method in a synthesis of a complex natural product whereas other, longer established methods such as the Yamaguchi procedure were unsuccessful. Although our overall synthetic route was relatively short and highly convergent with a longest linear sequence of 13 steps, it lacked good efficiency as reflected in an overall yield of 3%, due in no small part to the difficult macrolactonization proceeding in no more than 35% yield. Herein, we present a full account of a significantly enhanced second-generation synthesis of iejimalide B in which the key macrocyclization is accomplished by an efficient intramolecular Stille coupling reaction of a richly functionalized alkenylstannane/alkenyl iodide substrate.
Results and Discussion
The rationale for improving the total synthesis of iejimalides is focused on providing a more practical source of the iejimalides that would also permit facile access to novel structural analogues for drug development through a modular convergent approach. In order to have the greatest flexibility in controlling the absolute configurations of the chiral centers and to permit modification of individual regions of the structures of the natural products, we adopted a highly convergent strategy based upon the generation of fragments carrying individual stereochemical elements,12a,b which could be derived either from the chiral pool or from use of enantioselective transformations (Figure 2). Fürstner and coworkers have also adopted a fragment-based strategy in their work but using distinct methods of assembly.12c–e
Figure 2.
Retrosynthetic analysis of iejimalide B
For the synthesis of macrolides, the Yamaguchi macrolactonization is quite commonly the method of choice.15 We had therefore originally targeted the seco-acid 2 as a macrolactonization substrate. Disconnection of 2 at C19-C20 within a diene moiety reveals two fragments 4 and 5, which could be joined by an intermolecular Suzuki, Stille, or related coupling reaction. The alternative reverse order of these late-stage steps were considered whereby the ring closure would be accomplished by an intramolecular coupling reaction of a richly functionalized organometallic substrate 3. In turn, the precursor would be obtained by an intermolecular esterification of the same two fragments 2 and 5 employed in the original strategy.12f Construction of the alkenyl iodide fragment 4 was envisioned using two Julia-Kocienski olefination reactions based upon 6, 7, and 8 as intermediates. The first two of these fragments could be derived from the chiral pool compounds, Roche ester (R)-methyl 2-methyl-3-hydroxypropionate and l-malic acid, respectively, whereas the last of them could be obtained by various enantioselective processes. The organometallic component 5 could be derived from alkyne 9, which in turn could be prepared by an enantioselective Marshall propargylation reaction of aldehyde 10. We have previously reported the synthesis of this final fragment through use of a Heck coupling reaction.12a
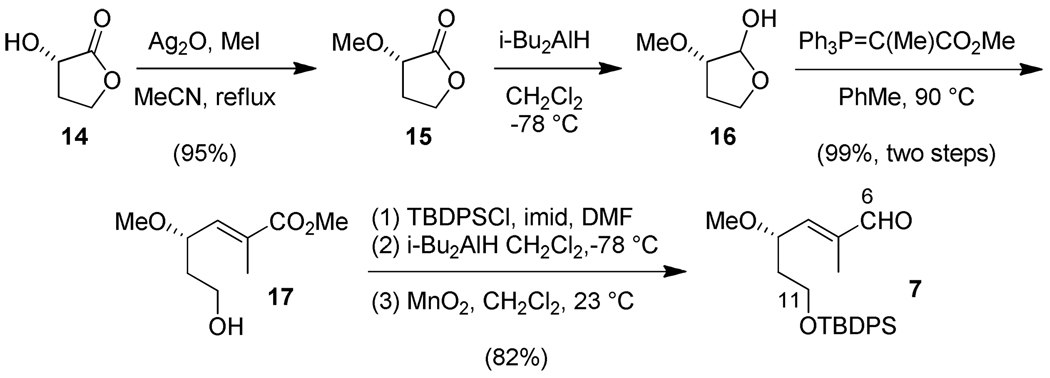
The synthesis began with the C(1)–C(5) subunit 6 based upon use of the R-enantiomer of the Roche ester 11 as described previously (Scheme 1).12a,b A sequence of alcohol protection, DIBAL-H reduction, Wittig reaction, and alcohol deprotection led to alcohol 13, which was purified by column chromatography to give the pure E isomer in an overall yield of 68%. This alcohol was converted into the desired sulfone 6 via a Mitsunobu reaction with 1-phenyltetrazole-5-thiol (PTSH) and molybdate-catalyzed oxidation.
Scheme 1.
The synthesis of the C(6)–C(11) subunit makes use of the commercially available hydroxylactone 14 derived from malic acid (Scheme 2).12b O-methylation provides methoxylactone 15. Subsequent DIBAL-H reduction and Wittig reaction afford key intermediate 17 with 10:1 E:Z-selectivity. Protection as the TBDPS derivative, reduction with DIBAL-H, separation of the E/Z mixture, and MnO2 oxidation provides enal 7 as the desired C(6)–C(11) subunit.
Scheme 2.
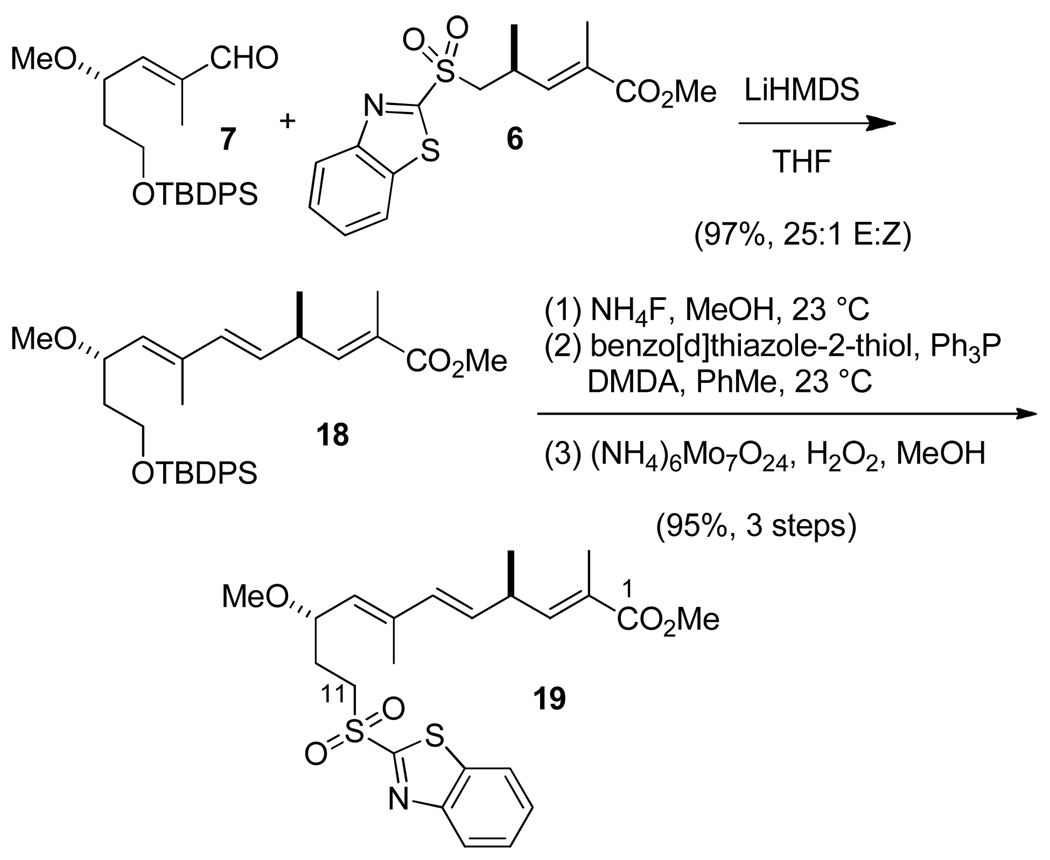
With the first two subunits 6 and 7 in hand, we were positioned to investigate their coupling by the Julia-Kocienski12f,16 olefination en route to the C(1)–C(11) subassembly (Scheme 3). We explored the use of both the sulfonylbenzothiazole derivative depicted as 6 and the corresponding 1-phenyltetrazole derivative (not pictured), but under comparable conditions using either LiHMDS or KHMDS as the base in THF, the former sulfone outperformed the latter both in terms of yield and stereoselectivity. Under the optimum conditions investigated, enal 7 underwent condensation with 1.2 mol-equiv of sulfone 6 treated with 1.4 mol-equiv of LiHMDS in THF to give 18 in nearly quantitative yield and with 25:1 E:Z selectivity. On the other hand, in HMPA/THF,16e both sulfones exhibited an preference for formation of the undesired Z alkene with a ratio of 7.7/1 in 48% yield for 6, which would in principal permit the synthesis of 5Z analogues of the iejimalides. Further elaboration of 18 led to the C(1)–C(11) sulfone 19.
Scheme 3.
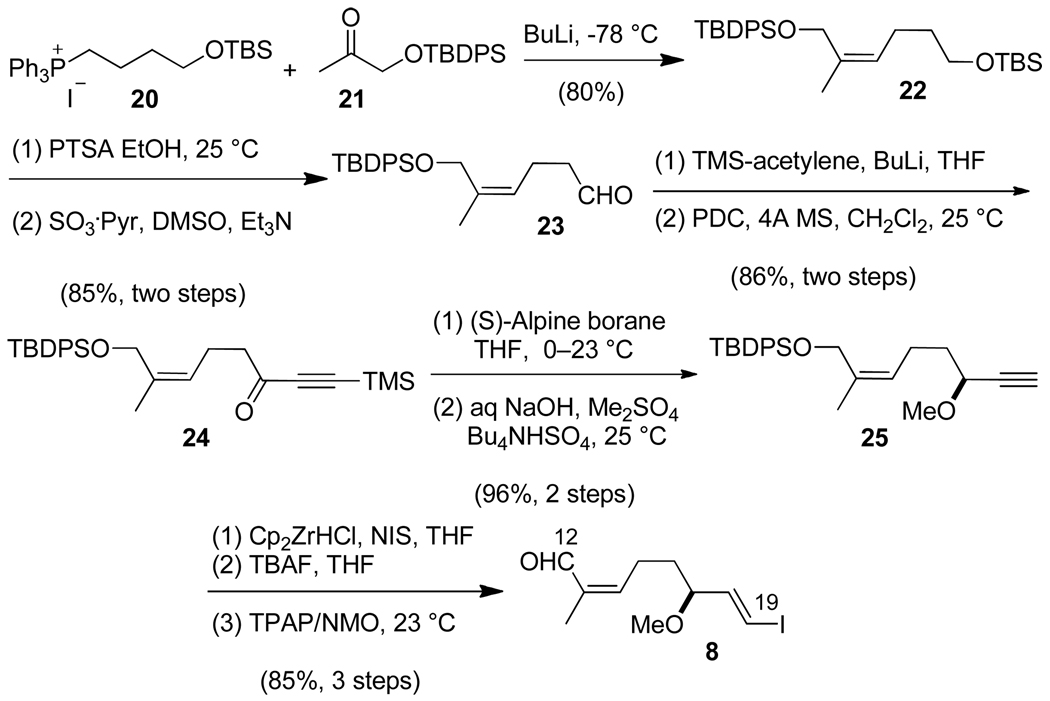
Construction of the C(12)–C(19) subunit began with generation of the C(13)–C(14) trisubstituted Z-alkene by a Wittig reaction of the ylide generated from phosphonium salt 2017 and the TBDPS-protected acetol 2118 (Scheme 4). Selective TBS deprotection of 22 and oxidation with sulfur trioxide·pyridine afforded aldehyde 23. Although generation of the (17S)-stereocenter could be accomplished through a one-step Carreira enantioselective addition of TMS-acetylene to 23,19 the yield was disappointingly low (ca. 40%), and isolation of pure product from the complex reaction mixture was tedious. Consequently, we turned our attention to an alternative three-step approach: introduction of the alkynyl group through non-stereoselective addition of TMS-acetylene to 23, PDC oxidation to give the ketone 24, and subsequent enantioselective reduction with (S)-Alpine borane. The resulting enantiomerically enriched propargylic alcohol (91% ee) was subjected to phase transfer-catalyzed, one-pot O-methylation and alkynyl TMS removal20 to provide the terminal alkyne 25. Hydrozirconation-iodination21, removal of the TBDPS group, and allylic alcohol oxidation with TPAP/NMO22 gave the C(12)–C(19) subunit 8 containing the trisubstituted unsaturated aldehyde and E-alkenyl iodide moieties required for further coupling reactions.
Scheme 4.
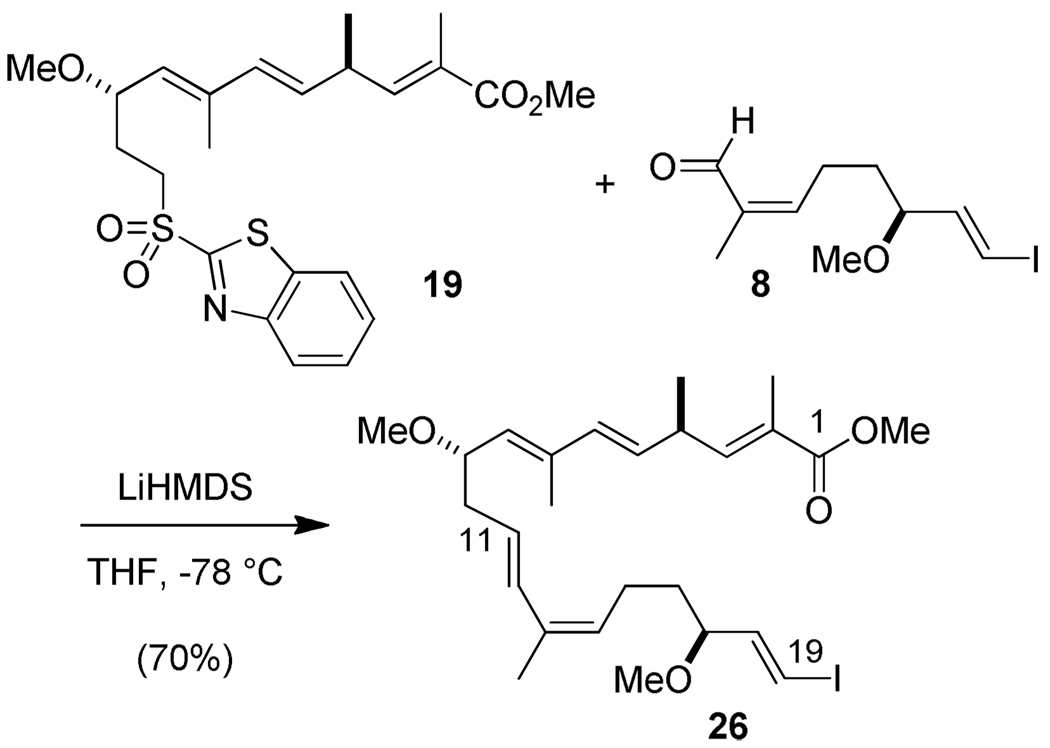
With the two preceding major subunits available, we explored the second Julia-Kocienski olefination between aldehyde 8 and sulfone 19 (Scheme 5). This step proceeded smoothly under conditions similar to those above to form the C(11)–C(12) E-double bond and to afford the fully functionalized C(1)–C(19) segment as the alkenyl iodide-terminated ester 26.
Scheme 5.
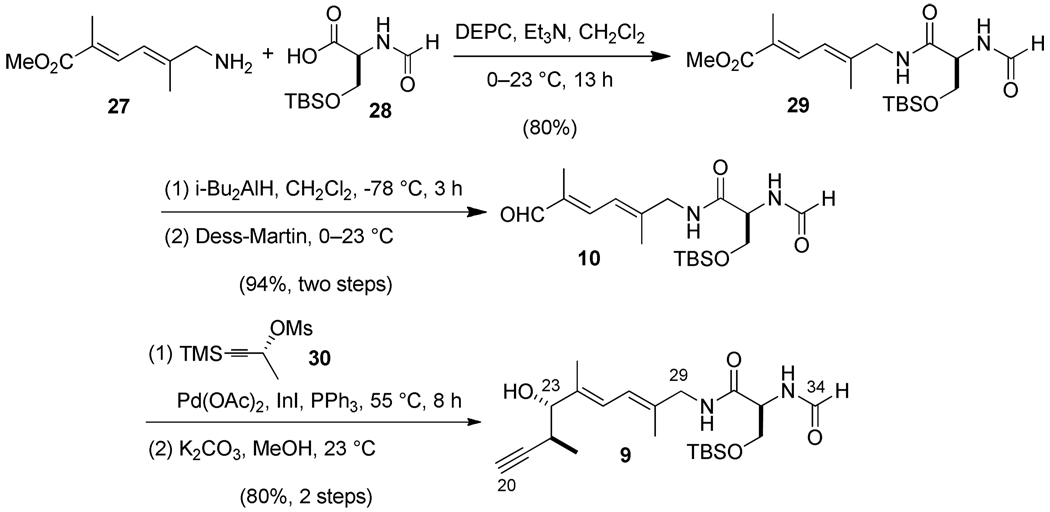
Synthesis of the C(20)–C(34) subunit was based upon use of the aminodienoate ester 27, which we had previously obtained through use of a Heck coupling.12a,23 DEPC-promoted amidation24 with the O-TBS-protected N-formylserine derivative 2825 produced the amide 29 (Scheme 6). Reduction of the ester with DIBAL-H followed by Dess-Martin oxidation afforded the aldehyde 10. A diastereoselective Marshall propargylation reaction26,12d,e of an enantioenriched allenylindium reagent derived from (R)-1-trimethylsilyl-1-butyn-3-yl mesylate generated the two 22S,23S chiral centers with good anti/syn selectivity (5:1 dr). Selective cleavage of the alkynyl TMS group by K2CO3 in MeOH gave the desired subunit 9.
Scheme 6.
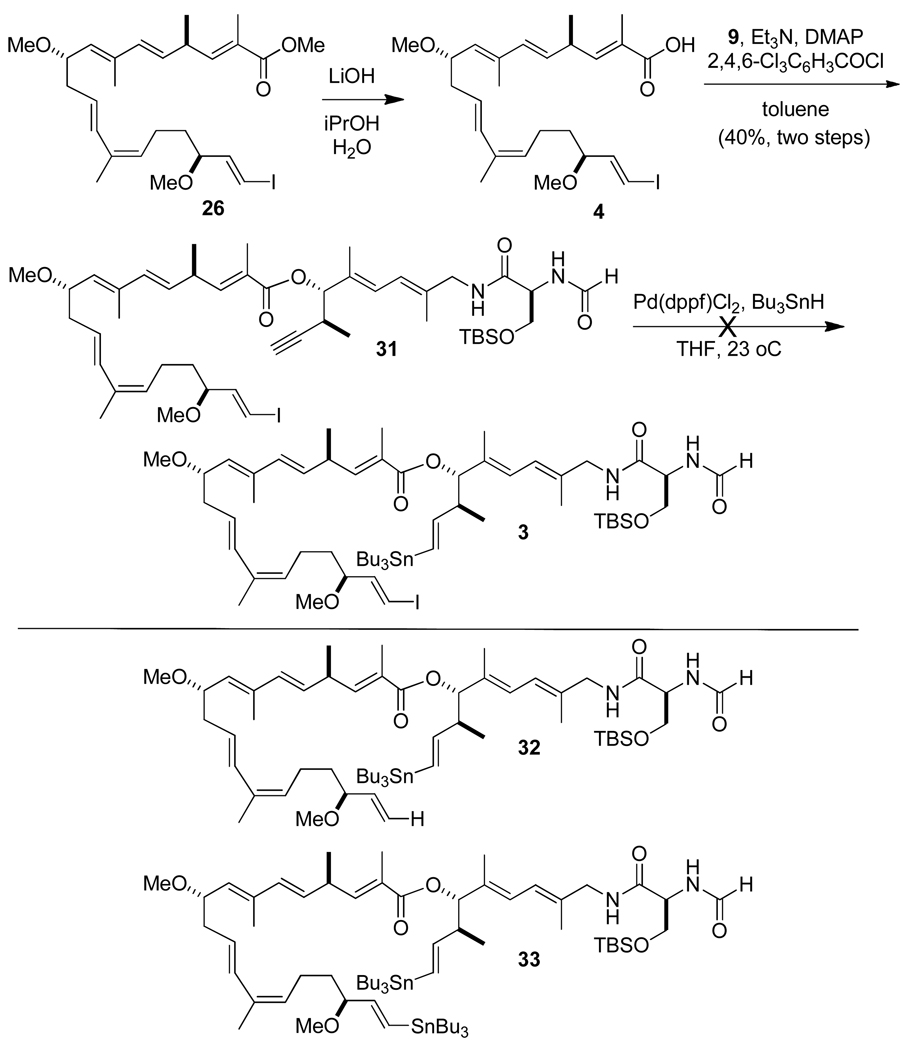
In an initial approach for joining the two major subassemblies, we performed a saponification of the ester 26 followed by a Yamaguchi esterification15 of the crude acid 4 and the alcohol 9 to give 31 in ca. 40% yield (Scheme 7). The 1H NMR and mass spectra of the crude product indicated that all of the skeletal atoms of the iejimalide system were joined in one overall assembly. However, further use of this material was abandoned due to a subsequent problem. In an attempt to prepare the substrate for the desired intramolecular Stille coupling reaction, we failed to obtain any of the desired alkenylstannane 3 by a standard hydrostannylation procedure that we had employed in our first-generation synthesis.12c Among the reaction products suggested by a combination of NMR and ESI-MS studies of the crude project mixture were the de-iodination product 32 and the bis(alkenylstannane) 33.
Scheme 7.
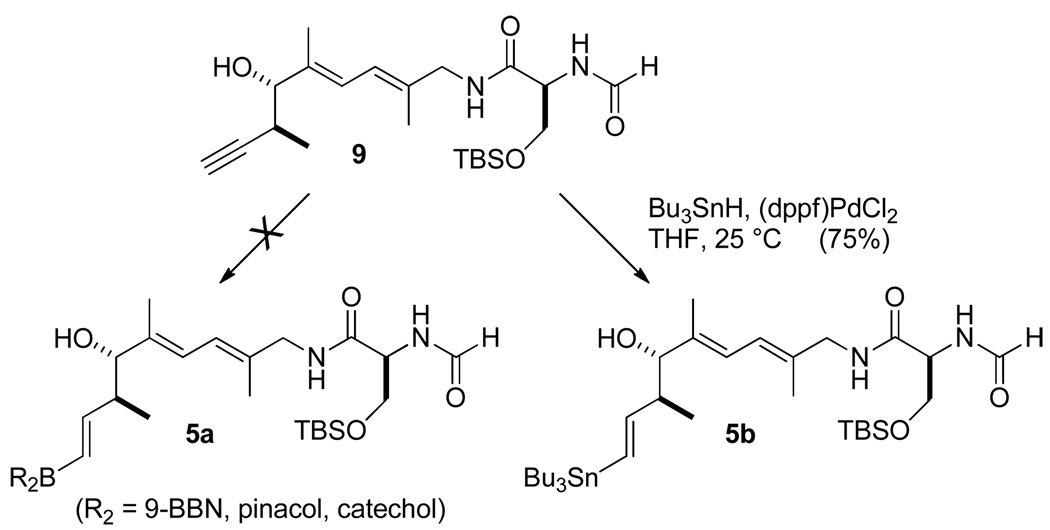
This difficulty in functionalizing the intact skeleton prompted a reverse order of key steps leading to the macrocyclization precursor whereby the alkyne hydrometalation would precede the esterification step. As there have been many elegant applications of the Suzuki-Miyaura coupling reaction under mild conditions in very complex natural products syntheses,27 we attempted to convert the unprotected homopropargyl alcohol 9 into an alkenylboron derivative 5a (Scheme 8). However, all of our efforts at effecting this conversion were unsuccessful. Neither uncatalyzed 28 nor palladium- or platinum-catalyzed hydroborations29 were effective. Although we did not characterize the resulting products, we concluded that the multiple functional groups present in the substrate (two amides, one diene and a free hydroxyl groups) interfered with the desired reaction. Considering that alkyne hydrostannylation is tolerant of many functional groups, we altered our approach slightly to explore formation of the corresponding alkenylstannane and its use in a ring-closing Stille coupling.30 Evaluation of conditions revealed that Pd(dppf)Cl2 is a superior catalyst for conversion of 9 to alkenylstannane 5b.
Scheme 8.
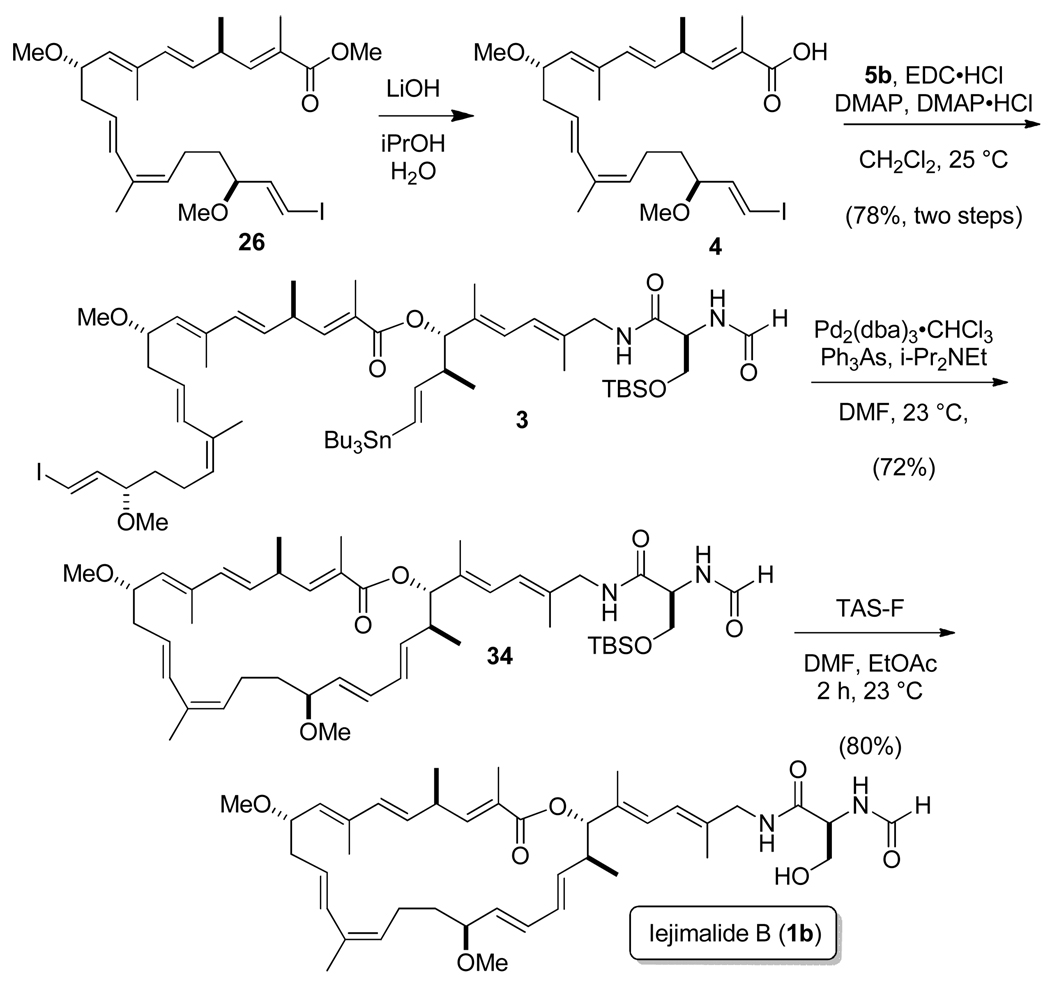
With the final subunits now in hand, the end game of the synthesis could be pursued (Scheme 9). As depicted in the retrosynthetic analysis in Figure 2, the macrolide ring of iejimalide B could be constructed from 4 and 5b by employing the esterification and Stille coupling steps (a and b, respectively) in either order. In our previously reported synthesis, performing the Stille reaction first led to difficulties in the subsequent intramolecular esterification whereby only a low to modest yield of the macrolide was obtained. Therefore, in the presently reported synthesis, we focused on the opposite order of these steps. As shown above, the methyl ester 26 was saponified to give the free acid 4 with which we attempted the esterification using 5b under several conditions. However, the use the Yamaguchi conditions, Mukaiyama’s salt,31 DCC-DMAP,32 EDC-DMAP, and EDC-DMAP-Sc(OTf)333 failed in our hands. Promisingly, when we applied the Keck protocol employing DCC (1 mol-equiv), DMAP (cat.), and DMAP·HCl (cat.),34 4 and 5b did undergo the desired reaction, albeit in low yield and with formation of a mixture from which the ester 3 was difficult to separate from the dicyclohexylurea byproduct. As is well known, the urea byproduct from use of EDC is water-soluble, which facilitates the isolation and purification of products. Accordingly, when we first employed EDC (1 mol-equiv), DMAP (cat.), and DMAP·HCl (cat.), the reaction was successful but again proceeded in low yield (ca. 20%). Optimization efforts based upon varying the stoichiometry of the reactants led to progressively higher yields. For example, the use of acid 4 and alcohol 5b in a 1:1 ratio along with EDC (2 mol-equiv), DMAP (2 mol-equiv), and DMAP·HCl (2.1 mol-equiv) gave ester 3 in ca. 60% yield. Finally, when the ratio of 4 and 5b was changed to 1.3:1, the yield increased quite satisfactorily to ca. 80%, which permitted procurement of conveniently serviceable quantities of the final cyclization candidate.
Scheme 9.
The critical and potentially quite challenging intramolecular Stille coupling reaction required careful consideration of conditions to obtain good results. We systematically evaluated some of the most often employed procedures, including the use of (CH3CN)2PdCl2,35 (Ph3P)4Pd-CuCl,36 and Pd2(dba)3-Ph3As-DIPEA37 catalytic systems. Of the conditions that we examined for our substrate 3, Pd2(dba)3·CHCl3-Ph3As-DIPEA proved to be most effective. Optimization studies revealed that employing Pd2(dba)3·CHCl3, Ph3As, and DIPEA in a ratio of 1:4:65 in DMF (c = 7 × 10−4 M) at 23 °C for 48 h under argon gave reproducibly quite good yields for this complex system in the range of 60 to 80%, with 72% being typical. The synthesis was completed by removal of the TBS protecting group through use of TAS-F38 to give iejimalide B (1b) in 80% yield. The product proved to be identical to an authentic sample39 by direct comparison by 1H NMR, 13C NMR, HPLC, optical rotation, and activity in the MCF-7 breast cancer cell line.2b,4a
Conclusions
The Fürstner laboratory and our laboratory had previously succeeded in the synthesis of iejimalides. The practicality and efficiency of our previous route was hampered by a very difficult macrolactonization in the penultimate step. We have greatly improved upon this earlier synthesis by accomplishing the construction of the macrocycle by a Keck-type intermolecular esterification of the two major subassemblies followed by a remarkably efficient intramolecular Stille coupling reaction in a complex, highly functionalized system. Iejimalide B is obtained by means of a highly convergent strategy in an overall yield of 19.5% for the longest linear pathway of 15 steps from the commercial available chiral lactone 14. The convergent fragment strategy enables the practical incorporation of structural analogues into the total synthesis. Other key features of this synthesis include the use of two Julia-Kocienski olefinations of complex substrates, a diastereoselective Marshall propargylation, and the use of Pd(dppf)Cl2 as a superior catalyst for the hydrostannylation of a richly functionalized alkyne. The efficiency of this route provides us with the opportunity to obtain sufficient quantities of iejimalide B itself and analogues for our ongoing studies of activity and drug discovery efforts upon which we will report in due course.
Experimental Section
General Methods
Unless stated otherwise, all reagents and solvents were used as received from commercial suppliers. Unless stated otherwise, all reactions were conducted in dried, anhydrous solvents. Triethylamine (Et3N) was distilled from CaH2 under an Ar atmosphere and stored over CaH2. All moisture sensitive reactions were performed using dried solvent in flame-dried glassware under an atmosphere of Ar. All low temperature reactions were performed under an atmosphere of Ar. Filtration was generally performed through a pad of Celite. TLC analyses were performed on aluminum-backed F254 silica gel plates. Flash chromatography was performed using silica gel (60 Å silica gel, 32–63 µ). NMR spectra were measured on a 300 MHz, 400 MHz, or 500 MHz instrument. All 1H chemical shifts (δ) are relative to residual protic solvent (CHCl3: δ 7.26 ppm), and all 13C chemical shifts (δ) are relative to the solvent (CDCl3: δ 77.23 ppm). Mass spectral data were measured with FAB or ESI positive ion mode. Infrared spectra were recorded on FT-IR spectrophotometer as neat thin films between NaCl plates in the case of liquid substances or as KBr pellets in the case of solids. Optical rotations were determined using the sodium D line (589 nm). All HPLC separations were performed using a C18 reverse phase column.
Synthesis of the C(1)–C(5) Subunit
(4S,2E)-methyl 5-hydroxy-2,4-dimethylpent-2-enoate (13)
To a stirred solution of methyl-(R)-Roche ester 11 (10.7 g, 90.6 mmol), imidazole (9.2 g, 135.4 mmol, 1.5 eq), and DMF (90 mL) in a 250 mL round-bottom flask, at 0 °C was added TBSCl (20.4 g, 135.4 mmol, 1.5 eq) in one portion. The reaction mixture was warmed to 23 °C and stirred overnight. To facilitate removal of excess TBSCl, anhyd methanol (20 mL) was added to the reaction mixture, and the mixture was allowed to stir for 30 min. The reaction was diluted with 20% EtOAc/hexanes (500 mL) and water (500 mL), and the layers were separated. The organic phase was washed with water (2 × 150 mL). The combined aq layers were back extracted with 20% EtOAc/hexanes (250 mL). The organic extracts were combined, washed with brine (200 mL), dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. Purification of the material was accomplished by flash column chromatography SiO2 column, eluting with 10% EtOAc/hexanes. The product-containing fractions were combined and then concentrated under reduced pressure to give (2R)-methyl 2-methyl-3-(1,1,2,2-tetramethyl-1-silapropoxy)-propanoate (21.0 g, quantitative) as a clear, colorless oil.
1H NMR (300 MHz, CDCl3, 23 °C) δ = 3.77 (dd, J = 9.5, 7.1 Hz, 1 H), 3.67 (s, 3 H), 3.65 (dd, J = 9.5, 5.8 Hz, 1 H), 2.65 (tq, J = 7.1, 6.6 Hz, 1 H), 1.14 (d, J = 7.1 Hz, 3 H), 0.87 (s, 9 H), 0.03 (s, 3 H), 0.02 (s, 3 H). (c = 0.4, CH2Cl2). (lit.40 1H NMR).
To a stirred solution of the preceding product (4.0 g, 17.2 mmol) and hexane (150 mL) in a 500 mL round-bottom flask under a argon atmosphere, at −78 °C, was added a 1.0 M solution of DIBAL (17.2 mL, 17.2 mmol, 1.0 eq) in toluene dropwise via additional funnel into the flask. After the addition was complete, the reaction mixture was kept at −78 °C and stirred for another 30 min. When the reaction was complete (monitored by TLC), it was quenched by the dropwise addition of MeOH (20 mL). The reaction mixture was allowed to stir at −78 °C for an additional 10 min and then transferred (cold) directly into a 1 L Erlenmeyer flask that contained a vigorously stirred mixture of Et2O (250 mL) and a satd aq solution of Rochelle’s salt (potassium sodium tartrate) (250 mL). The resulting emulsion was stirred for 3 h, by which time two layers formed. The mixture was filtered through celite using a Buchner funnel, and the filtrate was collected. The layers were separated, and the aq phase was extracted with Et2O (3 × 75 mL). The combined organic extracts were dried over anhyd Na2SO4, filtered, and then concentrated under reduced pressure to afford the 3-OTBS protected aldehyde 12. All of this crude product was dissolved in toluene (100 mL), and then methyl 2-(triphenylphosphoranylidene)propanoate (7.79 g, 20.6 mmol, 1.2 eq.) was added in one portion under Ar. The mixture was stirred at 70 °C for 8 h. The solvent was removed under reduced pressure, and the residue was purified by flash column chromatography (SiO2), eluting with hexane/EtOAc = 20/1, to afford the pure (4S,2E)-methyl 5-((tert-butyldimethylsilyl)oxy)-2,4-dimethylpent-2-enoate (3.93 g, 84 % for 2 steps, E/Z = 14/1) as a light yellow oil.
1H NMR (300 MHz, CDCl3, 23 °C) δ = 6.55 (dd, J = 9.9, 1.2 Hz, 1 H), 3.73 (s, 3 H), 3.57-3.49 (m, 2 H), 2.76-2.62 (m, 1 H), 1.86 (d, J = 1.2, 3 H), 1.01 (d, J = 7.1 Hz, 3 H), 0.87 (s, 9 H), 0.03 (s, 3 H), 0.02 (s, 3 H); 13C NMR (75 MHz, CDCl3, 23 °C): δ = 168.9, 145.1, 127.9, 67.3, 51.9, 36.5, 26.1, 16.4, 12.9, - 0.53 (lit.41 1H NMR).
The previous product (3.81 g, 13.98 mmol) was dissolved in THF (50 mL), to which TBAF (1.0 M in THF, 15.38 mL, 15.38 mmol) was added dropwise. The reaction was stirred at 23 °C and monitored by TLC. When the reaction was complete, solvent was removed under reduced pressure, and the residue was purified by flash column chromatography (SiO2), eluting with gradient hexane/EtOAc = 10/1 ~ 1/1, to afford pure (4S,2E)-methyl 5-hydroxy-2,4-dimethylpent-2-enoate (1.78 g, 81 %) as a clear colorless oil.
1H NMR (300 MHz, CDCl3, 23 °C) δ = 6.56 (d, J = 9.7 Hz, 1 H), 3.70 (s, 3 H), 3.59-3.42 (m, 2 H), 2.80-2.65 (m, 1 H), 2.12 (s, br, 1 H), 1.85 (s, 3 H), 0.99 (d, J = 6.9 Hz, 3 H). 13C NMR (75 MHz, CDCl3, 23 °C): δ = 168.8, 144.6, 128.8, 67.2, 51.9, 36.3, 16.2, 12.9. (c = 0.22, CH2Cl2). (lit.12e 1H NMR).
(4S,2E)-methyl 5-(benzo[d]thiazol-2-ylsulfonyl)-2,4-dimethylpent-2-enoate (6)
The primary alcohol 13 (4.14 g, 26.17 mmol), PPh3 (7.62 g, 29.05 mmol), and benzo[d]thiazole-2-thiol (4.86 g, 29.05 mmol) were dissolved in anhyd toluene (70 mL, Aldrich) in a water bath at 23 °C. Diisopropyl azodicarboxylate (DIAD, 5.72 mL, 29.05 mmol) was slowly added dropwise to the above rapidly stirring solution. After the mixture was stirred overnight at 23 °C, all solvent was removed on a rotary evaporator and the crude material was purified by flash column chromatography (SiO2), eluting with gradient hexane/EtOAc = 10/1 ~ 2/1, to afford the intermediate benzothiazole-sulfide in quantitative yield, which was used immediately for the oxidation. The product was dissolved in 120 mL MeOH and cooled to 0 °C. In a separate flask, (NH4)6Mo7O24·4H2O (32.34 g, 26.17 mmol) and 30% aq H2O2 (53.4 mL, 20 eq.) were combined, cooled to 0 °C, and added dropwise to the above MeOH solution. The reaction mixture was stirred for 11 h at 0 °C, diluted with 120 mL H2O, and stirred vigorously for 30 min. The mixture was then extracted with CH2Cl2 (3 × 200 mL). The combined organic phases were dried over MgSO4, filtered, and concentrated under vacuum. The remaining residue was purified by flash column chromatography (SiO2), eluting with gradient hexane/EtOAc/DCM = 4/1/0.5 ~ 2/1/0.5, to afford the sulfone 6 (8.8 g, 99 % for two steps).
1H NMR (300 MHz, CDCl3, 23 °C): δ = 8.20 (d, J = 7.8 1 H), 7.73 (d, J = 7.8 1 H), 7.67-7.55 (m, 2 H), 6.28 (dd, J = 10.2, 1.5 Hz, 1 H), 3.78 (dd, J = 14.7, 8.7 Hz, 1 H), 3.50 (dd, J = 14.7, 5.1 Hz, 1 H), 3.35 (s, 3 H), 3.35-3.27 (m, 1 H), 1.80 (d, J = 1.5 Hz, 3 H), 1.20 (d, J = 6.6 Hz, 3 H). 13C NMR (75 MHz, CDCl3, 23 °C): δ = 167.7, 166.5, 152.8, 142.1, 136.9, 128.4, 128.2, 127.8, 125.5, 122.5, 60.1, 51.7, 29.2, 20.1, 12.6. IR: δ = 1704, 1468, 1437, 1337, 1310, 1267, 1141, 763 cm−1. (c = 0.25, CH2Cl2). HR-MS (ESI) m/z ([M+Na]+): Calcd for C15H17NNaO4S2: 362.0497, Found: 362.0488. (lit.12f 1H NMR).
Synthesis of the C(6)–C(11) Subunit
(2S)-3-methoxydihydrofuran-2(3H)-one
The hydroxylactone 14 (2.4 g, 23.5 mmol) was dissolved in anhyd acetonitrile (60 mL) under an argon atmosphere, and methyl iodide (17.1 g, 120.47 mmol) was added. Silver (I) oxide (6.12 g, 26.41 mmol) was added, and the resulting mixture heated to reflux (75 °C oil bath) in the absence of light (aluminum foil cover). After 12 h, the mixture was filtered through celite, and the filter rinsed with diethyl ether (50 mL). The filtrate was concentrated in vacuo to afford a black oil. Purification by flash column chromatography (SiO2, hexane/EtOAc = 3/1 - 1/1) provided 2.59 g (95 %) of the (S)-3-methoxydihydrofuran-2(3H)-one 15 as a light yellow oil.
1H NMR (300 MHz, CDCl3, 23 °C) δ = 4.43-4.36 (m, 1 H), 4.26-4.18 (m, 1 H), 4.02 (t, J = 7.8 Hz, 1 H), 3.54 (s, 3 H), 2.56-2.46 (m, 1 H), 2.27-2.15 (m, 1 H) (lit.12b).
4S,2E)-methyl 6-hydroxy-4-methoxy-2-methylhex-2-enoate (17)12b
To a solution of the lactone 15 (4.06 g, 34.97 mmol) in anhyd diethyl ether (160 mL) at −78 °C under argon gas was added diisobutylaluminum hydride (1.0 M in hexane, 38.46 mL) via an addition funnel over 1 h. After 2 h, the reaction was quenched by careful dropwise addition of satd ethanolic tartaric acid (18 mL) followed by a mixture of sodium sulfate and silica (1:1 v/v, 20 mL). The mixture was stirred at 0°C, and 8.7 mL H2O was added dropwise. The mixture was then warmed to 23 °C slowly and stirred vigorously for 8 h. The mixture was filtered, and the solids rinsed with excess ether. The filtrate was concentrated in vacuo to yield the lactol 16 (4.13 g, quantitative yield) as clear colorless oil, which was used without further purification.
The lactol (6.48 g, 54.8 mmol) was dissolved in toluene (300 mL) to which was added methyl 2-(triphenylphosphoranylidene)propanoate (38.2 g, 109.7 mmol). The mixture was placed in an oil bath at 90 °C for 3 h under Ar. When the reaction was complete, as monitored by TLC, the solvent was evaporated in vacuo to give a yellow oil, which was then purified by flash column chromatography (SiO2, 30% EtOAc/Hexane) to provide (4S,2E)-methyl 6-hydroxy-4-methoxy-2-methylhex-2-enoate 17 in almost quantitative yield (~ 10.2 g, 99 %), with selectivity of E/Z = 10/1. The pure E isomer could not be obtained readily at this point by silica gel chromatography.
1H NMR (300 MHz, CDCl3, 23 °C) δ = 6.65 (d, J = 9.3 Hz, 1 H), 4.15–4.32 (m, 2 H), 3.78 (m, 1 H), 3.77 (s, 3 H), 3.27 (s, 3 H), 2.50 (s, 1H, br, OH), 1.87 (d, J = 1.5 Hz, 3 H), 1.80–1.90 (m, 1 H), 1.65–1.75 (m, 1 H).
(4S,2E)-6-((tert-butyldiphenylsilyl)oxy)-4-methoxy-2-methylhex-2-enal (7)
The alcohol 17 (54.86 mmol) was dissolved in 45 mL anhydrous DMF to which imidazole (8.68 g, 127.5 mmol) was added at 23 °C, and TBDPSCl (16.6 mL, 63.8 mmol) was added dropwise. The mixture was stirred at 23 °C under Ar for 8 h. The mixture was diluted with Et2O and washed successively with H2O (3 × 50 mL) and brine. The aq solution was combined and back extracted with Et2O (3 × 50 mL). The organic phases were combined and dried over MgSO4. After filtration, the solvent was concentrated in vacuo, and the residue was purified by flash column chromatography (SiO2) with gradient eluting (Hexane/EtOAc = 10/1 ~1/1), to provide the OTBDPS-protected intermediate (~ 23.5 g, quantitative, as a 10/1 E/Z mixture).
1H NMR (300 MHz, CDCl3, 23 °C): δ = 7.65–7.70 (m, 4 H), 7.35–7.46 (m, 6 H), 6.36 (dq, J = 9.5, 1.4 Hz, 1 H), 4.28–4.37 (m, 1 H), 3.85-3.79 (m, 1 H), 3.76 (s, 3 H), 3.76-3.68 (m, 1 H), 3.26 (s, 3 H), 1.93 (d, J = 1.5 Hz, 3 H), 1.93-1.65 (m, 2 H), 1.07 (s, 9 H). 13C NMR (75 MHz, CDCl3, 23 °C): δ = 168.3, 142.7, 136.2, 135.73, 135.71, 133.9, 129.8, 127.9, 74.4, 59.9, 56.9, 52.1, 27.0, 37.9, 19.4, 13.1. IR: δ = 3074, 3050, 2952, 2929, 2893, 2860, 1764, 1429, 1261, 1110, 737, 704 cm−1. (c = 0.6, CH2Cl2).
A solution of the previous product (1.1 g, 2.56 mmol) in toluene (20 mL) was cooled to −78 °C under Ar, to which DIBAL (1.0 M in toluene, 2.82 mL, 14.6 mmol) was added dropwise over 30 min. The resulting mixture was stirred for another 30 min at −78 °C and was quenched by dropwise addition of saturated ethanolic tartaric acid (3.2 mL) followed by a mixture of sodium sulfate and silica (1:1 v/v, 7 mL). The mixture was warmed to 0 °C, and 2.3 mL H2O was added dropwise. The mixture was then warmed slowly to 23 °C, and the slurry was stirred vigorously for 8 h. The mixture was filtered, and the solids were rinsed with excess ether. The filtrate was concentrated in vacuo to yield the crude material, which was purified by flash chromatography (Hex/EtOAc = 4/1~3/1) to provide (4S,2E)-6-((tert-butyldiphenylsilyl)oxy)-4-methoxy-2-methylhex-2-en-1-ol (0.85g, 83 %), which was further purified by bulb to bulb distillation under vacuum to afford the pure E isomer.
1H NMR (300 MHz, CDCl3, 23 °C): δ = 7.65–7.70 (m, 4 H), 7.26–7.43 (m, 6 H), 5.28–5.32 (m, 1 H), 4.24–4.27 (m, 1 H), 4.03 (s, 2 H), 3.67–3.80 (m, 2 H), 3.24 (s, 3 H), 1.82–1.89 (m, 1 H), 1.73 (s, 3 H), 1.61–1.70 (m, 1 H), 1.06 (s, 9 H). 13C NMR (75 MHz, CDCl3, 23 °C): δ = 139.1, 135.76, 135.73, 134.11, 134.04, 129.8, 127.8, 126.2, 73.9, 68.3, 60.3, 56.2, 38.6, 27.0, 19.4, 14.3. IR: δ = 3430 (s, b), 3072, 2960, 2930, 2889, 2860, 1474, 1427, 1110, 823, 737, 704 cm−1. (c = 0.8, CH2Cl2).
The previous product (380 mg, 0.953 mmol) was dissolved in anhd DCM (10 mL). Activated MnO2 (826 mg, 10 eq.) was added in several portions. The reaction was stirred for 16 h and monitored by TLC until the reaction was complete. The mixture was diluted with DCM and filtered through celite to afford a clear filtrate. Removal of the solvent under reduced pressure gave the pure α,β-unsaturated aldehyde 7 (374 mg, 99 %), which was used immediately for the following Julia-Kocieński coupling, without further purification.
1H NMR (300 MHz, CDCl3, 23 °C): δ = 9.45 (s, 1 H), 7.71-7.62 (m, 4 H), 7.48-7.35 (m, 6 H), 6.36 (dq, J = 9.5, 1.4 Hz, 1 H), 4.61-4.45 (m, 1 H), 3.91-3.82 (m, 1 H), 3.77-3.68 (m, 1 H), 3.32 (s, 3 H), 1.93-1.66 (m, 2 H), 1.83 (d, J = 1.5 Hz, 3 H), 1.11 (s, 9 H). 13C NMR (75 MHz, CDCl3, 23 °C): δ = 195.0, 154.0, 140.6, 135.74, 135.69, 133.8, 129.9, 127.9, 74.4, 59.7, 57.3, 37.7, 27.0, 19.4, 9.8 (lit.12f 1H NMR).
Completion of the C(1)–C(11) Subunit
Sulfone 6 (369.2 mg, 1.088 mmol) and aldehyde 7 (359 mg, 0.905 mmol) were placed in a flask and azeotropically dried three times with anhydrous benzene. The mixture was further dried under high vacuum and placed under Ar. Freshly dried THF (22 mL) was added to dissolve the above mixture, which was cooled to −78 °C. LiHMDS (1.31 mL of an 1.0 M solution in THF, 1.31 mmol ) was added dropwise. The mixture was stirred at −78 °C for another 30 min and then was warmed to 23 °C over 1.5 h. After being stirred for 30 min, the mixture was cooled to −78 °C and was quenched by addition of 12 mL of satd aq NH4Cl. The solution was warmed to 23 °C over 30 min. The mixture was then extracted with ethyl acetate (3 ×), and the organic phases were combined, washed with brine, dried over Na2SO4, filtered, and evaporated to dryness. The crude product was purified by flash silica gel chromatography (Hex/EA = 20/1) to afford pure compound 18 (456 mg, 97 % with a selectivity of E/Z = 25/1).
1H NMR (300 MHz, CDCl3, 23 °C ): δ = 7.63–7.69 (m, 4 H), 7.36–7.43 (m, 6 H), 6.65 (dq, J = 9.6, 1.4 Hz, 1 H), 6.10 (d, J = 15.6 Hz, E H6), 5.81 (d, J = 11.7Hz, Z H, H6), 5.59 (dd, J = 15.9, 6.9 Hz, 1 H), 5.28 (d, J = 9.3 Hz, E H, H8), 5.19 (d, J = 8.7Hz, Z H, H8), 4.26- 4.37 (m, 1 H, H9), 3.73 (s, 3 H), 3.64–3.86 (m, 2 H), 3.22–3.32 (m, 1 H), 3.23 (s, 3 H), 1.90 (d, J = 1.2 Hz, 3 H), 1.82 (d, J = 0.9 Hz, 3 H), 1.88-1.78 (m, 1 H), 1.71-1.60 (m, 1 H), 1.18 (d, J = 6.9 Hz, 3 H), 1.07 (s, 9 H). 13C NMR (75 MHz, CDCl3, 23 °C): δ = 168.9, 145.4, 136.5, 135.73, 135.68, 134.06, 133.99, 133.7, 132.3, 131.3, 129.72, 129.71, 127.8, 126.5, 74.2, 60.3, 56.2, 52.0, 38.7, 36.5, 27.0, 20.5, 19.4, 13.1, 12.7. IR: δ = 3075, 3050, 2960, 2934, 2901, 2862, 1715, 1429, 1392, 1265, 1192, 1112, 824, 747, 704 cm−1. (c = 0.24, CH2Cl2). HR-MS (ESI) m/z ([M+Na]+): Calcd for C32H44NaO4Si: 543.2901, Found: 543.2908 (lit.12f 1H NMR).
C(1)–C(11) sulfone (19)
Compound 18 (456 mg, 0.876 mmol) was dissolved in MeOH (8.8 mL) to which NH4F (648 mg, 17.5 mmol, 20 equiv) was added. The mixture was stirred for 24 h at 23 °C and monitored by TLC. The reaction was quenched with satd aq NaHCO3 and extracted with CH2Cl2 (3 ×). The organic phases were combined, washed with brine, dried over Na2SO4, filtered, and evaporated to dryness. The crude product was purified by flash silica gel chromatography (SiO2, hexane/EtOAc = 5/1 − 2/1) to afford alcohol intermediate (247 mg, quantitative).
1H NMR (300 MHz, CDCl3, 23 °C): δ = 6.57 (dq, J = 9.9, 1.5 Hz, 1 H), 6.06 (d, J = 15.9 Hz, E H, H6), 5.78 (d, J = 11.1Hz, Z H, H6), 5.55 (dd, J = 15.6, 6.9 Hz, 1 H), 5.26 (d, J = 9.0 Hz, E H, H8), 4.19- 4.26 (m, 1 H), 3.70 (s, 3H), 3.62–3.77 (m, 2 H), 3.18–3.32 (m, 1 H), 3.21 (s, 3 H), 2.78 (s, br 1 H, OH), 1.84 (d, J = 1.2 Hz, 3 H), 1.75 (d, J = 0.9 Hz, 3 H), 1.74–1.86 (m, 1 H), 1.59–1.69 (m, 1 H), 1.12 (d, J = 6.9 Hz, 3 H). 13C NMR (75 MHz, CDCl3, 23 °C): δ = 168.9, 145.2, 136.6, 133.4, 131.7, 131.3, 126.5, 77.1, 60.8, 56.2, 51.9, 37.8, 36.4, 20.4, 13.0, 12.6. IR: δ = 3450 (s, b), 2952, 2933, 1725, 1443, 1380, 1268, 1196, 1100 cm−1. (c = 0.18, CH2Cl2). HR-MS (ESI+) m/z ([M+Na]+): Calcd for C16H26NaO4: 305.1723, Found: 305.1708 (lit.12f 1H NMR).
The previous product (247 mg, 0.876 mmol), PPh3 (255 mg, 0.972 mmol), and benzo[d]thiazole-2-thiol (163 mg, 0.972 mmol) were dissolved in anhyd toluene (8 mL, Aldrich). To the above solution, DIAD (192 µL, 0.972 mmol) was added dropwise. After the mixture was vigorously stirred for 8 h at 23 °C, solvent was removed on a rotary evaporator. The crude material was purified by flash silica gel chromatography (SiO2, hexane/EtOAc = 20/1 ~ 15/1) to afford the benzothiazole-sulfide (359 mg, 95%).
1H NMR (300 MHz, CDCl3, 23 °C): δ = 7.82–7.88 (m, 1 H), 7.71–7.76 (m, 1 H), 7.36–7.44 (m, 1 H), 7.25–7.31 (m, 1 H), 6.60 (dd, J = 9.9, 1.2 Hz, 1 H), 6.07 (d, J = 15.9 Hz, 1 H), 5.57 (dd, J = 15.9, 6.9 Hz, 1 H), 5.30 (d, J = 8.1 Hz, 1 H), 4.17–4.25 (m, 1 H), 3.73 (s, 3 H), 3.36–3.44 (m, 2 H), 3.20–3.33 (m, 1 H), 3.26 (s, 3 H), 2.04–2.16 (m, 1 H), 1.90–2.01 (m, 1 H), 1.86 (d, J = 1.5 Hz, 3 H), 1.78 (d, J = 0.6 Hz, 3 H), 1.15 (d, J = 6.9 Hz, 3 H). IR: δ = 2928, 1715, 1466, 1433, 1270, 1241, 1098, 998, 751 cm−1. (c = 0.12, CH2Cl2).
The material (239 mg, 0.554 mmol) from the previous reaction was dissolved in MeOH (3.6 mL) and cooled to 0 °C. In a separate flask, (NH4)6Mo7O24·4H2O (178.2 mg, 0.144 mmol) and 30% aq H2O2 (1.47 mL, 25 eq.) were combined and cooled to 0 °C, which were added dropwise to the above MeOH solution. The reaction mixture was warmed to 0 °C slowly and stirred vigorously for 8 h. When the reaction was complete, monitored by TLC, the mixture was diluted with 8 mL H2O, stirred vigorously for 30 min and extracted with CH2Cl2 (3 × 20 mL). The combined organic phases were dried over MgSO4, filtered, and evaporated under vacuum. The remaining residue was purified by flash column chromatography (SiO2), eluting with gradient hexane/EtOAc = 5/1 ~ 4/1, to afford the sulfone 19 (~260 mg, quantitative).
1H NMR (500 MHz, CDCl3, 23 °C): δ = 8.20 (ddd, J = 8.2, 1.3, 0.7 Hz, 1 H), 8.00 (ddd, J = 7.9, 1.4, 0.7 Hz, 1 H), 7.50–7.63 (m, 2 H), 6.58 (dq, J = 9.8, 1.4 Hz, 1 H), 6.01 (d, J = 15.8 Hz, 1 H), 5.58 (dd, J = 15.6, 6.7 Hz, 1 H), 5.18 (d, J = 8.9 Hz, 1 H), 4.08–4.16 (m, 1 H), 3.72 (s, 3 H), 3.55–3.63 (m, 2 H), 3.19–3.26 (m, 1 H), 3.15 (s, 3 H), 1.97–2.07 (m, 2 H), 1.85 (d, J = 1.5 Hz, 3 H), 1.75 (d, J = 1.2 Hz, 3 H), 1.13 (d, J = 6.8 Hz, 3 H). 13C NMR (75 MHz, CDCl3, 23 °C): δ = 168.9, 165.8, 152.9, 145.0, 137.9, 136.9, 133.1, 132.4, 129.9, 128.2, 127.8, 126.7, 125.6, 122.5, 75.0, 56.2, 52.0, 51.4, 36.4, 28.5, 20.4, 13.2, 12.7. IR: δ = 2928, 1712, 1473, 1330, 1151, 1093, 763 cm−1. (c = 0.55, CHCl3). HR-MS (FAB+) m/z ([M-CH3O]+): Calcd for C22H26NO4S2: 432.1303, Found: 432.1307 (lit.12f 1H NMR).
Synthesis of the C(12)–C(19) Subunit
(2Z)-1-((tert-butyldiphenylsilyl)oxy)-6-((tert-butyldimethyl)silyloxy)-2-methylhexene (22)
4-O-TBS-butyltriphenylphosphonium iodide 2017a,b (6.34 g, 11.0 mmol) was added to a 250-mL flask and dried under high vacuum for 1 h. After freshly dried THF (45 mL) was added under Ar, n-BuLi (4.4 mL of a 2.4 M solution in THF, 10.5 mmol) was added dropwise. After being stirred for 1 h at 23 °C, the deep-red colored suspension was cooled to −78 °C, and a pre-cooled (−78 °C) solution of 1-O-TBS-propan- 2-one 21 (3.45 g, 11.0 mmol) in THF (75 mL) was added through a cannula. After being stirred for 8 h at −78 °C, the mixture was warmed to 23 °C and stirred for 8 h. During that time, a large amount of pale-white solid separated. The reaction was quenched with satd aq NH4Cl at 0 °C and stirred vigorously for 30 min. The solid was filtered off through celite and washed thoroughly with Et2O. The organic phase was separated, and the aq phase was extracted with Et2O (3 ×). The combined organic phase was washed with brine, dried over MgSO4, filtered, and concentrated. The residue was purified by flash chromatography (SiO2, hexane/EtOAc = 50/1−30/1) to provide the desired olefinic product 22 as a nearly colorless oil (4.04 g, E/Z = 1/12.5, 80% yield based on BuLi), which was used directly for the next step.
1H NMR (300 MHz, CDCl3, 23 °C): δ = 7.67–7.73 (m, 4 H), 7.37–7.46 (m, 6 H), 5.22 (t, J = 7.2 Hz, 1 H), 4.21 (s, 2 H), 3.52 (t, J = 6.3 Hz, 1 H), 1.90 (q, J = 7.2 Hz, 2 H), 1.85 (s, 3 H), 1.42–1.52 (m, 2 H), 1.07 (s, 9 H), 0.87 (s, 9 H), 0.02 (s, 6 H). 13C NMR (75 MHz, CDCl3, 23 °C): δ = 135.8, 134.9, 134.1, 129.7, 127.8, 126.4, 62.9, 62.7, 33.3, 27.0, 26.2, 24.0, 21.4, 19.5, 18.5, −5.1. IR: δ = 3074, 2960, 2929, 2891, 2856, 1474, 1433, 1257, 1108, 837, 781, 739, 700 cm−1. HR-MS (ESI+) m/z ([M+Na]+): Calcd for C29H46NaO2Si2: 505.2929, Found: 505.2909 (lit.12f 1H NMR).
(4Z)-6-((tert-Butyldiphenylsilyl)oxy)-5-methylhex-4-enal (23)
The olefinic product 22 (1.7 g, 3.52 mmol) was dissolved in 40 mL absolute EtOH, and pyridinium p-toluenesulfonate (180 mg, 0.72 mmol) was added in one portion. After being stirred for 24 h at 23 °C, the mixture was concentrated under vacuum, and the remaining residue was purified by a flash chromatography column (SiO2, gradient: Hexane/EA = 10/1 ~ 5/1) to provide the TBS- selectively deprotected alcohol as a colorless oil (1.23 g, 95%), which was used directly for the following oxidation.
1H NMR (300 MHz, CDCl3, 23 °C): δ = 7.68–7.73 (m, 4 H), 7.37–7.45 (m, 6 H), 5.25 (tq, J = 7.5, 0.9 Hz, 1 H), 4.20 (s, 2 H), 3.54 (t, J = 6.3 Hz, 1 H), 1.92 (q, J = 7.5 Hz, 2 H), 1.82 (d, J = 1.2 Hz, 3 H), 1.47–1.56 (m, 2 H), 1.07 (s, 9 H). 13C NMR (75 MHz, CDCl3, 23 °C): δ = 135.8, 135.2, 133.9, 129.8, 127.8, 126.4, 62.7, 62.3, 32.7, 27.0, 23.9, 21.5, 19.4. IR: δ = 2931, 2857, 1722, 1471, 1112, 832, 701 cm−1. HR-MS (ESI+) m/z ([M+Na]+): Calcd for C23H32NaO2Si: 391.2064, Found: 391.2040.
The previously prepared alcohol (1.0 g, 2.7 mmol) was dissolved in DMSO (8 mL) to which NEt3 (2.4 mL) was added. The mixture was placed in a water bath, and a solution of SO3·Py (1.30 g, 8.2 mmol) in DMSO (4 mL) was added dropwise. After being stirred for 20 min at 23 °C, the reaction was quenched by addition of ice-water, and extracted with Et2O. The organic phase was washed with water and brine, dried (MgSO4), filtered, and concentrated to dryness. The remaining residue was purified by flash chromatography (SiO2, hexane/EtOAc = 30/1) to provide the desired aldehyde 23 as a colorless oil (890 mg, 90%, E/Z = 1/12.5).
1H NMR (300 MHz, CDCl3, 23 °C): δ = 9.66 (t, J = 1.8 Hz, 1 H), 7.68–7.74 (m, 4 H), 7.37–7.47 (m, 6 H), 5.45 (t, J = 6.9 Hz, 1 H, E = minor), 5.16 (tq, J = 7.2, 1.2 Hz, 1 H, Z = major), 4.22 (s, 2 H, Z = major), 4.07 (s, 2 H, E = minor), 2.32 (td, J = 7.3, 1.0 Hz, 2 H), 2.14 (app. q, J = 6.9 Hz, 2 H), 1.85 (d, J = 1.2 Hz, 3 H), 1.08 (s, 9 H). 13C NMR (75 MHz, CDCl3, 23 °C): δ = 202.3, 136.4, 135.8, 135.7, 133.9, 129.8, 127.9, 127.8, 124.2, 62.7, 44.1, 27.0, 21.4, 20.4, 19.5. IR: δ = 2931, 2857, 1722, 1471, 1112, 832, 701 cm−1. HR-MS (ESI) m/z ([M+Na]+): Calcd for C23H30NaO2Si: 389.1907, Found: 389.1898.
(Z)-8-((tert-butyldiphenylsilyl)oxy)-7-methyl-1-(trimethylsilyl)oct-6-en-1-yn-3-one (24)
A solution of trimethylsilylacetylene (873 µL, 6.17 mmol) in THF (13 mL) was cooled to 0 °C under Ar, and n-BuLi (2.6 mL of a 1.6 M solution in THF, 4.1 mmol) was added dropwise. After the solution was stirred for 30 min at 0 °C, the aldehyde 23 (505 mg, 1.38 mmol) in THF (15 mL) was added dropwise. The mixture was stirred for 4.5 h and monitored by TLC. When the reaction was complete, it was quenched by addition of satd aq NH4Cl and stirred for 30 min. The mixture was extracted with ethyl acetate (3 ×), washed with brine, dried over MgSO4, filtered, and concentrated under vacuum. The remaining residue was purified by flash chromatography (SiO2) using hexane/EtOAc = 30/1 to elute the less polar impurities and hexane/EtOAc = 20/1 to afford the pure product as a colorless oil (641 mg, quantitative), which was used directly for the next step.
1H NMR (300 MHz, CDCl3, 23 °C): δ = 7.71–7.74 (m, 4 H), 7.41- 7.46 (m, 6 H), 5.22 (t, J = 7.5 Hz, 1 H, Z = major), 4.25 (dd, J = 12.0, 5.4 Hz, 3 H), 1.96–2.03 (m, 3 H), 1.85 (s, 3 H), 1.66 (qd, J = 6.9, 3 Hz 2 H), 1.09 (s, 9 H), 0.15 (s, 9 H). 13C NMR (75 MHz, CDCl3, 23 °C): δ = 135.81, 135.79, 135.72, 133.94, 133.91, 129.8, 127.8, 125.5, 106.9, 89.4, 62.7, 62.2, 37.9, 27.0, 23.4, 21.4, 19.5, 0.05. IR: δ = 3400, 3071, 2959, 2857, 2171, 1472, 1428, 1250, 1112, 1071, 843, 702, 615 cm−1. HR-MS (ESI) m/z ([M+Na]+): Calcd for C28H40NaO2Si2: 487.2459, Found: 487.2463.
The previously prepared racemic C(12)–C(19) propargylic alcohol (641 mg, 1.38 mmol) was dissolved in dry CH2Cl2 (50 mL) to which freshly dried molecular sieves (4 Å, 700 mg) were added. After the mixture was stirred for 15 min at 23 °C under Ar, pyridinium dichromate (PDC, 1.09 g, 2.89 mmol) was added, and the mixture was stirred for 8 h. After being filtered through a short neutral aluminum oxide column with celite on the top and washed with CH2Cl2, the filtrate was concentrated under vacuum. The remaining residue was purified by flash chromatography (SiO2), eluting with hexane/EtOAc = 50/1 to afford the pure product 24 as a colorless oil (549 mg, 86 % for two steps, E/Z = 1/12.5).
1H NMR (300 MHz, CDCl3, 23 °C): δ = 7.65–7.72 (m, 4 H), 7.35–7.48 (m, 6 H), 5.13 (tq, J = 7.2, 1.2 Hz, 1 H, Z = major), 4.19 (s, 2 H, Z = major), 4.03 (s, 2 H, E = minor), 2.60 (t, 2 H, J = 7.5 Hz, E = minor), 2.47 (t, 2 H, J = 7.8 Hz, Z = major), 2.15 (app. q, 2 H, J = 7.5 Hz), 1.81 (d, J = 1.2 Hz, 3 H), 1.05 (s, 9 H), 0.21 (s, 9 H). 13C NMR (75 MHz, CDCl3, 23 °C): δ = 187.2, 136.3, 135.8, 133.9, 129.8, 127.9, 124.1, 102.1, 97.9, 62.6, 45.6, 27.0, 22.3, 21.4, 19.5, −0.6. IR: δ = 2969, 2151, 1683, 1428, 1253, 1112, 847, 702 cm−1. HR-MS (ESI) m/z ([M+Na]+): Calcd for C28H38NaO2Si2: 485.2303, Found: 485.2310.
(3S,6Z)-8-((tert-Butyldiphenylsilyl)oxy)-7-methyl-3-methoxy-oct-6-en-1-yne (25)
The alkynyl ketone 24 (2.36 g, 5.10 mmol) was azeotropically dried two times with anhyd toluene, further dried under high vacuum for another 15 min, and placed under Ar. After the ketone was cooled to 0 °C, (S)-Alpine borane (21 mL of 0.5 M solution in THF, 10.5 mmol) was added, and most of the solvent was removed immediately under vacuum to ensure that the reaction was performed at high concentration. The oily mixture was stirred at 23 °C for 8 h, diluted with Et2O, and cooled to 0 °C, and acetaldehyde (8.8 mL) was added dropwise followed by ethanolamine (8.8 mL) while gas was evolved vigorously. The mixture was stirred for 30 min and was washed successively with water and brine. The aq phases were combined and back extracted with Et2O (2 ×). The organic phases were combined, washed with brine, dried (MgSO4), filtered, and concentrated. The remaining residue was purified by flash chromatography (SiO2, eluting with hexane/EtOAc = 20/1) to afford the pure product as a colorless oil (2.37 g, quantitative).
1H NMR (300 MHz, CDCl3, 23 °C): δ = 7.71–7.74 (m, 4 H), 7.41- 7.46 (m, 6 H), 5.22 (t, J = 7.5 Hz, 1 H, Z = major), 4.25 (dd, J = 12.0, 5.4 Hz, 3 H), 1.96–2.03 (m, 3 H), 1.85 (s, 3 H), 1.66 (qd, J = 6.9, 3 Hz 2 H), 1.09 (s, 9 H), 0.15 (s, 9 H). 13C NMR (75 MHz, CDCl3, 23 °C): δ = 135.81, 135.79, 135.72, 133.94, 133.91, 129.8, 127.8, 125.5, 106.9, 89.4, 62.7, 62.2, 37.9, 27.0, 23.4, 21.4, 19.5, 0.05. IR: δ = 3400, 3071, 2959, 2857, 2171, 1472, 1428, 1250, 1112, 1071, 843, 702, 615 cm−1. (c = 0.37, CH2Cl2). HR-MS (ESI) m/z ([M+Na]+): Calcd for C28H40NaO2Si2: 487.2459, Found: 487.2446 (lit.12f 1H NMR).
The previously prepared propargylic alcohol (2.37 g, 5.10 mmol) was dissolved in 22 mL PhMe (4 mL) and cooled to 0 °C, to which was added NaOH (22 mL, 50 wt% aq) followed by addition of tetrabutylammonium hydrogen sulfate (620 mg, 1.83 mmol) and dimethyl sulfate (3.9 mL, 41.1 mmol). The resulting two-phase mixture was stirred for 5 min at 0 °C and warmed to 23 °C. After being stirred for 3 h, the reaction was quenched by addition of MeOH (12 mL), followed by vigorous stirring for 10 min. The resulting mixture was poured into ice water and partitioned with Et2O. The organic phase was separated, and the aq phase was extracted with Et2O (3 ×). The combined organic phases were washed successively with water and brine, dried over MgSO4, filtered, and concentrated under vacuum. The remaining residue was purified by flash chromatography (SiO2, eluting with hexane/EtOAc = 30/1) to afford the desired product 25 as a clear colorless oil (1.99 g, 96 % for two steps E/Z = 1/13.6)
1H NMR (300 MHz, CDCl3, 23 °C): δ = 7.67–7.76 (m, 4 H), 7.36–7.49 (m, 6 H), 5.43 (t, J = 7.9 Hz, 1 H, E = minor), 5.17 (tq, J = 7.5, 1.2 Hz, 1 H, Z = major), 4.20 (s, 2 H, Z = major), 4.05 (s, 2 H, E = minor), 3.80 (dt, J = 6.9, 2.1 Hz, 1 H), 3.33 (s, 3 H), 2.32 (d, J = 1.8 Hz, 1 H), 1.99 (app. q, J = 7.5, 2 H), 1.83 (d, J = 0.9 Hz, 3 H), 1.60–1.72 (m, 2 H), 1.05 (s, 9 H). 13C NMR (75 MHz, CDCl3, 23 °C): δ = 135.79, 135.72, 134.0, 129.7, 127.8, 125.3, 82.6, 74.0, 70.4, 62.7, 56.5, 35.8, 27.0, 23.4, 21.4, 19.5. IR: δ = 3303, 3071, 3048, 2955, 2930, 2857, 2113, 1472, 1428, 1255, 1112, 836, 701 cm−1. (c = 0.78, CH2Cl2). HR-MS (ESI) m/z ([M+Na]+): Calcd for C26H34NaO2Si: 429.2220, Found: 429.2210.
(S,2Z,7E)-8-iodo-6-methoxy-2-methylocta-2,7-dienal (8)
The alkyne 25 (260 mg, 0.64 mmol) was dissolved in freshly dried THF (6 mL) at 23 °C, to which solid Cp2ZrHCl (174 mg, 0.672 mmol, 1.1 eq.) was added followed by N-iodo-succinimide (158 mg, 0.704 mmol, 1.1 eq.) 30 min later. After being stirred for 1 h at 23 °C, the mixture was diluted with Et2O and EtOAc. The organic phase was separated and washed successively with satd aq NaHCO3 and brine. After the organic phase was dried over MgSO4 and filtered, the solvent was concentrated under vacuum, and the remaining residue was purified by flash chromatography (SiO2, eluting with hexane/EtOAc = 15/1) to provide the desired TBDPS-protected vinyl iodide as a colorless oil (342 mg, quantitative)
1H NMR (300 MHz, CDCl3, 23 °C): δ = 7.68–7.72 (m, 4 H), 7.36–7.48 (m, 6 H), 6.31 (dd, J = 14.6, 7.3 Hz, 1 H), 6.20 (d, J = 14.6 Hz, 1 H ), 5.10–5.20 (m, 1 H), 4.18 (s, 2 H), 3.32–41(m, 1 H), 3.19 (s, 3 H ), 1.91-1.82 (m, 2 H), 1.85 (s, 3 H), 1.32–1.61 (m, 2 H), 1.06 (s, 9 H). 13C NMR (75 MHz, CDCl3, 23 °C): δ = 146.8, 135.9, 135.6, 134.06, 134.04, 129.8, 127.9, 125.7, 83.4, 78.2, 62.7, 56.7, 35.0, 27.0, 23.3, 21.4, 19.5. IR: δ = 3070, 3048, 2959, 2931, 2892, 2856, 1604, 1471, 1458, 1428, 1389, 1263, 1170, 1110, 949 cm−1. (c = 0.15, CH2Cl2). HR-MS (ESI) m/z ([M+Na]+): Calcd for C26H35INaO2Si: 557.1343, Found: 557.1356.
The previously prepared TBDPS-protected vinyl iodide (342 mg, 0.64 mmol) was dissolved in THF (7.0 mL), and 1.0 M TBAF/THF (1.28 mL, 1.28 mmol, 2 eq.) was added dropwise at 23 °C. After 6 h, TLC indicated that conversion was complete, the mixture was concentrated on a rotary evaporator, and the remaining oil was purified by flash chromatography (SiO2, gradient: hexane/EtOAc = 6/1 ~ 3/1) to provide the desired allylic alcohol as a colorless oil (162 mg, 85 % for two steps)
1H NMR (300 MHz, CDCl3, 23 °C): δ = 6.38 (dd, J = 12.3, 6.5 Hz, 1 H), 6.29 (d, J = 12.3 Hz, 1 H), 5.19–5.30 (m, 1 H), 4.09 (s, 2 H), 3.46–3.57 (m, 1 H), 3.25 (s, 3 H), 2.05–2.19 (m, 2 H), 1.79 (s, 3 H), 1.45–1.71 (m, 3 H). 13C NMR (75 MHz, CDCl3, 23 °C): δ = 146.7, 135.5, 127.4, 83.1, 78.6, 61.6, 56.6, 34.8, 23.3, 21.6. IR: δ = 3384 (s, br), 2969, 2929, 2859, 1604, 1449, 1171, 1102, 1006, 948 cm−1. (c = 0.3, CH2Cl2). HR-MS (ESI) m/z ([M+Na]+): Calcd for C10H17INaO2: 319.0165, Found: 319.0157.
The above allylic alcohol (130 mg, 0.44 mmol) was dissolved in CH2Cl2 (7 mL), to which freshly flame-dried molecular sieves (4 Å) were added followed by N-methyl-morpholine N-oxide (NMO) (78 mg, 0.67 mmol). 10 min later, tetrapropylammonium perruthenate (TPAP) (7.8 mg, 0.022 mmol) was added, and the mixture was stirred for 11 h at 23 °C under Ar. The mixture was diluted with CH2Cl2 and washed with satd aq Na2SO3 containing some brine. After separation, the aq phase was back extracted with CH2Cl2 (2 ×). The combined organic phases were dried (MgSO4), filtered, and carefully concentrated. Purification by flash chromatography (SiO2, eluting with hexane/EtOAc = 4/1) provided the fully functionalized aldehyde 8 containing the terminal vinyl iodide (ca. 129 mg) as a colorless oil in quantitative yield. This product was used immediately for the following Julia-Kocieński coupling reaction.
1H NMR (300 MHz, CDCl3, 23 °C): δ = 10.12 (s, 1 H, Z ), 6.46 (tq, J = 7.8, 1.2 Hz, 1 H), 6.41 (dd, J = 14.4, 6.6 Hz, 1 H), 6.32 (d, J = 14.4 Hz, 1 H), 3.49–3.56 (m, 1 H), 3.25 (s, 3 H), 2.52–2.77 (m, 2 H), 1.76 (q, J = 1.2 Hz, 3 H), 1.57–1.75 (m, 2 H). 13C NMR (75 MHz, CDCl3, 23 °C): δ = 191.3, 148.3, 146.2, 136.8, 82.8, 79.0, 56.8, 34.6, 22.6, 16.8. IR: δ = 3048, 2924, 2850, 1677, 1604, 1447, 1351, 1253, 1170, 1103, 950 cm−1. (c = 0.23, CH2Cl2). HRMS (FAB) m/z ([M+H]+): Calcd for C10H16IO2: 295.0195, Found: 295.0188.
Completion of the C(1)–C(19) Subunit
C(1)–C(11) sulfone 19 (165 mg, 0.356 mmol) and C(12)–C(19) aldehyde (S)-8 (129 mg, 0.44 mmol) were combined in the same flask, azeotropically dried with benzene three times, and dissolved in freshly dried THF (7.5 mL). After the solution was cooled to −78 °C, LiHMDS (0.48 mL, 1.0 M in THF, 0.48 mmol) was added dropwise. After the solution was stirred for 2 h at −78 °C, the cooling bath was removed, and the reaction was warmed to 23 °C over 1.5 h. After 30 min, it was cooled with dry-ice and quenched with satd aq NH4Cl. The mixture was warmed to 23 °C, stirred vigorously for 30 min, and extracted with CH2Cl2 (3 ×). The combined organic phase was washed successively with water and brine, dried over MgSO4, filtered, and concentrated in vacuo. The remaining residue was purified by flash chromatography (SiO2, gradient eluting with DCM/hexane = 2/1~5/1) to provide the desired product 26 (135 mg, 70 %) as a clear oil.
1H NMR (300 MHz, CDCl3, 23 °C): δ = 6.61 (dq, J = 9.8, 1.4 Hz, 1 H), 6.44 (d, J = 15.6 Hz, 1 H), 6.39 (dd, J = 14.5, 7.7 Hz, 1 H), 6.27 (d, J = 14.5 Hz, 1 H), 6.08 (d, J = 15.7 Hz, 1 H), 5.53–5.68 (m, 2 H), 5.29 (d, J = 9.2 Hz, 1 H), 5.20 (t, J = 7.6 Hz, 1 H), 4.01–4.08 (m, 1 H), 3.74 (s, 3 H), 3.48 (app q, J = 6.0 Hz, 1 H), 3.25 (s, 6 H), 3.23–3.28 (m, 1 H), 2.05–2.50 (m, 4 H), 1.87 (d, J = 1.5 Hz, 3 H), 1.78 (d, J = 1.1 Hz, 3 H), 1.77 (d, J = 1.2 Hz, 3 H), 1.58–1.70 (m, 1 H), 1.47–1.60 (m, 1 H), 1.15 (d, J = 6.6 Hz, 3 H) 13C NMR (75 MHz, CDCl3, 23 °C): δ = 169.0, 146.8, 145.4, 136.6, 133.7, 132.9, 131.9, 131.5, 129.4, 128.2, 126.6, 126.4, 83.3, 78.5, 77.5, 56.8, 56.3, 52.0, 39.7, 36.5, 35.0, 23.1, 20.9, 20.5, 13.3, 12.7. IR: δ = 2960, 2927, 1715, 1647, 1606, 1435, 1261, 1099, 1018, 965, 873, 800, 750 cm−1. (c = 0.2, CHCl3). HRMS (FAB+) m/z ([M-H]+): Calcd for C26H38IO4: 541.1815, Found: 541.1838.
Synthesis of the C(20)–C(34) Subunit
O-TBS-protected Iejimalide Side Chain Ester (29)
Diene-amine 27 (718 mg, 4.24 mmol)23a,b and N-formyl-O-TBS-(S)-serine 28 (1.05 g, 4.24 mmol)25 were azeotropically dried with toluene three times and further dried under high vacuum for 1 h. Freshly dried CH2Cl2 (25 mL) was added to the mixture under Ar, the resulting suspension was cooled to 0 °C, and diethylcyanophosphonate (diethylphosphoryl cyanide, DEPC, 713 µL, 93 %, 4.37 mmol) was added dropwise followed by CaH2-distilled NEt3 (652 µL, 4.67 mmol). The solution was warmed to 23 °C and stirred 10 h. When the reaction was complete as monitored by TLC, the mixture, without workup, was directly purified by flash chromatography (SiO2, eluting with DCM containing 1 % NEt3) to provide the desired of O-TBS protected iejimalide side chain ester 29 (1.35 g, 80 % ).
1H NMR (300 MHz, CDCl3, 23 °C): δ = 8.22 (s, 1 H), 7.38 (d, J = 11.8 Hz, 1 H), 6.82–6.90 (m, 2 H), 6.20 (d, J = 11.8 Hz, 1 H), 4.47–4.53 (m, 1 H), 4.03 (dd, J = 9.8, 4.2 Hz, 1 H), 3.90–3.96 (m, 2 H), 3.73 (s, 3 H), 3.62 (dd, J = 9.7, 8.0 Hz, 1 H), 1.89 (s, 3 H), 1.83 (s, 3 H), 0.84 (s, 9 H), 0.07 (s, 3 H), 0.04 (s, 3 H). 13C NMR (75 MHz, CDCl3, 23 °C): δ = 170.0, 169.2, 161.3, 142.2, 133.4, 127.0, 121.4, 62.8, 53.1, 52.0, 47.1, 25.9, 18.2, 15.7, 12.7, −5.3, −5.4. IR: δ = 3275, 2953, 2931, 1708, 1666, 1641 cm−1. (c = 0.14, CHCl3). mp: 112 °C. HR-MS (ESI) m/z ([M+H]+): Calcd for C19H35N2O5Si: 399.2310, Found: 399.2311.
Iejimalide Side Chain Aldehyde (10)
A solution of O-TBS protected iejimalide side chain ester 29 (630 mg, 1.58 mmol) in CH2Cl2 (15 mL) was cooled to −78 °C, and DIBAL-H (4.74 mL, 1.0 M in hexanes, 4.74 mmol, 3.0 equiv) was added dropwise. After 3 h, another portion of DIBAL-H (1.5 mL, 1.0 M in hexanes) was added dropwise to assure complete reaction as monitored by TLC. After being stirred for another 3 h, the reaction was quenched at −78 °C by addition of MeOH (30 mL), and the cooling bath was removed 10 min later. Satd aq Rochelle's salt solution (50 mL) was added dropwise once the mixture warmed to 0 °C. After being vigorously stirred over 10 h at 23 °C, the mixture was extracted with CH2Cl2 (4 ×). The combined organic phases were dried over MgSO4, filtered, and concentrated under reduced pressure. The remaining crude material was purified by flash chromatography (SiO2, gradient: MeOH/CH2Cl2 = 1/100 ~ 3/100) to provide the intermediate allylic alcohol (585 mg, quantitative).
1H NMR (300 MHz, CDCl3, 23 °C): δ = 8.15 (s, 1 H), 7.04 (d, J = 7.2 Hz, 1 H), 6.89 (t, J = 5.7 Hz, 1 H), 6.18 (d, J = 11.1 Hz, 1 H), 6.08 (d, J = 11.1 Hz, 1 H), 4.46–4.52 (m, 1 H), 4.02 (s, 2 H), 3.96 (dd, J = 9.9, 4.2 Hz, 1 H), 3.83 (br, d, J = 4.8 Hz, 2 H), 3.62 (dd, J = 9.6, 7.8 Hz, 1 H), 2.69 (br, s, 1 H), 1.70 (s, 3 H), 1.69 (s, 3 H), 0.83 (s, 9 H), 0.05 (s, 3 H), 0.03 (s, 3 H). 13C NMR (75 MHz, CDCl3, 23 °C): δ = 169.9, 161.5, 137.7, 133.3, 122.3, 119.9, 68.4, 62.9, 53.2, 47.5, 25.9, 18.2, 15.0, 14.2, −5.4, −5.5 (lit.[4] 1H NMR).
The previously prepared intermediate alcohol (354 mg, 0.96 mmol) was dissolved in CH2Cl2 (10 mL) and cooled to 0 °C. Dess-Martin periodinane (486 mg, 1.15 mmol, 1.2 equiv) was added in one portion. After being stirred for 15 min at 0 °C, the mixture was warmed to 23 °C and stirred for 45 min. The reaction was quenched by addition of a basic aq Na2S2O3 solution, and the resulting mixture was vigorously stirred for 15 min. After being extracted with CH2Cl2 (3 ×), the organic phases were washed successively with water and brine, dried over Na2SO4, filtered and concentrated in vacuo. The remaining residue was purified by flash chromatography (SiO2, gradient eluting with MeOH/DCM = 0.5/100 ~ 1.5/100) to provide the desired aldehyde 10 (330 img, 94 %) as a light grayish white solid.
1H NMR (300 MHz, CDCl3, 23 °C): δ = 9.42 (s, 1 H), 8.22 (s, 1 H), 7.04 (d, J = 11.6 Hz, 1 H), 6.98 (t, J = 5.9 Hz, 1 H), 6.85 (d, J = 6.4 Hz, 1 H), 6.39 (d, J = 11.6 Hz, 1 H), 4.48–4.58 (m, 1 H), 4.03 (dd, J = 9.8, 4.1 Hz, 1 H), 3.93–4.01 (m, 2 H), 3.63 (dd, J = 9.8, 7.9 Hz, 1 H), 1.88 (s, 3 H), 1.78 (s, 3 H), 0.83 (s, 9 H), 0.06 (s, 3 H), 0.04 (s, 3 H). 13C NMR (75 MHz, CDCl3, 23 °C): δ = 195.3, 170.0, 161.4, 145.3, 143.7, 137.7, 120.9, 62.8, 53.2, 47.0, 25.8, 18.2, 15.8, 9.5, −5.4, −5.5. IR: δ = 3276, 2951, 2930, 2860, 1665, 1646, 1561, 1385, 1229, 1127, 839 cm−1. (c = 0.2, CH2Cl2). mp: 110 °C. HR-MS (FAB) m/z ([M+H]+): Calcd for C18H33N2O4Si: 369.2210, Found: 369.2231.
C(20)–C(34) Alkyne (9)
C(23)–C(34) aldehyde 10 (328 mg, 0.89 mmol) and PPh3 (19.4 mg, 0.074 mmol) were dissolved in THF (8 mL) and HMPA (2 mL) to which (R)-4-TMS-3-butyn-2-ylmethanesulfonate 30 (246 mg, 1.12 mmol) was added. The mixture was placed into a preheated oil bath a 55 °C, and Pd(OAc)2 (14.7 mg, 0.065 mmol) was added in one portion followed by powdered InI (272 mg, 1.13 mmol) one minute later. After being stirred for 4 h at 55 °C under Ar, the mixture was removed from the oil bath and cooled to 23 °C. The reaction was quenched by being poured into satd aq NH4Cl in an ice-water cooling bath, stirred for 5 min, and extracted with CH2Cl2 (3 ×). The combined organic phases were dried over MgSO4, filtered, and concentrated. The remaining residue was purified by flash chromatography (SiO2, gradient eluting with DCM/MeOH = 100/0~100/1.5) to provide the desired TMS-alkyne intermediate contaminated with HMPA.
HR-MS (ESI) m/z ([M+H]+): Calcd for C25H46N2NaO4Si2: 517.2888, Found: 517.2864. The above HMPA-contaminated intermediate TMS-alkyne was dissolved in MeOH (15 mL), and K2CO3 (271 mg, 1.96 mmol) was added in one portion. After being stirred for 6 h at 23 °C, the mixture was poured into satd aq NH4Cl and stirred vigorously for 30 min and then extracted with CH2Cl2 (3 ×). The organic phases were combined, dried over MgSO4, filtered, and evaporated to dryness. The remaining residue was purified by flash chromatography (SiO2, gradient eluting with DCM/MeOH = 100/1~ 100/2, to provide the desired C(20)–C(34) alkyne 9 (301 mg, 80 % for 2 steps ) with a ratio of diastereomers of this batch: 4.97/1.
1H NMR (400 MHz, CDCl3, 23 °C ): δ = 8.26 (s, 1 H), 6.59 (br s, 2 H), 6.23 (d, J = 11.1 Hz, 1 H), 6.15 (d, J = 11.1 Hz, 1 H), 4.46 (m, 1 H), 4.09 (dd, J = 9.7, 4.1 Hz, 1 H), 3.91 (m, 3 H), 3.58 (dd, J = 9.7, 8.3 Hz, 1 H), 2.65–2.73 (m, 1 H), 2.22 (d, J = 3.6 Hz, 1 H), 2.17 (d, J = 2.4 Hz, 1 H), 1.76 (s, 3 H), 1.73 (s, 3 H), 1.12 (d, J = 7.0 Hz, 3 H), 0.88 (s, 9 H), 0.11 (s, 3 H), 0.08 (s, 3 H). 13C NMR (75 MHz, CDCl3, 23 °C): δ = 169.8, 161.2, 136.8, 134.6, 123.5, 122.2, 85.8, 81.1, 71.1, 62.7, 53.1, 47.5, 31.5, 26.0, 18.3, 17.8, 15.2, 12.0, −5.3, −5.4. IR: δ = 3307, 2929, 2857, 2112, 1659, 1550, 1462, 1388, 1254, 1112, 1028, 837 cm−1. (c = 0.22, CHCl3). HR-MS (ESI) m/z ([M+Na]+): Calcd for C22H38N2NaO4Si: 445.2493, Found: 445.2490.
Fully Functionalized C(20)–C(34) Segment (5b)
C(20)–C(34) alkyne 9 (98 mg, 0.23 mmol) was dissolved in freshly dried THF (10 mL), and Pd(dppf)Cl2·CH2Cl2 (8.5 mg, 0.012 mmol, 0.05 equiv) was added at 23 °C under Ar. After 5 min, Bu3SnH (0.25 mL, 0.93 mmol, 4 equiv) was added dropwise over 20 min, and during the addition, vigorous bubbling occurred, and the color of the mixture changed from red to orange and then to deep red-orange. After the addition, the mixture was stirred for 30 min at 23 °C and concentrated on a rotary evaporator. 1H NMR of the crude material indicated a 16/1 E/Z isomer ratio. The crude product was purified by flash chromatography. The prepared column was first washed with 1 % Et3N-hexane to basify the column; and the crude material was loaded on the column with toluene and eluted with DCM/Et3N = 100/1 to afford the product contaminated with colloidal SiO2 and adsorbed NEt3. The presence of NEt3 in the eluent was found to avoid the partial decomposition of vinyl stannane 5b during silica gel chromatography. Further purification was accomplished by dissolving the product in toluene and filtering the mixture through Celite to remove the colloidal SiO2 and adsorbed NEt3 to give the pure alkenyl stannane 5b (123 mg, 75%) as an oil.
1H NMR (300 MHz, CDCl3, 23 °C): δ = 8.26 (s, 1 H), 6.56–6.65 (m, 2 H), 6.08–6.18 (m, 3 H), 5.82 (dd, J = 19.0, 8.0 Hz, 1 H), 4.42–4.50 (m, 1 H), 4.10 (dd, J = 9.6, 4.1 Hz, 1 H), 3.84- 3.98 (m, 2 H), 3.70 (d, J = 8.9 Hz, 1 H), 3.58 (dd, J = 9.7, 8.4 Hz, 1 H), 2.28–2.40 (m, 1 H), 1.75 (s, 3 H), 1.74 (s, 3 H), 1.43–1.54 (m, 6 H), 1.24–1.36 (m, 6 H), 0.85–0.93 (m, 27 H), 0.12 (s, 3 H), 0.08 (s, 3 H). 13C NMR (75 MHz, CDCl3, 23 °C): δ = 169.8, 161.2, 151.4, 138.0, 133.7, 131.8, 123.4, 122.7, 81.1, 62.8, 53.1, 47.7, 46.9, 29.4, 27.5, 26.0, 18.3, 17.0, 15.2, 13.9, 11.9, 9.8, - 5.2, - 5.3. IR: δ = 3297, 2956, 2855, 1652, 1556, 1464, 1386, 1258, 1108, 1006, 838, 779 cm−1. (c = 0.4, CHCl3). HR-MS (FAB) m/z ([M-OH]+): Calcd for C34H65N2O3SiSn: 697.3793, Found: 697.3776.
End Game: Ester Hydrolysis, Keck Esterification, Intramolecular Stille Coupling Reaction, and Deprotection. Iejimalide B (1b)
The methyl ester 26 of the C(1)–C(19) subunit (53 mg, 0.098 mmol) was suspended in a freshly prepared LiOH solution (0.1 M LiOH in 1.2/1 i-PrOH/H2O, 10 equiv, 0.982 mmol, 9.8 mL), which was stirred at 23 °C for 10 h. The mixture was neutralized with aq NaHSO4 (1.04 eq, 0.15 M), diluted with EtOAc, and washed with brine (2 × 7.5 mL). After the organic phase was dried over MgSO4 and filtered, the solvent was evaporated under vacuum to give the free acid 4 of the fully functionalized C(1)–C(19) segment (52 mg), which was immediately used in the subsequent Keck intermolecular esterification. A portion was purified by flash chromatography with a short SiO2 column (hexane/EtOAc/MeOH = 4/1/1% to 4/1/2%).
1H NMR (300 MHz, CDCl3, 23 °C): δ = 6.76 (dq, J = 9.6, 1.2 Hz, 1 H), 6.44 (d, J = 15.0 Hz, 1 H), 6.39 (dd, J = 14.7, 7.5 Hz, 1 H), 6.27 (d, J = 14.7 Hz, 1 H), 6.08 (d, J = 15.6 Hz, 1 H), 5.53–5.68 (m, 2 H), 5.30 (d, J = 9.0 Hz, 1 H), 5.20 (t, J = 7.2 Hz, 1 H), 4.02–4.09 (m, 1 H), 3.45–3.52 (m, 1 H), 3.26 (s, 3 H), 3.25 (s, 3 H), 3.23–3.35 (m, 1 H), 2.14–2.52 (m, 4 H), 1.87 (d, J = 1.2 Hz, 3 H), 1.78 (d, J = 1.1 Hz, 3 H), 1.77 (d, J = 1.2 Hz, 3 H), 1.59–1.70 (m, 1 H), 1.47–1.59 (m, 1 H), 1.17 (d, J = 6.9 Hz, 3 H). 13C NMR (75 MHz, CDCl3, 23 °C): δ = 173.5, 147.6, 146.8, 136.6, 133.9, 132.9, 131.9, 131.2, 129.4, 128.2, 126.3, 126.1, 83.3, 78.5, 77.6, 56.7, 56.3, 39.6, 36.7, 34.9, 23.1, 20.9, 20.4, 13.3, 12.4. IR: δ = 3450 (b), 2960, 2926, 2852, 1690, 1453, 1267, 1096, 965, 802 cm−1. (c = 0.06, CH2Cl2). HR-MS (ESI+) m/z ([M+Na]+): Calcd for C25H37INaO4: 551.1629, Found: 551.1617.
To a flame dried 5-mL flask was added EDCI (29.7 mg, 0.155 mmol), DMAP (18.9 mg, 0.155 mmol), and DMAP.HCl (25.8 mg, 0.162 mmol), which was further dried under high vacuum for 3.5 h and placed under Ar. After the mixture was cooled to 0 °C, 4 (53.1 mg, 0.101 mmol) was added with 0.8 mL DCM. After the mixture was stirred for 10 min at 0 °C, 5b (55.2 mg, 0.077 mmol) in 0.8 mL DCM was added dropwise. The reaction flask was sealed under Ar and stirred for 48 h in the dark at 23 °C. When the reaction was complete as monitored by TLC, the mixture was diluted with DCM, washed with water, and back extracted with DCM. The organic phases were combined, washed with brine (2 × 5 mL), dried over Na2SO4, filtered, and concentrated under vacuum. The remaining residue was purified by flash chromatography (SiO2, eluting with hexane/EtOAc/MeOH = 4/1/0.5% ~ 4/1/1%) to afford the desired inter-molecular Keck esterification product 3 (74 mg, 78%) as an oil, which was used immediately in the following intramolecular Stille coupling reaction.
1H NMR (300 MHz, CDCl3, 23 °C): δ = 8.25 (s, 1 H), 6.53–6.68 (m, 3 H), 6.45 (d, J = 16.5 Hz, 1 H), 6.39 (dd, J = 14.4, 7.5 Hz, 1 H), 6.27 (d, J = 14.4 Hz, 1 H), 6.05–6.23 (m, 3 H), 5.96 (d, J = 15.6 Hz, 1 H), 5.82 (dd, J = 19.0, 8.0 Hz, 1 H), 5.53–5.68 (m, 2 H), 5.28 (d, J = 9.0 Hz, 1 H), 5.20 (t, J = 6.6 Hz, 1 H), 5.09 (dd, J = 8.7, 5.4 Hz, 1 H), 4.42–4.50 (m, 1 H), 4.09 (dd, J = 9.6, 3.9 Hz, 1 H), 4.01–4.09 (m, 1 H), 3.81- 3.97 (m, 2 H), 3.57 (dd, J = 9.3, 8.4 Hz, 1 H), 3.45–3.52 (m, 1 H), 3.25 (s, 6 H), 3.18–3.30 (m, 1 H), 2.12–2.65 (m, 5 H), 1.84 (d, J = 0.6 Hz, 3 H), 1.78 (s, 3 H), 1.77 (s, 3 H), 1.73 (br, s, 6 H), 1.59–1.70 (m, 1 H), 1.50–1.59 (m, 1 H), 1.39–1.50 (m, 6 H), 1.22–1.32 (m, 6 H), 1.13 (d, J = 6.9 Hz, 3 H), 0.79–0.93 (m, 27 H), 0.10 (s, 3 H), 0.07 (s, 3 H). 13C NMR (75 MHz, CDCl3, 23 °C) δ = 169.7, 167.3, 161.2, 150.5, 146.8, 145.0, 136.6, 134.9, 134.4, 133.5, 132.9, 131.7, 131.6, 129.3, 128.6, 128.2, 126.9, 126.4, 124.1, 122.3, 83.2, 82.9, 78.5, 77.5, 62.7, 56.7, 56.3, 53.1, 47.6, 44.1, 39.7, 36.5, 34.9, 29.2, 27.4, 25.9, 23.1, 20.9, 20.5, 18.2, 16.9, 15.3, 13.9, 13.3, 12.8, 12.7, 9.6, −5.3, −5.4. IR: δ = 3300 (m, b), 2958, 2927, 2872, 2856, 1708, 1655, 1600, 1553, 1465, 1387, 1259, 1223, 1104, 1008, 990, 965, 839, 779 cm−1. (c = 0.65, CH2Cl2). HR-MS (ESI) m/z ([M+Na]+): Calcd for C59H101IN2NaO7SiSn: 1247.5342, found: 1247.5338
The previous product 3 (24 mg, 0.0196 mmol) was dissolved in anhyd DMF (28 mL, c ≈ 7 × 10−4) and transferred to a flame dried Schlenk tube. The mixture was degassed under high vacuum at −78 °C 3 times, and the tube was back filled with Ar. After the mixture was warmed to 23 °C, Pd2(dba)3·CHCl3 (6.5 mg, 0.0063 mmol, 0.32 eq.), Ph3As (7.7 mg, 0.0251 mmol, 1.28 eq.), and DIPEA (71 µL, 0.408 mmol, 20.8 eq.) were added successively to the mixture, which was stirred at 23 °C under Ar. After 24 h, another portion of Pd2(dba)3·CHCl3 (6.5 mg, 0.0063 mmol, 0.32 eq.), Ph3As (7.7 mg, 0.0251 mmol, 1.28 eq.), and DIPEA (71 µL, 0.408 mmol, 20.8 eq.) were added and stirred for another 24 h. When the reaction was complete as monitored by TLC, the mixture was diluted with DCM, washed with water (3 × 20mL), and back extracted with DCM. The organic phases were combined, dried over Na2SO4, filtered, and concentrated under vacuum. The remaining crude material was purified by flash chromatography (SiO2, DCM/MeOH = 100/0.3~100/1) to afford the desired O-TBS-protected iejimalide B 34 (11.3 mg, 72 %) as an oil, which was used in the following deprotection step. A portion was further purified by HPLC using a C18 reverse phase column (semi-prep, 9.4 × 250, 5 µ; gradient: MeCN/H2O = 85/15 ~ 98/2, 3 mL/min, tR = 21 min).
1H NMR (600 MHz, CDCl3, 23 °C): δ = 8.28 (s, 1 H), 6.62 (dd, 1 H, J = 1.4, 10.4 Hz), 6.57 (m, 2 H), 6.50 (d, 1 H, J = 15.8 Hz), 6.28 (d, 1 H, J = 11.2 Hz), 6.14 (d, 1 H, J = 11.2 Hz), 6.02 (m, 2 H), 5.86 (d, 1 H, J = 15.4 Hz), 5.54 - 5.35 (m, 4 H), 5.19 (dd, 1 H, J = 5.8, 11.2 Hz), 5.15 (d, 1 H, J = 10.4 Hz), 5.09 (d, 1 H, J = 9.6 Hz), 4.46 (m, 1 H), 4.12 (m, 2 H), 3.91 (m, 2 H), 3.59 (app. t, 1 H), 3.28 (m, 1 H), 3.26 (s, 3 H), 3.14 (m, 1 H), 2.95 (s, 3 H), 2.67 (m, 1 H), 2.55 (m, 2 H), 2.32 (m, 1 H), 1.90 (m, 1 H), 1.81 −1.75 (5 overlapping s, 15 H), 1.63 (m, 1 H), 1.33 (m, 1 H), 1.05 (d, 3 H, J = 6.8 Hz), 0.92 (d, 3 H, J = 7.4 Hz), 0.88 (s, 9 H), 0.12 (s, 3 H), 0.09 (s, 3 H). 13C NMR (125 MHz, CDCl3, 23 °C) δ = 169.8, 167.8, 161.2, 145.8, 137.1, 136.1, 135.2, 134.0, 133.55, 133.34, 133.25, 132.3, 131.97, 131.96, 131.1, 129.8, 128.7, 125.6, 125.5, 125.0, 122.1, 83.1, 79.7, 77.0, 62.7, 56.4, 56.1, 53.1, 47.5, 41.2, 40.9, 38.4, 35.0, 26.0, 23.0, 21.6, 21.0, 18.3, 16.9, 15.3, 13.2, 12.2, 12.1, −5.3, −5.4. IR: δ = 3290 (m, b), 2925, 2856, 1715, 1674, 1557, 1484, 1449, 1386, 1331, 1261, 1184, 1100, 1023, 968, 916, 700 cm−1. HR-MS (ESI) m/z ([M+Na]+): Calcd for C47H74N2NaO7Si: 829.5158, found: 829.5188
O-TBS protected iejimalide B 34 (7.5 mg, 9.3 µmol) was transferred into a plastic vial by using EtOAc (600 µL) and DMF (600 µL, to which TAS-F (10 equiv, 0.5 M in DMF, 186 µL, 0.093 mmol) was added. The mixture was stirred and monitored by TLC (silica gel). After being stirred for 2 h at 23 °C, the mixture was diluted with pH 7 buffer, and extracted with EtOAc (3 ×). The organic phases were combined, dried over Na2SO4, filtered and concentrated under vacuum. The resulting crude material was purified by flash chromatography (SiO2, gradient DCM/MeOH = 50/1~30/1) to afford the synthetic iejimalide B (1b) (5 mg, 80 %), which was further purified by HPLC using a C18 reverse phase column (semi-prep, 9.4 × 250, 5 µ; isocratic elution: MeCN/H2O = 80/20, 3 mL/min, tR = 8.6 min).
1H NMR (600 MHz, CDCl3, 23 °C): δ = 8.28 (s, 1 H), 6.81 (d, 1 H, J = 6.6 Hz), 6.80 (m, 1 H), 6.63 (dd, 1 H, J = 1.5, 10.3 Hz), 6.48 (d, 1 H, J = 15.6 Hz), 6.28 (d, 1 H, J = 11.4 Hz), 6.13 (d, 1 H, J = 11.2 Hz), 6.03 (m, 2 H), 5.86 (d, 1 H, J = 15.4 Hz), 5.54 - 5.37 (m, 4 H), 5.19 (dd, 1 H, J = 6.2, 11.0 Hz), 5.15 (d, 1 H, J = 10.0 Hz), 5.08 (d, 1 H, J = 9.0 Hz), 4.50 (m, 1 H), 4.23 (dd, 1 H, J = 2.5, 11.5 Hz), 4.13 (m, 1 H), 3.90 (m, 2 H), 3.65 (m, 1 H), 3.28 (m, 1 H), 3.27 (s, 3 H), 3.14 (m, 1 H), 2.97 (s, 3 H), 2.68 (m, 1 H), 2.55 (m, 2 H), 2.32 (m, 1 H), 1.90 (m, 1 H), 1.80 - 1.75 (5 overlapping s, 15 H), 1.62 (m, 1 H), 1.33 (m, 1 H), 1.05 (d, 3 H, J = 6.8 Hz), 0.94 (d, 3 H, J = 6.6 Hz). 13C NMR (125 MHz, CDCl3, 23 °C) δ = 170.6, 167.7, 161.9, 145.8, 137.1, 136.0, 134.9, 134.0, 133.5, 133.3, 133.2, 132.3, 132.0, 131.9, 131.1, 129.8, 128.7, 125.6, 125.3, 124.9, 121.5, 83.0, 79.8, 76.9, 62.5, 56.4, 56.1, 52.6, 47.2, 41.0, 40.8, 38.2, 35.0, 23.1, 21.6, 20.9, 16.9, 15.3, 13.3, 12.3, 12.2. IR: δ = 3320 (br), 2964, 2926, 1651 (br), 1538, 1448, 1386, 1260, 1217, 1098, 1023, 967, 871, 800 cm−1. (c = 0.2, CH2Cl2). HR-MS (ESI) m/z ([M+Na]+): Calcd for C41H60N2NaO7: 715.4298, Found: 715.4283.
Supplementary Material
Acknowledgment
We are grateful for the financial support of this research provided by the Walther Cancer Institute and the Indiana Clinical and Translational Sciences Institute funded by Grant # RR025761 from the National Institutes of Health, National Center for Research Resources, Clinical and Translational Sciences Award. We also wish to express our deepest appreciation to many previous co-workers and collaborators who have made key contributions to earlier stages of this work, including (in alphabetical order) Dr. Muriel Cottard, Prof. Tatsuo Higa, Mr. Jakob Jensen, Dr. Peter McHenry, Dr. Matthew Mendlik, Mr. David Miller, Prof. Rudolph Navari, Prof. Per-Ola Norrby, Dr. Torben Pedersen, Prof. George R. Pettit, Prof. Tobias Rein, Prof. Daniel Strand, Prof. Junichi Tanaka, Prof. Martin Tenniswood, Dr. James Ulager, Prof. Olaf Wiest, and Dr. David Young.
Footnotes
This paper is dedicated to Professor Elias J. Corey
Supporting Information Available: 1H and 13C NMR spectra of all key reaction products. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Kobayashi J, Cheng J, Ohta T, Nakamura H, Nozoe S, Hirata Y, Ohizumi Y, Sasaki T. J. Org. Chem. 1988;53:6147–6150. [Google Scholar]; (b) Kikuchi Y, Ishibashi M, Sasaki T, Kobayashi J. Tetrahedron Lett. 1991;32 797-796. [Google Scholar]; (c) Tsuda M, Nozawa K, Shimbo K, Ishiyama H, Fukushi E, Kawabata J, Kobayashi J. Tetrahedron Lett. 2003;44:1395–1399. [Google Scholar]; (d) Nozawa K, Tsuda M, Ishiyama H, Sasaki T, Tsuruo T, Kobayashi J. Bioorg. Med. Chem. 2006;14:1063–1067. doi: 10.1016/j.bmc.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 2.(a) Kazami S, Muroi M, Kawatani M, Kubota T, Usui T, Kobayashi J, Osada H. Biosci. Biotechnol. Biochem. 2006;70:1364–1370. doi: 10.1271/bbb.50644. [DOI] [PubMed] [Google Scholar]; (b) Schweitzer Dirk, Zhu Junyi, Jarori Gotam, Tanaka Junichi, Higa Tatsuo, Davisson VJ, Helquist P. Bioorg. Med. Chem. 2007;15:3208–3216. doi: 10.1016/j.bmc.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 3. http://dtp.nci.nih.gov/dtpstandard/servlet/MeanGraphSummary?testshortname=NCI+Cancer+Screen+Current+Data&searchtype=NSC&searchlist=727699.
- 4.(a) Wang WLW, McHenry P, Jeffrey R, Schweitzer D, Helquist P, Tenniswood M. J. Cell. Biochem. 2008;105:998–1007. doi: 10.1002/jcb.21898. [DOI] [PubMed] [Google Scholar]; (b) McHenry P, Wang WLW, Devitt E, Kluesner N, Davisson VJ, McKee E, Schweitzer D, Helquist P, Tenniswood M. J. Cell. Biochem. 2010;109:634–642. doi: 10.1002/jcb.22438. [DOI] [PubMed] [Google Scholar]
- 5.Forgac M. Nat. Rev. Mol. Cell Biol. 2007;8:917–929. doi: 10.1038/nrm2272. [DOI] [PubMed] [Google Scholar]
- 6.Beyenbach KW, Wieczorek H. J. Exp. Biol. 2006;209:577–589. doi: 10.1242/jeb.02014. [DOI] [PubMed] [Google Scholar]
- 7.Pérez-Sayáns M, Somoza-Martín JM, Barros-Angueira F, Rey JM, García-García A. Cancer Treat Rev. 2009;35:707–713. doi: 10.1016/j.ctrv.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 8.(a) Huss M, Wieczorek H. J. Exp. Biol. 2009;212:341–346. doi: 10.1242/jeb.024067. [DOI] [PubMed] [Google Scholar]; (b) Beutler JA, McKee TC. Curr. Med. Chem. 2003;10:787–796. doi: 10.2174/0929867033457827. [DOI] [PubMed] [Google Scholar]
- 9.Muroi M, Kazami S, Noda K, Kondo H, Takayama H, Kawatani M, Usui T, Osada H. Chem. Biol. 2010;17:460–470. doi: 10.1016/j.chembiol.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Dechant R, Binda M, Lee SS, Pelet S, Winderickx J, Peter M. EMBO J. 2010;29:2515–2526. doi: 10.1038/emboj.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruciat C-M, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, Boutros M, Niehrs C. Science. 2010;327:459–463. doi: 10.1126/science.1179802. [DOI] [PubMed] [Google Scholar]
- 12.(a) Cottard M, Kann N, Rein T, Åkermark B, Helquist P. Tetrahedron Lett. 1995;36:3115–3118. [Google Scholar]; (b) Mendlik MT, Cottard M, Rein T, Helquist P. Tetrahedron Lett. 1997;38:6375–6378. [Google Scholar]; (c) Fürstner A, Aïssa C, Chevrier C, Teply F, Nevado C, Tremblay M. Angew. Chem. Int. Ed. 2006;45:5832–5837. doi: 10.1002/anie.200601859. [DOI] [PubMed] [Google Scholar]; (d) Fürstner A, Nevado C, Tremblay M, Chevrier C, Teply F, Aïssa C, Waser M. Angew. Chem. Int. Ed. 2006;45:5837–5842. doi: 10.1002/anie.200601860. [DOI] [PubMed] [Google Scholar]; (e) Fürstner A, Nevado C, Waser M, Tremblay M, Chevrier C, Teply F, Aïssa C, Moulin E, Müller O. J. Am. Chem. Soc. 2007;129:9150–9161. doi: 10.1021/ja072334v. [DOI] [PubMed] [Google Scholar]; (f) Schweitzer D, Kane J, Strand D, McHenry P, Tenniswood M, Helquist P. Org. Lett. 2007;9:4619–4622. doi: 10.1021/ol702129w. [DOI] [PubMed] [Google Scholar]; (g) Moulin E, Nevado C, Gagnepain J, Kelter G, Fiebig H-H, Fürstner A. Tetrahedron. 2010;66:6421–6428. [Google Scholar]
- 13.Simmons TL, Coates RC, Clark BR, Engene N, Gonzalez D, Esquenazi E, Dorrestein PC, Gerwick WH. Proc Nat Sci Acad USA. 2008;105:4587–4594. doi: 10.1073/pnas.0709851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt EW, Donia MS. Curr. Opin. Biotech. 2010;21:827–833. doi: 10.1016/j.copbio.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) Inanaga J, Hirata K, Saeki H, Katsuki T, Yamaguchi M. Bull. Chem. Soc. Jpn. 1979;52:1989–1993. [Google Scholar]; (b) Kawanami Y, Dainobu Y, Inanaga J, Katsuki T, Yamaguchi M. Bull. Chem. Soc.Jpn. 1981;54:943–944. [Google Scholar]; (c) Lepage O, Kattnig E, Fürstner A. J. Am. Chem. Soc. 2004;126:15970–15971. doi: 10.1021/ja044130+. [DOI] [PubMed] [Google Scholar]
- 16.(a) Baudin JB, Hareau G, Julia SA, Ruel O. Tetrahedron Lett. 1991;32:1175–1178. [Google Scholar]; (b) Blakemore PR, Cole WJ, Kocienski PJ, Morley A. Synlett. 1998:26–28. [Google Scholar]; (c) Blakemore PR, Kocienski PJ, Morley A, Muir K. J. Chem. Soc., Perkin Trans. 1999;1:955–968. [Google Scholar]; (d) Blakemore PR. J. Chem. Soc., Perkin Trans. 2002;1:2563–2585. [Google Scholar]; (e) Liu J, Xu K, He J, Zhang L, Pan X, She X. J. Org. Chem. 2009;74:5063–5066. doi: 10.1021/jo900820f. [DOI] [PubMed] [Google Scholar]
- 17.(a) Nyström J-E, McCanna TD, Helquist P, Amouroux R. Synthesis. 1988:56–58. [Google Scholar]; (b) Avery TD, Caiazza D, Culbert JA, Taylor DK, Tiekink ERT. J. Org. Chem. 2005;70:8344–8351. doi: 10.1021/jo050806n. [DOI] [PubMed] [Google Scholar]
- 18.Sreekumar C, Darst KP, Still WC. J. Org. Chem. 1980;45:4260–4262. [Google Scholar]
- 19.Anand NK, Carreira EM. J. Am. Chem. Soc. 2001;123:9687–9688. doi: 10.1021/ja016378u. [DOI] [PubMed] [Google Scholar]
- 20.Wipf P, Graham TH. J. Am. Chem. Soc. 2004;126:15346–15347. doi: 10.1021/ja0443068. [DOI] [PubMed] [Google Scholar]
- 21.Kalish VJ, Shone RL, Kramer SW, Collins PW, Babiak KA, McLaughlin KT, Ng JS. Synth. Commun. 1990;20:1641–1645. [Google Scholar]
- 22.(a) Griffith WP, Ley SV, Withcombe GP, White AD. J. Chem. Soc. Chem. Comm. 1987:1625–1627. [Google Scholar]; (b) Alvarez R, Domínguez M, Pazos Y, Sussman F, de Lera AR. Chem. Eur. J. 2003;9:5821–5831. doi: 10.1002/chem.200304847. [DOI] [PubMed] [Google Scholar]
- 23.(a) Kao L-C, Stakem FG, Patel BA, Heck RF. J. Org. Chem. 1982;47:1267–1277. [Google Scholar]; (b) Heck RF. Org. React. 1982;27:345–390. [Google Scholar]
- 24.Ando A, Shioiri T. Tetrahedron. 1989;45:4969–4988. [Google Scholar]
- 25.Hill DR, Hsiao C-N, Kurukulasuriya R, Wittenberger SJ. Org. Lett. 2002;4:111–113. doi: 10.1021/ol016976d. [DOI] [PubMed] [Google Scholar]
- 26.(a) Marshall JA, Grant CM. J. Org. Chem. 1999;64:696–697. doi: 10.1021/jo982255p. [DOI] [PubMed] [Google Scholar]; (b) Marshall JA, Chobanian HR, Yanik MM. Org. Lett. 2001;3:3369–3372. doi: 10.1021/ol016605x. [DOI] [PubMed] [Google Scholar]
- 27.(a) Chemler SR, Trauner D, Danishefsky SJ. Angew. Chem. Int. Ed. 2001;40:4544–4568. doi: 10.1002/1521-3773(20011217)40:24<4544::aid-anie4544>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]; (b) Kotha S, Lahiri K, Kashinath D. Tetrahedron. 2002;58:9633–9695. [Google Scholar]; (c) Lee SJ, Gray Kaitlyn C, Paek JS, Burke MD. J. Am. Chem. Soc. 2008;130:466. doi: 10.1021/ja078129x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Tortosa M, Yakelis NA, Roush WR. J. Org. Chem. 2008;73:9657–9667. doi: 10.1021/jo801794s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Li P, Li J, Arikan F, Ahlbrecht W, Dieckmann M, Menche D. J. Org. Chem. 2010;75:2429–2444. doi: 10.1021/jo100201f. [DOI] [PubMed] [Google Scholar]; (f) Macklin TK, Micalizio GC. J. Am. Chem. Soc. 2009;131:1392–1393. doi: 10.1021/ja809491b. [DOI] [PubMed] [Google Scholar]; (g) Smith AB, III, Foley MA, Dong S, Orbin A. J. Org. Chem. 2009;74:5987–6001. doi: 10.1021/jo900765p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Rahn A, Kalesse M. Angew. Chem. Int. Ed. 2008;47:597–599. doi: 10.1002/anie.200703930. [DOI] [PubMed] [Google Scholar]
- 28.(a) Tucker CE, Davidson J, Knochel P. J. Org. Chem. 1992;57:3482–3485. [Google Scholar]; (b) Kabalka GW. Org. Prep. Proced. Int. 1977;9:131–147. [Google Scholar]
- 29.(a) Pereira S, Srebnik M. J. Am. Chem. Soc. 1996;118:909–910. [Google Scholar]; (b) Yamamoto Y, Fujikawa R, Yamada A, Miyaura N. Chem. Lett. 1999:1069–1070. [Google Scholar]; (c) Yamamoto Y, Kurihara K, Yamada A, Takahashi M, Takahashi Y, Miyaura N. Tetrahedron. 2003;59:537–542. [Google Scholar]
- 30.Stille JK, Sweet MP. Tetrahedron Lett. 1989;30:3645–3648. [Google Scholar]
- 31.(a) Mukaiyama T, Usui M, Saigo K. Chem. Lett. 1976:49–50. [Google Scholar]; (b) Narasaka K, Maruyama K, Mukaiyama T. Chem. Lett. 1978:885–888. [Google Scholar]; (c) Convers E, Tye H, Whittaker M. Tetrahedron Lett. 2004;45:3401–3404. [Google Scholar]
- 32.(a) Neises B, Steglich W. Angew. Chem., Int. Ed. Engl. 1978;17:522–524. [Google Scholar]; (b) Hassner A, Alexanian V. Tetrahedron Lett. 1978;19:4475–4478. [Google Scholar]
- 33.Zhao H, Pendri A, Greenwald RB. J. Org.Chem. 1998;63:7559–7562. doi: 10.1021/jo981161c. [DOI] [PubMed] [Google Scholar]
- 34.(a) Boden EP, Keck GE. J. Org. Chem. 1985;50:2394–2395. [Google Scholar]; (b) Keck GE, Sanchez C, Wager CA. Tetrahedron Lett. 2000;41:8673–8676. [Google Scholar]; (c) Paterson I, Yeung K-S, Ward RA, Cumming JG, Smith JD. J. Am. Chem. Soc. 1994;116:9391–9392. [Google Scholar]; (d) Tsai C-Y, Huang X, Wong C-H. Tetrahedron Lett. 2000;41:9499–9503. [Google Scholar]; (e) Hanessian S, Ma J, Wang W. J. Am. Chem. Soc. 2001;123:10200–10206. doi: 10.1021/ja011452u. [DOI] [PubMed] [Google Scholar]; (f) Lewis A, Stefanuti I, Swain SA, Smith SA, Taylor RJK. Org. Biomol. Chem. 2003;1:104–116. doi: 10.1039/b209637b. [DOI] [PubMed] [Google Scholar]; (g) Christie HS, Heathcock CH. Proc. Nat. Acad. Sci. 2004;101:12079–12084. doi: 10.1073/pnas.0403887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masse CE, Yang M, Solomon J, Panek JS. J. Am. Chem. Soc. 1998;120:4123–4134. [Google Scholar]
- 36.Munakata R, Katakai H, Ueki T, Kurosaka J, Takao K-i, Tadano K-i. J. Am. Chem. Soc. 2004;126:11254–11267. doi: 10.1021/ja048320w. [DOI] [PubMed] [Google Scholar]
- 37.(a) Nicolaou KC, Murphy F, Barluenga S, Ohshima T, Wei H, Xu J, Gray DLF, Baudoin O. J. Am. Chem. Soc. 2000;122:3830–3838. [Google Scholar]; (b) Toshima K, Jyojima T, Miyamoto N, Katohno M, Nakata M, Matsumura S. J. Org. Chem. 2001;66:1708–1715. doi: 10.1021/jo001377q. [DOI] [PubMed] [Google Scholar]
- 38.Noyori R, Nishida I, Sakata J, Nishizawa M. J. Am. Chem. Soc. 1980;102:1223–1225. [Google Scholar]
- 39.The authentic sample was obtained as previously reported in reference 2b.
- 40.Keck GE, Giles RL, Cee VJ, Wager CA, Yu T, Kraft MB. J. Org. Chem. 2008;73:9675–9691. doi: 10.1021/jo802215v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsui K, Zheng B-Z, Kusaka S-i, Kuroda M, Yoshimoto K, Yamada H, Yonemitsu O. Eur J. Org. Chem. 2001;19:3615–3624. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.