Abstract
Background
A rise in circulating dehydroepiandrosterone sulfate (DHEAS) concentration occurs during the menopausal transition (MT) that is ovarian-stage but not age-related. The objective of this study was to determine the source of the rise in circulating DHEAS.
Methods
Circulating DS concentrations in women that had undergone bilateral salpingo-oophorectomy (BSO) were compared to the pattern of circulating DHEAS in women that progressed through the MT naturally. Annual serum samples from the Study of Women's Health Across the Nation (SWAN) over a ten year study period were used. From1272 women in the SWAN cohort that were eligible for longitudinal evaluation of DHEAS annual samples, eighty one underwent BSO during the pre- or early-perimenopause stage of the menopausal transition and were potentially available for study. Of these eighty one BSO participants, twenty had sufficient annual samples for evaluation of the post-BSO trajectory of circulating DHEAS. SWAN women not having previous hormone replacement therapy those with intact ovaries were compared to women that underwent a BSO immediately after a pre- or early perimenopausal annual visit. There were no intervention and circulating concentrations of DHEAS was the main outcome.
Results
A detectable rise in DHEAS was observed in fourteen (70%) of the twenty BSO women which is similar to the proportion (85%) of women with intact ovaries that had a detectable DHEAS rise. The mean rise in DHEAS (5-8%) was similar in both BSO and non-BSO women.
Conclusion
The MT rise in DHEAS (5-8%) occurring in the absence of ovaries is largely of adrenal origin.
Keywords: Dehydroepiandrosterone sulfate, menopause, adrenal, ovary
Introduction
Previous and recent reports reveal a positive inflection in circulating dehydroepiandrosterone sulfate (DHEAS) that occurs during the menopausal transition (MT) of most women 1, 2. This rise in DHEAS is detectable in annual samples of 85% of middle-aged women and is associated with changes in ovarian status, with the positive slope beginning in the early perimenopause and continuing until the early post menopause, ultimately declining to pre menopausal levels in the late post-menopause.
The positive inflection of DHEAS is common across five ethnic groups, that is White, African American, Hispanic, Chinese, and Japanese 1, 2 and has the potential to significantly impact the circulating estrogen-to-androgen balance 2. The peripheral conversion of DHEAS to more bioactive steroid hormones contributes to an individual woman's endocrine status and may determine her symptoms and health. While potentially important, neither the source nor the mechanism by which this rise of DHEAS is propagated is known. The necessity in understanding the dynamics of DHEAS changes in perimenopausal women is underscored by the accumulating data on the increasing clinical use of dehydroepiandrosterone (DHEA) for the treatment of postmenopausal vaginal atrophy 3 and premature ovarian failure 4.
Both the adrenals and the ovaries have the capacity to synthesize and secrete DHEA, which can then be sulfated by the liver. Thus, the increased production from either or both of these steroidogenic organs could contribute to the shift in circulating DS that is observed during the MT 5, 6. An increase in ovarian DHEA can be induced by the administration of follicle stimulating hormone (FSH) in women with polycystic ovary syndrome 7 and an ovarian-to-peripheral vein gradient of DHEA is observed in postmenopausal women 6 indicating that the increase in circulating DHEAS observed during the menopause transition (MT) could be attributable to increased steroid production by the ovaries, the adrenals or both.
Investigating the endocrine profile of women who have undergone surgical menopause may therefore provide insight into the source of the increase in circulating DHEAS. If women who had bilateral salpingo-oophorectomy (BSO) during the early peri-menopause did not exhibit a rise in DS during the subsequent MT, then this would indicate that the ovaries were primarily responsible for the increase in DS production that is observed during the MT. However, if women who had undergone BSO prior to the late peri-menopause continued to exhibit a robust increases in DHEAS during the remainder of the MT, in a manner similar to the rise in DHEAS observed in women undergoing natural menopause, then the source of the rise in DHEAS would be attributable primarily to the adrenals.
The purpose of this investigation was to identify and characterize participants in the Study of Women's Health Across the Nation (SWAN) who had undergone BSO during the pre- and early peri-menopause and were followed with subsequent annual blood collections. Using these annual serum samples, the mean DHEAS profiles of these women were compared to the mean DHEAS profiles of women undergoing natural menopause during the same MT stages. The result of this comparison was used to clarify whether or not the presence of the ovaries is necessary for the inflection and rise of DHEAS in the MT transition.
Materials and Methods
Participants
In a previous report 2, within-woman changes in annual circulating DHEAS concentrations occurring during natural (non-surgical) menopause transitions were assessed, omitting women with a simple hysterectomy or BSO and women who did not reach late perimenopause. This study compares women with intact ovaries to those with a BSO occurring after a pre- or early perimenopausal annual visit. Of the 81 participants meeting this criterion, DHEAS measurements post surgery were concurrent with hormone therapy (HT) for 38 women, and another 23 women had HT use at two or more consecutive visits post-surgery, leaving 20 women with at least one HT-free DHEAS measurement post-surgery available for these analyses. This relatively small number of well-defined participants results from the requirements that a woman be pre- or early perimenopausal at the visit preceding the BSO surgery (of 74 other BSOs, 24 had already had a simple hysterectomy, 30 were late peri- or postmenopausal, and 20 were using HT at the visit prior to surgery) and have HT-free DHEAS measurements following surgery. For comparison, we also included data from participants with intact ovaries. Of the 3221 women without a BSO during pre-/early- perimenopause, 184 had a hysterectomy or a BSO during HT use or after early perimenopause, 1870 had no DHEAS measurements during late perimenopause, and 8 were missing outcome or covariate data, yielding a sample of 1159 women with intact ovaries.
Assays
An automated, ACS:180-based chemiluminescent assay was developed using the Bayer Diagnostics ACS:180 to determine the levels of DHEAS in human serum. This chemiluminescent immunoassay involves the competitive binding of a Dimethylacridinuim ester (DMAE) labeled DHEAS derivative to a rabbit anti-DHEAS antibody. The solid phase is Goat anti-rabbit IgG conjugated to superparamagnetic particles (PMP). 10 uL of unextracted serum is required for the assay in addition to sufficient dead volume for aspiration and repeat analysis. The assay range is 1.52 to 1020 ug/dL and the assay is standardized against DHEAS obtained from Steraloids. The detection level of this assay is approximately 1.9 ug/dL. Closely related steroids (danazol, cholesterol, pregnenolone, 17a-hydroxyprogesterone, corticosterone, 17a-estradiol, 17b-estradiol, testosterone, estriol, 11-deoxycortisol, norethindrone, estrone, aldosterone, estradiol glucuronide and cortisol) show little cross reactivity in the assay indicating high specificity. The assay demonstrates acceptable parallelism of diluted serum samples and recovery of added exogenous hormone. DHEA cross reacts 192%, but the low levels of serum DHEA render this inconsequential. The expected values are 150-500 ug/dL for normally cycling females and 10-350 ug/dL for postmenopausal females. The intra assay coefficient of variation is 8.02% (n=261) and inter assay coefficient of variation is 11.34% (53.32 ug/dL, n=37) and 9.74% (250.21 ug/dL, n=37).
Analysis Plan
The outcome variable, DHEAS, was natural log tranformed to normalize its skewed distribution. Independent variables included baseline and concurrent menopause status according to the STRAW Convention 8, categorized as follows: pre- or early perimenopausal (<3 months amenorrhea), late peri- or early postmenopausal (more than 3 months of amenorrhea but within 2 years of final menstrual period, late postmenopausal (>2 years after FMP)), and BSO. Covariates included ethnicity, clinical site, baseline weight, baseline height, age, baseline smoking (never, current, or past), and HT use at a previous SWAN visit. Analyses included height and weight rather than BMI, because within-woman changes in BMI are due primarily to changes in weight 9. Observations concurrent with exogenous hormone use or within a 6-month washout period after hormone discontinuation were omitted from analyses.
BSO and intact participants were compared with respect to baseline characteristics using Fisher's exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables. For within-woman changes in log DHEAS associated with the menopause transition, we estimated a linear mixed model (PROC MIXED in SAS) as a function of baseline and concurrent menopause status, adjusting for the covariates listed above. The time-varying covariates age and weight were separated into baseline value and change since baseline, to distinguish between-woman (cross-sectional) and within-woman (longitudinal) effects.
Mean within-woman changes in log DHEAS associated with transitions from one menopause stage to another, particularly pre-/early perimenopause to BSO, and pre-/early perimenopause to late peri-/early postmenopause, as well as the difference in these two within-woman changes, were estimated from the model using appropriate contrasts. Next, we estimated woman-specific changes in log DHEAS by including random effects (i.e., woman-specific estimates) for the intercept and time-varying menopause status.
Results
The characteristics of the 20 BSO participants were similar to the participants with intact ovaries, with the exception of body mass index (BMI) (Table 1). The higher BMI in BSO women was not attributable to a disproportionate number of African American women in the BSO group, as adjustment for ethnicity had little impact on the magnitude of this difference and the difference remained statistically significant. On average, the BSO occurred 8.0 months (range 1.3 months – 15.3 months) after the last pre-surgery annual visit. BSO surgery date was unknown for two women without a hysterectomy, as only the date of hysterectomy – and not BSO – was ascertained. Total number of visits included in analyses was similar for BSO (mean 7.3 visits, range 2 – 11 visits) and intact participants (mean 8.0 visits, range 2 – 11 visits). Among BSO participants, 90% had at least two post-surgery observations included in analyses (mean 3.4, range 1 – 9).
Table 1. Baseline characteristics of women with a BSO (N=20) and intact women with 1+visit during late perimenopause (N=1159).
| % (N) or Mean (SD) | |||
|---|---|---|---|
| Baseline characteristic | BSO women | Intact women | p-value |
| Ethnicity: | 0.0850 | ||
| African American | 50.0 (10) | 30.2 (350) | |
| White | 35.0 (7) | 45.3 (525) | |
| Chinese | 5.0 (1) | 10.0 (116) | |
| Hispanic | 10.0 (2) | 3.6 (42) | |
| Japanese | 0.0 (0) | 10.9 (126) | |
| Site: | 0.0831 | ||
| Detroit | 15.0 (3) | 17.3 (201) | |
| Boston | 25.0 (5) | 15.5 (180) | |
| Chicago | 30.0 (6) | 12.3 (142) | |
| Oakland | 5.0 (1) | 17.3 (201) | |
| Los Angeles | 5.0 (1) | 17.0 (197) | |
| Newark | 10.0 (2) | 5.3 (61) | |
| Pittsburgh | 10.0 (2) | 15.3 (177) | |
| Menopause status: | 1.0000 | ||
| Premenopausal | 55.0 (11) | 53.1 (615) | |
| Early perimenopausal | 45.0 (9) | 46.9 (544) | |
| Smoking status: | 0.9488 | ||
| Never | 65.0 (13) | 59.9 (694) | |
| Past | 20.0 (4) | 24.4 (283) | |
| Current | 15.0 (3) | 15.7 (182) | |
| Age | 45.1 (2.7) | 46.7 (2.6) | 0.0871 |
| Body mass index (kg/m2) | 35.8 (10.6) | 28.2 (7.3) | 0.0004 |
| Height (m) | 1.6 (0.06) | 1.6 (0.07) | 0.3910 |
| Weight (kg) | 95.4 (26.6) | 70.4 (21.1) | 0.0002 |
| Age at BSO | 50.4 (4.1) | -- | -- |
Among the BSO women the mean within-woman change in log DHEAS for pre/early peri-menopause to BSO was 0.074, corresponding to a 7.69% increase in DHEAS, while among the women with intact ovaries the mean within-woman change in log DHEAS for pre/early perimenopause to late peri-/early post-menopause was 0.048, corresponding to a 4.93% increase in DHEAS. Comparing these two estimated mean changes in log DHEAS, the between-group difference was -0.026 (SE 0.065, p=0.69). In short, the mean within-woman increase in DHEAS from pre-/early perimenopause to after BSO was larger than the corresponding increase from pre-/early perimenopause to late peri-/early postmenopause in the intact group, but this difference was not statistically significant, probably due to a small sample size for the BSO group
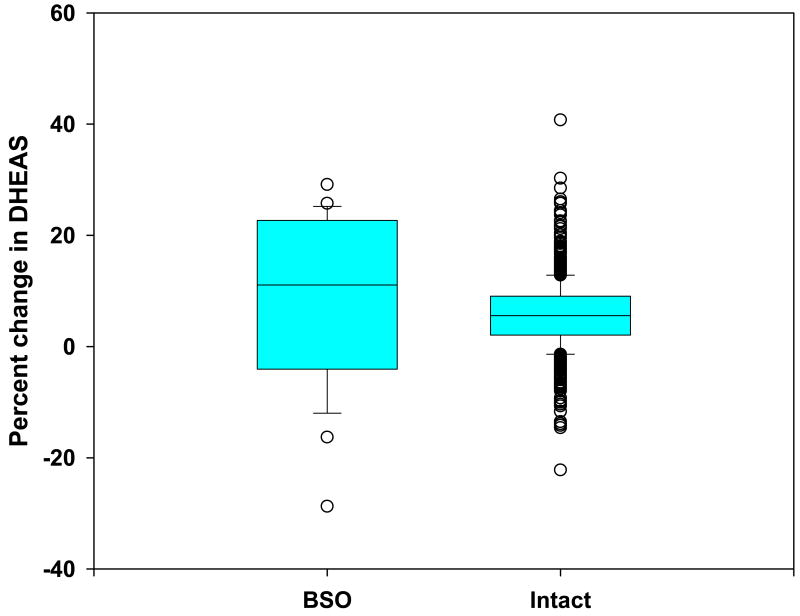
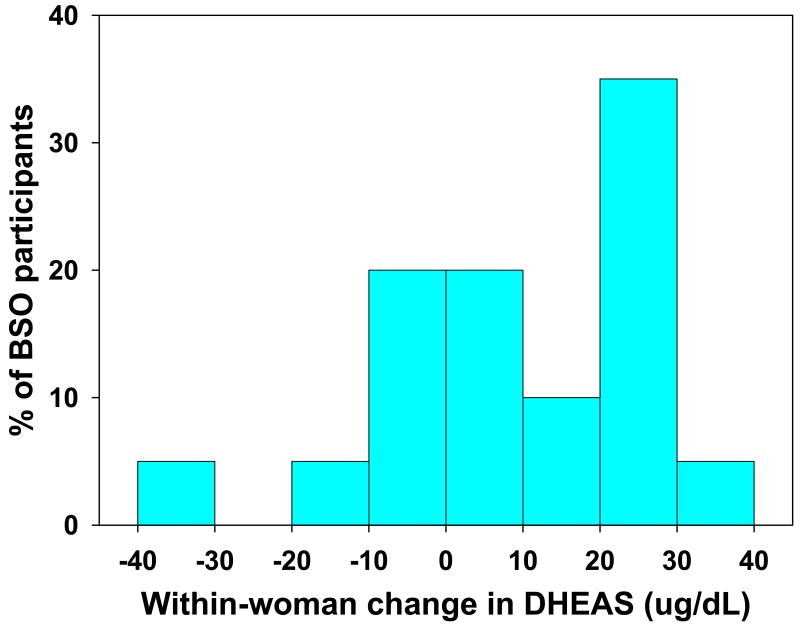
The adjusted within-woman change pre- and post-BSO (Figure 1) was positive for 70% (N=14) of the twenty BSO participants, compared with a positive adjusted within-woman change in DHEAS between pre- and early perimenopause and late peri-/early postmenopause occurring in 85% of women with intact ovaries (2). The range of increase for the fourteen women that exhibited a rise in DHEAS following surgery was from 2.5 to 31.6 ug/dL and the decline in DS for the six women that exhibited a fall in DHEAS ranged from 2.0 to 31.2 ug/dL (Figure 2).
Figure 1.
Distribution of within-woman percent change in circulating DHEAS in SWAN women who underwent bilateral salpingo oophorectomy (BSO) and those who traversed a natural menopause (intact).
Figure 2.
Distribution of amount of within-woman change in circulating DHEAS in the 20 women with BSO surgery at the pre-or early perimenopausal stage who were followed for at least one year post-surgery (n=20). Six of these women (30%) exhibited declines in DHEAS while 14 (70%) had a rise in DHEAS. Estimated within-woman changes were adjusted for age, ethnicity, clinical site, smoking, height and weight, and prior HT use.
Discussion
The present data demonstrate that the previously observed rise in DHEAS during the MT transition is evident even in the absence of the ovaries in 70% (14 of the 20) BSO women. These data suggest strongly that the menopause transition-associated rise in DHEAS is, in part, an adrenal phenomenon. While the observed increases in DHEAS levels are small on an annual basis, they represent a potentially large contribution of substrate for peripheral conversion to more bioactive sex steroids when the total sex steroid milieu of the menopausal transition is considered 10. Furthermore, these data suggest that individual differences in adrenal steroid production may be primarily responsible for individual differences in total circulating sex steroid levels during the menopausal transition and therefore contribute to the range of phenotypes that are observed at this time.
The predominant peripheral conversion products of DHEA in women are androgens and not estrogens 11, 12. Our data therefore also suggests that the androgen-estrogen balance may be determined by adrenal function as well as ovarian function in the years just preceding and at menopause. The decline in ovarian function, which appears to be requisite for inducing an increase in adrenal steroid production, can be either gradual as with natural menopause or abrupt as with surgical menopause as demonstrated here. Furthermore, once initiated, this induction is sustained for several years and continues past the menopause in women with intact ovaries. It would appear therefore that the initial decline of ovarian function provides a trigger for an event that may include years of subsequent increased adrenal steroidogenesis.
While these findings show that the ovaries are not required for the sustained rise of DHEAS through the MT, they do not exclude the ovaries from participating in that event. The source of circulating androgens in mid-aged women has been a contentious issue for decades and the present study provides some clarity to that controversy. Since the circulating levels of DHEAS are maintained after menopause at levels similar to premenopause 13, comparisons to earlier studies of postmenopausal women may provide useful information regarding the nature of ovarian steroid production. In one of the earliest studies, positive ovarian arterio-venous gradients for testosterone (T), androstenedione (Adione) and DHEA were documented in postmenopausal women and demonstrate the ability of the senescent ovary to continue to produce androgens including DHEA 5.
The pre-menopausal ovary contributes approximately half of the circulating T and Adione 14 and continues to produce androgens past the menopause (Longcope et al., 1980). However, there is controversy regarding the amount of androgens the human ovary produces at different life stages. Couzinet et al 15 found the necessary steroidogenic machinery for androgen production to be missing in post menopausal ovaries of women with adrenal insufficiency. This is in conflict with an earlier study 16 that reported DHEAS levels were more than 2-fold lower in women without ovaries vs. age-matched pre- and postmenopausal women with intact ovaries. In contrast, DHEAS levels were similar in 610 intact and 125 BSO women in the Rancho Bernardo Study and displayed a similar decline with age 17. These results do not support an ovarian contribution to circulating DHEAS in older women, and are consistent with the observations of an absence of a change in DHEAS levels in 20 postmenopausal women during the 6 weeks following BSO 18. The bulk of the data to date do not favor an ovarian contribution to the circulating pool of DHEAS.
There is limited but positive evidence to suggest that the human premenopausal ovary can produce significant amounts of DHEAS under specific conditions. Barnes and co-workers 19 treated a woman with isolated follicle stimulating hormone (FSH) deficiency with FSH, followed by human chorionic gondadotropin (hCG) to show that FSH exposure resulted in the ability of the ovary to respond to a luteinizing hormone stimulation by increasing DHEA production. Similarly, Wachs et al.7 demonstrated an increase in circulating DHEA following treatment of women with polycystic ovarian syndrome with recombinant FSH. More recently ovarian arterio-venous samples reveal a DHEA gradient several years after menopause 6. While all of these studies demonstrate the potential for the ovary to secrete DHEA, none of the study participants were normal, pre-menopausal, mid-aged women. Until there is additional evidence or a better understanding of the mechanism(s) involved, it seems reasonable to conclude that most of the increase in DHEAS observed during the menopausal transition is a result of increased adrenal steroid production.
There are at least two weaknesses in our study. Foremost is that there was no intention or experimental design to investigate adrenal function in the original SWAN design because at that time there was no rationale to do so. In fact, DHEAS was included in SWAN as a measure of somatic aging, with the assumption that circulating levels would continuously decline with chronological age and therefore provide a between-woman, independent reference for ovarian aging which was in line with conventional thinking at that time. Second, is the absence of any measure to determine if either the clearance or sulfation of DHEA changes in middle-age women. Fortunately, there is at least one study that indicates this is not the case 20.
Conclusion
In summary, we have demonstrated that the majority of middle aged women who are undergoing the menopause transition demonstrate a transient increase in circulating DHEAS that appears to be independent of ovarian function and evident in a similar proportion of women whether or not their ovaries had been surgically removed. These findings highlight the importance of acknowledging adrenal androgen production as a potentially important source of circulating sex steroids in peri- and post-menopausal women.
Acknowledgments
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women's Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Clinical Centers: University of Michigan, Ann Arbor – MaryFran Sowers, PI; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx – Rachel Wildman, PI 2010; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Sherry Sherman 1994 – present; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Linda Weglicki (pending approval).
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Kim Sutton-Tyrrell, PI 2001 – present; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
Funding: The Study of Women's Health Across the Nation (SWAN) was funded by the National Institute on Aging, the National Institute of Nursing Research and the NIH Office of Research on Women's Health.
Footnotes
Disclosure information: The authors declare they have no competing financial interests.
References
- 1.Lasley BL, Santoro N, Randolf JF, Gold EB, Crawford S, Weiss G, McConnell DS, Sowers MF. The relationship of circulating dehydroepiandrosterone, testosterone, and estradiol to stages of the menopausal transition and ethnicity. J Clin Endocrinol Metab. 2002;87:3760–3767. doi: 10.1210/jcem.87.8.8741. [DOI] [PubMed] [Google Scholar]
- 2.Crawford S, Santoro N, Laughlin GA, Sowers MF, McConnell D, Sutton-Tyrrell K, Weiss G, Vuga M, Randolph J, Lasley B. Circulating dehydroepiandrosterone sulfate concentrations during the menopausal transition. J Clin Endocrinol Metab. 2009;94:2945–2951. doi: 10.1210/jc.2009-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Labrie F, Archer D, Bouchard C, Fortier M, Cusan L, Gomez JL, Girard G, Baron M, Ayotte N, Moreau M, Dube R, Cote I, Labrie C, Lavoie L, Berger L, Gilbert L, Martel C, Balser J. Intravaginal dehydroepiandrosterone (Prasterone), a physiological and highly efficient treatment of vaginal atrophy. Menopause. 2009;16:907–922. doi: 10.1097/gme.0b013e31819e8e2d. [DOI] [PubMed] [Google Scholar]
- 4.Mamas L, Mamas E. Premature ovarian failure and dehydroepiandrosterone. Fertil Steril. 2009;91:644–646. doi: 10.1016/j.fertnstert.2007.11.055. [DOI] [PubMed] [Google Scholar]
- 5.Longcope C, Hunter R, Franz C. Steroid secretion by the postmenopausal ovary. Am J Obstet Gynecol. 1980;138:564–568. doi: 10.1016/0002-9378(80)90287-2. [DOI] [PubMed] [Google Scholar]
- 6.Fogle RH, Stanczyk FZ, Zhang X, Paulson RJ. Ovarian androgen production in postmenopausal women. J Clin Endocrinol Metab. 2007;92:3040–3043. doi: 10.1210/jc.2007-0581. [DOI] [PubMed] [Google Scholar]
- 7.Wachs DS, Coffler MS, Malcom PJ, Shimasaki S, Chang RJ. Increased androgen response to follicle-stimulating hormone administration in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:1827–1833. doi: 10.1210/jc.2007-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, Woods N. Executive summary: Stages of Reproductive Aging Workshop (STRAW) Fertil Steril. 2001;76:874–878. doi: 10.1016/s0015-0282(01)02909-0. [DOI] [PubMed] [Google Scholar]
- 9.Sternfeld B, Wang H, Quesenberry CP, Jr, Abrams B, Everson-Rose SA, Greendale GA, Matthews KA, Torrens JI, Sowers M. Physical activity and changes in weight and waist circumference in midlife women: findings from the Study of Women's Health Across the Nation. Am J Epidemiol. 2004;160:912–922. doi: 10.1093/aje/kwh299. [DOI] [PubMed] [Google Scholar]
- 10.Labrie F, Cusan L, Gomez JL, Martel C, Berube R, Belanger P, Chaussade V, Deloche C, Leclaire J. Changes in serum DHEA and eleven of its metabolites during 12-month percutaneous administration of DHEA. J Steroid Biochem Mol Biol. 2008;110:1–9. doi: 10.1016/j.jsbmb.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Bird CE, Murphy J, Boroomand K, Finnis W, Dressel D, Clark AF. Dehydroepiandrosterone: kinetics of metabolism in normal men and women. J Clin Endocrinol Metab. 1978;47:818–822. doi: 10.1210/jcem-47-4-818. [DOI] [PubMed] [Google Scholar]
- 12.Burger HG. Androgen production in women. Fertil Steril. 1978;77 4:S3–5. doi: 10.1016/s0015-0282(02)02985-0. 2002. [DOI] [PubMed] [Google Scholar]
- 13.Longcope C, Franz C, Morello C, Baker R, Johnston CC., Jr Steroid and gonadotropin levels in women during the peri-menopausal years. Maturitas. 1986;8:189–196. doi: 10.1016/0378-5122(86)90025-3. [DOI] [PubMed] [Google Scholar]
- 14.Judd HL, Judd GE, Lucas WE, Yen SS. Endocrine function of the postmenopausal ovary: concentration of androgens and estrogens in ovarian and peripheral vein blood. J Clin Endocrinol Metab. 1974;39:1020–1024. doi: 10.1210/jcem-39-6-1020. [DOI] [PubMed] [Google Scholar]
- 15.Couzinet B, Meduri G, Lecce MG, Young J, Brailly S, Loosfelt H, Milgrom E, Schaison G. The postmenopausal ovary is not a major androgen-producing gland. J Clin Endocrinol Metab. 2001;86:5060–5066. doi: 10.1210/jcem.86.10.7900. [DOI] [PubMed] [Google Scholar]
- 16.Cumming DC, Rebar RW, Hopper BR, Yen SS. Evidence for an influence of the ovary on circulating dehydroepiandrosterone sulfate levels. J Clin Endocrinol Metab. 1982;54:1069–1071. doi: 10.1210/jcem-54-5-1069. [DOI] [PubMed] [Google Scholar]
- 17.Laughlin GA, Barrett-Connor E. Sexual dimorphism in the influence of advanced aging on adrenal hormone levels: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2000;85:3561–3568. doi: 10.1210/jcem.85.10.6861. [DOI] [PubMed] [Google Scholar]
- 18.Sluijmer AV, Heineman MJ, De Jong FH, Evers JL. Endocrine activity of the postmenopausal ovary: the effects of pituitary down-regulation and oophorectomy. J Clin Endocrinol Metab. 1995;80:2163–2167. doi: 10.1210/jcem.80.7.7608272. [DOI] [PubMed] [Google Scholar]
- 19.Barnes RB, Rosenfield RL, Namnoum A, Layman LC. Effect of follicle-stimulating hormone on ovarian androgen production in a woman with isolated follicle-stimulating hormone deficiency. N Engl J Med. 2000;343:1197–1198. doi: 10.1056/NEJM200010193431614. [DOI] [PubMed] [Google Scholar]
- 20.Longcope C. Dehydroepiandrosterone metabolism. J Endocrinol. 1996;150 Suppl:S125–127. [PubMed] [Google Scholar]