Abstract
The common marmoset is a small, arboreal, New World primate that has emerged as a promising non-human model system in auditory neuroscience. A complete understanding of the neuroethology of auditory processing in marmosets will include behavioral work examining how sounds are perceived by these animals. However, there have been few studies of the marmoset’s hearing and perceptual abilities and the audiogram of this species has not been measured using modern psychophysical methods. The present experiment pairs psychophysics with an operant conditioning technique to examine perception of pure tone stimuli by marmosets using an active behavioral paradigm. Subjects were trained to lick at a feeding tube when they detected a sound. Correct responses provided access to a food reward. Pure tones of varying intensities were presented to subjects using the method of constant stimuli. Behavioral thresholds were calculated for each animal based on hit rate - threshold was defined by the tone intensity that the animal correctly identified 50% of the time. Results show that marmoset hearing is comparable to that of other New World monkeys, with a hearing range extending from about 125 Hz up to 36 kHz and a sensitivity peak around 7 kHz.
Keywords: Marmoset, audiogram, threshold, behavior, psychophysics, non-human primate
INTRODUCTION
The common marmoset (Callithrix jacchus) is a small-bodied New World primate that has emerged as a promising model system for studies of the neural underpinnings of mammalian auditory and vocal processing (e.g., Wang, 2000; Wang, 2007). Recent advances in transgenic studies in the marmoset (Sasaki et al., 2009) also makes this species an attractive non-human primate model for research across a number of fields both within and outside of neuroscience. Marmosets are particularly tractable experimental animals for neurophysiological studies for a number of reasons, including an easily accessible, relatively flat auditory cortex and a propensity to vocalize readily in captivity. Recent studies have described neurophysiological and behavioral mechanisms supporting auditory perception in these animals including, for example, the cortical coding of periodicity and representation of pitch (Bendor & Wang, 2005, 2006, 2010), the coding of sound intensity (Sadagopan & Wang, 2008; Watkins & Barbour, 2008, 2011), auditory responses to species-specific vocalization stimuli (Wang, 2000; Wang & Kadia, 2001), sensory-motor activity during self-initiated vocal behavior (Eliades & Wang, 2003, 2005, 2008), and antiphonal calling behaviors (Miller & Wang, 2006; Miller et al., 2009a, 2009b, 2010). A richer and more complete understanding of the cortical physiology supporting auditory behavior requires knowledge of how these animals actually perceive sounds, beginning with basic measures such as the acuity and limits of the auditory system. Such questions can be addressed using proper behavioral techniques which allow an animal to directly report on perceived changes in its acoustic environment.
One of the most fundamental problems in auditory perception, and perhaps the most basic function of the auditory system, is detecting the presence of a sound. This aspect of hearing is commonly measured psychophysically in animals by presenting a specific target sound at various intensities against a silent background and measuring a behavioral response. The intensity at which an animal responds 50% of the time is typically called the subject’s absolute threshold for that sound (Gescheider, 1985). Measuring such thresholds at a number of different frequencies gives rise to an audibility curve, or audiogram, which describes an animal’s relative sensitivity to sound across its range of hearing.
Audiograms have been measured in multiple Old World primate species, including blue monkeys (Brown & Wasser, 1984), chimpanzees (Elder, 1933, 1934, 1935), macaques (Harris, 1943; Jackson et al., 1999; Owren et al., 1988; Pfingst et al., 1978; Stebbins, 1973; Stebbins, Green & Miller, 1966), vervet monkeys (Owren et al., 1988; Stebbins, 1973), and yellow baboons (Heinz et al., 1982). This body of work has provided us with a good understanding of the limits and sensitivity of primate auditory systems and has shown that hearing sensitivity in different primate species is similar across frequency (for reviews, see Fay, 1988; Heffner, 2004; Stebbins, 1971, 1978). These animals generally have an 8–10 octave range of hearing with audibility curves shifted along the frequency axis toward higher frequencies compared to typical human audiograms.
Almost all of the psychoacoustic work in New World monkeys comes from studies of absolute thresholds or frequency difference limens measured in two species, the squirrel monkey (e.g., Beecher, 1974b; Fujita & Elliott, 1965; Green, 1975; Serafin et al., 1982; Wiernicke et al., 2001) and the owl monkey (Beecher, 1974a; Recanzone et al., 1991). Hearing sensitivity in these animals is similar to other primates, with a hearing range extending from around 100 Hz to over 30 kHz. Both species exhibit a W-shaped audiogram with heightened sensitivity around 2 kHz and 8 kHz and reduced sensitivity around 4 kHz, a threshold profile not seen in either apes or Old World monkeys. To our knowledge, the only psychoacoustic study of common marmosets is a behavioral audiogram measured over 50 years ago in an unpublished report (Seiden, 1957). In that experiment, absolute thresholds were measured for five animals using a conditioned avoidance task in which a tone reliably preceded presentation of an electric shock. Marmosets in that study were reported to have a hearing range typical of primate species and also showed the W-shaped audiogram common to other New World monkeys.
In the present study, we paired operant conditioning techniques with an anticipatory licking task in order to measure tone detection thresholds in common marmosets. Our goal was to confirm and update the previously observed marmoset audibility curve of Seiden (1957) using more current psychophysical methods and positive reinforcement. We also wanted to test the feasibility of this task for future studies in which behavioral testing can be used to better understand the relationship between auditory perception and its anatomical and neurophysiological underpinnings in this species.
MATERIAL AND METHODS
Subjects
The subjects in this experiment were four common marmosets (one male, three female) between two and five years of age. Subjects were housed in individual cages in a large colony at The Johns Hopkins University School of Medicine. All animals were maintained at approximately 90% of their free-feeding weight on a diet consisting of a combination of monkey chow, fruit, and yogurt and had ad libitum access to water. Subjects were tested twice a day, five days per week between the hours of 0900 and 1800. All experimental procedures were approved by the Johns Hopkins University Animal Care and Use Committee and were in compliance with the guidelines of the National Institutes of Health.
Operant testing apparatus
Marmosets were tested while seated in a Plexiglas restraint chair mounted in the center of a single-walled sound isolation chamber (Industrial Acoustic Company, New York, NY, model 400A [101 × 124 × 230 cm interior dimensions]). The interior walls and ceiling of the chamber were lined with 3-inch acoustic absorption foam (Sonex, Pinta Acoustics, Minneapolis, MN) in order to minimize both sound reflections inside the chamber and ambient sounds from outside the chamber. A food delivery tube was attached to a plate at the top of the restraint chair and positioned so that the tube end was within reach of the animal’s tongue. Reward food consisted of a mixture of Gerber single-grain rice cereal, strawberry and/or banana-flavored Nesquik, and a protein powder supplement. Reward was delivered via a syringe pump (New Era Pump Systems, Inc., Farmingdale, NY, model NE-500) mounted to the base of the chair. A custom bracket, on which one infrared (IR) light emitter and one IR light detector was positioned, was mounted to the end of the feeding tube. This arrangement created an IR photobeam passing directly across the end of the feeding tube. Subject behavior was recorded by monitoring when the photobeam was broken by the animal licking at the feeding tube. A red light-emitting diode (LED) was mounted on the wall of the chamber in front of the animal’s head and served as a secondary reinforcer by flashing at 10 Hz whenever the animal produced a correct response.
All target sound stimuli were generated offline using Matlab software (Mathworks, Inc., Natick, MA) and delivered at a nominal sampling rate of 100 kHz through a digital signal processor and programmable attenuator (Tucker-Davis Technologies, Gainesville, FL; Models RX6 and PA5) followed by an audio amplifier (Crown Instruments, Model D-75). Stimuli were played from a loudspeaker (Tannoy Ltd., Scotland, UK, Arena series; frequency response from 80 Hz - 54 kHz) mounted 40 cm directly in front of the animal. Stimuli were calibrated before a test session using a 1/2” free-field microphone (Brüel & Kjaer, Type 4191) positioned in the chamber at the same location as the animal’s head. The output of the microphone was amplified using a custom preamplifier, sent directly into a digital signal processor (Tucker-Davis Technologies, RX6), and analyzed using a custom Matlab calibration program written specifically for this hardware configuration. The reference amplitude was set by a 1 kHz tone calibrated at 90 dB SPL (sound pressure level; re. 20 µPa) with 0 dB attenuation. Additionally, once a week the calibration was validated using a commercially-available hand held sound level meter (Brüel & Kjaer, Type 2250). All experimental events were computer-controlled and an animal’s behavior was monitored during each test session via closed-circuit video (Watec Ltd., model WAT-902A CCD camera).
Stimuli and operant testing procedures
The testing procedures used in this experiment are based on operant conditioning methods previously described for both primates (Niemiec and Moody, 1995; Sinnott et al., 1985; Stebbins, 1973) and birds (Dooling and Okanoya, 1995; Okanoya and Dooling, 1987). Marmosets were first trained to detect a pure tone target sound against a background of silence. This sound was a 500 ms long 6 kHz pure tone presented at a level of 70 dB SPL. Animals were required to withhold licking until this target stimulus was presented. The time between the start of a trial and the presentation of the target sound was randomized between 3 and 10 s. After this interval had ended, targets were alternated with equal-duration periods of silence during a 5 s response interval. Marmosets were to lick the feeding tube within this response interval if they detected the target stimulus in order to receive access to approximately 0.2 mL of food reward.
Marmosets were tested in two daily sessions of 100 trials each. Each session was comprised of 70 target trials in which the target sound was presented and 30 sham trials in which no stimulus was presented. The order of target trials and sham trials was pseudo-randomized such that sham trials could not occur on consecutive trials. Licking the feeding tube during a sham trial was recorded as a “false alarm”, during which the chamber lights were extinguished (a “blackout”) for a period of 5 s. Also, a false alarm caused the inter-trial interval to be reset, thus extending the period of time before the next stimulus. Licking the feeding tube outside of the response interval of a trial also resulted in a blackout period. A failure to respond during presentation of the target sound (i.e., during the 5 s response interval) was recorded as a “miss”, and a new trial sequence was then initiated. A hit rate was calculated based on the proportion of correct responses during those trials that involved the target sound. Training was complete once animals completed three consecutive sessions with an average hit rate greater than 80% and a false alarm rate below 20%.
Testing sessions followed the same general procedures and methods as above. For these sessions, pure tone stimuli ranging in frequency from 125 Hz to 36 kHz (125 Hz, 250 Hz, 500 Hz, 1 kHz, 2 kHz, 4 kHz, 6 kHz, 7 kHz, 8 kHz, 10 kHz, 12 kHz, 16 kHz, 32 kHz, 36 kHz) were generated and stored offline. All stimuli began at zero phase and were 500 ms in duration with a 5 ms rise/fall time. One frequency was tested at a time and a series of seven intensities was selected for each test session. Initially, the stimulus series progressed in 10 dB steps and covered a 60 dB range from 10 to 70 dB SPL. Once the location of the threshold was found (usually after one or two sessions), stimulus intensities were adjusted so that they covered a 30 dB range (in 5 dB steps) in order to give a more precise threshold estimate. The specific levels of this second stimulus series were chosen such that they bracketed an animal’s presumed threshold. If thresholds still remained above the lowest target amplitude (i.e., 5 dB SPL), animals were tested with a third stimulus series covering an even more restricted 18 dB range (3 to 21 dB SPL). This third series was necessary for three frequencies across two subjects (i.e., 7 kHz for marmoset 1; 7 and 8 kHz for marmoset 4).
As in training, subjects were tested in two daily sessions of 100 trials each. Stimuli were presented in blocks of 10 trials. Each trial block was composed of all 7 target intensities and 3 sham trials in which no stimulus was presented. As before, licking the feeding tube during a sham trial was recorded as a “false alarm”, which resulted in 5 s “blackout” and a resetting of the inter-trial interval. Failing to respond to a target presentation during the 5 s response interval was recorded as a “miss”.
At the end of a session, a hit rate was calculated for each target. Thresholds for pure tone detection were defined as the amplitude that was detected 50% of the time, after being corrected for false alarm rate according to the high threshold theory [Pc*=(Pc-FA)/(1-FA)] (Gescheider, 1985). Animals were tested until their threshold values stabilized (i.e., three successive sessions in which the obtained threshold value was within +/− 1/3 of the step size). Final threshold estimates are based on a minimum of 300 trials. Sessions with a false alarm rate higher than 25% were discarded (<10% of all sessions).
RESULTS
All four experimental subjects learned to perform the operant task. Marmosets responded to tone presentations during the training sessions by licking at the feeding tube and withheld licking in the absence of a tone stimulus. All animals became proficient at this task and reached criterion performance (i.e., >80% hit rate, <20% false alarm rate for three consecutive sessions) within three to six weeks of the initial training session. Animals generalized easily to threshold testing sessions in which the tone amplitude was varied. Across all subjects, the average false alarm rate in test sessions was 14.3 +/− 3.61%. The average discriminability index (d’) in those same sessions was 1.68 +/− 0.18.
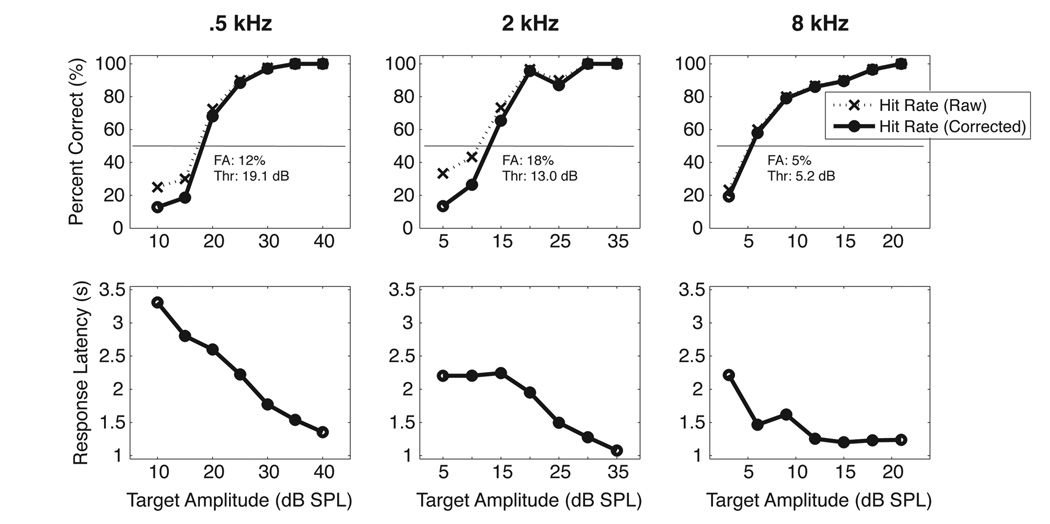
Representative psychometric functions and response latencies from one subject are shown in the panels of Figure 1. The three upper panels show changes in raw and corrected hit rates (dotted vs. solid lines) from sessions using .5 kHz (left panel), 2 kHz (center panel), and 8 kHz (right panel) tones as a function of stimulus amplitude. The step size for these sessions was 5 dB, 5 dB, and 3 dB, respectively. As tone intensity decreased, hit rates also decreased. False alarm rates for these sessions were 12%, 18%, and 5%, respectively. The 50% threshold mark is denoted by a horizontal solid line. The lower three panels show concomitant changes in response latency as a function of stimulus amplitude. The latency to lick was longer when the tones were lower in amplitude, reflecting a greater difficulty in hearing out those stimuli against a silent background. Additionally, response latencies at .5 kHz were higher than those measured at either 2 kHz or 8 kHz, likely underscoring the relative difficulty in hearing lower frequency sounds in these animals (see below). Across all animals, the average difference in latency between the highest and lowest amplitude in a test session was .89 +/− .31 s.
Figure 1.
Representative psychometric functions and response latencies from one subject (Marmoset 4) at three different frequencies. Upper panels show changes in hit rate as a function of stimulus amplitude. Dotted lines show raw hit rates while solid lines show hit rates after a correction for false alarm rate has been applied (see text for a description). Lower panels show concomitant changes in the animal’s latency to lick.
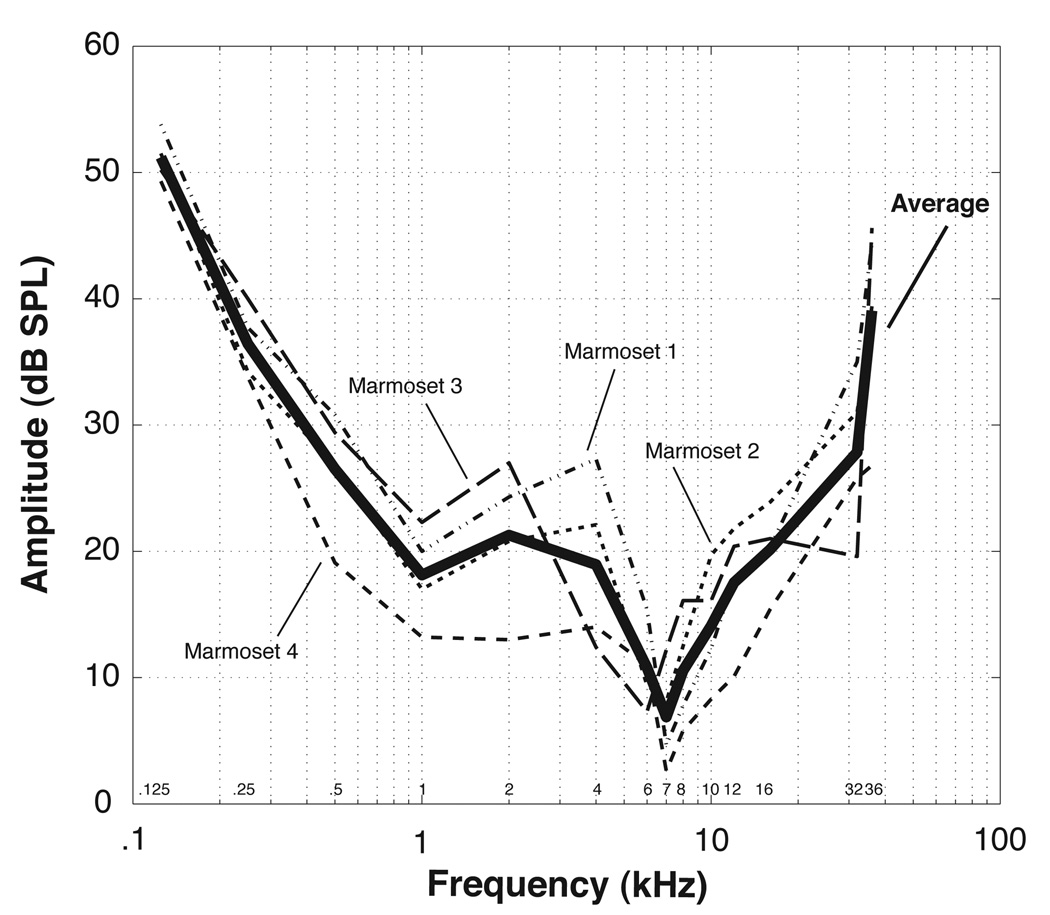
Separate audibility curves for each animal are shown in Figure 2A. There is general agreement in thresholds among the four subjects across frequency, with all animals showing sensitivities ranging from 125 Hz up to 36 kHz. The typical W-shaped audiogram common in New World monkeys was seen in three out of the four subjects tested. In these animals, there were areas of relatively heightened sensitivity at approximately 1 kHz and 7 kHz and a region of reduced sensitivity from 2 to 4 kHz.
Figure 2.
The average marmoset audiogram (bold solid line) is plotted along with the separate curves for each individual monkey (dashed/dotted lines). Specific frequencies used in the current experiment are labeled on the horizontal axis.
Marmoset 1 showed decreasing thresholds from 53.8 dB SPL to 20.0 dB SPL as frequency increased from 125 Hz to 1 kHz. Thresholds for this animal then increased to 27.3 dB SPL at 4 kHz before decreasing again to 4.6 dB at 7 kHz. Above 7 kHz, sensitivity decreased from 7.8 dB SPL at 8 kHz to 44.4 dB SPL at 36 kHz.
Sensitivities measured in marmoset 2 were similar to marmoset 1. This animal had a threshold of 51.5 dB SPL at 125 Hz, which decreased to 17.0 dB SPL at 1 kHz. Thresholds increased to 22.1 dB SPL at 4 kHz and then dropped rather abruptly to 9.4 and 8.1 dB SPL at 6 and 7 kHz, respectively. Sensitivity decreased with increasing frequencies above 7 kHz, eventually reaching 39.4 dB SPL at 36 kHz.
Thresholds for marmoset 3 agreed with those from marmosets 1 and 2, especially at lower frequencies. Sensitivities for this animal decreased from 50.2 dB SPL at 125 Hz to 22.3 dB SPL at 1 kHz before increasing to 27 dB SPL at 2 kHz. Thresholds then decreased to a minimum of 7.3 dB SPL at 6 kHz before gradually rising to 21 dB SPL at 16 kHz. Unlike marmosets 1 and 2, this animal did not show a decrease in sensitivity between 16 and 32 kHz, instead showing a slight increase between these frequencies before finally decreasing again to 45.6 dB SPL at 36 kHz.
Marmoset 4 had much better overall sensitivity compared to the other three monkeys. Thresholds decreased from 49.3 dB SPL at 125 Hz to 13.2 dB SPL at 1 kHz, a pattern consistent among all four marmosets. However, this animal showed approximately equal sensitivity at all frequencies between 1 kHz and 6 kHz, with thresholds changing only from 13.2 dB SPL to 11 dB SPL across this range. Thresholds then decreased to a sensitivity peak of 2.6 dB SPL at 7 kHz and gradually rose to 26.8 dB SPL at 36 kHz. Thus, this animal had much lower thresholds across most of its hearing range and also lacked the W-shaped audiogram seen in the other three animals.
The averaged audiogram from the four monkeys is shown in Figure 2B. Overall sensitivity in these animals tended to be fairly good, with average thresholds below 15 dB measured across a one octave range between 5 and 10 kHz and with a peak in average sensitivity around 7 kHz. Thresholds below 20–25 dB were measured across several octaves from about .5 kHz to 20 kHz. Specifically, average thresholds decreased from 51.2 dB SPL to 18.1 between 125 Hz and 1 kHz before increasing again to 21.3 dB SPL at 2 kHz. Sensitivity then improved down to 7 kHz, with an average threshold of 6.9 dB SPL, and finally became poorer as frequency increased beyond 7 kHz, eventually reaching 39.1 dB SPL at 36 kHz.
DISCUSSION
We measured audiograms in four common marmosets using operant conditioning techniques paired with an anticipatory licking task. Subjects were trained to lick at a feeding tube when they detected a target sound. Correct responses provided access to a food reward. Pure tones of varying intensities were presented to subjects using the method of constant stimuli and behavioral thresholds were calculated for each animal based on its hit rate. Thresholds for each frequency were defined by the target intensity that the animal correctly responded to 50% of the time.
Overall, hearing sensitivity in these animals is comparable to other New World monkeys, with a hearing range extending over nine octaves from 125 Hz up to 36 kHz. Lowest thresholds were seen in all four animals around 7 kHz. Three of the four subjects exhibited the W-shaped audiogram typical of New-World monkeys with sensitivity peaks at 1 kHz and 7 kHz and a region of reduced sensitivity between 2 and 4 kHz. The fourth subject showed greater sensitivity across most of its hearing range and lacked a W-shaped audiogram. It is unclear why this particular subject showed differences in sensitivity. There is evidence that spectral notches caused by pinna-based frequency selectivity aid in directional discrimination and sound localization abilities in many species, including bats (Heffner et al., 2003; Jen & Chen, 1988; Koay et al, 2003; Wotton et al., 1995), cats (Rice et al., 1992), and chinchillas (Heffner et al., 1996). However, this marmoset’s pinnae did not appear noticeably different from those of the other subjects. Also, this animal was one of the three female monkeys and was neither the youngest nor the oldest subject. Thus, differences in frequency sensitivity in this animal are likely due to individual variability rather than physical, age or sex differences.
Comparison with previous behavioral threshold data
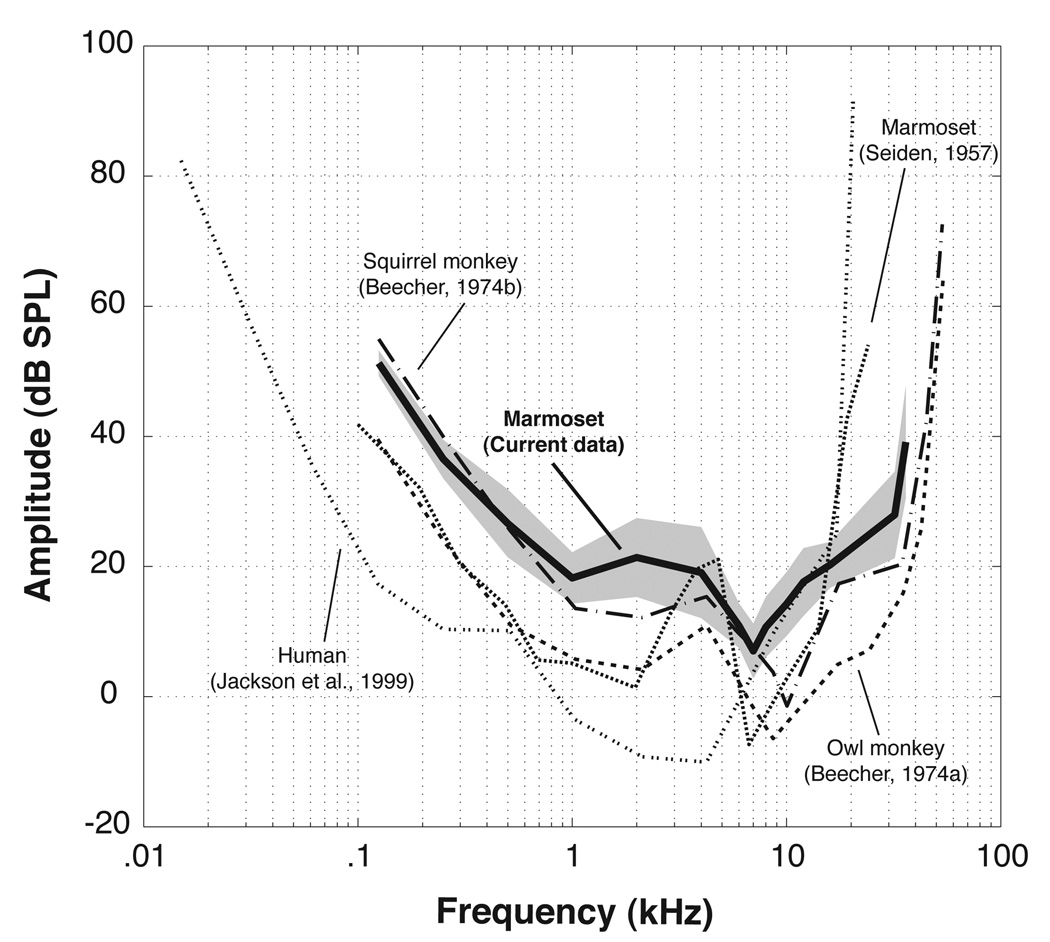
Figure 3 compares the audiogram data from the current study with previously measured New World monkey audibility curves (i.e., common marmosets [Seiden, 1957], squirrel monkeys [Beecher, 1974b], owl monkeys [Beecher, 1974a]) and also with recent data from humans (Jackson et al., 1999). As with other monkey audiograms, audibility curves here were shifted towards higher frequencies compared to the typical human audiogram. Marmosets had greatly reduced sensitivity below 6 kHz compared to humans, but better sensitivity than humans above 10 kHz. Current thresholds also differed in significant ways from the prior marmoset data reported by Seiden (1957), especially with regard to both the overall audiogram shape and the threshold values at the upper and lower extremes of the species’ hearing range. Thresholds from the current study were as much as 15–20 dB higher than those reported by Seiden (1957) for frequencies below 2 kHz and over 20 dB lower for frequencies over 15 kHz. Additionally, Seiden (1957) observed a much more pronounced notch in sensitivity around 4 kHz compared to the subjects in this experiment.
Figure 3.
Comparison of audiograms obtained from the current study (bold solid line, shaded area denotes one standard deviation from the mean) and several previous studies (common marmosets [Seiden, 1957], squirrel monkeys [Beecher, 1974b], owl monkeys [Beecher, 1974a], and humans [Jackson et al., 1999]).
We discuss three possible reasons for the difference between the present audiogram data and those of Seiden (1957), along with the relative likelihood of each differentially affecting estimates of threshold between the two studies. First, the present work used positive reinforcement while Seiden (1957) used a shock procedure and there has been some argument that negative reinforcement can result in different threshold estimates compared to positive reinforcement (e.g., Stebbins, 1970; Stebbins, 1971). However, the data collected to date suggest that this is not the case. For example, there is general agreement in reported thresholds in both squirrel monkeys (Beecher, 1974b) and macaque monkeys (Harris, 1943) using both positive and negative reinforcement. Similarly, the audiogram of the big brown bat showed little difference between a task using food reward (Dalland, 1965) and one using a conditioned avoidance procedure (Koay et al., 1997). Thus, it seems that the use of a particular reinforcement contingency should have little effect on measured thresholds.
Second, the present study differs from Seiden (1957) in whether the experimenter had manual, online control over how stimuli were presented to the subjects. Both the inter-trial interval and the duration of stimulus presentation in the current experiment were predetermined and could not be changed or adjusted during testing. In Seiden (1957), the amount of time before a trial was initiated was subjectively chosen by the experimenter on a trial by trial basis depending on an animal’s perceived quiescence. Also, the interval between the onset of the sound stimulus and delivery of the shock could be manually extended if an animal failed to respond within a preset 6 second time window. Seiden (1957) reported a low false alarm rate in his study, which suggests that increasing trial duration may not have been a problem. However, the effect of increasing stimulus duration is unclear and such interventions can potentially result in biased threshold estimates in psychophysical tasks.
Finally, the two studies may have differed in how loud the stimuli were at the subject’s ears. Animals were placed in a restraint chair in the present study, which essentially holds their heads in a fixed position in space relative to the loudspeaker. Additionally, all stimulus calibration was performed with a microphone located at roughly the same position as the animal’s head. Seiden (1957), however, averaged across several amplitude measurements taken at various points in the animals’ testing cage. Subjects in that study were also able to move around freely during testing, which means that a particular animal’s threshold would depend on its location in the sound field and the direction it was facing relative to the loudspeaker. Thus, the directionality of high frequency stimuli could have resulted in higher sensitivity estimates at those frequencies while the comparatively good low-frequency hearing reported by Seiden (1957) could have resulted from animals placing themselves inside of a standing wave.
Species-specific processing of vocal communication sounds
Lowest thresholds in the present study were measured in a narrow frequency band between 6 and 10 kHz with peak sensitivity around 7 kHz. This range overlaps with the power spectra of several of the marmosets’ primary vocalizations, especially the long distance ‘phee’ call (Agamaite, 1997; DiMattina and Wang, 2006; Pistorio et al., 2006; Epple, 1968; Norcross and Newman, 1993; Norcross et al., 1999). Phees are the most common call-type in these animals and likely serve as a species-specific contact call. They are produced when marmosets are isolated from their social group and during antiphonal exchanges with visually-occluded individuals (e.g., Miller and Wang, 2006; Norcross et al., 1994). There has been little quantitative work done on owl monkey vocalizations (although see Andrew, 1963), but we do know something about vocalizations and vocal behavior in squirrel monkeys. These animals produce calls known as isolation peeps which are functionally and structurally equivalent to marmoset phee calls (Newman, J.D., 2003; Winter et al., 1966). Most of the spectral energy in isolation peeps is in the 9–12 kHz range, which is also the region in which squirrel monkey audiograms show lowest thresholds. Thus, the regions of best hearing in these two New World monkeys may reflect a specialization for processing species-specific vocalizations. Many other species show similar increases in sensitivity at frequencies present in their vocalizations, including bats (Dalland, 1965; Koay et al., 1997; Long & Schnitzler, 1975), birds (Dooling, 1982; Dooling et al., 2000), and frogs (Gerhardt & Schwartz, 2001; Narins, 1992; Narins & Capranica, 1976; Ryan et al., 1992). This sort of coupling between a species’ frequency of best hearing and the spectral content of their vocalizations appears to be a common mechanism in many animals for perceiving behaviorally meaningful social signals in a complex, noisy environment.
Overall, these results are the first to describe the hearing sensitivity of common marmosets using operant conditioning methods with positive reinforcement. We have shown that these animals can 1) be easily trained to respond to sounds for access to a food reward, 2) hear across a roughly nine octave range, and 3) hear best at those frequencies which are also present in their species-specific vocalizations. We hope to use these kinds of operant and psychophysical methods to test more complex aspects of this species’ auditory perception in the future and to draw meaningful behavioral conclusions from marmoset neural data. It is hoped that this work will provide new insight into how sounds are processed in marmosets and, when used in conjunction with established neurophysiological techniques, will provide a new understanding of the neuroethology of auditory processing and perception in both human and nonhuman primates.
Acknowledgements
We would like to thank Jenny Estes and Nathaniel Sotuyo for their generous help with animal care. We also thank Marcus Jeschke, Amanda Lauer, and an anonymous reviewer for their many helpful and insightful comments on the manuscript. Finally, we thank Rhiannon Desideri, Meredith Maguire, and Smita Mohan for their assistance in performing the experiments. This work was supported by NIH grants DC003180, DC005808, and DC008578.
Abbreviations
- IR
infrared
- LED
light-emitting diode
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michael S. Osmanski, Email: michael.osmanski@jhu.edu.
Xiaoqin Wang, Email: xiaoqin.wang@jhu.edu.
REFERENCES
- Agamaite JA. M.S.E. Thesis. Baltimore, MD: Johns Hopkins University; 1997. A quantitative classification of the vocal repertoire of the common marmoset (Callithrix jacchus jacchus) [Google Scholar]
- Aitkin LM, Merzenich MM, Irvine DRF, Clarey JC, Nelson JE. Frequency representation in auditory cortex of the common marmoset (Callithrix jacchus jacchus) Journal of Comparative Neurology. 1986;252:1750185. doi: 10.1002/cne.902520204. [DOI] [PubMed] [Google Scholar]
- Andrew RJ. Origin and evolution of the calls and facial expressions of the primates. Behaviour. 1963;20:1–109. [Google Scholar]
- Beecher MD. Hearing in the owl monkey (Aotus trivirgatus): I. Auditory sensitivity. Journal of Comparative and Physiological Psychology. 1974a;86:898–901. doi: 10.1037/h0036416. [DOI] [PubMed] [Google Scholar]
- Beecher MD. Pure tone thresholds of the squirrel monkey (Saimiri sciureus) Journal of the Acoustical Society of America. 1974b;55:196–198. doi: 10.1121/1.1928152. [DOI] [PubMed] [Google Scholar]
- Bendor DA, Wang X. The neuronal representation of pitch in primate auditory cortex. Nature. 2005;436:1161–1165. doi: 10.1038/nature03867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendor DA, Wang X. Cortical representations of pitch in monkeys and humans. Current Opinion in Neurobiology. 2006;16:391–399. doi: 10.1016/j.conb.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendor D, Wang X. Neural response properties of primary, rostral, and rostrotemporal fields in the auditory cortex of marmoset monkeys. Journal of Neurophysiology. 2008;100:888–906. doi: 10.1152/jn.00884.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendor D, Wang X. Neural coding of periodicity in marmoset auditory cortex. Journal of Neurophysiology. 2010;103:1809–1822. doi: 10.1152/jn.00281.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CH, Wasser PM. Hearing and communication in blue monkeys (Cercopithecus mitis) Animal Behavior. 1984;32:66–75. [Google Scholar]
- Dalland JI. Auditory thresholds in the bat: A behavioral technique. Journal of Auditory Research. 1965;5:95–108. [Google Scholar]
- DiMattina C, Wang X. Virtual vocalization stimuli for investigating neural representations of species-specific vocalizations. Journal of Neurophysiology. 2006;95:1244–1262. doi: 10.1152/jn.00818.2005. [DOI] [PubMed] [Google Scholar]
- Dooling RJ. Auditory perception in birds. In: Kroodsma DE, Miller EH, editors. Acoustic communication in birds, Vol. 1. Production, perception, and design features of sounds. New York: Academic Press; 1982. pp. 95–130. [Google Scholar]
- Dooling RJ, Lohr B, Dent ML. Hearing in birds and reptiles. In: Dooling RJ, Popper AN, Fay RR, editors. Comparative hearing: Birds and reptiles. New York: Springer-Verlag; 2000. pp. 308–359. [Google Scholar]
- Dooling RJ, Okanoya K. The method of constant stimuli in testing auditory sensitivity in small birds. In: Klump GM, Dooling RJ, Fay RR, Stebbins WC, editors. Methods in Comparative Psychoacoustics. Basel: Birkhauser-Verlag; 1995. pp. 161–169. [Google Scholar]
- Elder JH. Audiometric studies with the chimpanzee. Psychological Bulletin. 1933;30:547–548. [Google Scholar]
- Elder JH. Auditory acuity of the chimpanzee. Journal of Comparative and Physiological Psychology. 1934;17:157–183. [Google Scholar]
- Elder JH. The upper limit of hearing in the chimpanzee. American Journal of Physiology. 1935;112:109–115. [Google Scholar]
- Eliades SJ, Wang X. Sensory-motor interaction in the primate auditory cortex during self-Initiated vocalizations. Journal of Neurophysiology. 2003;89:2194–2207. doi: 10.1152/jn.00627.2002. [DOI] [PubMed] [Google Scholar]
- Eliades SJ, Wang X. Dynamics of auditory-vocal interaction in monkey auditory cortex. Cerebral Cortex. 2005;15:1510–1523. doi: 10.1093/cercor/bhi030. [DOI] [PubMed] [Google Scholar]
- Eliades SJ, Wang X. Neural substrates of vocalization feedback monitoring in primate auditory cortex. Nature. 2008;453:1102–1106. doi: 10.1038/nature06910. [DOI] [PubMed] [Google Scholar]
- Epple G. Comparative studies on vocalizations in marmoset monkeys. Folia Primatologica. 1968;8:1–40. doi: 10.1159/000155129. [DOI] [PubMed] [Google Scholar]
- Fay RR. Hearing in vertebrates: A psychophysics databook. Winnetka, IL: Hill-Fay Associates; 1988. [Google Scholar]
- Fujita S, Elliott DN. Thresholds of audition for three species of monkeys. Journal of the Acoustical Society of America. 1968;37:139–144. doi: 10.1121/1.2143403. [DOI] [PubMed] [Google Scholar]
- Gerhardt HC, Schwartz JJ. Auditory tuning and frequency preferences in anurans. In: Ryan MJ, editor. Anuran communication. Washington: Smithsonian Institution Press; 2001. pp. 73–85. [Google Scholar]
- Gescheider GA. Psychophysics: Method, theory, and application. New York: Lawrence Erlbaum & Assoc.; 1985. [Google Scholar]
- Green S. Auditory sensitivity and equal loudness in the squirrel monkey (Saimiri sciureus) Journal of the Experimental Analysis of Behavior. 1975;23:255–264. doi: 10.1901/jeab.1975.23-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JD. The auditory acuity of preadolescent monkeys. Journal of Comparative Psychology. 1943;35:255–265. [Google Scholar]
- Heffner RS. Primate hearing from a mammalian perspective. Anatomical Record A. 2004;281A:1111–1122. doi: 10.1002/ar.a.20117. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Koay G, Heffner HE. Sound localization in chinchillas: III, effect of pinna removal. Hearing Research. 1996;99:13–21. doi: 10.1016/s0378-5955(96)00074-3. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Koay G, Heffner HE. Hearing in American leafnosed bats. III: Artibeus jamaicensis. Hearing Research. 2003;184:113–122. doi: 10.1016/s0378-5955(03)00233-8. [DOI] [PubMed] [Google Scholar]
- Heinz RD, Turkkan JS, Harris AH. Pure tone thresholds in the yellow baboon (Papio cynocephalus) Hearing Research. 1982;8:71–75. doi: 10.1016/0378-5955(82)90035-1. [DOI] [PubMed] [Google Scholar]
- Jackson LS, Heffner RS, Heffner HE. Free-field audiogram of the Japanese macaque (Macaca fuscata) Journal of the Acoustical Society of America. 1999;106:3017–3023. doi: 10.1121/1.428121. [DOI] [PubMed] [Google Scholar]
- Jen PH-S, Chen D. Directionality of sound pressure transformation at the pinna of echolocating bats. Hearing Research. 1988;34:101–118. doi: 10.1016/0378-5955(88)90098-6. [DOI] [PubMed] [Google Scholar]
- Koay G, Heffner RS, Bitter KS, Heffner HE. Hearing in American leaf-nosed bats: II, Carollia perspicillata. Hearing Research. 2003;178:27–34. doi: 10.1016/s0378-5955(03)00025-x. [DOI] [PubMed] [Google Scholar]
- Koay G, Heffner HE, Heffner RS. Audiogram of the big brown bat (Eptesicus fuscus) Hearing Research. 1997;105:202–210. doi: 10.1016/s0378-5955(96)00208-0. [DOI] [PubMed] [Google Scholar]
- Long GR, Schnitzler HU. Behavioral audiograms from the bat, Rhinolophus ferrumequinum. Journal of Comparative Physiology. 1975;100:211–219. [Google Scholar]
- Miller CT, Beck K, Meade B, Wang X. Antiphonal call timing in marmosets is behaviorally significant: Interactive playback experiments. Journal of Comparative Neurobiology A. 2009a;195:783–789. doi: 10.1007/s00359-009-0456-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CT, Eliades SJ, Wang X. Motor planning for vocal production in common marmosets. Animal Behavior. 2009b;78:1195–1203. doi: 10.1016/j.anbehav.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CT, Mandel K, Wang X. The communicative content of the common marmoset phee call during antiphonal calling. American Journal of Primatology. 2010;72:974–980. doi: 10.1002/ajp.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CT, Wang X. Sensory-motor interactions modulate a primate vocal behavior: Antiphonal calling in common marmosets. Journal of Comparative Neurobiology A. 2006;192:27–38. doi: 10.1007/s00359-005-0043-z. [DOI] [PubMed] [Google Scholar]
- Narins PM. Biological constraints on anuran acoustic communication: Auditory capabilities of naturally-behaving animals. In: Webster DB, Fay RF, Popper AN, editors. The evolutionary biology of hearing. New York: Springer-Verlag; 1992. pp. 439–454. [Google Scholar]
- Narins PM, Capranica RR. Sexual differences in the auditory system of the treefrog, Eleutherodactylus coqui. Science. 1976;192:378–380. doi: 10.1126/science.1257772. [DOI] [PubMed] [Google Scholar]
- Newman JD. Auditory communication and central auditory mechanisms in the squirrel monkey: Past and present. In: Ghazanfar AA, editor. Primate Audition: Ethology and Neurobiology. Vol. 13. 2003. pp. 198–203. [Google Scholar]
- Niemiec AJ, Moody DB. Constant stimulus and tracking procedures for measuring sensitivity. In: Klump GM, Dooling RJ, Fay RR, Stebbins WC, editors. Methods in Comparative Psychoacoustics. Basel: Birkhauser-Verlag; 1995. pp. 65–77. [Google Scholar]
- Norcross JL, Newman JD. Context and gender specific differences in the acoustic structure of common marmoset (Callithrix jacchus) phee calls. American Journal of Primatology. 1993;30:37–54. doi: 10.1002/ajp.1350300104. [DOI] [PubMed] [Google Scholar]
- Norcross JL, Newman JD, CoFrancesco LM. Context and sex differences exist in the acoustic structure of phee calls by newly-paired common marmosets (Callithrix jacchus) American Journal of Primatology. 1999;49:165–181. doi: 10.1002/(SICI)1098-2345(199910)49:2<165::AID-AJP7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Norcross JL, Newman JD, Fitch WT. Responses to natural and synthetic phee calls by common marmosets. American Journal of Primatology. 1994;33:15–29. doi: 10.1002/ajp.1350330103. [DOI] [PubMed] [Google Scholar]
- Okanoya K, Dooling RJ. Hearing in passerine and psittacine birds: A comparative study of absolute and masked auditory thresholds. Journal of Comparative Psychology. 1987;101:7–15. [PubMed] [Google Scholar]
- Owren MJ, Hopp SL, Sinnott JM, Petersen MR. Absolute auditory thresholds in three old world monkey species (Cercopithecus aethiops, C. neglectus, Macaca fuscata) Journal of Comparative Psychology. 1988;102:99–107. doi: 10.1037/0735-7036.102.2.99. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Laycock J, Flammino F, Lonsbury-Martin B, Martin G. Pure tone thresholds for the rhesus monkey. Hearing Research. 1978;1:43–47. doi: 10.1016/0378-5955(78)90008-4. [DOI] [PubMed] [Google Scholar]
- Pistorio AL, Vintch B, Wang X. Acoustical analysis of vocal development in a New World primate, the common marmoset (Callithrix jacchus) Journal of the Acoustical Society of America. 2006;120:1655–1670. doi: 10.1121/1.2225899. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Jenkins WM, Hradek GT, Merzenich MM. A behavioral frequency discrimination paradigm for use in adult primates. Behavior Research Methods, Instruments, and Computers. 1991;23:357–369. [Google Scholar]
- Rice JJ, May BJ, Spirou GA, Young ED. Pinna-based spectral cues for sound localization in the cat. Hearing Research. 1992;58:132–152. doi: 10.1016/0378-5955(92)90123-5. [DOI] [PubMed] [Google Scholar]
- Ryan MJ, Perrill SA, Wilczynski W. Auditory tuning and call frequency predict population based mating preferences in the cricket frog, Acris crepitans. American Naturalist. 1992;139:1370–1383. [Google Scholar]
- Sadagopan S, Wang X. Level invariant representation of sounds by populations of neurons in primary auditory cortex. Journal of Neuroscience. 2008;28:3415–3426. doi: 10.1523/JNEUROSCI.2743-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki E, Suemizu H, Shimada A, Hanazawa K, Oiwa R, Kamioka M, Tomioka I, Sotomaru Y, Hirakawa R, Eto T, Shiozawa S, Maeda T, Ito M, Ito R, Kito C, Yagihashi C, Kawai K, Miyoshi H, Tanioka Y, Tamaoki N, Habu S, Okano H, Nomura T. Generation of transgenic non-human primates with germline transmission. Nature. 2009;459:523–527. doi: 10.1038/nature08090. [DOI] [PubMed] [Google Scholar]
- Seiden HR. Ph.D. Thesis. Princeton, NJ: Princeton University; 1957. Auditory acuity of the marmoset monkey (Hapale jacchus) [Google Scholar]
- Serafin JV, Moody DB, Stebbins WC. Frequency selectivity of the monkey’s auditory system: Psychophysical tuning curves. Journal of the Acoustical Society of America. 1982;71:1513–1519. doi: 10.1121/1.387851. [DOI] [PubMed] [Google Scholar]
- Stebbins WC. Animal Psychophysics: The Design and Conduct of Sensory Experiments. New York, NY: Appleton-Century-Crofts; 1970. [Google Scholar]
- Stebbins WC. Hearing. In: Schrier AM, Stollnitz F, editors. Behavior of Non-Human Primates. Vol. 3. New York: Academic Press; 1971. pp. 159–192. [Google Scholar]
- Stebbins WC. Hearing in old world monkeys. American Journal of Physical Anthropology. 1973;38:357–364. doi: 10.1002/ajpa.1330380233. [DOI] [PubMed] [Google Scholar]
- Stebbins WC. Hearing of the primates. In: Chivers DJ, Herbert J, editors. Recent Advances in Primatology. New York, NY: Academic Press; 1978. pp. 703–720. [Google Scholar]
- Stebbins WC, Green S, Miller FL. Auditory sensitivity of the monkey. Science. 1966;153:1646–1647. doi: 10.1126/science.153.3744.1646-a. [DOI] [PubMed] [Google Scholar]
- Wang X. On cortical coding of vocal communication sounds in primates. Proceedings of the National Academy of Sciences. 2000;97:11843–11849. doi: 10.1073/pnas.97.22.11843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Neural coding strategies in auditory cortex. Hearing Research. 2007;229:81–93. doi: 10.1016/j.heares.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Wang X, Kadia SC. Differential representation of species-specific primate vocalizations in the auditory cortices of marmoset and cat. Journal of Neurophysiology. 2001;86:2616–2620. doi: 10.1152/jn.2001.86.5.2616. [DOI] [PubMed] [Google Scholar]
- Watkins PV, Barbour DL. Specialized neuronal adaptation for preserving input sensitivity. Nature Neuroscience. 2008;11:1259–1261. doi: 10.1038/nn.2201. [DOI] [PubMed] [Google Scholar]
- Watkins PV, Barbour DL. Level-tuned neurons in primary auditory cortex adapt differently to loud versus soft sounds. Cerebral Cortex. 2011;21:178–190. doi: 10.1093/cercor/bhq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiernicke A, Haüsler U, Jürgens U. Auditory frequency discrimination in the squirrel monkey. Journal of Comparative Physiology A. 2001;187:189–195. doi: 10.1007/s003590100189. [DOI] [PubMed] [Google Scholar]
- Winter P, Ploog D, Latta J. Vocal repertoire of the squirrel monkey (Saimiri sciureus), its analysis and significance. Experimental Brain Research. 1966;1:359–384. doi: 10.1007/BF00237707. [DOI] [PubMed] [Google Scholar]
- Wotton JM, Haresign T, Simmons JA. Spatially dependent acoustic cues generated by the external ear of the big brown bat, Eptesicus fuscus. The Journal of the Acoustical Society of America. 1995;98:1423–1445. doi: 10.1121/1.413410. [DOI] [PubMed] [Google Scholar]