Abstract
Osteoporosis is a major health problem in the elderly. Epidemiological evidence has shown an association between tea consumption and the prevention of bone loss in the elderly population. Ingestion of green tea and green tea bioactive compounds may be beneficial in mitigating bone loss of this population and decreasing their risk of osteoporotic fractures. This review describes the effect of green tea with its bioactive components on bone health with an emphasis on the following: (i) the etiology of osteoporosis, (ii) evidence of osteo-protective impacts of green tea on bone mass and microarchitecture in various bone loss models in which induced by aging, sex hormone deficiency, and chronic inflammation, (iii) discussion of impacts of green tea on bone mass in two obesity models, (iv) observation of short-term green tea supplementation given to postmenopausal women with low bone mass, (v) possible mechanisms for the osteo-protective effects of green tea bioactive compounds, and (vi) a summary and future research direction of green tea and bone health.
Keywords: tea polyphenols, antioxidant, bone mineral density, osteoporosis, human, rat, cell
1. Introduction
The trend of increased life expectancy is accompanied with an increase in the prevalence of osteoporosis and concomitant complications in the elderly population. Osteoporosis, a degenerative bone disease, is characterized by low bone mass and microstructural deterioration of bone tissue that results in bone fragility and an increased susceptibility to fractures [1]. Hip fracture is the most severe consequence of osteoporosis, resulting in decreased activities of daily living, lowered quality of life, and increased mortality [2].
Osteoporosis occurring in postmenopausal women and elderly men represents a major health and economic burden in our fast growing elderly population. In the United States, approximately 44 million or 55 percent of the people 50 and older have osteoporosis or low bone mass [3]. It is estimated that by 2020, there will be over 61 million women and men in this age category that are affected [3]. By the year 2025, experts predict that the costs of osteoporosis-related expenses will rise to approximately $25.3 billion [4].
Although there are a variety of agents available for the prevention and/or treatment of low bone mass (also called osteopenia) and osteoporosis, some patients select complementary and alternative therapies, such as dietary supplements or functional foods, for this purpose [5]. Tea, the dried leaves of the Camellia sinensis species of the Theaceae family, is a popular beverage with an annual production of three billion kilograms worldwide [6]. In the past decade, epidemiological evidence has shown an association between tea consumption and the prevention of age-related bone loss in the elderly population. The impact of tea consumption on bone mass and risk of osteoporotic fractures in humans has been comprehensively reported in our previous review paper and we found that among different forms of tea (green tea, black tea, white tea, and Oolong tea), drinking green tea and/or ingesting green tea bioactive compounds may mitigate bone loss in elderly women and men, thereby decreasing their risk of osteoporotic fractures [7]. Therefore, in this review, we focus on animal studies in various models with an emphasis on bone health. In addition, a short-term translational study employing green tea supplement given to postmenopausal women with low bone mass is also discussed.
2. Bone biology and metabolic disorders
Bone is a highly specialized support tissue which is characterized by its rigidity and hardness. As a material, bone has strength similar to cast iron, while its density is as low as wood. Calcium and phosphorus mineral crystals are deposited around the protein strands. The flexible protein strands provide the tensile strength that holds the structure together and the brittle minerals provide the solid structure. The two main categories of bone cells are osteoblasts that form the bone and osteoclasts that resorb (dissolve) the bone. The combined and cooperative activities of osteoblasts and osteoclasts result in a bone architecture that provides mechanical support and protection for the body. In addition, bone serves as a vital reservoir of minerals, principally calcium and phosphorus, necessary for maintaining normal cellular, neurologic, and vascular activities of the body [8].
Both osteoblastic and osteoclastic cells regulate bone metabolism, and both cell types are involved in the development of osteoporosis [8]. Osteoblasts, the bone-forming cells, locate near the surface of the bone and produce cytokines that affect osteoclasts. Cytokines, including macrophage-colony stimulating factor (M-CSF) and receptor activator of nuclear factor-κB (NF-κB) ligand (RANKL), are both essential for osteoclast differentiation, function, and survival [9, 10]. Osteoclasts, the bone-resorbing multinucleated cells, are tightly attached to mineralized bone surfaces through their integrins and form resorption lacuna by secreting protons, proteases, and superoxide through ruffled borders [11, 12]. Bone resorption by activated osteoclasts with subsequent deposition of a new matrix by osteoblasts causes the formation of bone structure and bone remodeling [8]. Imbalance between bone formation and bone resorption is the key pathophysiological event in many metabolic bone disorders in adult humans, including osteoporosis, which results in bone loss [13].
3. Evidence of osteo-protective effects of green tea in animals
All but one animal studies support that green tea may benefit bone health by mitigating bone loss due to aging, aging plus sexual hormone deficiency, or chronic inflammation, or by preserving bone mass due to obesity, as summarized in Table 1.
Table 1.
Effect of green tea supplementation on bone health in various animal models
| First author, yr Reference | Model | Animals | Treatments | Main Results |
|---|---|---|---|---|
| Shen, 2008 [16] Shen, 2010 [21] |
|
Sham and OVX, 14-mo-old female F344×BFN1/NIA rats | GTP: 0.1% or 0.5% (wt/vol) in drinking water for 16 weeks | ↑ Femur BMDa ↑ Serum OC, ↓ serum TRAP, ↓ urinary Ca via ↓ 8-OHdG (a marker of oxidative stress DNA damage), ↑ GPX activity (a marker of antioxidant capacity), and ↑ SOD1 expression (a marker of antioxidant capacity) |
| Shen, 2009 [17] |
|
Sham and OVX, 14-mo-old female F344×BFN1/NIA rats | GTP: 0.1% or 0.5% (wt/vol) in drinking water for 16 weeks | ↑ BV/TV, BFR, Tb.N and Tb.Th of proximal tibiab ↑ Cortical thickness and area of femurd ↓ Tb.Sp and bone erosion of proximal tibiab ↓ Endocortical bone eroded surface of tibia shaftb |
| Shen, 2010 [23] |
|
Sham and ORX, 15-mo-old male F344 rats | GTP: 0.5% (wt/vol) in drinking water for 16 weeks | ↑ BV/TV, Tb.Th and bone formation rates in both proximal tibia and periosteal tibial shaftsb ↓ Eroded surface in both proximal tibia and endocortical tibial shaftsb via ↑ GPX activity |
| Shen, 2010 [25] | Chronic inflammation (LPS)-induced bone loss model | 3-month-old female CD rats | GTP: 0.5% (wt/vol) in drinking water for 12 weeks | ↑ Femur BMDa ↑ Serum OC, ↓ serum TRAP via ↓ 8-OHdG production and ↓ COX-2 and TNF-α mRNA expression (makers of pro- inflammatory cytokine) |
| Shen, 2010 [26] | Chronic inflammation (LPS)-induced bone loss model | 3-month-old female rats | GTP: 0.5% (wt/vol) in drinking water for 12 weeks | ↑ BV/TV, Tb.N and Tb.Th of femurd and tibiab ↑ Periosteal bone formation rate in tibial shaftsb ↓ Tb.Sp in proximal tibia and eroded surface in endocortical tibial shaftsb ↓ Osteoclastic number of tibia shaftsb ↑ Bone strength of femur via ↓ TNF-α protein expression in trabecular bone |
| Nakamura, 2010 [27] | Topically LPS- infected gingival inflammation model | 7-wk-old male BALB/c mice | GTC: injection (10 μg/mL) into infected gingival, once every 48 hr for 10 times | ↓ LPS-induced alveolar bone resorptionb,c (as shown by increased TRAP-positive osteoclasts) via ↓ IL-1β production (a marker of pro- inflammatory cytokine) or ↓ osteoclastogenesis directly |
| Maruyama, 2010 [28] | Topically LPS- infected periodontal inflammation model | 8-wk-old male Wistar rats | GTC: 1% in dentifrice for the last 4 weeks in a total of 8 weeks study period | ↓ LPS-induced inflammation cell infiltration in connective tissue via ↓ hexanoyl-lysine (a maker of lipid peroxidation), nitrotyrosine (a maker of oxidative protein damage), and TNF-α (a maker of pro- inflammatory cytokines) No impact on LPS-induced bone resorption |
| Shen, 2010 [30] | High-fat-diet induced obesity model | 3-mo-old CD female rats fed a high-fat diet for 4 months | GTP: 0.5% (wt/vol) in drinking water 16 weeks | ↑ Femur BMC and BMDa ↓ Weight gain (↓ fat mass and ↑ fat-free mass) ↓ Serum leptin production via ↑ GPX activity |
| Iwaniec, 2009 [31] | Ggenetically obese, leptin-deficient model | 5-wk-old lean C57BL/6 wild type and leptin-deficient (ob/ob) male mice | GTE: 0, 1%, or 2% (wt/wt) in diet for 6 weeks | ↓ Femur length, BV/TV, mineral content, cortical volume, and cortical thicknessd ↓ Cancellous bone volume/tissue volume and Tb.Th in lumbar vertebraed |
, Measured by DEXA;
, measured by histomorphometry;
, measured by histology;
, measured by micro-computed tomography.
Abbreviation: BFR, bone formation rate; BMC, bone mineral content; BMD, bone mineral density; BV/TV, bone total volume; BW, body weight; Ca, calcium; Cd, cadmium; COX-2, cyclooxygenase-2; DYD, deoxypyridinoline; GPX, glutathione peroxidase; GTC, green tea catechin; GTE, green tea extract; GTP, green tea polyphenols; IL-1β, interleukin-1β; LPS, lipopolysaccharide; OC, osteocalcin; 8-OHdG, 8-hydroxy-2′-deoxyguanosine; ORX, orichdectomized; OVX, ovariectomized; SOD, superoxide dismutase; Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness; TNF-α, tumor necrosis factorα; TRAP, tartrate-resistant acid phosphatase.
3.1. Female rat models of osteoporosis
The models of intact and ovariectomized (OVX) aged female rats have been widely used to study both aging and ovarian hormone deficiency-induced bone loss, respectively. A number of studies show that both intact and OVX models have been recognized as appropriate female rat models to study premenopausal and postmenopausal women with low bone loss [14] for the following reasons: (i) aged female rats have completed bone growth and commenced bone loss, while bone growth is still in progress in young rats [14]. Sham-operated aged female rats were used as an established model of premenopausal women, and (ii) an OVX rat properly simulates estrogen-deficient condition and has been widely used as an animal model for postmenopausal osteoporosis [15].
Animal studies conducted in our laboratory have shown that green tea polyphenols (GTP) have positive effects on bone mass and microarchitecture in both models of bone loss [16, 17]. The findings indicated that GTP prevented the aging-induced as well as aging plus estrogen deficiency-induced reduction in femoral bone mass (i.e., bone mineral content (BMC) and density (BMD)) in sham rats and OVX rats, respectively. Serum osteocalcin (a bone formation biomarker) was elevated by the GTP treatment, while serum tartrate-resistant acid phosphatase (TRAP, a bone resorption biomarker) was suppressed by the GTP treatment.
In that study, female F344×BFN1/NIA rats (14-month-old) were divided into seven groups: baseline group (euthanized at the beginning of study), sham operated (SH), SH+0.1% GTP (weight/volume), SH+0.5% GTP, OVX, OVX+0.1% GTP, and OVX+0.5% GTP. Treatments were started two weeks after surgery and continued for 16 weeks. Rats were given 0.1% or 0.5% concentration of GTP in drinking water daily to mimic human consumption of green tea of 1 or 4 cups a day, respectively [18–20]. These dosages (1 and 4 servings a day, 1 serving = 120 mL) are feasible for human daily consumption. GTP has purity higher than 98%. Every 1000 mg of GTP contained 480 mg of EGCG, 160 mg of epicatechin gallate (ECG), 60 mg of epicatechin (EC), 103 mg of epigallocatechin (EGC), and 30 mg of Catechin according to the high performance liquid chromatography coupled with electrochemical detection (HPLC-ECD) and HPLC-UV analyses [16].
Aging significantly reduced the bone mass of the femur and deteriorated the bone microarchitecture of the tibia. Aging plus estrogen deficiency produced a more detrimental effect on bone mass and microarchitecture than aging alone [16]. The same study demonstrated beneficial effects of GTP supplementation in preserving bone mass of the femur [16] and improving cancellous and cortical microstructure of the tibia in both SH and OVX rats by enhancing bone formation and suppressing bone erosion [17]. The impact of GTP on bone parameters was dose-dependent. For example, GTP, as low as 0.1%, was effective in restoring femoral BMD in the SH group, when compared to the baseline group. SH+0.5% GTP treatment even completely prevented aging-induced bone loss. The results showed that urinary 8-hydroxy-2′-deoxyguanosine (a biomarker of oxidative stress) and urinary calcium were decreased, and the liver glutathione peroxidase activities [16] and superoxide dismutase 1 expression (indicator of antioxidant capacity) [21] were increased as a result of SH and OVX rats receiving GTP supplementation [16, 17]. The authors concluded that the bone protective mechanism of GTP, in part, may be due to the ability of GTP to suppress oxidative stress and increase antioxidant capacity. Moreover, it was noted by the authors that such an osteoprotective effect of GTP on OVX rats appears to be mild compared to that on SH rats.
From the same study, the impact of GTP on bone mass in OVX rats may be independent of estrogen level and showed no changes in serum estradiol concentration of rats [16]. Interestingly, the sham-operated groups (estrogen adequate) with GTP supplementation only showed an effect on uterine weight, not on serum estradiol concentration, a result possibly associated with its weak binding affinity for estrogen receptor(s) (ER), especially ER-α and ER-β [22].
3.2. Male rat models of osteoporosis
Similar to the above findings observed in an animal model for postmenopausal osteoporosis, Shen et al. also reported that GTP supplementation improves bone microstructure in aged orchidectomized (ORX) rat model, a model for senile male osteoporosis [23]. In that study, fifty 15-month-old aged F344 male rats were assigned into SH, SH+0.5% GTP, ORX, and ORX+0.5% GTP groups with 10 rats per treatment group. Same type and concentration of GTP (0.5%, wt/vol in drinking water) has been used in our female rat models of osteoporosis [16, 17]. The remaining 10 rats without any surgical procedures formed the baseline group and were euthanized before the beginning of the study. GTP supplementation was started two weeks after surgery and continued for 16 weeks. Femoral bones were collected for bone scan using dual energy X-ray absorptiometry and micro-computed tomography, as well as for bone strength, and tibial bones were collected for microartictural properties using bone histomorphometric analyses. Orchidectomy reduced serum testosterone, BMD, and bone strength. Orchidectomy also decreased trabecular bone volume, number and thickness in distal femur and proximal tibia, and bone formation rate in trabecular bone of proximal tibia, but increased bone formation rates in endocortical tibial shaft [23].
The results of this study showed that supplementation of GTP was effective in preventing both the aging- and aging plus ORX-induced loss of bone volume in both distal femur and tibia, resulting in improved bone strength. Similar observations were made in trabecular number and thickness where GTP supplementation was protective against the loss of trabecular number and thickness as a result of aging or aging plus ORX. From the same study, authors also reported that GTP supplementation significantly increased bone formation rates, but suppressed bone erosion in both proximal tibia and endocortical tibial shaft of aged ORX rats. Such a bone-protective effect of GTP may, in part, due to the elevation of antioxidant potential, as shown in the greater activity of glutathione peroxidase in liver. These observations were similar to the earlier findings in a female rat model of bone loss [16[.
3.3. Chronic inflammation-induced bone loss models
Emerging data also indicate that GTP is very effective in preserving bone mass in various models of chronic inflammation-induced bone loss. Green tea polyphenols have been found to counteract inflammation-induced bone loss in a recent animal study by Shen et al. [25, 26]. In Shen et al.’s study, a 2 (placebo vs. lipopolysaccharide, LPS) × 2 (no GTP vs. 0.5% GTP in drinking water) factorial design was used with 40 female rats (3-month-old) assigned to 4 groups for 12 weeks. Same type and concentration of GTP (0.5%, wt/vol in drinking water) has been used in our female rat models of osteoporosis [16, 17]. They reported that LPS administration resulted in a significant increase in the inflammation index as determined by the total white blood cell count, and accompanied by significant bone loss and bone microstructure deterioration. Bone loss was demonstrated by a decrease in BMD [25], accompanied by lower trabecular volume fraction, number, and thickness in the proximal tibia, and increased eroded surface and osteoclast number in the endocortical tibial shafts, as assessed by bone histomorphometry [26]. In addition, LPS administration also resulted in a lower osteocalcin concentration and a higher TRAP concentration compared to the placebo treatment [26].
After 12 weeks of treatment, supplementation of GTP (400 mg/kg body weight) in the drinking water significantly increased BMD, serum osteocalcin, as well as trabecular volume fraction and number in both femur and tibia, but decreased serum TRAP, eroded surface, and osteoclast number in endocortical tibial shafts [25, 26]. This study demonstrated that GTP supplementation in drinking water for 12 weeks prevented trabecular bone loss due to increased bone turnover by LPS administration. From the same study, GTP supplementation was shown to suppress oxidative stress (as shown by decreased urinary 8-hydroxy-2′-deoxyguanosine levels) and inflammation (as shown by suppressed mRNA expression of tumor necrosis factor-α and cyclooxygenase-2 in spleen). Authors concluded that GTP mitigates bone loss in a chronic-inflammation-induced bone loss model by reducing oxidative stress-induced damage and inflammation [25, 26].
3.4. LPS-induced alveolar bone resorption model
Shen et al.’s study [25] is corroborated by the findings of Nakamura et al. [27] that topical application of green tea catechin (GTC) into LPS-treated gingivae of BALB/c mice significantly inhibited LPS-induced alveolar bone resorption. In that study, LPS or LPS with 1% Sunphenon BG (containing 91.3% polyphenol and 76.6% GTC, consisting of 45.9% EGCG, 9.6% EGC, 8.6% ECG, 5.3% EC, and others, Taiyo Kagaku, Mie, Japan) was injected a total of 10 times, once every 48 h, into the gingivae of BALB/c mice [27]. Another group of mice, housed with free access to water containing 1% Sunphenon BG throughout the experimental period, were also injected with LPS in a similar manner.
LPS induced the alveolar bone resorption (as shown in increased TRAP-positive osteoclasts by immunohistological staining and quantification) and interleukin-1 beta (IL-1β) expression in gingival tissue. GTC by injection or oral administration to mice significantly inhibited LPS-induced alveolar bone resorption in mice. Authors concluded that such inhibitory impact of GTC on bone resorption is mediated by suppressing IL-1β expression or by directly inhibiting RANKL-induced osteoclastogenesis.
A recent study published by Maruyama et al. [28] using male Wistar rats also showed that supplementation of GTC in dentifrices suppresses gingival oxidative stress and periodontal inflammation. In that study, twenty-four 8-week-old male Wistar rats were randomly assigned into four groups of six rats each group: (1) control group receiving no treatment for 8 weeks, (2) periodontal inflammation group receiving inflammation for 8 weeks, (3) periodontal inflammation plus control dentifrice for 8 weeks and receiving control dentifrice to the gingival sulcus daily for the last 4 weeks, and (4) periodontal inflammation + 1% GTC (Sunphenon 100S; Taiyo Kagaku, Mie, Japan)-containing dentifrice group having experimental periodontal inflammation for 8 weeks and receiving topical application of a GTC-containing dentifrice to the gingival sulcus daily for the last 4 weeks. The Sunphenon 100S was composed of 18% EGCG, 11.6% GCG, 4.6% ECG, 15.0% EGC, 14.8% GC, 7.0% EC, and 3% catechin.
After an 8-week inflammation period, periodontal inflammation caused alveolar bone loss and inflammatory cell infiltration in the connective tissue subjacent to the juntional epithelium of rats. Topical application of GTC-containing dentifrice suppressed such inflammatory cell infiltration, but had no effect on alveolar bone loss. The inhibitory effect on inflammation was demonstrated by decreased gingival oxidative stress and expression of pro-inflammatory cytokines.
Although green tea supplementation was shown to inhibit inflammation in these three different inflammation models, only two studies [25, 27] demonstrated GTP’s ability to suppress bone resorption. The discrepancy may be explained by various animal models, various doses and length of green tea supplementation, different types of bones, and assessment of bone resorption (histomorphometric analysis in Shen et al., immunohistochemistric analysis in Nakamura et al., and histological analysis in Maruyama et al.).
3.5. Obesity models
Studies have shown that increased central body fat had a negative association with BMD in postmenopausal women, suggesting prevention of menopause-related osteoporosis through reducing centralized fat deposition [29]. Shen et al. [30] demonstrated that supplementation of GTP (0.5% vol/wt) in drinking water for 16 weeks provided both anti-obesity and osteo-protective benefits in middle-aged, high-fat-diet-induced obese female rats. In that study, 3-month-old CD female rats were fed either a low-fat (LF) diet (n = 12) or a high-fat (HF) diet (n= 24) at libitum for 4 months. Animals in the LF diet group continued on a LF diet for additional 4 months, while those in the HF diet group were divided into groups with or without 0.5% GTP in drinking water, in addition to a HF diet for another 4 months. Same type and concentration of GTP (0.5%, wt/vol in drinking water) has been used in our female rat models of osteoporosis [16, 17]. Body composition including fat mass and fat-free mass was measured by bioimpedance spectroscopy. Femoral BMC and BMD were measured by dual-energy x-ray absorptiometry. Serum leptin and adiponectin (fat hormones) were also determined in these rats. After 4 months of treatment, compared to the LF diet, the HF diet increased body weight and fat mass and leptin, decreased fat-free mass, and had no impact on BMC, BMD, and serum adiponectin in rats. GTP supplementation was able to prevent weight gain (shown by decreased fat mass and increased fat-free mass), increased BMC and BMD, suppressed serum leptin, but had no influence on serum adiponectin in HF diet-induced obese rats. Authors concluded that GTP supplementation in drinking water for 4 months prevents weight gain and enhances bone matrix in rats, through improving antioxidant capacity (increased liver glutathione peroxidase) and modulating fat hormones (suppressed leptin) [30].
In an earlier study, Iwaniec et al. [31] also reported that green tea extract (GTE) supplementation to lean C57BL/6 wild type (WT) and genetically obese, leptin-deficient (ob/ob) male mice prevented weight gain. In that study, both five-week-old lean and ob/ob mice were assigned to powder diets containing GTE at 0, 1, or 2% (wt/wt) for 6 weeks. According to the results of HPLC-UV, the GTE contained 30% (wt/wt) catechins [consisting of 48% EGCG, 31% EGC, 13% ECG, and 8% EC] and 5.6 mg caffeine/100 mg GTE [31]. Rats were given 1% or 2% GTE in diet was equivalent to human consumption of green tea of 7 or 14 servings a day, respectively.
Femoral and lumbar vertebral bone volume and architecture were assessed by microcomputed tomography. Then, femoral bones were ashed to measure BMC and BMD. Compared to the WT mice, ob/ob mice had lower values for bone length, bone volume, and BMC of femur, but higher values for cancellous bone volume of lumbar vertebrae. GTE supplementation in the diets suppressed the rate of bone accumulation during growth, as shown by shorter length, reduced total and cortical bone volume, and cortical thickness, as well as decreased BMC of femur. The same study also showed that neither genotype nor treatment influenced femoral BMD, indicating normal mineralization during growth.
The discrepancy between these two models (Shen et al. [30] and Iwaniec et al. [31]) may be due to different dosage of green tea (~ 4 servings/day in Shen’s study vs. ~ 7–14 servings/day in Iwaniec’ study), different model of obesity (high-fat-diet induced obese model vs. genetically obese, leptin-deficient ob/ob mice model), and different age of animals (18 months old vs. 3 months old) reflecting different stages of bone metabolism (remodeling during aging vs. modeling during growth). Although green tea has been considered as a relatively safe beverage, a rich source of antioxidants, showing no serious side effect for up to 8 servings per day in humans, a high dosage of green tea may become a source of prooxidants that has a detrimental impact on bone matrix.
4. Implication of green tea’s osteo-protective effect in humans
In addition to the animal studies, the findings of our short-term 6-month clinical trial indicated that the consumption of GTP (500 mg per day) by postmenopausal women appeared to be safe, particularly in terms of liver and kidney functions [32]. In that study, 171 postmenopausal women with low bone mass (57.4±6.8 yr, BMI 28.4±5.3 kg/m2) were randomly assigned into 4 treatment groups for 24 weeks: (1) Placebo (500 mg medicinal starch/day), (2) GTP (500 mg GTP/day), (3) Placebo + Tai Chi (placebo plus Tai Chi exercise at 60 min/session, 3 sessions/week), and (4) GTP + Tai Chi (GTP plus Tai Chi exercise). After 24 weeks intervention, 21 participants dropped out (12% attrition rate) due to loss of interest or any other condition unrelated to the current study intervention. The compliance rates for study agents (both placebo and GTP) and TC exercise were 89% and 83%, respectively.
Data show that GTP supplementation provided higher values for serum bone-specific alkaline phosphatase (bone formation biomarker) after 4 weeks (P = 0.03), while Tai Chi exercise provided higher values for BAP after 12 weeks (P = 0.04) and marginally (P = 0.09) after 24 weeks. Neither GTP supplementation nor Tai Chi exercise had any effect on serum TRAP (bone resorption biomarker) levels. BMD could not be justified as an outcome in such a short-term study where changes in BMD were not expected to be detectable. Although the effects of GTP on bone biomarkers are encouraging and promising, a longer-term clinical study in which BMC and BMD are assessed is needed to confirm the bone protective effects of GTP in postmenopausal women.
5. Mechanisms of action
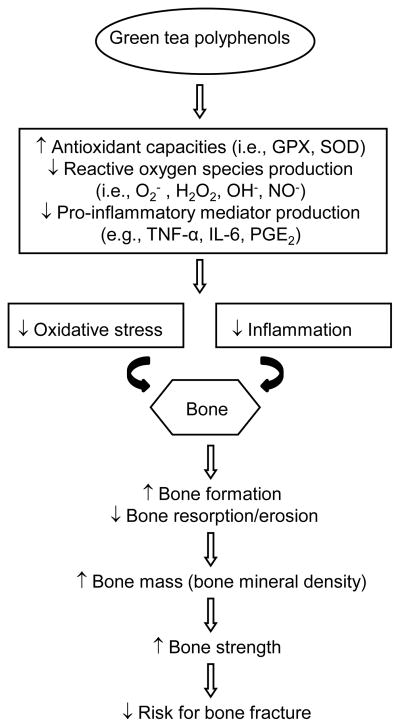
Numerous studies have indicated that excessive oxidative stress is a pivotal pathogenic factor [33] for age-related bone loss in mice [34], rats [16], and the elderly population [35, 36] resulting in increased osteoblast and osteocyte apoptosis, and decreased osteoblast population and bone formation rate [34]. Oxidative stress suppresses osteoblastic differentiation [37, 38] via extracellular signal-regulated kinases (ERK) and ERK-dependent NF-κB signaling pathways [39]. Osteoblasts can produce antioxidants, such as glutathione peroxidase, to protect against reactive oxygen species (ROS) [40], and transforming growth factor-β involved in reduction of bone resorption [41]. ROS are also involved in bone resorption by directly contributing osteoclast-generated superoxide to bone degradation [42], and oxidative stress increases differentiation and function of osteoclasts [43]. A tight association between excessive production of oxidative stress and the pathogenesis of osteoporosis in humans was shown in a variety of bone loss models induced by aging [36], estrogen deficit [35, 36], obesity [44], advanced chronic kidney disease [45], atherosclerosis [44], and diabetes [44, 46].
Similar to the impact of oxidative stress on bone loss, systemic chronic inflammation also results in the development of osteoporosis. Chronic inflammation causes continuous activation of both the innate and adaptive immune systems that disturb the balance between bone formation and resorption, and then triggers inflammatory bone loss [47]. Several inflammatory mediators, such as tumor necrosis factor (TNF)-α, IL-1, IL-6, IL-7, IL-17, prostaglandin E2 produced by macrophages, T cells or fibroblasts, stimulate osteoclast formation and bone resorption by inducing the expression of M-CSF and RANKL. In addition, TNF induces Dickkopf 1, a Wnt antagonist, which blocks bone formation through inhibiting the differentiation of osteoblasts from mesencymal precursors. On the other hand, TNF also induces the expression of sclerostin in osteocytes, a potent down-regulator of bone formation [47]. An epidemiologic study demonstrated a positive association between inflammatory markers and incident non-traumatic fractures in an elderly population [48]. Clinical sequelae of systemic chronic inflammation on bone loss have also been shown in different rheumatic diseases, such as rheumatoid arthritis, systemic lupus erythematosus and spondyloarthritides [47]. Since excessive oxidative stress and chronic inflammation contribute to bone loss, it is important to understand the role of antioxidants and anti-inflammatory agents in dietary supplements or functional foods, such as green tea, in mitigating bone loss during the development of osteoporosis. Green tea extract, a non-oxidized and non-fermented product, is rich in phenolic compounds, such as EGCG (most abundant component), ECG, EC, EGC, and catechins [6], which act as antioxidant and anti-inflammatory agents. Therefore, the beneficial effects of green tea on bone may be mediated, in part, through its antioxidant and anti-inflammatory properties.
The abilities of green tea or its active components to increase indices of bone formation (osteoblastogenesis) have been supported by the following evidences: (1) When osteoblastic MC3T3-E1 cells treated with (+)-catechin at 10−4 to 10−5 mol/L for 48 hours, (+) catechin increased osteoblastic survival and decreased osteoblastic apoptosis through an inhibition of TNF-α and IL-6 production [49]. (2) When antler progenitor cells treated with EGCG at 25 μmol/L for 24 hours, EGCG enhanced proliferation and differentiation of bone cells, as shown an elevation in alkaline phosphatase activity, via Wnt signaling pathway [50]. (3) When osteoblastic MC3T3-E1 cells treated with EGCG at 30 μmol/L for 60 minutes, EGCG favored osteoblastogenesis via suppressing transforming growth factor-β-stimulated heat shock protein 27 induction that mediated via SAPK/JNK pathway [51]. (4) When MC3T3-E1 cells treated with EGCG at 100 μmol/L for 60 minutes, EGCG increased osteoblastogenesis via inhibiting prostaglandin D2-induced heat shock protein 27 induction that mediated through p44/p42 MAPK pathway [52]. (5) When MC3T3-E1 cells treated with EGCG at 10–100 μmol/L for 60 minutes, EGCG stimulated osteoblastogenesis via increasing prostaglandin-F2-induced vascular endothelial growth factor synthesis that involved in SAPK/JNK activation [53, 54]. (6) When SaOS-2 cells treated with EGCG at 1–5 μmol/L for up to 17 days, ECGC promoted formation of mineralized bone nodules including area and number, via suppressing Runt-related transcription factor-2 (Runx2) expression [55, 56]. In addition, similar results in mineralization findings were also observed in D1 cells treated with EGCG at 10 μmol/L for up to 4 weeks [57].
On the other hand, the abilities of green tea and its bioactive components to suppress indices of bone resorption (osteoclastogenesis) have been demonstrated by the following evidences: (1) When osteoclast-like multinucleated cells treated with ECGC at 25–100 μmol/L for 3 days, ECGC increased apoptosis of osteoclasts but without affecting osteoblasts via enhancing single strand DNA damage [58, 60], via inducing the Fenton reaction [58, 59], or via increasing caspase-3 activation [61]. (2) When RAW264.7 cells treated with EGCG at 10–100 μmol/L for 24 hours, EGCG decreased survival of osteoclasts, increased apoptosis of osteoclasts, and decreased osteoclastic differentiation via by decreasing RANKL-induced NF-κB transcriptional and nuclear translocation [62, 64–66]. (3) When bone marrow with primary osteoclasts cells treated with EGCG at 20 μmol/L for 3 days, EGCG suppressed the formation of osteoclasts via inhibiting expression of matrix metalloproteinase-9 [63]. (4) When embryonic mouse calvaria treated with EGCG at 0.1–1 mmol/L for 18 hours, EGCG inhibited bone resorption and prevented osteoclast activation by acting on bone collagen that could well render bone tissue less prone to resorption [67].
6. Summary and future research
Osteoporosis is the result of a metabolic imbalance of faster resorption than formation. To date our animal studies strongly suggest that green tea has a pronounced effect on bone in terms of bone preservation as shown by higher bone mass (BMC and BMD), trabecular bone volume, number, and thickness, and lower trabecular separation through enhancing bone formation and suppressing bone resorption, resulting in greater bone strength. Although these results are mostly obtained from rat studies, we anticipate that the observations of these animal studies will be confirmed in human studies. Our short-term translational study showing GTP favored bone formation at an early intervention stage would extend the findings from animals to the human population. In future human studies, green tea and its active ingredients should be tested long term through evaluating the bioavailability via validated biofluid biomarkers and through assessing the efficacy in terms of bone mass (BMC and BMD), micro-architecture (cancellous and endocortical bones), and bone strength via advanced imaging technology in order to confirm their benefits to osteoporosis.
Acknowledgments
The preparation of this review was supported by NIH/NCCAM grant R21AT003735, Laura W. Bush Institute for Women’s Health, and Winthrop-University Hospital.
Abbreviation
- BMC
bone mineral content
- BMD
bone mineral density
- EC
(−) epicatechin
- ECG
(−) epicatechin gallate
- EGC
(−) epigallocatechin
- EGCG
(−) epigallocatechin gallate
- ER
estrogen receptor
- ERK
extracellular signal-regulated kinases
- LF
low fat
- GTC
green tea catechins
- GTP
green tea polyphenols
- HF
high fat
- IL
interleukin
- LPS
lipopolysaccharide
- M-CSF
macrophage-colony stimulating factor
- NF-kB
receptor activator of nuclear factor-kB
- ORX
orchidectomized
- OVX
ovariectomized
- RANKL
receptor activator of nuclear factor-kB ligand
- ROS
reactive oxygen species
- Runx2
Runt-related transcription factor-2
- SH
sham
- TNF-α
tumor necrosis factor-α
- TRAP
tartrate-resistant acid phosphatase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. JAMA. 2001;285(6):785–95. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- 2.Holroyd C, Cooper C, Dennison E. Epidemiology of osteoporosis. Best Pract Res Clin Endocrinol Metab. 2008;22(5):671–85. doi: 10.1016/j.beem.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Boonen S, Dejaeger E, Vanderschueren D, Venken K, Bogaerts A, Verschueren S, Milisen K. Osteoporosis and osteoporotic fracture occurrence and prevention in the elderly: a geriatric perspective. Best Pract Res Clin Endocrinol Metab. 2008;22(5):765–85. doi: 10.1016/j.beem.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–75. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 5.Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States. Adv Data. 2002:1–19. [PubMed] [Google Scholar]
- 6.Yang CS, Landau JM. Effects of tea consumption on nutrition and health. J Nutr. 2000;130:2409–12. doi: 10.1093/jn/130.10.2409. [DOI] [PubMed] [Google Scholar]
- 7.Shen CL, Yeh JK, Cao JJ, Wang JS. Green tea and bone metabolism. Nutr Res. 2009;29(7):437–56. doi: 10.1016/j.nutres.2009.06.008. Review Erratum in: Nutr Res 2009;29(9):684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nijweide PJ, Burger EH, Feyen JH. Cells of bone: proliferation, differentiation, and hormonal regulation. Physiol Rev. 1986;66(4):855–86. doi: 10.1152/physrev.1986.66.4.855. Review. [DOI] [PubMed] [Google Scholar]
- 9.Ando K, Mori K, Rédini F, Heymann D. RANKL/RANK/OPG: key therapeutic target in bone oncology. Curr Drug Discov Technol. 2008;5(3):263–8. doi: 10.2174/157016308785739857. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuo K, Irie N. Osteoclast-osteoblast communication. Arch Biochem Biophys. 2008;473(2):201–9. doi: 10.1016/j.abb.2008.03.027. Review. [DOI] [PubMed] [Google Scholar]
- 11.Suda T, Takahashi N, Martin TJ. Modulation of osteoclast differentiation. Endocr Rev. 1992;13(1):66–80. doi: 10.1210/edrv-13-1-66. Review. [DOI] [PubMed] [Google Scholar]
- 12.Yavropoulou MP, Yovos JG. Osteoclastogenesis- Current knowledge and future perspectives. J Musculoskelet Neuronal Interact. 2008;8(3):204–16. [PubMed] [Google Scholar]
- 13.Fazzalari NL. Bone remodeling: A review of the bone microenvironment perspective for fragility fracture (osteoporosis) of the hip. Semin Cell Dev Biol. 2008;19(5):467–72. doi: 10.1016/j.semcdb.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Kalu DN, Liu CC, Hardin RR, Hollis BW. The aged rat model of ovarian hormone deficiency bone loss. J Endocrinol. 1989;124:7–16. doi: 10.1210/endo-124-1-7. [DOI] [PubMed] [Google Scholar]
- 15.Kimmel DB. Animal models for in vivo experimentation in osteoporosis research. In: Marcus R, Feldman D, Kelsey J, editors. Osteoporosis. San Diego, CA: Academic Press; 1996. pp. 671–83. [Google Scholar]
- 16.Shen CL, Wang P, Guerrieri J, Yeh J, Wang JS. Protective effect of green tea polyphenols on bone loss in middle-aged female rats. Osteoporosis Int. 2008;19(7):979–90. doi: 10.1007/s00198-007-0527-5. [DOI] [PubMed] [Google Scholar]
- 17.Shen CL, Yeh JK, Stoecker BJ, Chyu MC, Wang JS. Green tea polyphenols mitigate deterioration of bone microarchiteture in middle-aged female rats. Bone. 2009;44(4):684–90. doi: 10.1016/j.bone.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Luo H, Tang L, tang M, Billam M, Huang T, Yu J, Wei Z, Liang Y, Wang K, Zhang ZQ, Zhang L, Wang JS. Phase IIa chemoprevention trial of green tea polyphenols in high-risk individuals of liver cancer: modulation of urinary excretion of green tea polyphenols and 8-hydroxydeoxyguanosine. Carcinogenesis. 2006;27(2):262–8. doi: 10.1093/carcin/bgi147. [DOI] [PubMed] [Google Scholar]
- 19.Wang JS, Luo H, Wang P, Tang L, Yu J, Huang T, et al. Validation of green tea polyphenol biomarkers in a phase II human intervention trial. Food Chem Toxicol. 2008;46(1):232–40. doi: 10.1016/j.fct.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haque AM, Hashimoto M, Katakura M, Tanabe Y, Hara Y, Shido O. Long-term administration of green tea catechins improves spatial cognition learning ability in rats. J Nutr. 2006;136(4):1043–7. doi: 10.1093/jn/136.4.1043. [DOI] [PubMed] [Google Scholar]
- 21.Shao C, Chen L, Lu C, Shen CL, Gao W. A gel-based proteomic analysis of the effects of green tea polyphenols on ovariectomized rats. Nutrition. 2010 Aug 12; doi: 10.1016/j.nut.2010.05.019. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Kuruto-Niwa R, Inoue S, Ogawa S, Muramatsu M, Nozawa R. Effects of tea catechins on the ERE-regulated estrogenic activity. J Agric Food Chem. 2000;48(12):6355–61. doi: 10.1021/jf0008487. [DOI] [PubMed] [Google Scholar]
- 23.Shen CL, Yeh JK, Cao JJ, Tenner T, Wang JS. Green tea polyphenols supplementation improves bone microstructure in orchidectomized middle-aged rats. Proceeding of American Society for Bone and Mineral Research. 2010 Abstract: A10003829. [Google Scholar]
- 24.Blouin S, Libouban H, Moreau MF, Chappard D. Orchidectomy models of osteoporosis. Methods Mol Biol. 2008;455:125–34. doi: 10.1007/978-1-59745-104-8_9. [DOI] [PubMed] [Google Scholar]
- 25.Shen CL, Yeh JK, Cao JJ, Tatum OL, Dagda RY, Wang J-S. Green tea polyphenols mitigate bone loss of female rats in a chronic inflammation-induced bone loss model. J Nutr Biochem. 2009;21:968–74. doi: 10.1016/j.jnutbio.2009.08.002. 2010. [DOI] [PubMed] [Google Scholar]
- 26.Shen CL, Yeh JK, Samathanam C, Cao JJ, Stoecker BJ, Dagda RY, Chyu MC, Dunn DM, Wang JS. Green tea polyphenols attenuate deterioration of bone microarchitecture in female rats with systemic chronic inflammation. Osteoporosis Int. 2010 Mar 20; doi: 10.1007/s00198-010-1209-2. [Epub ahead of print]. PMID: 20306019. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura H, Ukai T, Yoshimura A, Kozuka Y, Yoshioka H, Yoshinaga Y, Abe Y, Hara Y. Green tea catechin inhibits lipopolysaccharide-induced bone resorption in vivo. J Periodontal Res. 2010;45(1):23–30. doi: 10.1111/j.1600-0765.2008.01198.x. [DOI] [PubMed] [Google Scholar]
- 28.Maruyama T, Tomofuji T, Endo Y, Irie K, Azuma T, Ekuni D, Tamaki N, Yamamoto T, Morita M. Supplementation of green tea catechins in dentifrices suppresses gingival oxidative stress and periodontal inflammation. Arch Oral Biol. 2011;56(1):48–53. doi: 10.1016/j.archoralbio.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Fu X, Ma X, Lu H, He W, Wang Z, Zhu S. Associations of fat mass and fat distribution with bone mineral density in pre- and postmenopausal Chinese women. Osteoporos Int. 2010 Mar 20; doi: 10.1007/s00198-010-1210-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Shen CL, Chanjaplammootil S, Yeh JK, Cao JJ, Chyu MC, Dagda RY, Wang JS. Anti-obesity and osteo-protective effect of green tea polyphenols on long-term high-fat-diet-induced obesity in rats. Submitted to EB 2011 annual meeting. [Google Scholar]
- 31.Iwaniec UT, Turner RT, Koo SI, Kaur R, Ho E, Wong CP, Bruno RS. Consumption of green tea extract results in osteopenia in growing male mice. J Nutr. 2009;139(10):1914–9. doi: 10.3945/jn.109.107201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen CL, Chyu MC, Yeh JK, Zhang Y, Pence BC, Felton CK, Dagda RY, Doctolero S, Wang JS. Effect of 24-week green tea polyphenols supplementation and Tai Chi exercise on bone biomarkers in postmenopausal osteopenic women. Proceeding of American Society for Bone and Mineral Research. 2010 Abstract: A10005208. [Google Scholar]
- 33.Banfi G, Iorio EL, Corsi MM. Oxidative stress, free radicals and bone remodeling. Clin Chem Lab Med. 2008;46(11):1550–5. doi: 10.1515/CCLM.2008.302. Review. [DOI] [PubMed] [Google Scholar]
- 34.Manolagas SC. De-fense! De-fense! De-fense: scavenging H2O2 while making cholesterol. Endocrinology. 2008;149(7):3264–6. doi: 10.1210/en.2008-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basu K, Michaëlsson H, Olofsson H, Johansson S, Melhus H. Association between oxidative stress and bone mineral density. Biochem Biophys Res Commun. 2001;288:275–9. doi: 10.1006/bbrc.2001.5747. [DOI] [PubMed] [Google Scholar]
- 36.Manolagas SC, Parfitt AM. What old means to bone. Trends Endocrinol Metab. 2010;21(6):369–74. doi: 10.1016/j.tem.2010.01.010. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mody N, Parhami F, Saraflan TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001;31:509–19. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 38.Fatokun AA, Stone TW, Smith RA. Responses of differentiated MC3T3-E1 osteoblast-like cells to reactive oxygen species. Eur J Pharmacol. 2008;587(1–3):35–41. doi: 10.1016/j.ejphar.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 39.Bai X-C, Lu D, Bai J, Zheng H, Ke ZY, Li XM, et al. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-κB. Biochem Biophys Res Commun. 2004;314(1):197–207. doi: 10.1016/j.bbrc.2003.12.073. [DOI] [PubMed] [Google Scholar]
- 40.Dreher I, Schütze N, Baur A, Hesse K, Schneider D, Köhrle J, et al. Selenoproteins are expressed in fetal human osteoblast-like cells. Biochem Biophys Res Commun. 1998;245:101–7. doi: 10.1006/bbrc.1998.8393. [DOI] [PubMed] [Google Scholar]
- 41.Fuller K, Lean JM, Bayley KE, Wani MR, Chambers TJ. A role for TGF-β in osteoclast differentiation and survival. J Cell Sci. 2000;113:2445–53. doi: 10.1242/jcs.113.13.2445. [DOI] [PubMed] [Google Scholar]
- 42.Sontakke AN, Tare RS. A duality in the roles of reactive oxygen species with respect to bone metabolism. Clin Chim Acta. 2002;318(1–2):145–8. doi: 10.1016/s0009-8981(01)00766-5. [DOI] [PubMed] [Google Scholar]
- 43.Garrett JR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest. 1990;85:632–9. doi: 10.1172/JCI114485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crepaldi G, Maggi S. Epidemiologic link between osteoporosis and cardiovascular disease. J Endocrinol Invest. 2009;32(4 Suppl):2–5. Review. [PubMed] [Google Scholar]
- 45.Johnson DW, Armstrong K, Campbell SB, Mudge DW, Hawley CM, Coombes JS, Prins JB, Isbel NM. Metabolic syndrome in severe chronic kidney disease: Prevalence, predictors, prognostic significance and effects of risk factor modification. Nephrology (Carlton) 2007;12(4):391–8. doi: 10.1111/j.1440-1797.2007.00804.x. [DOI] [PubMed] [Google Scholar]
- 46.Saito M, Marumo K. Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int. 2010;21(2):195–214. doi: 10.1007/s00198-009-1066-z. [DOI] [PubMed] [Google Scholar]
- 47.Schett G, Sieper J. Inflammation and repair mechanisms. Clin Exp Rheumatol. 2009;7(4 Suppl 55):S33–5. [PubMed] [Google Scholar]
- 48.Nakamura K, Saito T, Kobayashi R, Oshiki R, Oyama M, Nishiwaki T, Nashimoto M, Tsuchiya Y. C-reactive protein predicts incident fracture in community-dwelling elderly Japanese women: the Muramatsu study. Osteoporos Int. 2010 Oct 9; doi: 10.1007/s00198-010-1425-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 49.Choi EM, Hwang JK. Effects of (+)-catechin on the function of osteoblastic cells. Biol Pharm Bull. 2003;26(4):523–6. doi: 10.1248/bpb.26.523. [DOI] [PubMed] [Google Scholar]
- 50.Mount JG, Muzylak M, Allen S, Althnaian T, McGonnell IM, Price JS. Evidence that the canonical Wnt signalling pathway regulates deer antler regeneration. Dev Dyn. 2006;235(5):1390–9. doi: 10.1002/dvdy.20742. [DOI] [PubMed] [Google Scholar]
- 51.Hayashi K, Takai S, Matsushima-Nishiwaki R, Hanai Y, Kato K, Tokuda H, et al. (−)-Epigallocatechin gallate reduces transforming growth factor beta-stimulated HSP27 induction through the suppression of stress-activated protein kinase/c-Jun N-terminal kinase in osteoblasts. Life Sci. 2008;82(19–20):1012–7. doi: 10.1016/j.lfs.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 52.Yamauchi J, Takai S, Matsushima-Nishiwaki R, Hanai Y, Doi T, Kato H, et al. (−)-epigallocatechin gallate inhibits prostaglandin D2-stimulated HSP27 induction via suppression of the p44/p42 MAP kinase pathway in osteoblasts. Prostaglandins Leukot Essent Fatty Acids. 2007;77(3–4):173–9. doi: 10.1016/j.plefa.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Tokuda H, Harada A, Hirade K, Matsuno H, Ito H, Kato K, et al. Incadronate amplifies prostaglandin F2α-induced vascular endothelial growth factor synthesis in osteoblasts. Enhancement of MAPK activity. J Biol Chem. 2003;278:18930–7. doi: 10.1074/jbc.M209159200. [DOI] [PubMed] [Google Scholar]
- 54.Tokuda H, Takai S, Matsushima-Nishiwaki R, Akamatsu S, Hanai Y, Hosoi T, Harada A, Ohta T, Kozawa O. (−)-epigallocatechin gallate enhances prostaglandin F2alpha-induced VEGF synthesis via upregulating SAPK/JNK activation in osteoblasts. J Cell Biochem. 2007;100(5):1146–53. doi: 10.1002/jcb.21104. [DOI] [PubMed] [Google Scholar]
- 55.Kamon M, Zhao R, Sakamoto K. Green tea polyphenol (−)-epigallocatechin gallate suppressed the differentiation of murine osteoblastic MC3T3-E1 cells. Cell Biol Int. 2009;34(1):109–16. doi: 10.1042/CBI20090011. [DOI] [PubMed] [Google Scholar]
- 56.Vali B, Rao LG, El-Sohemy A. Epigallocatechin-3-gallate increases the formation of mineralized bone nodules by human osteoblast-like cells. J Nutr Biochem. 2007;18(5):341–7. doi: 10.1016/j.jnutbio.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 57.Chen CH, Ho ML, Chang JK, Hung SH, Wang GJ. Green tea catechin enhances osteogenesis in a bone marrow mesenchymal stem cell line. Osteoporos Int. 2005;16(12):2039–45. doi: 10.1007/s00198-005-1995-0. [DOI] [PubMed] [Google Scholar]
- 58.Nakagawa H, Wachi M, Woo JT, Kato M, Kasai S, Takahashi F, et al. Fenton reaction is primarily involved in a mechanism of (−)-epigallocatechin-3-gallate to induce osteoclastic cell death. Biochem Biophys Res Commun. 2002;292(1):94–101. doi: 10.1006/bbrc.2002.6622. [DOI] [PubMed] [Google Scholar]
- 59.Nakagawa H, Hasumi K, Takami M, Aida-Hyugaji S, Woo JT, Nagai K, Ishikawa T, Wachi M. Identification of two biologically crucial hydroxyl groups of (−)-epigallocatechin gallate in osteoclast culture. Biochem Pharmacol. 2007;73(1):34–43. doi: 10.1016/j.bcp.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 60.Nakagawa H, Hasumi K, Woo JT, Nagai K, Wachi M. Generation of hydrogen peroxide primarily contributes to the induction of Fe(II)-dependent apoptosis in Jurkat cells by (−)-epigallocatechin gallate. Carcinogenesis. 2004;25(9):1567–74. doi: 10.1093/carcin/bgh168. [DOI] [PubMed] [Google Scholar]
- 61.Islam S, Islam N, Kermode T, Johnstone B, Mukhtar H, Moskowitz RW, Goldberg VM, Malemud CJ, Haqqi TM. Involvement of caspase-3 in epigallocatechin-3-gallate-mediated apoptosis of human chondrosarcoma cells. Biochem Biophys Res Commun. 2000;270(3):793–7. doi: 10.1006/bbrc.2000.2536. [DOI] [PubMed] [Google Scholar]
- 62.Lin RW, Chen CH, Wang YH, Ho ML, Hung SH, Chen IS, Wang GJ. (−)-Epigallocatechin gallate inhibition of osteoclastic differentiation via NF-kappaB. Biochem Biophys Res Commun. 2009;379(4):1033–7. doi: 10.1016/j.bbrc.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 63.Yun JH, Pang EK, Kim CS, Yoo YJ, Cho KS, Chai JK, Kim CK, Choi SH. Inhibitory effects of green tea polyphenol (−)-epigallocatechin gallate on the expression of matrix metalloproteinase-9 and on the formation of osteoclasts. J Periodontal Res. 2004;39(5):300–7. doi: 10.1111/j.1600-0765.2004.00743.x. [DOI] [PubMed] [Google Scholar]
- 64.Ahn SC, Kim GY, Kim JH, Baik SW, Han MK, Lee HJ, et al. Epigallocatechin-3-gallate, constituent of green tea, suppresses the LPS-induced phenotypic and functional maturation of murine dendritic cells through inhibition of mitogen-activated protein kinases and NF-kappaB. Biochem Biophys Res Commun. 2004;313(1):148–55. doi: 10.1016/j.bbrc.2003.11.108. [DOI] [PubMed] [Google Scholar]
- 65.Lee JH, Jin H, Shim HE, Kim HN, Ha H, Lee ZH. Epigallocatechin-3-gallate inhibits osteoclastogenesis by down-regulating c-Fos expression and suppressing the nuclear factor-kappaB signal. Mol Pharmacol. 2010;77(1):17–25. doi: 10.1124/mol.109.057877. [DOI] [PubMed] [Google Scholar]
- 66.Ishida I, Kohda C, Yanagawa Y, Miyaoka H, Shimamura T. Epigallocatechin gallate suppresses expression of receptor activator of NF-kappaB ligand (RANKL) in Staphylococcus aureus infection in osteoblast-like NRG cells. J Med Microbiol. 2007;56(Pt 8):1042–6. doi: 10.1099/jmm.0.47029-0. [DOI] [PubMed] [Google Scholar]
- 67.Delaissé JM, Eeckhout Y, Vaes G. Inhibition of bone resorption in culture by (+)-catechin. Biochem Pharmacol. 1986;35(18):3091–4. doi: 10.1016/0006-2952(86)90391-6. [DOI] [PubMed] [Google Scholar]
- 68.Tokuda H, Takai S, Hanai Y, Matsushima-Nishiwaki R, Hosoi T, Harada A, et al. (−)-Epigallocatechin gallate suppresses endothelin-1-induced interleukin-6 synthesis in osteoblasts: inhibition of p44/p42 MAP kinase activation. FEBS Lett. 2007;581(7):1311–6. doi: 10.1016/j.febslet.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 69.Tokuda H, Takai S, Hanai Y, Matsushima-Nishiwaki R, Yamauchi J, Harada A, et al. (−)-Epigallocatechin gallate inhibits basic fibroblast growth factor-stimulated interleukin-6 synthesis in osteoblasts. Horm Metab Res. 2008;40(10):674–8. doi: 10.1055/s-2008-1073164. [DOI] [PubMed] [Google Scholar]
- 70.Takai S, Matsushima-Nishiwaki R, Adachi S, Natsume H, Minamitani C, Mizutani J, et al. (−)-Epigallocatechin gallate reduces platelet-derived growth factor-BB-stimulated interleukin-6 synthesis in osteoblasts: suppression of SAPK/JNK. Mediators Inflamm. 2008:291808. doi: 10.1155/2008/291808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu H, Zhu B, Shimoishi Y, Murata Y, Nakamura Y. (−)-Epigallocatechin-3-gallate induces up-regulation of Th1 and Th2 cytokine genes in Jurkat T cells. Arch Biochem Biophys. 2009;483(1):99–105. doi: 10.1016/j.abb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 72.Matsunaga K, Klein TW, Friedman H, Yamamoto Y. In vitro therapeutic effect of epigallocatechin gallate on nicotine-induced impairment of resistance to Legionella pneumophila infection of established MH-S alveolar macrophages. J Infect Dis. 2002;185(2):229–36. doi: 10.1086/338449. [DOI] [PubMed] [Google Scholar]