Abstract
Efficacy of donor lymphocyte infusion (DLI) for relapsed acute leukemia after allogeneic hematopoietic cell transplantation is limited. We hypothesized that interleukin-2 (IL-2) combined with DLI after chemotherapy might augment graft-versus-leukemia effects. To identify a safe and effective IL-2 regimen, a phase I/II study of DLI plus IL-2 therapy was performed for such patients. After chemotherapy, 17 patients received DLI (1×108 CD3/kg for patients with related donors, and 0.1×108 CD3/kg for those with unrelated donors) and an escalating dose of induction IL-2 (1.0, 2.0, or 3.0×106 IU/m2/day representing levels I (n=7), Ia (n=9), and II (n=1)) for 5 days followed by maintenance (1.0×106 IU/m2/day) for 10 days as a continuous intravenous infusion. Unacceptable IL-2-related toxicities developed in 1 patient at level I, 2 at level Ia and 1 at level II. Grades III–IV acute GVHD developed in 5 patients, and extensive chronic GVHD developed in 8. Eight patients had a complete remission after chemotherapy prior to DLI, and 2 additional patients had a complete remission after DLI plus IL-2 therapy. In conclusion, the maximal tolerated induction dose of IL-2 combined with DLI appears to be 1.0×106 IU/m2/day. IL-2 administration after DLI might increase the incidence of chronic GVHD.
Keywords: Hematopoietic cell transplantation, Acute leukemia, Recurrent malignancy, Donor lymphocyte infusion, Interleukin-2, Treatment
INTRODUCTION
Recurrent acute leukemia after allogeneic hematopoietic cell transplantation (HCT) poses a challenging clinical problem. Relapse tends to occur within 1 year after HCT, and the prognosis is poor [1]. Although remission can be induced in some patients, the median survival for these patients is usually 6 months or less [2]. Efficacy of a second transplantation is limited by refractory disease and high transplant-related mortality, especially in adults [3, 4]. Donor lymphocyte infusion (DLI) has been used to treat recurrent malignancy after HCT. Although the rate of response after DLI is more than 70% in patients with relapsed or persistent chronic myeloid leukemia, it is only 15–29% in those with acute leukemia after HCT [5–8]. Chemotherapy followed by DLI appears to improve response rates and disease-free survival (DFS) in patients with relapsed acute leukemia [9, 10].
Interleukin-2 (IL-2), a 15kd protein secreted by antigen-activated T cells, is critical for the generation and maintenance of an effective immune response. IL-2 stimulates the proliferation of antigen-specific or activated T cells and B cells, induces expansion of NK cells, induces the secretion of other lymphokines such as interferon gamma, granulocyte macrophage colony-stimulating factor and tumor necrosis factor, and augments the cytolytic function of cytotoxic T cells and NK cells [11, 12]. Moreover, lymphocytes incubated with pharmacologic concentrations of IL-2 can become non-specific cytolytic effector cells, termed lymphokine-activated killer cells, which are largely activated NK cells capable of killing fresh and cultured tumor targets in an MHC non-restricted fashion [13].
The failure of DLI in most patients with acute leukemia may reflect an absent or inadequate graft-versus-leukemia (GVL) effect. The potential effector mechanisms of GVL, which may not be the same for all tumors, include cytotoxic and noncytotoxic T cells, NK cells and secreted cytokines [14, 15]. Since these mechanisms may be enhanced by IL-2, it is postulated that IL-2 therapy in combination with DLI might augment a GVL effect, especially after cytoreduction by chemotherapy. The major concern, however, is that IL-2 therapy might also increase the incidence and severity of graft-versus-host disease (GVHD) associated with DLI, since GVL and GVHD might share similar effector mechanisms.
Several studies explored the use of IL-2 therapy after allogeneic HCT [16–18]. The results of a phase I study in children indicated that the maximum tolerated dose (MTD) of IL-2 for induction was 3.0 × 106/m2/day by continuous intravenous infusion for 5 days, which could be administered to prevent leukemic relapse after allogeneic HCT [18]. Results from 2 encouraging reports suggested that the combination of IL-2 with DLI was effective for treatment of diseases refractory to DLI alone [7, 19]. The feasibility and efficacy of an escalating dose of IL-2 regimen combined with a standardized dose of DLI have not yet been determined. Here we report the results of a prospective phase I/II study of chemotherapy followed by donor lymphocyte infusion plus IL-2 therapy for adult patients with relapsed acute leukemia after a T cell-replete allogeneic HCT.
MATERIALS AND METHODS
Patient and Donor Eligibility
Relapse in the current study was defined by morphologic, flow cytometric, cytogenetic, or molecular relapse. Patients with relapsed acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL) after allogeneic HCT were eligible for this study. Other eligibility criteria at study registration included a SWOG performance status ≤ 2, serum creatinine concentration ≤ 2 mg/dl, and serum total bilirubin concentration ≤ 2 mg/dl. Exclusion criteria included the absence of complete remission after HCT, the presence of preexisting congestive heart failure or arrhythmia not manageable with medical therapy, pulmonary dysfunction requiring O2 therapy, pneumonia or severe obstruction (FEV1 <50% predicted value or 50% decline from baseline) or severe restrictive lung disease (TLC < 60% or 50% declined from baseline) not due to leukemia, and the presence of sepsis, aspergillosis or other active infections likely to cause death within 3 months. A prior history of DLI was not an exclusion criterion if other study eligibility criteria were met.
The transplant donor used for DLI had to meet the eligibility criteria for apheresis. HLA-A or B allele mismatch was allowed for unrelated donors. Other mismatching was not allowed. The treatment protocol was approved by the IRB of the Fred Hutchinson Cancer Research Center. Written informed consent was obtained from all study participants in accordance with the Declaration of Helsinki.
Treatment Plan
Chemotherapy
Before DLI, patients received a chemotherapy regimen that was appropriate for type of leukemia, prior treatment and response history. Patients with extramedullary lesions also received local irradiation, and those with ALL or CNS relapse were treated with intrathecal chemotherapy.
Immunosuppressive drugs
Administration of systemic immunosuppressive medications was withdrawn when the chemotherapy induction regimen was started. If no flare of GVHD was observed after 1 week from discontinuation of immunosuppressive medications, steroid treatment was also tapered within 5 weeks as tolerated. Treatment with prednisone at doses less than 0.5 mg/kd/day could be continued, but the protocol required complete withdrawal of all other systemic immunosuppressive medications without GVHD exacerbation for at least 1 week before DLI. If GVHD flared, patients were treated with high-dose prednisone (1–2 mg/kg/day), and if the response was inadequate, other systemic immunosuppressive medications were added.
DLI
Patients could not have active grades II–IV acute GVHD or extensive chronic GVHD at the time of DLI. Patients were treated with a single DLI between 27 to 180 days after chemotherapy regardless of remission status. The cell dose was standardized at 1.0 × 108 CD3+ cells/kg for patients with related donors and 0.1 × 108 CD3+ cells/kg for those with unrelated donors.
IL-2 administration
Recombinant IL-2 (Aldesleukin, Chiron, CA) was mixed in 500 ml 5% dextrose in water containing 0.1% human serum albumin and administered by 24-hour continuous intravenous infusion (CIV) daily for 5 days in the induction phase, using an ambulatory infusion pump starting immediately after DLI or on the following day. The induction phase was followed by maintenance treatment with IL-2 at 1.0 × 106 IU/m2/day by CIV daily for 10 days beginning on day 7. The original protocol defined escalating dose levels of IL-2 at 1.0 (level I) and 3.0 (level II) × 106 IU/m2/day for 5 days. After the unexpected death of the first patient treated at dose level II, the protocol was amended to treat subsequent patients at an intermediate induction dose of IL-2 (2.0 × 106 IU/m2/day; level Ia). Patients received acetaminophen 15mg/kg and diphenhydramine 25 to 50 mg orally before daily doses of IL-2 during induction. Prophylactic ciprofloxacin 500 mg orally was given throughout the entire course of IL-2 therapy. Toxicity during the administration of IL-2 was graded according to WHO criteria. Treatment with IL-2 was discontinued when grade 3 toxicity occurred in any organ or when grade 2 neurologic or cardiac toxicity occurred. If toxicity returned to baseline or grade 1 severity within 48 hours, administration of IL-2 was resumed at 50% of the previous dose. Any doses missed due to toxicity were not administered, and total duration of therapy was not extended beyond 19 days.
Statistical Considerations
The primary objective of this study was to test the hypothesis that an IL-2 regimen similar to our previous children’s study as prophylaxis for relapse after allogeneic HCT [18] was also tolerable when given to adult patients who have received chemotherapy and DLI for treatment of recurrent acute leukemia. Study toxicity was defined as any of the following: (1) death related to acute or chronic GVHD, (2) development of acute or chronic GVHD with inability to decrease the dose of prednisone to less than 1mg/kg/day within 1 month after initiating treatment for GVHD, or (3) life threatening or fatal organ toxicity attributable to IL-2. An excess study toxicity rate was defined as any incidence where the lower limit of the 80% one-sided confidence interval exceeded 25%. Acute GVHD was graded according to consensus criteria [20], and chronic GVHD was classified by traditional criteria [21]. IL-2 may produce side effects of skin rash, diarrhea, and cholestasis, which are similar clinically and pathologically to GVHD, but these side effects were expected to resolve soon after discontinuation of treatment with IL-2 [18, 22]. Therefore, the diagnosis of GVHD was based on clinical characteristics such as persistence or worsening of signs or symptoms despite cessation of IL-2 therapy.
The secondary objective of this study was to examine the potential efficacy of DLI plus IL-2 at the highest acceptable dose of IL-2. However, all patients with all dose levels were analyzed together, because the total number of patients within each stratum was too small for meaningful analysis. DFS was utilized for this purpose. Based on the prespecified study design, patients were analyzed separately according to diagnosis and remission status at time of DLI: patients with AML in complete remission (CR) at the time of DLI (group 1), and those with AML not in CR or ALL in any stage at the time of DLI (group 2). DFS was calculated according to the Kaplan–Meier method, beginning from the date of DLI in patients with disease in remission at the time of DLI or from the date of remission after DLI in patients with disease not in remission at the time of DLI, ending at the date of relapse or death from any cause, and censoring at the end of follow-up for patients who survived without relapse. Patients who never achieved complete remission were considered to have a relapse event at the date of DLI.
RESULTS
Patient Characteristics
Between June 1999 and February 2005, a total of 17 patients (median age 33 years, range, 19–61) were enrolled in this study. Patient characteristics are summarized in Table 1. The underlying disease was AML in 11 (65%) patients and ALL in 6 (35%). Two (12%) patients had biphenotypic AML, and 3 AML cases (18%) had emerged from myelodysplastic syndrome. The ALL in 2 patients (12%) had a Philadelphia chromosome. Sixteen patients patients (94%) had HCT with high-intensity conditioning, and 1 patient (6%) had HCT with reduced-intensity conditioning (case #17). Six (35%) (case #1, 2, 3, 7, 8 and 15) had extramedullary involvement at relapse. Four (24%) received bone marrow grafts, and 13 (76%) received growth-factor mobilized blood cell grafts. Donors were HLA-identical relatives for 13 (76%) patients, HLA-identical unrelated volunteers for 3 (18%) and an HLA-C allele-mismatched unrelated volunteer for 1 (6%) (case # 3). Two (12%) patients had received DLI previously (case # 7 and 14). All patients received chemotherapy before DLI plus IL-2 therapy. The median time from HCT to chemotherapy was 11.8 months (range, 3.2–121.3). The median time from chemotherapy to DLI was 61 days (range, 36–91). Chemotherapies for each patient are summarized in Table 1. All patients had systemic immunosuppression discontinued at the time of chemotherapy. The shortest interval between discontinuation of immunosuppression and the first dose of IL-2 was 36 days.
Table 1.
Patient Characteristics
| Study Case # | Age at DLI /Sex | Donor | HCT Graft | HCT to Chemotherapy (Months) | Chemotherapy to DLI (Days) | Chemotherapy | Remission at DLI |
|---|---|---|---|---|---|---|---|
| Acute myeloid leukemia | |||||||
| 2 | 24 M | Sib | PBSC | 8.4 | 68 | HCVAD, HDAC/MTX | N (Extramedullary) |
| 3 | 56 M | URD | BM | 6.4 | 61 | Mylotarg | N (Extramedullary) |
| 5 | 61 F | Sib | BM | 121.3 | 40 | IDR + AraC | Y |
| 17 | 40 F | Sib | PBSC | 16.8 | 53 | IDR + AraC | N (BM FCM <5%) |
| 7 | 38 M | Sib | BM | 63.8 | 50 | Mylotarg | N (Cytogenetic) |
| 8 | 20 M | Sib | PBSC | 5.8 | 75 | HDAC + MIT | N (Extramedullary) |
| 10 | 60 F | Sib | PBSC | 25.9 | 68 | IDR + AraC | Y |
| 11 | 33 M | Sib | PBSC | 6.0 | 50 | IDR + AraC + ETP | Y |
| 12 | 22 M | Sib | PBSC | 7.5 | 54 | MIT + ETP | Y |
| 14 | 35 F | Sib | PBSC | 17.9 | 77 | MIT + ETP | Y |
| 15 | 21 F | Sib | PBSC | 3.2 | 36 | FLAG + IDR | N (BM FCM 5%) |
| Acute lymphoblastic leukemia | |||||||
| 1* | 24 F | Sib | BM | 13.4 | 63 | HDAC + ETP | N (BM FCM 0.1%) |
| 4 | 34 M | URD | PBSC | 8.7 | 62 | HCVAD | Y |
| 16 | 19 F | Sib | PBSC | 18.5 | 83 | VCR + DNR + DEX + PEG-ASP | Y |
| 9* | 21 F | URD | PBSC | 11.9 | 91 | VCR + DNR + PSL, FLAG | N (BM FCM 0.1%) |
| 13 | 29 M | URD | PBSC | 8.4 | 52 | VCR + AraC + MTX | Y |
| 6 | 50 F | Sib | PBSC | 11.8 | 50 | VCR + DNR + PSL + L-ASP | N (Cytogenetic) |
HCT indicates hematopoietic cell transplantation; Sib, sibling donor; URD, unrelated donor; PBSC, peripheral blood stem cell; BM, bone marrow; HCVAD, fractionated cyclophosphamide + vincristine + dexamethasone + doxorubicin; HDAC, high-dose cytarabine; IDR, idarubicin; AraC, cytarabine; FCM, flow cytometry; MIT, mitoxantrone; ETP, etoposide; FLAG, fludarabine + cytarabine + granulocyte colony-stimulating factor; DNR, daunorubicin; PSL, prednisone; VCR, vincristine; L-ASP, L-asparaginase; DEX, dexamethasone; PEG-ASP, polyethylene glycosylated asparaginase.
Philadelphia chromosome-positive acute lymphoblastic leukemia
Although 16 (94%) patients had a prior history of grades II–IV acute GVHD or extensive chronic GVHD, no patients had active GVHD at the time of DLI. Two patients (case # 2 and 3) were receiving low-dose prednisone at the time of DLI, and the remaining 15 patients were not receiving any immunosuppressive medications at the time of DLI. At the time of DLI, 8 patients (47%) had CR and 9 (53%) had hematological, cytogenetic, flow cytometric or molecular evidence of residual malignancy. Chimerism tests showed 95% to 100% donor-derived T cells and myeloid cells in blood samples from all patients before DLI.
Toxicity of IL-2
All 17 patients could be evaluated for toxicity. The toxicities of IL-2 therapy are summarized in Table 2. Nausea, vomiting, diarrhea, body weight gain, skin rash and cardiovascular toxicities were frequent but reversible. The incidence of diarrhea and rash increased in a dose-dependent fashion. Overall, grades 3–4 toxicities were observed in 1 (14%) patient at level I, 2 (22%) at level Ia, and 1 (100%) at level II. Administration of IL-2 was discontinued in 2 (22%) patients at level Ia due to the onset of grade III acute GVHD (case # 13) and liver toxicity (case # 15), respectively, and in 1 (100%) patient at level II due to capillary leak syndrome (case # 6). One patient died with capillary leak syndrome attributable to IL-2 (case # 6). Study toxicity directly or indirectly related to IL-2 was observed in 1 (14%) patient at level I (GVHD), 2 (22%) at level Ia (GVHD and IL-2 related), and 1(100%) at level II (IL-2 related) (Table 3).
Table 2.
IL-2 Infusion Related Toxicity According to Dose Level
| Toxicity | IL-2 Induction Dose Level |
|||||
|---|---|---|---|---|---|---|
| I (N = 7) |
Ia (N = 9) |
II (N = 1) |
||||
| No. | % | No. | % | No. | % | |
| Nausea / vomiting | ||||||
| All grades | 4 | (57) | 6 | (67) | 1 | (100) |
| Grades 3 – 4 | 0 | (0) | 0 | (0) | 0 | (0) |
| Diarrhea | ||||||
| All grades | 2 | (29) | 4 | (44) | 0 | (0) |
| Grades 3 – 4 | 1 | (14) | 1 | (11) | 0 | (0) |
| Body weight gain | ||||||
| All grades | 6 | (86) | 7 | (78) | 1 | (100) |
| Grades 3 – 4 | 0 | (0) | 0 | (0) | 1 | (100) |
| Kidney | ||||||
| All grades | 2 | (29) | 1 | (11) | 1 | (100) |
| Grades 3 – 4 | 0 | (0) | 0 | (0) | 1 | (100) |
| Skin | ||||||
| All grades | 2 | (29) | 6 | (67) | 1 | (100) |
| Grades 3 – 4 | 0 | (0) | 1 | (11) | 0 | (0) |
| Liver | ||||||
| All grades | 1 | (14) | 1 | (11) | 1 | (100) |
| Grades 3 – 4 | 0 | (0) | 1 | (11) | 1 | (100) |
| Thrombocytopenia | ||||||
| All grades | 1 | (14) | 1 | (11) | 1 | (100) |
| Grades 3 – 4 | 0 | (0) | 0 | (0) | 1 | (100) |
| Lung | ||||||
| All grades | 0 | (0) | 0 | (0) | 1 | (100) |
| Grades 3 – 4 | 0 | (0) | 0 | (0) | 1 | (100) |
| Cardiovascular (including hypotension) | ||||||
| All grades | 3 | (43) | 3 | (33) | 1 | (100) |
| Grades 3 – 4 | 0 | (0) | 0 | (0) | 1 | (100) |
| Neurological | ||||||
| All grades | 1 | (14) | 0 | (0) | 1 | (100) |
| Grades 3 – 4 | 0 | (0) | 0 | (0) | 0 | (0) |
| Overall grades 3 – 4 toxicities | 1 | (14) | 2 | (22) | 1 | (100) |
Table 3.
Outcomes after DLI plus IL-2 Therapy
| Study Case # | IL-2 Dose Level | GVHD after DLI |
Study Toxicity* | Remission after DLI | Remission Duration after DLI (Months) | |
|---|---|---|---|---|---|---|
| Acute | Chronic | |||||
| Acute myeloid leukemia | ||||||
| 2 | I | 0 | NA | N | N | – |
| 3 | I | III | Extensive | N | Y | 33 |
| 5 | I | III | Extensive | N | Y† | 35 |
| 17 | I | 0 | Extensive | N | N | – |
| 7 | Ia | 0 | NA | N | N | – |
| 8 | Ia | 0 | 0 | N | N | – |
| 10 | Ia | II | Extensive | N | Y† | 9 |
| 11 | Ia | 0 | Extensive | N | Y† | 5 |
| 12 | Ia | IV | Extensive | Y (GVHD) | Y† | 47 |
| 14 | Ia | 0 | 0 | N | Y† | 6 |
| 15 | Ia | 0 | 0 | N | Y | 3 |
| Acute lymphoblastic leukemia | ||||||
| 1 | I | 0 | 0 | N | N | – |
| 4 | I | III | Extensive | Y (GVHD) | Y† | 8 |
| 16 | I | 0 | 0 | N | Y† | 5 |
| 9 | Ia | 0 | Limited | N | N | – |
| 13 | Ia | III | Extensive | Y (IL2) | Y† | 12 |
| 6 | II | 0 | NA | Y (IL-2) | N | – |
GVHD indicates graft-versus-host disease; DLI, donor lymphocyte infusion; N, no; Y, yes; NA, not assessable.
Death related to acute or chronic GVHD, development of acute or chronic GVHD with inability to decrease the dose of prednisone to less than 1mg/kg/day within 1 month after initiating treatment for GVHD, or life threatening or fatal organ toxicity attributable to IL-2.
Also remission before DLI following chemotherapy.
Graft-versus-host Disease
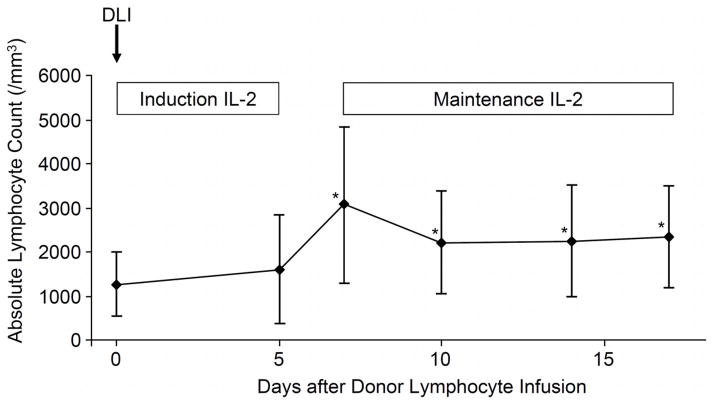
All patients were evaluated for acute GVHD (Table 3). Six of 17 (35%) patients developed grades II–IV acute GVHD after the IL-2 therapy, with a median onset at 55 days (range, 8–74) after DLI. One of the 6 patients (case # 13) developed grade III acute GVHD during IL-2 induction therapy beginning 8 days after DLI. This patient did not receive IL-2 maintenance therapy. The 5 patients who developed grades III–IV acute GVHD were all treated with high-dose prednisone with or without other immunosuppressive medications such as a calcineurin inhibitor and mycophenolate mofetil. In 2 of the 5 patients (case # 4 and 12), the dose of prednisone could not be decreased to less than 1mg/kg/day within 1 month. The incidence rate of grades III–IV acute GVHD was significantly higher in patients with unrelated donors than in those with related donors (3 of 4 patients vs. 2 of 13 patients, P = 0.022). Absolute lymphocyte counts at and after DLI are shown in Figure 2. Nine of 17 patients had lymphopenia (absolute lymphocyte count less than 1000/mm3) at the time of DLI. Grades III–IV acute GVHD after DLI was more prevalent in patients with lymphopenia than in those with higher lymphocyte counts (5 of 9 patients vs. 0 of 8 patients, P = 0.012). Fourteen patients could be evaluated for chronic GVHD. Nine (64%) of the 14 developed chronic GVHD, and 8 (89%) of the 9 had extensive chronic GVHD. The incidence rate of chronic GVHD was not statistically different between patients with related donors and those with unrelated donors (5 of 10 patients vs. 3 of 4 patients, P = 0.39). We found no statistically significant difference in the incidence of acute and chronic GVHD according to the dose level of IL-2.
Figure 2.
Absolute lymphocyte counts at and after DLI+IL-2 therapy. The * indicates statistically significant differences from values before DLI+IL-2, using a paired Mann-Whitney U test.
Patient Outcome
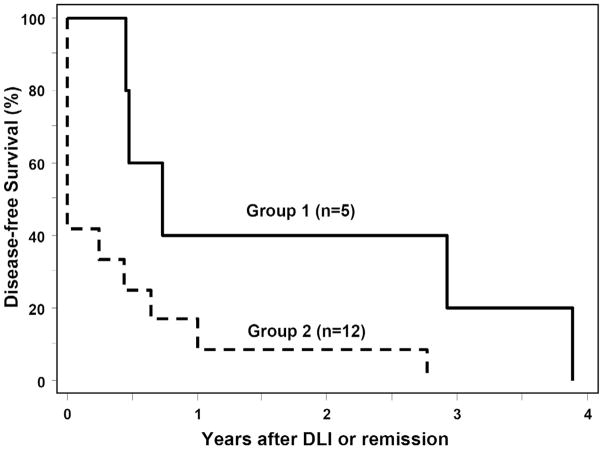
All 8 patients in CR at DLI remained in CR after DLI plus IL-2 therapy. Two of 9 patients (case # 3 and 15, both with AML) not in CR at DLI had a CR after DLI plus IL-2 therapy. Overall, 10 of the 17 patients had a CR after chemotherapy plus DLI plus IL-2 therapy. Remission duration exceeded 1 year in 3 of 11 patients with AML (range, 12–47 months), but all 10 patients with remission had subsequent relapse. The median disease-free survival was 8 months (range, 3–47) after DLI or the day of CR after DLI. Four patients received a second allogeneic HCT thereafter. To date, 1 patient (case # 14) is alive at 5.4 years after chemotherapy plus DLI plus IL-2 therapy after a second allogeneic HCT for recurrent malignancy at 11 months after DLI plus IL-2 therapy. The other 16 patients died at a median of 11 months (range, 0.8–74) after DLI plus IL-2 therapy. The cause of death was leukemia except in the 1 patient who died with capillary leak syndrome. As shown in Figure 1, DFS at 1 year was 40% (95% confidence interval, 5.2–75%) for patients with AML in remission at DLI (group 1) and 8% (95% confidence interval, 0.5–31%) for those with AML not in CR or ALL in any stage at DLI (group 2).
Figure 1.
Disease-free survival for patients with AML in complete remission at DLI (group 1) and for those with AML not in remission or ALL in any stage at DLI (group 2).
Protocol Amendments and termination of the study
The protocol was amended twice during the study period. First, the protocol was amended to treat subsequent patients at an intermediate dose of IL-2 (dose level Ia), due to an unexpected death in the first patient treated at dose level II. Second, while the toxicities observed with level Ia did not meet the stopping rule according to the original protocol definition, 2 of the 7 patients treated in this group developed clinically significant GVHD. Based on careful review and reflection, the investigators decided to amend the protocol to treat 20 additional patients at dose level I. The next 2 patients (case # 14 and 15), however, received IL-2 at dose level Ia, in violation of the protocol. The study was closed 3 years later because of slow patient accrual.
DISCUSSION
Although combination of IL-2 therapy with DLI is a potentially effective approach for treatment of relapsed leukemia after allogeneic HCT, only 2 prior studies have reported the results of this type of treatment [7, 19]. Both studies reported encouraging results after IL-2 administration in patients with recurrent malignancy that had not responded after DLI, but only 4 and 2 patients in the two studies, respectively, had acute leukemia. In addition, the safety and efficacy of this treatment could not be fully evaluated due to heterogeneity in DLI dose and schedule, the presence or absence of prior chemotherapy, dose and schedule of IL-2, and the use of T cell-depleted grafts. To the best of our knowledge, the current study is the first prospective phase I/II clinical trial of IL-2 plus DLI designed to treat a population that included only patients with AML or ALL that had relapsed after T cell-replete HCT. All patients were treated with chemotherapy followed by a single standardized dose of DLI plus 15 days of IL-2.
In our study, IL-2 therapy was associated with dose-dependent toxicities, consistent with previous reports of studies using IL-2 in various settings [17, 18, 22]. Although most of the toxicities were reversible, intolerable toxicities included severe GVHD and severe capillary leak syndrome. While stopping rules for excessive toxicity defined in the original protocol were not met before the study was closed, we observed study toxicities in 2 of 9 patients treated at dose level Ia (2.0 × 106 IU/m2/day) and a death associated with IL-2 administration at dose level II (3.0 × 106 IU/m2/day). From these results, we conclude that the MTD of IL-2 induction therapy by continuous infusion after DLI appears to be dose level I (1.0 × 106 IU/m2/day).. This MTD dose is much lower than IL-2 dose of 6–18 × 106 IU/day for 3 days by subcutaneous injection combined with DLI reported by others [7, 19], but comparison is difficult because half-life of IL-2 in plasma is as short as 2.4 hours after subcutaneous administration [23].
The effects of IL-2 depend on the dose, the route of administration, and the clinical setting. For example, subcutaneous administration of low dose IL-2 (0.6 – 1.0 × 106 IU/m2/day) has been found to increase the number of CD4+CD25+ FOXP3+ T regulatory cells [24] and may ameliorate GVHD in the setting of steroid-refractory chronic GVHD [25]. In contrast, higher IL-2 dose regimens have been found to increase alloreactivity, promote GVL responses [7, 19], and may also increase the severity of GVHD when given after DLI by continuous infusion at doses between 1.0 – 3.0 × 106 IU/m2/day, as observed in our study.
Our results can be interpreted in comparison with previous studies of chemotherapy followed by DLI without IL-2 (Table 4) [8–10]. First, the incidence rates of grades II–IV and III–IV acute GVHD in our study are similar to those reported after DLI without IL-2. Second, development of early grade III acute GVHD (day 8 after DLI) was observed in 1 patient (case # 13) in our study, but no such cases were observed in previous DLI studies without IL-2. Third, the 50% incidence of extensive chronic GVHD in our study was somewhat higher than the 14–32% incidence reported in previous studies. These findings suggest that our IL-2 regimen might induce early acute GVHD and might increase the incidence of chronic GVHD when combined with DLI after T cell-replete HCT.
Table 4.
Summary of Previous Reports of DLI without IL-2 Followed by Chemotherapy for Relapsed Acute Leukemia and Current Study
| Parameters | Study |
|||
|---|---|---|---|---|
| Collins et al.[8] | Schmidt et al.[10] | Levine et al.[9] | Current | |
| Design | Retrospective | Retrospective | Prospective | Prospective |
| Disease, no. of patients | ||||
| Acute myeloid leukemia | 7* | 124* | 50* | 11 |
| Acute lymphoblastic leukemia | 3* | 0 | 0 | 6 |
| DLI dose, ×108/kg CD3 | 2.2 (mean)† | 0.1 (median)‡ | 1 | 1 |
| Grades II–IV acute GVHD | 46%† | 34%‡ | 45%§ | 35% |
| Grades III–IV acute GVHD | 22%† | NA | 28%§ | 29% |
| Chronic GVHD | 61%† | 46%‡ | 36%§ | 64% |
| Chronic extensive GVHD | 32%† | 26%‡ | 14%§ | 50% |
| 1 year DFS after DLI | 54% in AML 33% in ALL |
NA | 34% among patients in CR at DLI§ | 40% in AML CR at DLI, 8.3% in others |
DLI indicates donor lymphocyte infusion; GVHD, graft-versus-host disease; NA, not assessable; DFS, disease-free survival; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; CR, complete remission.
Data abstracted from the entire cohort.
Data represent the entire study cohort of 140 patients treated for various diagnoses and irrespective of the presence of chemotherapy before DLI.
Data represent the entire study cohort of 171 AML patients. Of the 171 patients, 124 received chemotherapy before DLI and information was missing in 7 patients.
Data represent the entire study cohort of 65 patients including 50 with AML and 15 with other diagnoses.
Miller et al. [26] found that lymphodepletion enhanced immune responses, causing severe GVHD after DLI, possibly by increasing the concentrations of cytokines such as IL-15 and IL-7 [27]. In our study, the risk of grades III–IV acute GVHD was increased in patients who had lymphopenia at the time of DLI plus IL-2. Recent studies have shown that high-dose melphalan, fludarabine, and cyclophosphamide all cause lymphdepletion with increased concentrations of IL-15 and IL-7 [28–30]. Although these specific drugs were infrequently used in our patients, many types of chemotherapy before DLI might have similar effects.
Durable CD8+ T cell memory responses have a strict requirement for IL-2, but recent studies highlight a biological role of IL-2 in facilitating immune tolerance through generation of CD4+CD25+FOXP3+ T regulatory cells [31]. In fact, several studies have shown that IL-2 administration increases the number of T regulatory cells in cancer patients [32, 33] and after CD4+ DLI [24]. Therefore, IL-2 given after DLI for treatment of recurrent acute leukemia after HCT may not represent the best approach for augmenting GVL effects associated with DLI in the future. Other Γc-signaling cytokines such as IL-7, IL-9, IL-15 and IL-21 may offer alternative choices for augmenting GVL, since most of these cytokines have positive effects on the maintenance of CD8+ T cell survival and function in vivo but do not affect growth of T regulatory cells [34–36].
This study was closed due to slow patient accrual, and the small sample size limits the ability to draw definitive conclusions regarding the efficacy of DLI plus IL-2. Nonetheless, the 1-year DFS rates of 40% among patients with AML in remission at DLI were not inferior to the 34–54% DFS rates after chemotherapy followed by DLI without IL-2 in previous studies (Table 4) [8]. The remission duration exceeding 1 year in 3 patients treated for recurrent AML was notable. These results support the previous finding by Nadal et al.[19] that DLI plus IL-2 treatment was more effective for patients with AML than for those with other diseases. Previous studies using continuous intravenous IL-2 at doses as low as 0.2–0.6 IU/m2/day after allogeneic HCT showed that IL-2 promoted expansion of NK cells but did not increase the numbers of CD3+ T cells [17, 37]. Recent studies have shown that mismatches of killer-immunoglobulin-like-receptors (KIR) were associated with reduced rates of leukemia relapse through alloreactive NK cells, particularly for patients with AML [15, 38]. By augmenting NK alloreactivity, IL-2 may augment GVL effects, particularly in patients with AML. In conclusion, MTD of continuous intravenous IL-2 induction therapy combined with DLI appears to be 1.0 × 106 IU/m2/day. IL-2 after DLI might increase the incidence of chronic GVHD, although the anti-leukemia efficacy of this treatment remains to be determined.
Acknowledgments
We thank the staff of the Long-Term Follow-Up (LTFU) Clinical and Research program for their assistance with data collection and continued efforts to update our LTFU records. We also express our deep gratitude to our patients for their participation in clinical trials and the referring physicians and our medical staff for their collaborative efforts in the excellent care provided to our patients and families. This research was supported by grants CA18029, CA78902 and CA15074 from the National cancer Institute, and HL36444 from the National Heart, Lung, Blood Institute. Y.I. is a recipient of a fellowship grant from the Banyu Life Science Foundation International.
Footnotes
Financial disclosure: The IL-2 used in this study was provided free of charge by Chiron, California. The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mielcarek M, Storer BE, Flowers ME, Storb R, Sandmaier BM, Martin PJ. Outcomes among patients with recurrent high-risk hematologic malignancies after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2007;13:1160–1168. doi: 10.1016/j.bbmt.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Mortimer J, Blinder MA, Schulman S, et al. Relapse of acute leukemia after marrow transplantation: natural history and results of subsequent therapy. J Clin Oncol. 1989;7:50–57. doi: 10.1200/JCO.1989.7.1.50. [DOI] [PubMed] [Google Scholar]
- 3.Radich JP, Sanders JE, Buckner CD, et al. Second allogeneic marrow transplantation for patients with recurrent leukemia after initial transplant with total-body irradiation-containing regimens. J Clin Oncol. 1993;11:304–313. doi: 10.1200/JCO.1993.11.2.304. [DOI] [PubMed] [Google Scholar]
- 4.Bosi A, Laszlo D, Labopin M, et al. Second allogeneic bone marrow transplantation in acute leukemia: results of a survey by the European Cooperative Group for Blood and Marrow Transplantation. J Clin Oncol. 2001;19:3675–3684. doi: 10.1200/JCO.2001.19.16.3675. [DOI] [PubMed] [Google Scholar]
- 5.Bar BM, Schattenberg A, Mensink EJ, et al. Donor leukocyte infusions for chronic myeloid leukemia relapsed after allogeneic bone marrow transplantation. J Clin Oncol. 1993;11:513–519. doi: 10.1200/JCO.1993.11.3.513. [DOI] [PubMed] [Google Scholar]
- 6.Kolb HJ, Schattenberg A, Goldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86:2041–2050. [PubMed] [Google Scholar]
- 7.Slavin S, Naparstek E, Nagler A, et al. Allogeneic cell therapy with donor peripheral blood cells and recombinant human interleukin-2 to treat leukemia relapse after allogeneic bone marrow transplantation. Blood. 1996;87:2195–2204. [PubMed] [Google Scholar]
- 8.Collins RH, Jr, Shpilberg O, Drobyski WR, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997;15:433–444. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]
- 9.Levine JE, Braun T, Penza SL, et al. Prospective trial of chemotherapy and donor leukocyte infusions for relapse of advanced myeloid malignancies after allogeneic stem-cell transplantation. J Clin Oncol. 2002;20:405–412. doi: 10.1200/JCO.2002.20.2.405. [DOI] [PubMed] [Google Scholar]
- 10.Schmid C, Labopin M, Nagler A, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol. 2007;25:4938–4945. doi: 10.1200/JCO.2007.11.6053. [DOI] [PubMed] [Google Scholar]
- 11.Heslop HE, Gottlieb DJ, Reittie JE, et al. Spontaneous and interleukin 2 induced secretion of tumour necrosis factor and gamma interferon following autologous marrow transplantation or chemotherapy. Br J Haematol. 1989;72:122–126. doi: 10.1111/j.1365-2141.1989.tb07671.x. [DOI] [PubMed] [Google Scholar]
- 12.Caligiuri MA, Zmuidzinas A, Manley TJ, Levine H, Smith KA, Ritz J. Functional consequences of interleukin 2 receptor expression on resting human lymphocytes. Identification of a novel natural killer cell subset with high affinity receptors. J Exp Med. 1990;171:1509–1526. doi: 10.1084/jem.171.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ortaldo JR, Mason A, Overton R. Lymphokine-activated killer cells. Analysis of progenitors and effectors. J Exp Med. 1986;164:1193–1205. doi: 10.1084/jem.164.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 15.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 16.Przepiorka D, Ippoliti C, Koberda J, et al. Interleukin-2 for prevention of graft-versus-host disease after haploidentical marrow transplantation. Transplantation. 1994;58:858–860. [PubMed] [Google Scholar]
- 17.Soiffer RJ, Murray C, Gonin R, Ritz J. Effect of low-dose interleukin-2 on disease relapse after T-cell-depleted allogeneic bone marrow transplantation. Blood. 1994;84:964–971. [PubMed] [Google Scholar]
- 18.Robinson N, Sanders JE, Benyunes MC, et al. Phase I trial of interleukin-2 after unmodified HLA-matched sibling bone marrow transplantation for children with acute leukemia. Blood. 1996;87:1249–1254. [PubMed] [Google Scholar]
- 19.Nadal E, Fowler A, Kanfer E, Apperley J, Goldman J, Dazzi F. Adjuvant interleukin-2 therapy for patients refractory to donor lymphocyte infusions. Exp Hematol. 2004;32:218–223. doi: 10.1016/j.exphem.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 21.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 22.Higuchi CM, Thompson JA, Petersen FB, Buckner CD, Fefer A. Toxicity and immunomodulatory effects of interleukin-2 after autologous bone marrow transplantation for hematologic malignancies. Blood. 1991;77:2561–2568. [PubMed] [Google Scholar]
- 23.Shaker MA, Younes HM. Interleukin-2: evaluation of routes of administration and current delivery systems in cancer therapy. J Pharm Sci. 2009;98:2268–2298. doi: 10.1002/jps.21596. [DOI] [PubMed] [Google Scholar]
- 24.Zorn E, Mohseni M, Kim H, et al. Combined CD4+ donor lymphocyte infusion and low-dose recombinant IL-2 expand FOXP3+ regulatory T cells following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:382–388. doi: 10.1016/j.bbmt.2008.12.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koreth J, Stevenson KE, Kim H, et al. Feasibility, safety, efficacy and immunologic impact of daily untra-low-dose interleukin-2 for steroid-refractory chronic graft-versus-host disease: A phase I Study. Biol Blood Marrow Transplant. 2010;16 (Suppl):S169. [Google Scholar]
- 26.Miller JS, Weisdorf DJ, Burns LJ, et al. Lymphodepletion followed by donor lymphocyte infusion (DLI) causes significantly more acute graft-versus-host disease than DLI alone. Blood. 2007;110:2761–2763. doi: 10.1182/blood-2007-05-090340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucas PJ, Kim SJ, Mackall CL, et al. Dysregulation of IL-15-mediated T-cell homeostasis in TGF-beta dominant-negative receptor transgenic mice. Blood. 2006;108:2789–2795. doi: 10.1182/blood-2006-05-025676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 29.Wallen H, Thompson JA, Reilly JZ, Rodmyre RM, Cao J, Yee C. Fludarabine modulates immune response and extends in vivo survival of adoptively transferred CD8 T cells in patients with metastatic melanoma. PLoS One. 2009;4:e4749. doi: 10.1371/journal.pone.0004749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Condomines M, Veyrune JL, Larroque M, et al. Increased plasma-immune cytokines throughout the high-dose melphalan-induced lymphodepletion in patients with multiple myeloma: a window for adoptive immunotherapy. J Immunol. 2010;184:1079–1084. doi: 10.4049/jimmunol.0804159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 32.Zhang H, Chua KS, Guimond M, et al. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nat Med. 2005;11:1238–1243. doi: 10.1038/nm1312. [DOI] [PubMed] [Google Scholar]
- 33.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klebanoff CA, Finkelstein SE, Surman DR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antony PA, Restifo NP. CD4+CD25+ T regulatory cells, immunotherapy of cancer, and interleukin-2. J Immunother. 2005;28:120–128. doi: 10.1097/01.cji.0000155049.26787.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sportes C, Hakim FT, Memon SA, et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205:1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soiffer RJ, Murray C, Cochran K, et al. Clinical and immunologic effects of prolonged infusion of low-dose recombinant interleukin-2 after autologous and T-cell-depleted allogeneic bone marrow transplantation. Blood. 1992;79:517–526. [PubMed] [Google Scholar]
- 38.Stringaris K, Adams S, Uribe M, et al. Donor KIR Genes 2DL5A, 2DS1 and 3DS1 are associated with a reduced rate of leukemia relapse after HLA-identical sibling stem cell transplantation for acute myeloid leukemia but not other hematologic malignancies. Biol Blood Marrow Transplant. 2010;16:1257–1264. doi: 10.1016/j.bbmt.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]