Abstract
Objective
Cross-sectional studies suggest an association between hysterectomy and negative affect. Using prospective data, we examined the associations of negative affect, attitudes toward aging and menopause, premenstrual symptoms and vasomotor symptoms with elective hysterectomy in midlife.
Methods
Data were from the Study of Women's Health Across the Nation, a multi-site community-based prospective cohort study of the menopausal transition (n=2,818). Annually reported hysterectomy at visits 2-9 was verified with medical records when available (71%). Anxiety, perceived stress, depressive symptoms, attitudes toward aging and menopause, vasomotor symptoms, and premenstrual symptoms were assessed at baseline using standardized questions. Cox proportional hazards models were used to relate these variables to subsequent elective hysterectomy. Covariates included demographic variables, menstrual bleeding problems, body mass index, hormone levels, and self-rated health, also assessed at baseline.
Results
Elective hysterectomy was reported by 6% of participants (n=168) over an 8-year period. Women with hysterectomy were not higher in negative affect or negative attitudes toward aging and menopause compared to women without hysterectomy. Vasomotor symptoms (HR 1.44, 95% CI 1.03-2.01, p=.03) and positive attitudes toward aging and menopause (HR 1.74, 95% CI 1.04-2.93) at baseline predicted hysterectomy over the 8-year period, controlling for menstrual bleeding problems, site, race/ethnicity, follicle stimulating hormone, age, education, body mass index, and self-rated health. Menstrual bleeding problems at baseline were the strongest predictor of hysterectomy (HR 4.30, 95% CI 2.05-9.05).
Conclusions
In this prospective examination, negative affect and attitudes were not associated with subsequent hysterectomy. Menstrual bleeding problems were the major determinant of elective hysterectomy.
Keywords: hysterectomy, affect, menopause, vasomotor symptoms
Introduction
Six hundred thousand hysterectomies are performed annually in the United States, making it the most common non-obstetric surgery among women. Approximately 96% of hysterectomies are performed to treat benign conditions such as uterine fibroids and endometriosis,1 and symptoms such as dysfunctional menstrual bleeding.2 The annual incidence of hysterectomy is highest among women between the ages of 40-54,1 in women with lower educational attainment,3,4 high BMI, and among African-American women.5
Hysterectomy is effective at treating these conditions, but entails substantial risks, leading to long-articulated concerns about the frequency of its use and potential for inappropriate utilization. The mortality rate for hysterectomy is 1 in 1,000,6 with hospital readmission rates estimated at 7.2%.7 Morbidity rates have been estimated at 6.1%,8 with surgical complications including hemorrhage, infection, and internal injury occurring in 1.6-18% of patients.7,9 Long-term effects may include pain during sexual intercourse,10 reduced physical functioning,11 and in the estimated 55-80% of hysterectomies with cooccurring bilateral oopherectomy,12,13 increased risk of cardiovascular disease and osteoporosis.14
Considering these potential risks, there is interest in understanding the non-medical predictors of hysterectomy. Independent of medical comorbidities,15 individuals with affective disorders,16,17 premenstrual syndrome (PMS),18,19 and vasomotor symptoms (VMS)20 have a higher rate of overall health care utilization and report decreased health-related quality of life.21 Individuals with elevated negative affect, including anxiety and depressive symptoms, also report higher rates of somatic and pain symptoms, as well as more bothersome VMS, even controlling for VMS frequency.22 Women with PMS and negative attitudes about aging and menopause prior to entering the menopausal transition are more likely to later report menopausal symptoms including VMS, sleep disturbances, genitourinary problems, and depressed mood.23-28 The prevalence of menstrual bleeding problems, commonly linked to hysterectomy, increases during the menopausal transition, affecting 10–30% of women of reproductive age and up to 50% of perimenopausal women.29 Thus, among midlife women with negative affect, negative attitudes toward aging and menopause, VMS, and PMS, symptoms such as heavy menstrual bleeding may be more often reported, less tolerated, and thereby more likely to lead to elective hysterectomy during midlife.
An association between negative affect and hysterectomy is supported by cross-sectional studies, which suggest that hysterectomized women have more general psychological distress than their age-matched non-hysterectomized peers.30,31 Additionally, in studies comparing psychological symptoms pre- and post-hysterectomy, depressive symptoms, anxiety, and psychological morbidity were more common in women scheduled for hysterectomy than would be expected in the general population.32-36 However, because psychological symptoms were evaluated at the time of treatment-seeking, it is unclear whether reported psychological symptoms were associated with the presenting menstrual problems and/or anxiety about surgery.36 Little is known about the individual differences between women who eventually have hysterectomies and those who do not in the years preceding symptom presentation and treatment-seeking.
Prospective evaluations of negative affect, attitudes toward aging and menopause, and symptoms prior to seeking treatment for menstrual problems are needed to increase our understanding of the contribution of these factors to elective hysterectomy. This study enriches previous research by utilizing the large, multi-ethnic sample of the Study of Women's Health Across the Nation (SWAN). We hypothesized that negative affect (anxiety, depressive symptoms, and perceived stress), negative attitudes toward aging and menopause, and a high burden of VMS and PMS-like symptoms would be associated with an increased likelihood of elective hysterectomy over the menopausal transition.
Methods
Participants
Participants were from the Study of Women's Health Across the Nation (SWAN), a multi-site community-based prospective study designed to examine the physical and psychological health of women as they undergo the menopausal transition. Details of the SWAN design and recruitment procedures have been reported elsewhere.37
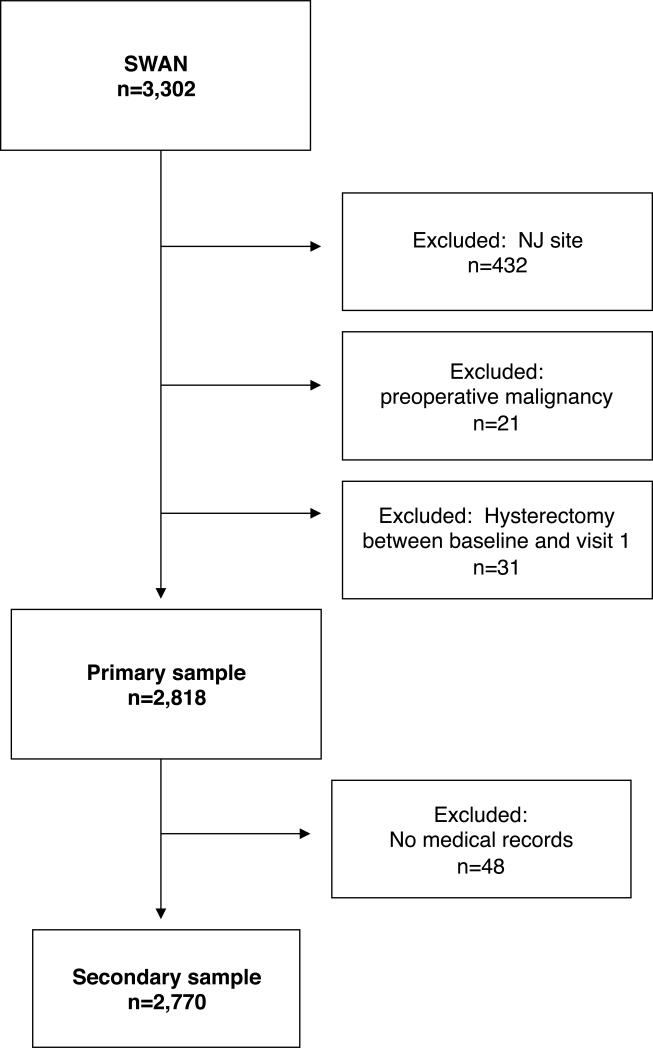
All SWAN cohort participants had an intact uterus and at least one ovary and met the additional eligibility criteria: aged 42–52 years, not being pregnant, not using reproductive hormones, and having 1 or more menstrual cycles in the 3 months prior to the interview. Each site recruited non-Hispanic White women as well as women belonging to a predetermined racial/ethnic minority group: African American women in Pittsburgh, Pennsylvania; Boston, Massachusetts; Detroit, Michigan; and Chicago, Illinois; Japanese women in Los Angeles, California; Hispanic women in Newark, New Jersey; and Chinese women in the Oakland area of California. Participants were recruited using established sampling techniques, random digit dialing, and random sampling from lists of names or household addresses. Select sites supplemented primary sampling frames to obtain adequate numbers of racial/ethnic minority women. Seventy-three percent of the women selected were contacted and provided information to determine eligibility; 51% (n = 3,302) of eligible women enrolled. Participants returned to their local site facility annually for interviewer- and self-administered questionnaires, a fasting blood draw, and reassessments of physical measures. Data collection for this analysis spanned from 1996-2007. SWAN was approved by the institutional review boards at each site, and each participant provided written, informed consent. Data collection ceased at the New Jersey SWAN site after 2001 for reasons unrelated to scientific aspects of the project but resulting in a more abbreviated follow-up, and the potential for less stable statistical models. Therefore, data from women at this site were excluded from the current analysis.
Design and procedures
Elective Hysterectomy
At annual follow-up visits 1-9, participants were asked whether they had a “hysterectomy (an operation to remove your uterus or womb)” since the previous study visit. Interviewers obtained written consents to request medical records for any reported hysterectomy from the surgery facility and these medical records were reviewed by a board certified gynecologist (GW) to verify hysterectomy and date of surgery. Of the 239 reports of hysterectomy, medical records were available for 166 women (68%), confirming self-reports in all but one case. Elective hysterectomy was defined as any hysterectomy occurring in the absence of known or suspected endometrial, uterine, or ovarian cancer; surgeries that did not meet this criteria (n=21) were excluded from data analysis. Surgeries that occurred within the first year were excluded (n=31) from data analyses because these may have occurred in response to symptoms present at the time of study entry.
Psychosocial and Symptom Measures
Negative affect
Negative affect, assessed at baseline, included measures of perceived stress, depressive symptoms, and anxiety. Perceived stress in the two weeks before the baseline interview was assessed with the four-item Perceived Stress scale. The extent of stress was measured using a 5 point scale with higher scores indicating greater perceived stress.38 Depressive symptoms were assessed using the Center for Epidemiological Studies Depression Scale (CES-D), a 20-item depression symptom scale. Participants indicated how many days in the last week they would agree with statements such as, “I felt lonely” and “I felt that everything I did was an effort”, A score of 16 or greater out of a possible 60 was used to define the presence of potentially significant depressive symptoms.39,40
Anxiety was assessed with four questions asking about the number of days (from 0= no days to 4= every day) in the two weeks prior to the baseline visit in which participants reported “irritability or grouchiness”, “feeling tense or nervous”, “heart pounding or racing”, or “feeling fearful for no reason”. The number of days that participants reported each item was summed into a score ranging from 0-16. Significant anxiety was categorized as anxiety scores in the top 20% of the sample (4 or greater).
Attitudes toward aging and menopause
Attitudes toward aging and menopause (neutral/negative, slightly positive, or highly positive) were based on participants’ extent of agreement with 7 positively correlated statements (Cronbach's α= 0.51) about individual and societal views about aging and the menopausal transition, such as “overall, going through the menopause or change of life will be, or was, a positive experience for me”. A mean score for each woman was calculated, with higher scores indicating more positive attitudes toward aging and menopause.41
Vasomotor symptoms (VMS)
Questions about how often (not at all, 1-5 days, 6-8 days, 9-13 days, every day) women experienced hot flashes and night sweats in the two weeks prior to the study visit were used to characterize VMS. Night sweats and hot flashes were combined into a single VMS variable. Responses were categorized as “none” if women reported neither hot flashes nor night sweats, and “any” if women reported hot flashes, night sweats, or both.
Premenstrual syndrome (PMS)-like symptoms
Baseline PMS-like symptoms were based on report of any of eleven psychological and physical symptoms including “anxious, jittery, nervous” and “breast pain, tenderness or swelling”, during or in the week before at least half of their menstrual periods in the last year. Women were categorized as having PMS-like symptoms if they reported at least one physical symptom, at least one psychological symptom, symptom interference with job or home activities, and dissolution of symptoms within 1-3 days of the onset of menses.
Covariates
All proposed covariates (from baseline) included in univariate analyses were selected on the basis of previously documented associations with hysterectomy3,5,29,42-44 and retained in multivariate analyses when univariate associations with hysterectomy were p < 0.20. Women were characterized as having menstrual bleeding problems at baseline if they reported heavy or very heavy menstrual flow, flooding or gushing, and clots. Menopausal status at baseline was categorized as premenopausal (bleeding in the previous 3 months with no change in cycle predictability in the past year) or early perimenopausal (bleeding in the previous 3 months with a decrease in cycle predictability in the past year). Race/ethnicity was self-reported in response to an open-ended question. Age was calculated using date of birth and the date of the baseline visit. Insurance status and education level were self-reported during the screening interview. Self-rated health was assessed by response to the following question: “In general, would you say your health is excellent, very good, good, fair or poor?”.45 Responses were categorized into four categories, with responses of “fair” and “poor” collapsed into a single category. Body mass index (BMI) was calculated using weight (kg) and height (m)2.
Reproductive hormones
Due to the relationship between increased estrogen secretion, concomitant FSH suppression, and factors related to hysterectomy, including fibroid growth, endometriosis, polyps, and abnormal uterine bleeding, reproductive hormones were considered as covariates. Estradiol (E2) and follicle stimulating hormone (FSH) were assessed at baseline and annually thereafter. Reproductive hormone levels were determined from fasting blood samples obtained during the baseline study visit in the 2- to 5-day window of the early follicular phase of the menstrual cycle. FSH was assayed on the ACS-180 automated analyzer (Bayer Diagnostics Corporation, Tarrytown, NY) with a 2-site chemiluminometric immunoassay. Interassay coefficients of variation were 11.4%, intraassay coefficients of variation were 3.8%, and the lower limit of detection (LLD) was 1.05 mIU/mL.46 Duplicate E2 assays were conducted with results reported as the arithmetic mean for each subject (CV of 3-12%). Other assays were single determinations. E2 was assayed using a rabbit anti-E2-6 ACS-180 immunoassay modified to increase sensitivity. The interassay coefficient of variation was 10.6%, intrassay coefficient of variation was 6.4%, and an LLD of 1.0 pg/mL.47 FSH and E2 were log transformed to normalize their distributions.
Analytic sample
A total of 2,818 women, including 168 women with hysterectomy not due to preoperative malignancy in visits 2 through 9, comprised the analytic sample. The analytic sample excluded women from the New Jersey site and women who had a hysterectomy in the first year of follow-up. Asubset analysis was conducted excluding the data from 48 women without medical records available to verify self-reported hysterectomy. The subset sample was thus comprised of 2,770 women, including 120 women with verified hysterectomy.
Statistical analyses
Differences in key baseline factors in women with and without self-reported hysterectomy at study visit 2-9 were assessed with chi-square tests for comparisons of categorical variables and t-tests for comparisons of continuous variables. All primary explanatory variables associated with hysterectomy at p <.05 and covariates associated with hysterectomy at p <.20 in univariate analysis were retained in the multiple variable analyses.
Associations of baseline attitudes toward aging and menopause, PMS-like symptoms, and vasomotor symptoms with hysterectomy were estimated with hazard ratios (HR) using Cox proportional hazards modeling. To determine the time to event, the self-reported date of hysterectomy was used if a date from medical records was not available. Women who did not report hysterectomy were censored at the date of their last visit; women with self-reported bilateral salingo-oopherectomy without hysterectomy were censored at the date of the visit at which the surgery was reported (n=15). The proportional hazards assumption was tested by including interactions between key predictors and time, which identified one violation of the assumption. A significant time by menstrual bleeding problems interaction indicated that the impact of baseline bleeding problems decreased over the follow-up period. This interaction was included in the primary model to address nonproportionality.48 Additional covariates adjusted for in modeling included menstrual bleeding problems, site, race, age, BMI, educational attainment, self-rated health, and FSH (log-transformed). All predictors and covariates were entered simultaneously in the final multivariate model. For all analyses, p values less than 0.05 (two-tailed) were considered statistically significant. Analyses were performed with SPSS, version 17.0.49
Results
Characteristics of sample
Compared with women in the analytic sample, women who were excluded because of early hysterectomy between baseline and first follow-up had a higher mean BMI (31.6 v. 28.1, p=.01), were more likely to report menstrual bleeding problems at baseline (48.1% v. 22.2%, p<.001), and were more likely to report PMS-like symptoms at baseline (23.9% v. 13.5%, p=.07, trend). There were no other differences on key baseline factors between women who were and were not included in the primary analytic sample (data not shown).
Over the observed 8-year period, 6.0% of participants (n=168) reported having a hysterectomy for a condition that was not cancer-related. This is comparable to expected rates for all-cause hysterectomy among women aged 45-54 in the general population.13 The most common preoperative symptoms and diagnoses identified in the available medical records, (n=120, 71%), were suspected or diagnosed uterine fibroids (n=82, 66.1%), suspected or diagnosed menorrhagia (n=71, 57.3%), and chronic pelvic pain (n=34, 27.4%). Suspected or diagnosed fibroids largely explained the presentation of menorrhagia (n=62, 87.3%) and chronic pelvic pain (n=30, 88.2%) in this sample.
According to medical records, 77 women (64%) had a hysterectomy with concomitant bilateral oopherectomy, 10 women (8%) had a hysterectomy with concomitant unilateral oopherectomy, and 33 (28%) had a hysterectomy with ovarian conservation. The median time to hysterectomy was 3.9 years from baseline (range 1.1- 9.4 years).
Baseline Predictors of Subsequent Elective Hysterectomy
Compared to women without hysterectomy, women who reported hysterectomy were more likely to report menstrual bleeding problems, were slightly younger (45.4 vs. 45.9, p=.04), had a higher BMI (29.6 vs. 28.0, p=.01), and had lower FSH values (18.4vs. 24.4, p<.001) at the study baseline. The groups also varied by site, race/ethnicity, and educational attainment (Table 1).
Table 1.
Reporting of Elective Hysterectomy by Sample Characteristics at Baseline: Univariate Associations
| Total (n=2,818) | Hysterectomy (n=168, 5.96%) | No hysterectomy (n=2,650, 94.04%) | p-value | |
|---|---|---|---|---|
| Race/ethnicity n, column % | <.001 | |||
| African American | 914 (32.4) | 78 (46.4) | 836 (31.5) | |
| White | 1378 (48.9) | 73 (43.5) | 1305 (49.2) | |
| Chinese | 246 (8.7) | 5 (3.0) | 241 (9.1) | |
| Japanese | 280 (9.9) | 12 (7.1) | 268 (10.1) | |
| Education n, row % | .04 | |||
| <=high school | 571 (20.4) | 30 (17.9) | 541 (20.5) | |
| some college | 937 (33.5) | 71 (42.3) | 866 (32.9) | |
| college/post-college | 1293 (46.2) | 67 (39.9) | 1226 (46.6) | |
| Menopausal status n, row % | .13 | |||
| Early perimenopausal | 1291 (46.5) | 88 (52.4) | 1203 (46.1) | |
| Premenopausal | 1487 (53.5) | 80 (47.6) | 1407 (53.9) | |
| Health insurance n, row % | .46 | |||
| No | 144 (5.1) | 6 (3.6) | 138 (95.8) | |
| Yes | 2670 (94.9) | 161 (96.4) | 2509 (94.8) | |
| Self-rated health n, row % | .11 | |||
| Excellent | 648 (23.3) | 33 (19.6) | 615 (23.5) | |
| Very good | 1040 (37.4) | 60 (35.7) | 980 (37.5) | |
| Good | 759 (27.3) | 59 (35.1) | 700 (26.8) | |
| Fair/poor | 333 (12.0) | 16 (9.5) | 317 (12.1) | |
| Smoking status n, row % | .46 | |||
| Never | 1588 (56.8) | 102 (60.7) | 1486 (56.6) | |
| Past | 725 (25.9) | 37 (22.0) | 688 (26.2) | |
| Current | 482 (17.2) | 29 (17.3) | 453 (17.2) | |
| Menstrual bleeding problems | <.001 | |||
| No | 2192 (77.8) | 102 (60.7) | 2090 (78.9) | |
| Yes | 625 (22.2) | 66 (39.3) | 559 (21.1) | |
| Age mean, SD | 45.9 (2.7) | 45.4 (2.6) | 45.9 (2.7) | .03 |
| BMI mean, SD | 28.1 (7.3) | 29.6 (7.4) | 28.0 (7.3) | .01 |
| FSH* mean, SD | 24.1 (25.4) | 18.4 (15.0) | 24.4 (25.9) | <.001 |
| Estradiol* mean, SD | 76.1 (80.6) | 70.1 (53.5) | 76.5 (82.0) | .98 |
| Time to hysterectomy mean, SD | 4.1 (2.0) |
Raw values shown; analysis performed with log-transformed values.
Abbreviations: body mass index (BMI), follicle-stimulating hormone (FSH)
The presence of VMS, a more positive attitude toward aging and menopause, significant anxiety, and PMS-like symptoms at baseline were each associated with hysterectomy in univariate analysis (Table 2). There was also a significant interaction between menstrual bleeding problems and time to hysterectomy; bleeding problems at baseline were associated with increased risk of subsequent hysterectomy, but this association decreased over time.
Table 2.
Reporting of Elective Hysterectomy by Affect, Attitudes and Symptoms at Baseline: Univariate Associations
| Total (n=2,818) | Hysterectomy (n=168, 5.96%) | No hysterectomy (n=2,650, 94.04%) | p-value | |
|---|---|---|---|---|
| Negative affect n, column% | ||||
| Depression | .67 | |||
| No | 2192 (77.8) | 128 (76.2) | 2064 (77.9) | |
| Yes | 625 (22.2) | 40 (23.8) | 585 (22.1) | |
| Significant anxiety | .02 | |||
| No | 2193 (78.9) | 119 (71.3) | 2074 (79.4) | |
| Yes | 585 (21.1) | 48 (28.7) | 537 (20.6) | |
| Anxiety mean, SD | 2.4 (2.3) | 2.7 (2.6) | 2.4 (2.3) | .13 |
| Perceived stress mean, SD | 8.4 (2.9) | 8.6 (3.0) | 8.4 (2.9) | .29 |
| Attitudes Toward Aging & Menopause n, row % | <.01 | |||
| Neutral/negative | 512 (18.4) | 20 (12.2) | 492 (18.8) | |
| Slightly positive | 892 (32.0) | 43 (26.2) | 849 (32.4) | |
| Highly positive | 1382 (49.6) | 101 (61.6) | 1281 (48.9) | |
| VMS n, row % | <.01 | |||
| None | 1721 (61.4) | 84 (50.0) | 1637 (62.2) | |
| Any | 1080 (38.6) | 84 (50.0) | 996 (37.8) | |
| PMS-like symptoms n, row % | .02 | |||
| No | 2426 (86.5) | 135 (80.4) | 2291 (86.8) | |
| Yes | 380 (13.5) | 33 (19.6) | 347 (13.2) |
Abbreviations: vasomotor symptoms (VMS), premenstrual syndrome-like symptoms (PMS-like symptoms)
VMS and a more positive attitude toward aging and menopause continued to predict subsequent hysterectomy after adjusting for known risk factors including menstrual bleeding problems, race/ethnicity, socioeconomic status, physical health, age, BMI, FSH levels, and the interaction of time by bleeding problems. The associations between PMS-like symptoms and significant anxiety and hysterectomy were no longer seen in the fully adjusted model. Having a low baseline FSH and being African American were also associated with an increased likelihood of subsequent hysterectomy (Table 3).
Table 3.
Risk of Elective Hysterectomy by Sample Characteristics, Attitudes, and Symptoms at Baseline: Multivariate Associations from Cox Proportional Hazard Models (n=2,818)*
| Hazard Ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|
| Attitudes Toward Aging & Menopause | .03 | ||
| Neutral/negative | -- | Referent | |
| Slightly positive | 1.18 | 0.68-2.04 | |
| Highly positive | 1.74 | 1.04-2.93 | |
| Significant Anxiety | .19 | ||
| No | -- | Referent | |
| Yes | 1.28 | .89-1.86 | |
| VMS | .03 | ||
| None | -- | Referent | |
| Any | 1.43 | 1.03-2.01 | |
| PMS-like symptoms | .38 | ||
| No | -- | Referent | |
| Yes | 1.20 | 0.80-1.80 | |
| Menstrual bleeding problems | <.001 | ||
| No | Referent | ||
| Yes | 4.30 | 2.05-9.05 | |
| Race/ethnicity | .08 | ||
| White | -- | Referent | |
| African American | 1.60 | 1.08-2.36 | |
| Chinese | 0.86 | 0.26-2.86 | |
| Japanese | 0.64 | 0.30-1.39 | |
| Education | .64 | ||
| College/post-college | -- | Referent | |
| Some college | 0.88 | 0.56-1.39 | |
| <High school | 1.08 | 0.76-1.55 | |
| Self-rated health | .25 | ||
| Excellent | -- | Referent | |
| Very good | 1.09 | 0.70-1.70 | |
| Good | 1.37 | 0.85-2.20 | |
| Fair/poor | 0.83 | 0.43-1.61 | |
| Age | 0.96 | 0.90-1.02 | .21 |
| BMI | 1.00 | 0.98-1.03 | .87 |
| FSH | 0.46 | 0.27-0.79 | <.01 |
Model adjusted by site, (time × menstrual bleeding problems) interaction term, and covariates as listed. Hazard ratios represent the strength of association with hysterectomy when all predictors and covariates are entered into the model simultaneously.
Abbreviations: vasomotor symptoms (VMS), premenstrual syndrome-like symptoms (PMS-like symptoms), body mass index (BMI), follicle-stimulating hormone (FSH)
Baseline Predictors of Medical Record-Verified Elective Hysterectomy
In the subset analysis limited to medical-record verified hysterectomies, only women with menstrual bleeding problems were at significantly increased risk for hysterectomy after adjusting for other known risk factors including menstrual bleeding problems, race/ethnicity, socioeconomic status, physical health, age, BMI, and FSH levels. There was no evidence of variability of the effect of menstrual bleeding problems over time in this sample so this interaction was not included in the subset analysis. The increased risk for hysterectomy among African-American women and women with lower FSH persisted in this subset, though the trend for positive attitudes toward aging and menopause and the association with VMS did not (Table 4). However, the hazard ratios were reduced only slightly, from 1.75 in the full sample to 1.67 in the subset analyses for highly positive attitudes. Power to detect these effects in the subset was 49% compared to 67% in the full sample, due to the smaller sample size and lower event rate.
Table 4.
Risk of Medical Record-Verified Elective Hysterectomy by Sample Characteristics, Attitudes, and Symptoms at Baseline: Multivariate Associations from Cox Proportional Hazard Models (n=2,770)*
| Hazard Ratio | 95 % Confidence Interval | p-value | |
|---|---|---|---|
| Attitudes Toward Aging & Menopause | .17 | ||
| Neutral/negative | -- | Referent | |
| Slightly positive | 1.24 | .66-2.34 | |
| Highly positive | 1.67 | .91-3.05 | |
| Significant Anxiety | .22 | ||
| No | -- | Referent | |
| Yes | 1.32 | .84-2.06 | |
| VMS | .25 | ||
| None | -- | Referent | |
| Any | 1.24 | .85-1.88 | |
| PMS | .71 | ||
| No | -- | Referent | |
| Yes | 1.10 | .67-1.82 | |
| Menstrual bleeding problems | <.01 | ||
| No | -- | Referent | |
| Yes | 1.70 | 1.14-2.53 | |
| Race/ethnicity | .07 | ||
| White | -- | Referent | |
| African American | 1.62 | 1.01-2.61 | |
| Chinese | .39 | .07-2.03 | |
| Japanese | .59 | .27-1.30 | |
| Education | .95 | ||
| College/post-college | -- | Referent | |
| Some college | .92 | .54-1.56 | |
| <High school | .96 | .62-1.47 | |
| Self-rated health | .32 | ||
| Excellent | -- | Referent | |
| Very good | .92 | .56-1.51 | |
| Good | 1.22 | .72-2.08 | |
| Fair/poor | .67 | .30-1.48 | |
| Age | .95 | .88-1.02 | .18 |
| BMI | .99 | .97-1.02 | .70 |
| FSH | .45 | .24-.89 | .02 |
Models adjusted for site and covariates as listed. Hazard ratios represent the strength of association with hysterectomy when all predictors and covariates are entered into the model simultaneously.
Abbreviations: vasomotor symptoms (VMS), premenstrual syndrome-like symptoms (PMS-like symptoms), body mass index (BMI), follicle-stimulating hormone (FSH)
Discussion
This study expanded the perspective of previous research of the relationship between hysterectomy and negative affect by addressing the question with prospective data as opposed to a cross-sectional approach. Although negative affect is generally high when assessed prior to hysterectomy,36 we found that measures of negative affect, including anxiety, depressive symptoms, and perceived stress, did not increase the likelihood of hysterectomy over the menopausal transition. Cross-sectional studies may suffer from a lack of non-hysterectomized comparison groups, and assessment close to the time of surgery. In contrast, we compared women with and without a subsequent hysterectomy using measures of affect assessed, on average, four years prior to hysterectomy. Our results suggest that negative affect in the years prior to treatment-seeking is not predictive of subsequent hysterectomy.
Negative attitudes toward aging and menopause were also not associated with elective hysterectomy. Contrary to our hypothesis, a more positive attitude towards aging and menopause was related to subsequent elective hysterectomy. While negative attitudes toward aging and menopause have not been specifically linked to hysterectomy, they have been associated with increased reporting of menopausal symptoms and depressed mood.23,25 It was assumed a priori that negative attitudes toward aging and menopause in the early transition would influence symptom sensitivity and tolerance over the transition and increase the likelihood of treatment-seeking leading to hysterectomy. Instead, women with a positive outlook towards their imminent menopause may be more willing to take action via surgical menopause in the face of problematic symptoms.
VMS was independently and positively associated with having a hysterectomy. After adjusting for risk factors including menstrual bleeding problems, women with VMS in the early menopausal transition were 43% more likely than those without to have an elective hysterectomy. This is the first known study to link VMS with subsequent hysterectomy. Hormonal variability in the menopausal transition may play a role in the underlying etiology of VMS as well as the development of menstrual symptoms associated with hysterectomy.50 Additionally, subjective reporting of VMS may be related to an increased awareness of all negative symptoms due to somatic sensitivity and symptom amplification,51 evidence of which has been seen in past studies linking VMS to increased symptom sensitivity.52,53 The negative effects of VMS on quality of life20 may also increase the likelihood of menstrual bleeding problems being less tolerated, and the effect of VMS on healthcare utilization may increase the risk of seeking and receiving treatment for the conditions and symptoms leading to a hysterectomy.
PMS has not been specifically linked to hysterectomy in the literature, but a connection was hypothesized based on its associations with increased reporting of menopausal symptoms,26-28 increased overall health care utilization, and decreased health-related quality of life.18,19 In this sample, PMS-like symptoms were independently associated with hysterectomy, but the association did not persist after adjusting for known risk factors. Women with PMS-like symptoms were also more likely to report VMS and menstrual bleeding problems, which had strong associations with hysterectomy. Women whose data were excluded from analysis because of preoperative malignancy or hysterectomy shortly after baseline measurement were also more likely to report PMS-like symptoms.
Occasional or persistent heavy menstrual bleeding is reported by one-third of women, with prevalence increasing to 50% of women in the years prior to menopause.29 Heavy bleeding is the main presenting symptom in 50-70% of all hysterectomies performed,2 and we anticipated that a high reported burden of menstrual bleeding problems would be associated with an elevated likelihood of elective hysterectomy over the menopausal transition. Consistent with this expectation, menstrual bleeding problems were predominant in the women whose data were excluded from analysis due to preoperative malignancy or hysterectomy within 12 months of the baseline measurement, and were consistently associated with elective hysterectomy, even following adjustment for risk factors. The association was retained in subset analyses excluding women without medical record confirmation of hysterectomy. The presence of menstrual bleeding problems assessed an average of four years prior to hysterectomy more than quadrupled the risk of subsequent hysterectomy over the observed period, even after accounting for the decreasing impact of menstrual bleeding over time.
Additional findings deserve mention. In our sample, there was an increased risk for hysterectomy among African-American women. This finding is consistent with the literature, in which hysterectomy rates are usually higher among African-American women compared to other groups.5,43,54,55 High BMI,5 smoking,44 and poor health56 are associated with past hysterectomy in community samples and often attributed in part to poor health prior to surgery,31 but there was no effect of self-rated health or of smoking status in univariate analysis, and no effect of BMI in the fully adjusted model. Women whose data were excluded from analysis due to preoperative malignancy or hysterectomy soon after baseline measurement had significantly higher BMI than women in the analytic sample, which may have obscured a relationship between these factors. Despite past findings that socioeconomic status (SES) is lower in women who have had a hysterectomy,3-5,30,44,54,57-61 the independent association of educational attainment with hysterectomy in our sample did not persist in adjusted analysis. Income, ability to pay for basics, and health insurance status were also not significantly associated with hysterectomy. Differences in demographic risk factors between our findings and those of past investigations may be due to the prospective design and older age of our sample. In light of our findings, midlife may be a time when some conventional risk factors for hysterectomy are less relevant.
In a subset analysis, excluding women reporting hysterectomy without available medical records, VMS no longer had an association with hysterectomy, while menstrual bleeding problems continued to contribute to hysterectomy risk. Being African American and having lower FSH levels at baseline also continued to be associated with increased risk. Self-report of hysterectomy has been shown to be accurate when validated against medical records.62 This was true in our sample of women as well. In our cohort, the differences in these analyses are likely due to a loss of statistical power , rather than a more accurate reflection of hysterectomy in the secondary analysis.
Several limitations of this study should be noted. As reflected in the fact that only half of those women who were eligible for SWAN chose to enroll in the study, women who chose to participate in SWAN and continue to adhere to the time commitment of annual visits are highly motivated and may differ from the general population. The effects of duration, frequency, and stability of time-varying measures, as well as their changing influences closer to the time of surgery, were not examined. While our interest was in examining these factors with distance from the event, there may be additional information to be gained in subsequent analyses that address the influence of time-varying characteristics of these factors. Because data heavily relied on self-report, information may exhibit response bias or subjective inaccuracies. As predictors of elective hysterectomy among women in midlife may differ from predictors among younger women, findings should not be generalized to younger populations. Further, women with prior hysterectomy were ineligible for SWAN enrollment, and therefore women who had a hysterectomy after enrollment may not be representative of all women with hysterectomy. This sample characteristic may have contributed to differences between the results of this study and others.
Despite these limitations, the study also had considerable strengths. This study utilized a large, well-characterized, multi-ethnic population-based sample of women in midlife. The incidence of hysterectomy was collected over an eight year period, allowing for observations across a range of a participant ages and stages in the menopausal transition. This study was unique in prospectively evaluating the influence of a variety of factors on subsequent elective hysterectomy.
Conclusions
This study contributes to our limited knowledge about the antecedents of elective hysterectomy among women in midlife. Previous findings of associations between negative affect and hysterectomy do not fully address the role of affect in prospectively influencing decisions by women and their physicians regarding elective hysterectomy. Our results should assure physicians that treatment-seeking is not always a sign of depression, anxiety, or negative expectations about menopause, while menstrual bleeding problems are the most influential factor for hysterectomy. However, the length of time between our assessment of menstrual bleeding problems and subsequent hysterectomy, a mean of four years, suggests that these problems are endured for a long period. Additionally, the association of VMS in the early menopausal transition with subsequent hysterectomy may indicate that a symptomatic menopause is linked to an increased occurrence of or sensitivity to the symptoms and conditions precipitating hysterectomy. Finally, our findings suggest that women who elect hysterectomy are not choosing this avenue because of negative affect and poor attitudes toward aging.
Figure 1.
Study sample selection.
ACKNOWLEDGMENTS
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women's Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Clinical Centers: University of Michigan, Ann Arbor - MaryFran Sowers, PI; Massachusetts General Hospital, Boston, MA - Robert Neer, PI 1994 - 1999; Joel Finkelstein, PI 1999- present; Rush University, Rush University Medical Center, Chicago, IL - Lynda Powell, PI 1994 – 2009; Howard Kravitz, PI 2009 – present; University of California, Davis/Kaiser - Ellen Gold, PI; University of California, Los Angeles - Gail Greendale, PI; University of Medicine and Dentistry - New Jersey Medical School, Newark –Gerson Weiss, PI 1994 – 2004; Nanette Santoro, PI 2004 – present; and the University of Pittsburgh, Pittsburgh, PA - Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD - Marcia Ory 1994 – 2001; Sherry Sherman 1994 – present; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor - Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001; University of Pittsburgh, Pittsburgh, PA – Kim Sutton-Tyrrell, PI 2001 – present.
Steering Committee: Chris Gallagher, Chair
Susan Johnson, Chair
We thank the study staff at each site and all the women who participated in SWAN.
Footnotes
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keshavarz H, Hillis SD, Kieke BA, Marchbanks PA. Hysterectomy surveillance. United States 1994-1999. MMWR CDC Surveill Summ. 2002;51:1–8. [PubMed] [Google Scholar]
- 2.El-Hemaidi I, Gharaibeh A, Shehata H. Menorrhagia and bleeding disorders. Curr Opin Obstet Gynecol. 2007;19:513–520. doi: 10.1097/GCO.0b013e3282f1ddbe. [DOI] [PubMed] [Google Scholar]
- 3.Kjerulff K, Langenberg P, Guzinski G. The socioeconomic correlates of hysterectomies in the United States. Am J Public Health. 1993;83:106–108. doi: 10.2105/ajph.83.1.106. (1993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brett KM, Marsh JV, Madans JH. Epidemiology of hysterectomy in the United States: Demographic and reproductive factors in a nationally representative sample. J Womens Health. 1997;6:309–316. doi: 10.1089/jwh.1997.6.309. [DOI] [PubMed] [Google Scholar]
- 5.Bower JK, Schreiner PJ, Sternfeld B, Lewis CE. Black-white differences in hysterectomy prevalence: The CARDIA study. Am J Public Health. 2009;99:300–307. doi: 10.2105/AJPH.2008.133702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlson KJ. Outcomes of hysterectomy. Clin Obstet Gynecol. 1997;40:939–946. doi: 10.1097/00003081-199712000-00029. [DOI] [PubMed] [Google Scholar]
- 7.Spilsbury K, Semmens JB, Hammond I, Bolck A. Persistent high rates of hysterectomy in Western Australia: A population-based study of 83,000 procedures over 23 years. BJOG. 2006;113:804–809. doi: 10.1111/j.1471-0528.2006.00962.x. [DOI] [PubMed] [Google Scholar]
- 8.Kafy S, Huang JYJ, Al-Sunaidi M, Wiener D, Tulandi T. Audit of morbidity and mortality rates of 1792 hysterectomies. J Minim Invasive Gynecol. 2006;13:55–59. doi: 10.1016/j.jmig.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Warren L, Ladapo JA, Borah BJ, Gunnarsson CL. Open abdominal versus laparoscopic and vaginal hysterectomy: Analysis of a large United States payer measuring quality and cost of care. J Minim Invasive Gynecol. 2009;16:581–588. doi: 10.1016/j.jmig.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Department of Health & Human Services, Office on Women's Health.. Hysterectomy [July 28, 2010]; Available at: http://womenshealth.gov/faq/hysterectomy.cfm.
- 11.Sowers M, Tomey K, Jannausch M, Eyvazzadeh A, Nan B, Randolph J., Jr Physical functioning and menopause states. Obstet Gynecol. 2007;110:1290–1296. doi: 10.1097/01.AOG.0000290693.78106.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brett KM. Can hysterectomy be considered a risk factor for cardiovascular disease? Circulation. 2005;111:1456–1458. doi: 10.1161/01.CIR.0000161141.92300.F3. [DOI] [PubMed] [Google Scholar]
- 13.Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1–34.e7. doi: 10.1016/j.ajog.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 14.Shoupe D, Parker WH, Broder MS, Liu Z, Farquhar C, Berek JSM. Elective oophorectomy for benign gynecological disorders. Menopause. 2007;14:580–585. doi: 10.1097/gme.0b013e31803c56a4. [DOI] [PubMed] [Google Scholar]
- 15.Thompson D, Richardson E. Current issues in the economics of depression management. Curr Psychiatry Rep. 1999;1:125–134. doi: 10.1007/s11920-999-0021-1. [DOI] [PubMed] [Google Scholar]
- 16.Katon W, Von Korff M, Lin E, et al. Distressed high utilizers of medical care. DSM-III-R diagnoses and treatment needs. Gen Hosp Psychiatry. 1990;12:355–362. doi: 10.1016/0163-8343(90)90002-t. [DOI] [PubMed] [Google Scholar]
- 17.Simon G, Ormel J, VonKorff M, Barlow W. Health care costs associated with depressive and anxiety disorders in primary care. Am J Psychiatry. 1995;152:352–357. doi: 10.1176/ajp.152.3.352. [DOI] [PubMed] [Google Scholar]
- 18.Borenstein JE, Dean BB, Endicott J, Wong J, et al. Health and economic impact of the premenstrual syndrome. J Reprod Med. 2003;48:515–524. [PubMed] [Google Scholar]
- 19.Hourani LL, Yuan H, Bray RM. Psychosocial and lifestyle correlates of premenstrual symptoms among military women. J Womens Health. 2002;13:812–821. doi: 10.1089/jwh.2004.13.812. [DOI] [PubMed] [Google Scholar]
- 20.Guthrie JR, Dennerstein L, Taffe JR, Donnelly V. Health care-seeking for menopausal problems. Climacteric. 2003;6:112–117. [PubMed] [Google Scholar]
- 21.Mancuso CA, Peterson MG, Charlson ME. Effects of depressive symptoms on health-related quality of life in asthma patients. J Gen Intern Med. 2000;15:301–310. doi: 10.1046/j.1525-1497.2000.07006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thurston RC, Bromberger JT, Joffe H, et al. Beyond frequency: who is most bothered by vasomotor symptoms? Menopause. 2008;15:841–847. doi: 10.1097/gme.0b013e318168f09b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avis NE, McKinlay SM. A longitudinal analysis of women's attitudes toward the menopause: Results from the Massachusetts Women's Health Study. Maturitas. 1991;13:65–79. doi: 10.1016/0378-5122(91)90286-y. [DOI] [PubMed] [Google Scholar]
- 24.Avis NE, Crawford SL, McKinlay SM. Psychosocial, behavioral, and health factors related to menopause symptomatology. Womens Health. 1997;3:103–120. [PubMed] [Google Scholar]
- 25.Olofsson AS, Collins A. Psychosocial factors, attitude to menopause and symptoms in Swedish perimenopausal women. Climacteric. 2000;3:33–42. doi: 10.3109/13697130009167597. [DOI] [PubMed] [Google Scholar]
- 26.Freeman EW, Sammel MD, Rinaudo PJ, Sheng L. Premenstrual syndrome as a predictor of menopausal symptoms. Obstet Gynecol. 2004;103:960–966. doi: 10.1097/01.AOG.0000124804.81095.7f. [DOI] [PubMed] [Google Scholar]
- 27.Morse CA, Dudley E, Guthrie J, Dennerstein L. Relationships between premenstrual complaints and perimenopausal experiences. J Psychosom Obstet Gynaecol. 1998;19:182–191. doi: 10.3109/01674829809025696. [DOI] [PubMed] [Google Scholar]
- 28.Stewart DE, Boydell KM. Psychologic distress during menopause: Associations across the reproductive life cycle. Int J Psychiatry Med. 1993;23:157–162. doi: 10.2190/026V-69M0-C0FF-7V7Y. [DOI] [PubMed] [Google Scholar]
- 29.Jabbour HN, Kelly RW, Fraser HM, Critchley HOD. Endocrine regulation of menstruation. Endocr Rev. 2006;27:17–46. doi: 10.1210/er.2004-0021. [DOI] [PubMed] [Google Scholar]
- 30.Byles JE, Mishra G, Schofield M. Factors associated with hysterectomy among women in Australia. Health Place. 2000;6:301–308. doi: 10.1016/s1353-8292(00)00011-3. [DOI] [PubMed] [Google Scholar]
- 31.Ceausu I, Shakir YA, Lidfeldt J, Samsioe G, Nerbrand C. The hysterectomized woman: Is she special? The Women's Health in the Lund Area (WHILA) study. Maturitas. 2006;53:201–209. doi: 10.1016/j.maturitas.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Ballinger CB. Psychiatric morbidity and the menopause: Survey of a gynaecological out-patient clinic. Br J Psychiatry. 1997;131:83–89. doi: 10.1192/bjp.131.1.83. [DOI] [PubMed] [Google Scholar]
- 33.Martin RL, Roberts WV, Clayton PJ, Wetzel R. Psychiatric illness and non-cancer hysterectomy. Dis Nerv Sys. 1977;38:974–980. [PubMed] [Google Scholar]
- 34.Gath D, Cooper P, Day A. Hysterectomy and psychiatric disorder: I. Levels of psychiatric morbidity before and after hysterectomy. Br J Psychiatry. 1982;140:335–342. doi: 10.1192/bjp.140.4.335. [DOI] [PubMed] [Google Scholar]
- 35.Ryan MM, Dennerstein L, Pepperell R. Psychological aspects of hysterectomy. A prospective study. Br J Psychiatry. 1989;154:516–522. doi: 10.1192/bjp.154.4.516. [DOI] [PubMed] [Google Scholar]
- 36.Khastgir G, Studd JW, Catalan J. The psychological outcome of hysterectomy. Gynecol Endocrinol. 2000;14:132–141. doi: 10.3109/09513590009167672. [DOI] [PubMed] [Google Scholar]
- 37.Sowers M, Crawford S, Sternfeld B, et al. SWAN: A multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, et al., editors. Menopause: Biology and pathology. Academic Press; New York, NY: 2000. pp. 175–180. [Google Scholar]
- 38.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 39.Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385. [Google Scholar]
- 40.Roberts R, Vernon S. The Center for Epidemiologic Studies Depression Scale: Its use in a community sample. Am J Psychiatry. 1983;140:41–46. doi: 10.1176/ajp.140.1.41. [DOI] [PubMed] [Google Scholar]
- 41.Sommer B, Avis N, Meyer P, et al. Attitudes toward menopause and aging across Ethnic/Racial groups. Psychosom Med. 1999;61:868–875. doi: 10.1097/00006842-199911000-00023. [DOI] [PubMed] [Google Scholar]
- 42.Materia E, Rossi L, Spadea T, et al. Hysterectomy and socioeconomic position in Rome, Italy. J Epidemiol Community Health. 2002;56:461–465. doi: 10.1136/jech.56.6.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kjerulff KH, Guzinski GM, Langenberg PW, Stolley PD, Moye NE, Kazandjian VA. Hysterectomy and race. Obstet Gynecol. 1993;82:757–764. [PubMed] [Google Scholar]
- 44.Treloar SA, Do KA, O'Connor VM, O'Connor DT, Yeo MA, Martin NG. Predictors of hysterectomy: An Australian study. Am J Obstet Gynecol. 1999;180:945–954. doi: 10.1016/s0002-9378(99)70666-6. [DOI] [PubMed] [Google Scholar]
- 45.Ware JE. SF-36 health survey 211. Manual and interpretation guide. The Health Institute, New England Medical Center; Boston, MA: 1993. [Google Scholar]
- 46.Randolph JF, Jr, Sowers M, Gold EB, et al. Reproductive hormones in the early menopausal transition: Relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab. 2003;88:1516–1522. doi: 10.1210/jc.2002-020777. [DOI] [PubMed] [Google Scholar]
- 47.England BG, Parsons GH, Possley RM, McConnell DS, Midgley AR. Ultrasensitive semiautomated chemiluminescent immunoassay for estradiol. Clin Chem. 2002;48:1584–1586. [PubMed] [Google Scholar]
- 48.Allison PD. Survival Analysis Using the SAS System: A Practical Guide. SAS Institute Inc; Cary, NC: 1995. [Google Scholar]
- 49.SPSS for Windows, Rel. 17.0.0. SPSS Inc.; Chicago: 2008. [Google Scholar]
- 50.Prior JC. Perimenopause: the complex endocrinology of the menopausal transition. Endocr Rev. 1998;19:397–428. doi: 10.1210/edrv.19.4.0341. [DOI] [PubMed] [Google Scholar]
- 51.Thurston RC, Blumenthal JA, Babyak MA, Sherwood A. Emotional antecedents of hot flashes during daily life. Psychosom Med. 2005;67:137–146. doi: 10.1097/01.psy.0000149255.04806.07. [DOI] [PubMed] [Google Scholar]
- 52.Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women's health across the nation. Am J Public Health. 2006;96:1226–1235. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barsky AJ, Goodson JD, Lane RS, Cleary PD. The amplification of somatic symptoms. Psychosom Med. 1988;50:510–519. doi: 10.1097/00006842-198809000-00007. [DOI] [PubMed] [Google Scholar]
- 54.Meilahn EN, Matthews KA, Egeland G, Kelsey SF. Characteristics of women with hysterectomy. Maturitas. 1989;11:319–329. doi: 10.1016/0378-5122(89)90028-5. [DOI] [PubMed] [Google Scholar]
- 55.Powell LH, Meyer P, Weiss G, et al. Ethnic differences in past hysterectomy for benign conditions. Womens Health Issues. 2005;15:179–186. doi: 10.1016/j.whi.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 56.Kharazmi E, Fallah M, Luoto R. Cardiovascular diseases attributable to hysterectomy: A population-based study. Acta Obstet Gynecol Scand. 2007;86:1476–1483. doi: 10.1080/00016340701698633. [DOI] [PubMed] [Google Scholar]
- 57.Marks NF, Shinberg DS. Socioeconomic differences in hysterectomy: The Wisconsin Longitudinal Study. Am J Public Health. 1997;87:1507–1514. doi: 10.2105/ajph.87.9.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koepsell TD, Weiss NS, Thompson DJ, Martin DP. Prevalence of prior hysterectomy in the Seattle-Tacoma area. Am J Public Health. 1980;70:40–47. doi: 10.2105/ajph.70.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Settnes A, Jorgensen T. Hysterectomy in a Danish cohort: Prevalence, incidence and socio-demographic characteristics. Acta Obstet Gynecol Scand. 1996;75:274–280. doi: 10.3109/00016349609047101. [DOI] [PubMed] [Google Scholar]
- 60.Harlow BL, Barbieri RL. Influence of education on risk of hysterectomy before age 45 years. Am J Epidemiol. 1999;150:843–847. doi: 10.1093/oxfordjournals.aje.a010089. [DOI] [PubMed] [Google Scholar]
- 61.Santow G. Education and hysterectomy. Aust N Z J Obstet Gynaecol. 1995;35:60–69. doi: 10.1111/j.1479-828x.1995.tb01833.x. [DOI] [PubMed] [Google Scholar]
- 62.Phipps AI, Buist DS. Validation of self-reported history of hysterectomy and oophorectomy among women in an integrated group practice setting. Menopause. 2009;16:576–581. doi: 10.1097/gme.0b013e31818ffe28. [DOI] [PMC free article] [PubMed] [Google Scholar]