Abstract
Objective
To evaluate recent trends and the adoption of practice recommendations for MHT use from 2001 to 2009, by formulation, dosage, patient age, and characteristics of physicians reporting MHT visits.
Design
National Disease and Therapeutic Index™ physician survey data from 2001 to 2009 were analyzed for visits at which MHT use was reported by U.S. office-based physicians. Estimated national volume of visits for which MHT use was reported.
Results
MHT use declined each year since 2002. Systemic MHT fell from 16.3 million (M) visits in 2001 to 6.1 M in 2009. Declines were greatest for women 60+ years old (64%) but were also substantial for women <50 years old (59%), and women 50-59 years old (60%). Women 60+ years old accounted for 37% of MHT use. Lower dose products increased modestly, from 0.7 M (2001) to 1.3 M (2009), as did vaginal MHT use, from 1.8 M (2001) to 2.4 M (2009). Declines in continuing systemic MHT (65%) were greater than for newly initiated MHT (51%). Compared to other physicians, Obstetrician/Gynecologists changed their practices less, thereby increasing their overall share of total MHT visits from 72% (2001) to 82% (2009).
Conclusions
Total MHT use has steadily declined. Increased use of lower dose and vaginal products reflect clinical recommendations. Uptake of these products, however, has been modest and substantial use of MHT continues in older women.
Keywords: menopausal hormone therapy, physician practice patterns
INTRODUCTION
The large randomized, placebo-controlled Women’s Health Initiative (WHI) Estrogen plus Progestin (E+P) trial in postmenopausal women was stopped in July 2002 because the cardiovascular and breast cancer risks of combined conjugated equine estrogens (CEE) and medroxyprogesterone (MPA) outweighed health benefits 1. Menopausal hormone therapy (MHT), particularly CEE plus MPA use, decreased dramatically after this report 2. Clinical guidance from the U.S. Food and Drug Administration (FDA) and other groups emphasized that MHT should not be used for prevention, but was still appropriate, at the lowest effective dose and for the shortest duration, for managing moderate to severe menopausal symptoms 3, 4. However, accumulating evidence beginning with the 2004 WHI CEE-only trial in women with prior hysterectomy reported a more balanced risk-benefit profile 5, and suggested possible cardiovascular benefit in women aged 50-59 years 6 or closer to menopause 7-9. This led to consensus statements 10, 11 stating that MHT initiated in women <60 years old does not increase coronary heart disease (CHD) risk and possibly decreases it. Conversely, other research showed no cardiovascular benefit in younger women 12 and has reinforced and extended evidence on MHT-associated harms, particularly for breast cancer 13-16 and cognitive function 17. Thus, we sought to determine whether national MHT use continues to decline in the context of this new evidence, the current clinical recommendations and recent consensus statements. We assessed changes in product type and dose, continuing or newly initiated use, patient age, and physician specialty using nationally representative data.
METHODS
Data Source
Data was extracted from the January 2000 through December 2009 NDTI™ that provides nationally representative estimates on practices of non-federally employed U.S. office-based physicians via a physician survey conducted by IMS HEALTH (Plymouth Meeting, PA) 18. The survey uses a two-stage stratified cluster design: physicians are sampled in the first stage with visits of each physician sampled at the second stage. Physicians providing direct patient care are selected from the master lists of all U.S. physicians compiled by the American Medical Association and American Osteopathic Association (both of Chicago Illinois) via a random, stratified sample by geographic region and specialty (designed to match national patterns). Physicians may participate in the survey continuously and are replaced when they discontinue participation. Each calendar quarter, approximately 1800 participating physicians report on all patients evaluated on 2 consecutive and randomly assigned workdays. In 2009, the survey included 340, 820 projected patient encounters as office visits (89%), hospital visits (7%), telephone calls (2%) and other contacts (2%).
For each patient encounter, physicians report all patient diagnoses and all medications prescribed to treat each unique diagnosis19. Medications include those available over-the-counter. Physicians note whether a medication is newly prescribed or is continued from a previous visit and each medication is recorded as a “drug use.” An individual patient encounter may generate more than one “drug use” if the same drug is used to treat more than one diagnosis. For 8% of uses, newly initiated versus continuing medication status was not available, and were categorized as continuing medications in this analysis. Detailed patient demographics, including age, gender, ethnicity, and insurance coverage, are recorded. Physician characteristics included age, gender, and specialty. NTDI survey questions include for each unique diagnosis (1) Describe the diagnosis or reason for visit, (2) For this diagnosis only, record all Rx, OTC products, and Vitamins exactly as issued or recommended for this diagnosis, and (3) For each product, check whether it was Previously Ordered and Continued OR Started this Visit. For much of our analysis, we aggregated information across all diagnoses that prompted MHT use.
Information collected from NDTI™ visits is projected to national estimates accounting for physician non-response and geographic patterns of physician practice. The 95% confidence intervals (95% CI) for these estimates were calculated from relative standard error formulas that varied with sample size 20.
The NDTI database has several advantages over alternative databases of outpatient visits. NDTI provides more current information compared to federal survey data and private and governmental health insurance data. NDTI also provides nationally representative data with large sample sizes. Larger samples are available only for data that are regionally and demographically distinct, while national coverage is only available from less current federal surveys. The NDTI database has also been cross-validated against other national sources of information on outpatient practice 21, 22.
Measures
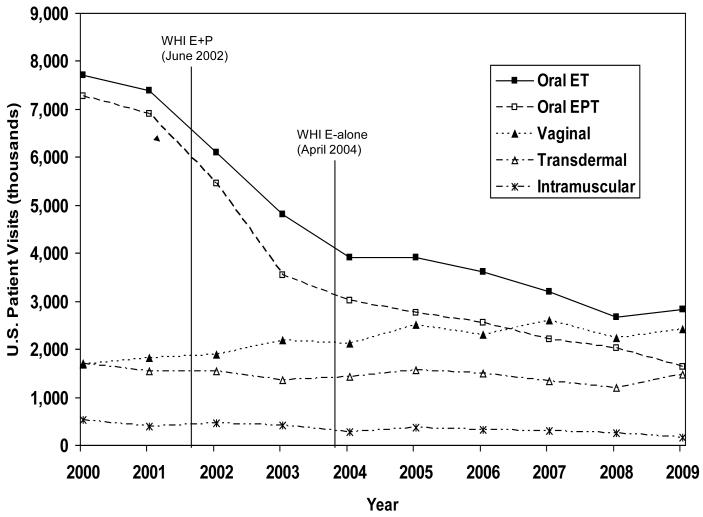
Using NDTI™ annual 2001 to 2009 data, we selected patient visits by women over the age of 18 years where MHT was mentioned. Annual survey responses meeting this definition decreased from 4,163 in 2001 to 1,936 in 2009. Figures 1 includes year 2000 data to highlight the relative stability in MHT use prior to 2002.
Figure 1.
U.S. Reported MHT Use by Formulation, January 2000 through December 2009, IMS Health, National Disease and Therapeutic Index™ (NDTI).
The primary outcome measure was the estimated national volume of visits for which MHT use was reported by office-based physicians. Trends in reported MHT use were differentiated by estrogen formulation, including route of administration, the presence of other hormones, and estrogen dosage. MHT was categorized as systemic (defined as oral, transdermal, and intramuscular) or vaginal. MHT was categorized as estrogen therapy alone (ET) or with a progestogen or androgen (EPT), noting that the estrogen and androgen combination was infrequently reported. EPT can be reported as either a combination product or separate products at the same visit. Standard estrogen dosages were equivalent to CEE ≥0.625 mg. Lower dosages were CEE 0.3 mg and 0.45 mg, and micronized estradiol 0.5 mg. We grouped patients as <50, 50-59, 60+ years of age and differentiated obstetrician/gynecologists (Ob/Gyns) from other physicians who were mostly primary care.
RESULTS
2001-2009 MHT Use
From 2001-2009, total reported MHT use decreased 52%, from 17.5 million (M) reported use to 8.3 M (Figure 1). Systemic MHT (ET+EPT) use declined from 16.3 M (95% CI 14.9-17.8) in 2001 to 8.6 M (95% CI 7.6-9.6) in 2004 to 6.1 M (95% CI 5.3-6.9) in 2009. The 62% decline included a 47% decline from 2001 to 2004 and a 28% decline from 2004 to 2009. Vaginal MHT increased 32% from 1.8 M (2001) (95% CI 1.4-2.2) to 2.1 M (2004) (95% CI 1.7-2.5) to 2.4 M (2009) (95% CI 2.0-2.8).
Decreases were particularly dramatic for oral MHT use (Figure 1). From 2001-2009, oral ET use dropped 62%, from 7.4 M (95% CI 6.5-8.3) in 2001 to 3.9 M (95% CI 3.3-4.5) in 2004 to 2.8 M (95% CI 2.3-3.3) in 2009. Oral EPT use declined 76%, from 6.9 M (95% CI 6.1-7.7) in 2001 to 3.0 M (95% CI 2.5-3.5) in 2004 to 1.6 M (95% CI 1.3-1.9) in 2009. Transdermal MHT use remained stable from 1.5 M (95% CI 1.2-1.8) in 2001 to 1.4 (95% CI 1.1-1.7)in 2004 to 1.5 M (95% CI 1.2-1.8) in 2009, while intramuscular MHT use declined 58% from its already low use of 0.4 M (95% CI 0.3-0.5) in 2001 to 0.2 M (95% CI 0.1-0.3) in 2009.
The proportion of MHT visits associated with menopause-related diagnoses remained relatively stable from 2001 (84%) to 2009 (81%). For these visits, in both 2001 and 2009, MHT was used more often (72.7%, 2001; 72.3%, 2009) than other therapies (27.3%, 2001; 27.7%, 2009), which included categories such as vitamins (4.5%, 2001; 7.4%, 2009), homeopathic and herbal products (3.2%, 2001; 4.3%, 2009), antidepressants (0.6%, 2001; 1.7%, 2009), and anxiolytics (0.3%, 2001; 0.1%, 2009).
Oral MHT Use: Standard and Lower dose
Standard dose ET use declined 71%, from 7.5 M (2001) (95% CI 6.6-8.4) to 3.3 M (2004) (95% CI 2.8-3.8) to 2.1 M (2009) (95% CI 1.7-2.5), and standard dose EPT use declined 83%, from 6.1 M (2001) (95% CI 5.3-6.9) to 2.0 M (2004) (95% CI 1.6-2.4) to 1.0 M (2009) (95% CI 0.8-1.2). Lower dose MHT use increased from 0.7 M (2001) (95% CI 0.5-0.9) to a peak of 1.9 M (2005) (95% CI 1.5-2.3), increasing from 5% to 28% of all oral MHT, respectively, then dropped to 1.3 M, 29% of all oral MHT in 2009.
Continuing and newly initiated MHT use
Continuing systemic MHT use declined 65%, from 12.7 M (95% CI 11.5-13.9) in 2001 to 4.4 M (95% CI 3.8-5.0) in 2009. Newly initiated systemic MHT use declined (51%) from 3.3 M (2001) (95% CI 2.8-3.8) to 2.2 M (2004) (95% CI 1.8-2.6) to 1.6 M (2009) (95% CI 1.3-1.9). The greater decreases in continuing over newly initiated systemic MHT were seen in all age groups. In contrast, continuing and newly initiated vaginal MHT use increased 30% and 23%, respectively from 2001-2009. Newly initiated vaginal MHT accounted for half of all reported vaginal MHT both years.
MHT use by Age
Women 50-59 years old used 39% of total MHT in 2001 and 41% in 2009. Systemic MHT visits had a median age of 55 years (interquartile range [IQR] 50-62 years) in 2001 and of 55 years (IQR 50-61 years) in 2009, while vaginal MHT visits had a median age of 67 years (IQR 57-75 years) in 2001 and 60 years (IQR 55-68 years) in 2009.
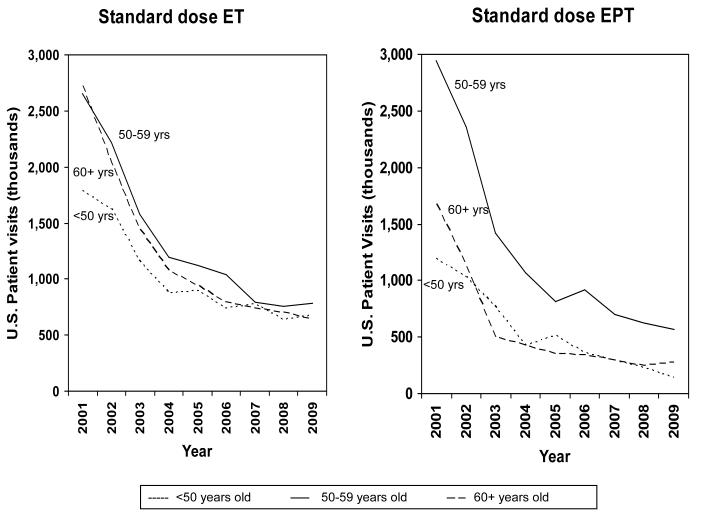
From 2001-2009, systemic MHT use decreased 64% in women 60+ years old, from 5.1 M (2001) (95% CI 4.4-5.8) to 2.4 M (2004) (95% CI 2.0-2.8) to 1.8 M (2009) (95% CI 1.4-2.2), 60% in those aged 50-59 years old, from 6.6 M (2001) (95% CI 5.8-7.4) to 3.6 M (2004) (95% CI 3.1-4.1) to 2.6 M (2009) (95% CI 2.2-3.0), and 59% in women aged <50 years, from 3.6 M (2001) (95% CI 3.1-4.1) to 2.1 M (2004) (95% CI 1.7-2.5) to 1.5 M (2009) (95% CI 1.2-1.8). The magnitude of the decline in standard dose oral ET increased with advancing age (i.e. 62% for those aged <50 years, 71% for 50-59 year olds, and 77% for 60+ year olds) while standard dose oral EPT use decreased more in younger and older women (88% for <50 year olds, 83% in 60+ year olds vs. 81% in 50-59 year olds) (Figure 2).
Figure 2.
U.S. Standard Dose Oral MHT Use by Age Group, January 2001 through December 2009, IMS Health, National Disease and Therapeutic Index™ (NDTI).
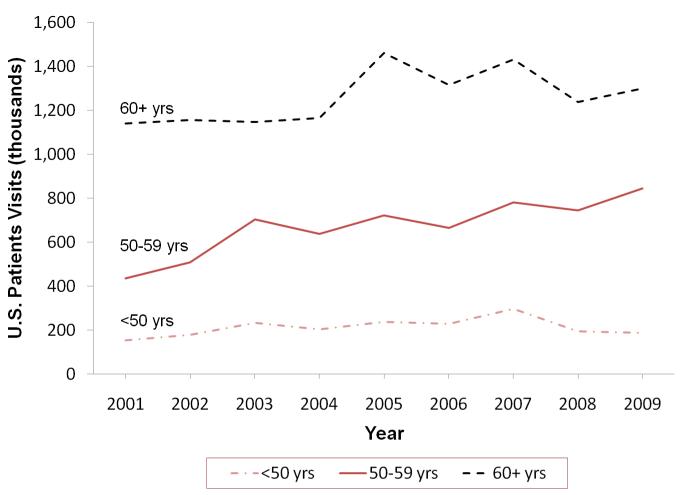
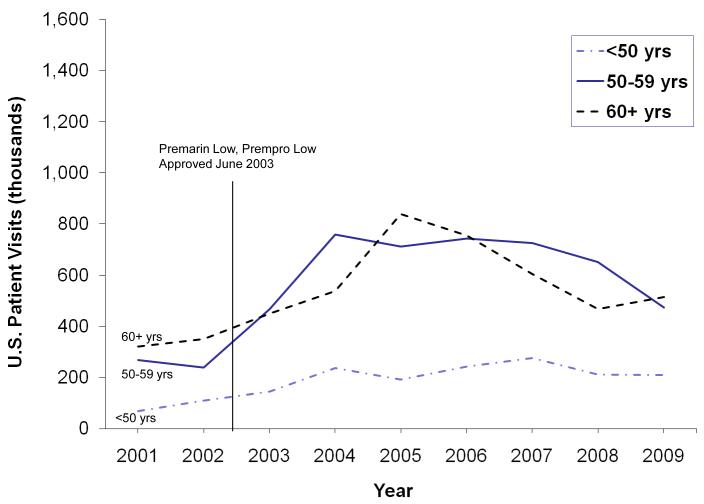
From 2001-2009, lower dose oral MHT use increased 102% in women <60 years, from 0.33 M (2001) (95% CI 0.21-0.45) to 1.0 M (2004) (95% CI 0.76-1.24) to 0.68 M (2009) (95% CI 0.49-0.87), compared to 61% increase for 60+ year olds, from 0.32 M (2001) (95% CI 0.21-0.44) to 0.54 M (2004) (95% CI 0.38-0.70) to 0.52 M (2009) (95% CI 0.36-0.68) (Figure 3). Vaginal MHT use increased 22% in women aged <50 years, from 0.15 M (2001) (95% CI 0.08-0.22) to 0.19 M (2009) (95% CI 0.12-0.27), 100% in those aged 50-59 years, from 0.44 M (2001) (95% CI 0.30-0.58) to 0.84 M (2009) (95% CI 0.63-1.06), and 21% in those aged 60+ years, from 1.1 M (2001) (95% CI 0.85-1.36) to 1.3 M (2009) (95% CI 1.02-1.58) (Figure 4).
Figure 3.
U.S. Lower Dose Oral Use by Age Group, January 2001 through December 2009, IMS Health, National Disease and Therapeutic Index™ (NDTI).
Figure 4.
U.S. Vaginal MHT Use by Age Group, January 2001 through December 2009, IMS Health, National Disease and Therapeutic Index™ (NDTI).
MHT Use by Other Patient Characteristics
In 2001, MHT visits comprised 5% (14 M (95% CI 12.7-15.3)) of a total 284 M physician visits by women 40 to 70 years old, of which 80% were for whites (5% of which were for MHT use) and 20% for non-whites (of which 4% were for MHT use). In 2009, MHT visits comprised 2.8% (7M (95% CI 6.2-7.8)) of a total 246 M physician visits, 76% for whites (3% for MHT use) and 24% for non-whites (2% for MHT use). For all patient visits associated with MHT, the proportion of MHT visits for whites and blacks was similar in 2001 (83.4% and 8.1 %, respectively) and 2009 (83.3% and 6.9%) (Table 1). The majority of MHT visits were associated with private insurance in both 2001 (70%) and 2009 (70.9%), with a small portion having no insurance coverage both years (2% and 2%).
Table 1.
Patient Characteristics for Patient Visits During Which MHT Was Reported
| Patient Characteristic | % of MHT visits | |
|---|---|---|
| 2001 | 2009 | |
| 17.5 Million Visits* | 8.3 Million Visits* | |
| Age | ||
| 19-29 | 0.5 | 0.6 |
| 30-39 | 3.1 | 3.2 |
| 40-49 | 17.7 | 16.1 |
| 50-59 | 39.6 | 40.7 |
| 60-69 | 20.2 | 25.7 |
| 70-79 | 10.9 | 8.1 |
| 80+ | 3.6 | 3.3 |
| Unspecified | 4.4 | 2.3 |
| Ethnicity | ||
| White | 83.4 | 83.3 |
| Black | 8.1 | 6.9 |
| Hispanic | 2.7 | 4.0 |
| Asian | 1.4 | 2.8 |
| Other | 4.4 | 3.1 |
| Insurance status | ||
| Private Insurance | 70.0 | 70.9 |
| Medicare | 18.5 | 18.0 |
| Medicaid | 2.7 | 2.7 |
| Other/unspecified | 8.8 | 8.4 |
NDTI estimate of patient visits (projection)
MHT Use by Physician Characteristics
Ob/Gyns were associated with a larger proportion of 2001 MHT visits (72%) compared with primary care physicians and other specialties (28%, Table 2). Smaller 2001-2009 declines in Ob/Gyns’ MHT visits (46%) compared to other physician specialties (69%) increased Ob/Gyns’ proportion to 82% in 2009. For both Ob/Gyn and other specialties, MHT patient visits were more likely to be associated with male not female physicians (Table 2). However, by 2009, this gap was smaller, more so for specialties other than Ob/Gyn. Ob/Gyn MHT visits were more often associated with older physicians (55+ years old) while other specialty MHT visits were more often associated with younger physicians (45-54 years old), particularly in2001. The specialty differential in decreasing MHT use was observed for all categories for which MHT use declined. Patterns of increase from 2001-2009 were similar for Ob/Gyns and other specialties for lower dose oral MHT (from 0.47 M (95% CI 0.32-0.62) to 0.91 M (95% CI 0.68-1.14) and from 0.21 M (95% CI 0.12-0.30) to 0.31 M (95% CI 0.20-0.42), respectively) and vaginal MHT (37% for Ob/Gyns, and 14% for other physicians).
Table 2.
Physician Characteristics for Patient Visits During Which MHT Was Reported
| % of MHT Visits | ||
|---|---|---|
| Physician Characteristic |
2001 | 2009 |
| 17.5 Million Visits* |
8.3 Million Visits* |
|
| Ob/Gyn | 72.6 | 81.9 |
| Age | ||
| <45 years | 10.7 | 3.0 |
| 45-54 years | 26.4 | 25.9 |
| 55-64 years | 28.7 | 38.7 |
| 65+ years | 34.3 | 32.4 |
| Physician Sex | ||
| Male | 83.7 | 77.4 |
| Female | 16.3 | 22.6 |
| Other Specialties | 27.4 | 18.1 |
| Physician Age | ||
| <45 years | 21.7 | 12.8 |
| 45-54 years | 39.0 | 36.3 |
| 55-64 years | 25.4 | 36.6 |
| 65+ years | 13.9 | 14.3 |
| Sex | ||
| Male | 72.5 | 57.9 |
| Female | 27.5 | 42.1 |
NDTI estimate of patient visits (projection)
DISCUSSION
Nationally representative data on MHT use among patients visiting U.S. outpatient physicians indicate that systemic MHT use continued to decline from 2004 to 2009 (28% decline), but at a slower rate than during the years immediately following the 2002 publication of the WHI E+P trial (47%). Overall, 2009 systemic MHT use was 37% of its 2001 level. MHT doses and route of administration also changed, with lower dose oral MHT increasing from 0.7 M (2001) to 1.3 M (2009), transdermal MHT use remained stable from 1.5 M to 1.5 M, vaginal MHT use increasing from 1.8 M to 2.4 M, and oral EPT use declining slightly more (76%) than ET use (62%). Women aged 60+ years old reduced systemic MHT use more (64%) than younger women (60%), yet continued to account for a sizable portion of MHT use (31% of systemic MHT in 2009, down from 32% in 2001). Few prescription (such as antidepressants) or non-prescription alternatives (such as dietary supplements) to MHT were used for menopause-related diagnoses.
These findings extend previously reported trends in U.S. and international studies 2, 23-27 and indicate no resurgence in MHT use through 2009. Declining MHT use largely reflects changes initiated after publication of WHI E+P trial results, including physicians’ increased awareness of net benefits versus risks of MHT use. In addition, new evidence, along with clinical guidelines, continues to help inform physicians and patients about MHT use. For example, the WHI CEE-only trial 5 and others 14-16, 28 found a temporal association of reduced breast cancer incidence with reduced MHT use, while other studies have assessed differential risk by age or years since menopause 7, 29, 30. Current guidelines and FDA recommendations support MHT starting at the lowest effective dosage for the shortest duration of time necessary for treatment of significant menopausal symptoms 3, 10, 31, 32. Despite the successful translation of clinical trial results into practice, further modification of MHT prescribing practices may be needed, particularly with respect to distinctions based on MHT dose, route of administration, co-administration of progestogens, and patient age.
Lower Dose MHT
Accumulating evidence suggests that lower dose MHT is effective for vasomotor symptoms and osteoporosis prevention and may have a better safety profile than MHT in standard doses 10, 33-36. In observational studies, lower dose MHT does not appear to raise cardiovascular risk in young, healthy, symptomatic women 34, 37. We observed, however, that standard dose oral MHT continues to exceed lower dose oral MHT use. Although use of lower dose formulations increased after the 2003 introduction of Premarin Low® (CEE of 0.45 mg) and Prempro Low® (CEE of 0.3 mg and 0.45 mg), their use decreased after 2005. Despite new lower dose MHT options in various routes of administration, we did not observe prominent use of these products. Current recommendations 3, 10, 31, 32 to use the lowest dose MHT effective for symptom relief should receive greater consideration.
Transdermal MHT
Transdermal MHT, which foregoes first-pass hepatic metabolism, is hypothesized to reduce cardiovascular and venous thromboembolic risk compared to oral MHT due to reduced stimulation of liver proteins and a more favorable metabolic profile 38. Given the value of transdermal MHT in controlling vasomotor symptoms 39, 40, it is surprising that transdermal MHT has not increased overall from 2001 to 2009.
Vaginal Formulations
The North American Menopause Society recommends that vaginal MHT be used if only vulvovaginal symptoms are present and lower dose oral estrogen be used for women with persistent vulvovaginal symptoms 32, consistent with 2003 FDA recommendations3. We report only modestly increased vaginal MHT use from 2001 to 2009, suggesting possible underutilization of this therapy despite increased availability of lower dose products.
EPT versus ET
In the WHI hormone trials, health risks of EPT exceeded the benefits, whereas a neutral risk-to-benefit ratio was found for ET alone 1, 5. It may be that some harms associated with EPT derive from progestogens 41. Our data indicate that absolute declines in EPT use continue to exceed those for ET from 2001-2009. Greater differentiation between ET and EPT use in older age groups may especially be warranted, as a lower threshold for MHT may be appropriate for younger, symptomatic post-hysterectomy patients with vasomotor symptoms.
Use in Younger Women
Cardiovascular disease is generally low in younger women and lower dose oral MHT or other non-oral routes of administration may be effective and safer for managing symptoms in peri-menopausal women 5, 7, 42. Thus, it seems appropriate that systemic MHT use decreased less in younger women than those aged 60+ years old. Younger symptomatic women provided with low dose, limited duration MHT may face limited increase in the absolute risk of adverse events.
Physicians are important disseminators of current evidence and guidelines. Risk-benefit discussions should be undertaken in any woman contemplating MHT. While most critical among women at higher risk of complications due to increased age or the presence of specific co-morbidities, such discussions should also take place in younger women who consider MHT for moderate to severe vasomotor symptoms. In the absence of such symptoms, MHT is not recommended because potential risks may outweigh benefits.
Use in Older Women
Concerns about MHT use in older women have increased since 2002 with new evidence of cardiovascular and cancer risks that highlight hazards in older women 7, 13, 29, 43. The temporal association of a population-wide decline in breast cancer and decreases in MHT use reinforce cautions regarding MHT use in women at high risk of breast cancer, which may include the substantial number of older women receiving MHT. Although lack of past MHT-related adverse events in older women is stated as justification for continuing MHT, this does not equate to a lack of future risk.
Limitations
Several limitations deserve attention. NDTI includes physicians in non-federal office-based private practices and excludes those in publicly-funded practices, who are more likely to serve lower income patients. Physicians may vary in how they classify new versus continued MHT use. In particular, re-initiation of prior MHT may not be coded consistently. NDTI patient visits may not represent the general population of women and their patterns of MHT use. In particular, women seeking MHT-specific care and those making more physician visits are likely over-represented. NDTI lacks patient interview information that might have added greater depth to the observed practice patterns, particularly for the limited uptake of low dose and topical products. Given our data’s lack of detailed information on patient symptoms and response to past therapies, there are inherent limitations in commenting on the appropriateness of current practice patterns.
CONCLUSION
Current guidelines stress tailoring MHT decisions to an individual’s risks and benefits. Although the continued decline and transformation of MHT has shifted in the direction of available evidence and guidelines; further refinements in practice may be warranted. Despite reduced use, standard dose oral MHT remains the dominant formulation, yet lower dose, transdermal and vaginal preparations may yield less harm. The potentially lower risk of ET may suggest a lower threshold for its use compared to EPT. Older women are at increased risk for CVD events and breast cancer; risks that are more likely to outweigh the benefits of systemic MHT. In healthy younger women, however, the benefits of MHT for treatment of moderate to severe symptoms may compensate for disease risks. While future research should seek to provide more detailed risk-benefit information on both subpopulations and different forms of MHT, greater recognition of distinctions based on MHT dose, route of administration, need for concomitant progestogens, and patient age may move clinical practice into better alignment with available evidence.
Acknowledgment
The statements, findings, conclusions, views, and opinions contained and expressed in this article are based in part on data obtained under license from the National Disease and Therapeutic Index ™ (2000-2009) IMS Health Incorporated. All Rights Reserved. The statements, findings, conclusions, views, and opinions contained and expressed herein are not necessarily those of IMS Health Incorporated or any of its affiliated or subsidiary entities.
Funding/Support: This study was supported by an institutional National Research Service Award (5T32-HL07034) and a Midcareer Investigator Awards in Patient-Oriented Research (5K24-HL086703) both funded by the National Heart, Lung, and Blood Institute.
Role of the Sponsor: The funding agency did not play a role in the design and conduct of the study, analysis and interpretation of the data, or the preparation and approval of the manuscript.
Funding: This research was supported by training grants from the National Heart, Lung, and Blood Institute (NHLBI) including an Institutional Training Award (T32-HL07034) and a Mid-career Mentoring Award (RSS, K24-HL086703).
Footnotes
Disclosure of Conflicts of Interest: none
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 2.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291(1):47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 3.Labeling Guidance for Noncontraceptive Estrogen Drug Products for the Treatment of Vasomotor Symptoms and Vulvar and Vaginal Atrophy Symptoms [Accessed July 20, 2010];Prescribing Information for Health Care Providers and Patient Labeling. 2005 Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/InformationbyDrugClass/UCM135336.
- 4.Stefanick ML. Estrogens and progestins: background and history, trends in use, and guidelines and regimens approved by the US Food and Drug Administration. Am J Med. 2005;118(Suppl 12B):64–73. doi: 10.1016/j.amjmed.2005.09.059. [DOI] [PubMed] [Google Scholar]
- 5.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 6.Hsia J, Langer RD, Manson JE, et al. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166(3):357–65. doi: 10.1001/archinte.166.3.357. [DOI] [PubMed] [Google Scholar]
- 7.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297(13):1465–77. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 8.Estrogen and progestogen use in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause. 17(2):242–55. doi: 10.1097/gme.0b013e3181d0f6b9. [DOI] [PubMed] [Google Scholar]
- 9.Santen RJ, Allred DC, Ardoin SP, et al. Postmenopausal Hormone Therapy: An Endocrine Society Scientific Statement. J Clin Endocrinol Metab. 2010 doi: 10.1210/jc.2009-2509. doi:10.1210/jc.2009-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pines A, Sturdee DW, Birkhauser MH, Schneider HP, Gambacciani M, Panay N. IMS updated recommendations on postmenopausal hormone therapy. Climacteric. 2007;10(3):181–94. doi: 10.1080/13697130701361657. [DOI] [PubMed] [Google Scholar]
- 11.Rees M, Stevenson J. Primary prevention of coronary heart disease in women. Menopause Int. 2008;14(1):40–5. doi: 10.1258/mi.2007.007037. [DOI] [PubMed] [Google Scholar]
- 12.Prentice RL, Manson JE, Langer RD, et al. Benefits and risks of postmenopausal hormone therapy when it is initiated soon after menopause. Am J Epidemiol. 2009;170(1):12–23. doi: 10.1093/aje/kwp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative Randomized Trial. JAMA. 2003;289(24):3243–53. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 14.Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med. 2007;356(16):1670–4. doi: 10.1056/NEJMsr070105. [DOI] [PubMed] [Google Scholar]
- 15.Krieger N. Hormone therapy and the rise and perhaps fall of US breast cancer incidence rates: critical reflections. Int J Epidemiol. 2008;37(3):627–37. doi: 10.1093/ije/dyn055. [DOI] [PubMed] [Google Scholar]
- 16.Clarke CA, Glaser SL, Uratsu CS, Selby JV, Kushi LH, Herrinton LJ. Recent declines in hormone therapy utilization and breast cancer incidence: clinical and population-based evidence. J Clin Oncol. 2006;24(33):e49–50. doi: 10.1200/JCO.2006.08.6504. [DOI] [PubMed] [Google Scholar]
- 17.Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289(20):2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 18.IMS Health Incorporated; National Disease and Therapeutic Index™, January, 2000-December, 2009. All Rights Reserved.
- 19. [Accessed July 20, 2010];National Disease and Therapeutic Index™ website. Available at: https://web01.imshealth.com/ndti/Demo/NDTIdemo.html.
- 20.2007 NDTI™ Precision Table: Diagnosis Visits (Estimated Sampling Error) Two-Staged Stratified Cluster Methodology, Dataview Software. IMS Health Incorporated; Plymouth Meeting, PA: 2007. [Google Scholar]
- 21.Stafford RS, Radley DC. The underutilization of cardiac medications of proven benefit, 1990 to 2002. J Am Coll Cardiol. 2003;41(1):56–61. doi: 10.1016/s0735-1097(02)02670-0. [DOI] [PubMed] [Google Scholar]
- 22.Zell ER, McCaig LF, Kupronis BA, Besser RE, Schuchat A. A comparison of the National Disease and Therapeutic Index and the National Ambulatory Medical Care Survey to evaluate antibiotic usage. Proceedings of the Section on Survey Research Methods American Statistical Association; Alexandria, VA. 2000. pp. 840–845. [Google Scholar]
- 23.Buist DS, Newton KM, Miglioretti DL, et al. Hormone therapy prescribing patterns in the United States. Obstet Gynecol. 2004;104(5 Pt 1):1042–50. doi: 10.1097/01.AOG.0000143826.38439.af. [DOI] [PubMed] [Google Scholar]
- 24.Hing E, Brett KM. Changes in U.S. prescribing patterns of menopausal hormone therapy, 2001-2003. Obstet Gynecol. 2006;108(1):33–40. doi: 10.1097/01.AOG.0000220502.77153.5a. [DOI] [PubMed] [Google Scholar]
- 25.Haas JS, Kaplan CP, Gerstenberger EP, Kerlikowske K. Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med. 2004;140(3):184–8. doi: 10.7326/0003-4819-140-3-200402030-00009. [DOI] [PubMed] [Google Scholar]
- 26.Du Y, Doren M, Melchert HU, Scheidt-Nave C, Knopf H. Differences in menopausal hormone therapy use among women in Germany between 1998 and 2003. BMC Women’s Health. 2007;7:19. doi: 10.1186/1472-6874-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morabia A, Costanza MC. Recent reversal of trends in hormone therapy use in a European population. Menopause. 2006;13(1):111–5. doi: 10.1097/01.gme.0000172595.68648.16. [DOI] [PubMed] [Google Scholar]
- 28.Chlebowski RT, Kuller LH, Prentice RL, et al. Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med. 2009;360(6):573–87. doi: 10.1056/NEJMoa0807684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grodstein F, Manson JE, Stampfer MJ. Hormone therapy and coronary heart disease: the role of time since menopause and age at hormone initiation. J Women’s Health. 2006;15(1):35–44. doi: 10.1089/jwh.2006.15.35. (2002) [DOI] [PubMed] [Google Scholar]
- 30.Manson JE, Bassuk SS, Harman SM, et al. Postmenopausal hormone therapy: new questions and the case for new clinical trials. Menopause. 2006;13(1):139–47. doi: 10.1097/01.gme.0000177906.94515.ff. [DOI] [PubMed] [Google Scholar]
- 31.Hormone Replacement Therapy for the Prevention of Chronic Conditions in Postmenopausal Women [Accessed July 20, 2010];2005 Available at: http://www.ahrq.gov/clinic/uspstf/uspspmho.htm#0related. [PubMed]
- 32.Estrogen and progestogen use in peri- and postmenopausal women: March 2007 position statement of The North American Menopause Society. Menopause. 2007;14(2):168–82. doi: 10.1097/gme.0b013e31803167ab. [DOI] [PubMed] [Google Scholar]
- 33.Wakatsuki A, Ikenoue N, Shinohara K, Watanabe K, Fukaya T. Effect of lower dosage of oral conjugated equine estrogen on inflammatory markers and endothelial function in healthy postmenopausal women. Arterioscler Thromb Vasc Biol. 2004;24(3):571–6. doi: 10.1161/01.ATV.0000115383.49802.0c. [DOI] [PubMed] [Google Scholar]
- 34.van de Weijer PH, Mattsson LA, Ylikorkala O. Benefits and risks of long-term low-dose oral continuous combined hormone therapy. Maturitas. 2007;56(3):231–48. doi: 10.1016/j.maturitas.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Jick H, Derby LE, Myers MW, Vasilakis C, Newton KM. Risk of hospital admission for idiopathic venous thromboembolism among users of postmenopausal oestrogens. Lancet. 1996;348(9033):981–3. doi: 10.1016/S0140-6736(96)07114-0. [DOI] [PubMed] [Google Scholar]
- 36.Appt SE, Clarkson TB, Lees CJ, Anthony MS. Low dose estrogens inhibit coronary artery atherosclerosis in postmenopausal monkeys. Maturitas. 2006;55(2):187–94. doi: 10.1016/j.maturitas.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Ettinger B. Rationale for use of lower estrogen doses for postmenopausal hormone therapy. Maturitas. 2007;57(1):81–4. doi: 10.1016/j.maturitas.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 38.Modena MG, Sismondi P, Mueck AO, et al. New evidence regarding hormone replacement therapies is urgently required transdermal postmenopausal hormone therapy differs from oral hormone therapy in risks and benefits. Maturitas. 2005;52(1):1–10. doi: 10.1016/j.maturitas.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Bachmann GA, Schaefers M, Uddin A, Utian WH. Lowest effective transdermal 17betaestradiol dose for relief of hot flushes in postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2007;110(4):771–9. doi: 10.1097/01.AOG.0000284450.51264.31. [DOI] [PubMed] [Google Scholar]
- 40.Haines C, Yu SL, Hiemeyer F, Schaefers M. Micro-dose transdermal estradiol for relief of hot flushes in postmenopausal Asian women: a randomized controlled trial. Climacteric. 2009;12(5):419–26. doi: 10.1080/13697130902748967. [DOI] [PubMed] [Google Scholar]
- 41.Colditz GA. Estrogen, estrogen plus progestin therapy, and risk of breast cancer. Clin Cancer Res. 2005;11(2 Pt 2):909s–17s. [PubMed] [Google Scholar]
- 42.Ettinger B. Vasomotor symptom relief versus unwanted effects: role of estrogen dosage. Am J Med. 2005;118(Suppl 12B):74–8. doi: 10.1016/j.amjmed.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 43.Wassertheil-Smoller S, Hendrix SL, Limacher M, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA. 2003;289(20):2673–84. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]