Abstract
Rhodium-catalyzed oxidative cyclization of allylic hydroxylamine-derived sulfamate esters furnishes a novel family of bicyclic aziridines that serve as functional precursors to substituted diamines. Investigations with the N4-Troc form of these heterocycles have led to manifold improvements in reaction performance and scope, and have revealed unique differences in the stability and reactivity of such compounds dictated by the choice of N4-protecting group.
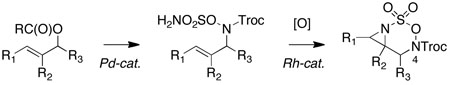
The rapid assembly of aza-heterocycles from sulfamate derivatives is made possible through selective C–H and π-bond amination methods.1 Our laboratories have recently reported that two catalytic processes, Pd π-allyl coupling and Rh-catalyzed alkene aziridination, may be used in sequence to generate a previously unknown class of strained heterobicyclic products.2 While the potential to synthesize differentially-substituted 1,2- and 1,3- diamines3,4,5,6 from these unique oxathiadiazinane derivatives was evident, little was known about the factors that govern their stability and reactivity. Herein, we detail new protocols to prepare highly substituted forms of these heterocycles, and highlight for the first time the unusual stability and utility of Troc-protected oxathiadiazinanes for 1,2-diamine synthesis (Figure 1).
Figure 1.
Novel heterocyclic substrates as precursors to substituted diamines.
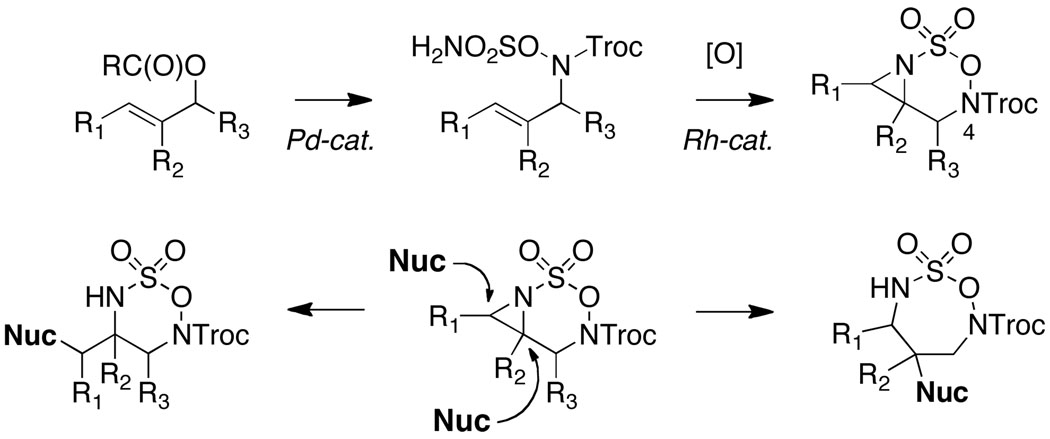
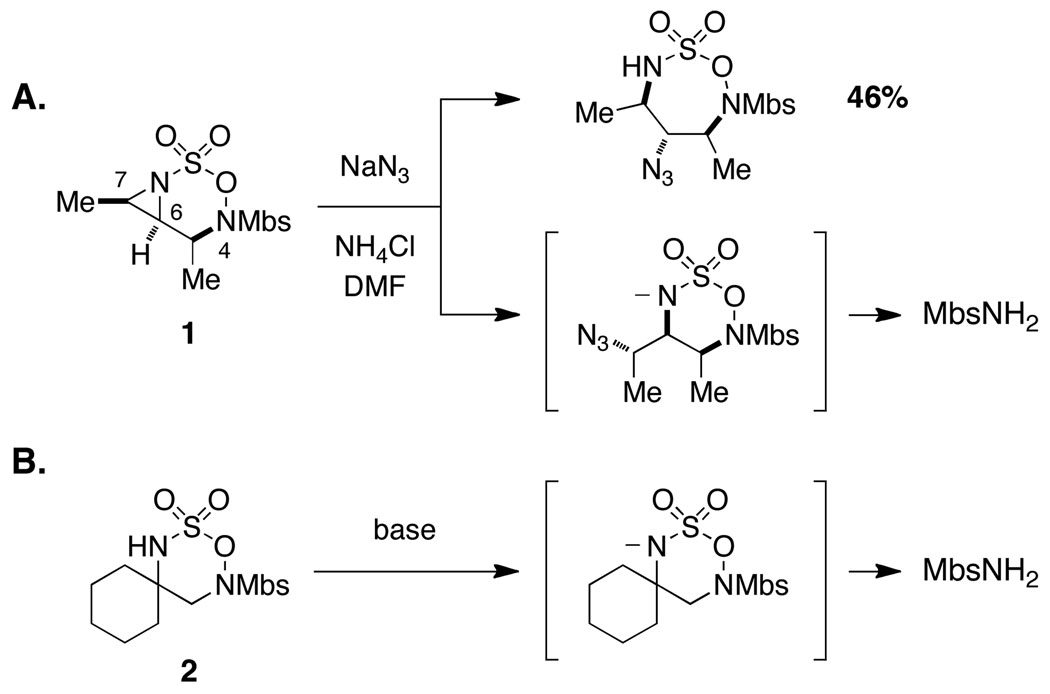
Our initial studies described the synthesis and reactivity of aziridine-fused N-Mbs-[1,2,3,6]-oxathiadiazinane-2,2-dioxide heterocycles, which were found, in select cases, to undergo nucleophilic ring opening with modest efficiency (Figure 2a).7,8,9 Studies to further optimize these addition reactions and to determine the origin of material loss led us to conclude that aziridine displacement occurred with little regiocontrol. The intermediate generated from nucleophilic attack at C7 was, however, unstable to the reaction conditions and decomposed to liberate MbsNH2. A series of control experiments using oxathiadiazinane 210 demonstrated that base additives (e.g., KN(SiMe3)2, pyridine, or NaH) would promote rapid ring fragmentation (Figure 2b). The unusual and unprecedented reactivity of the N-Mbs oxathiadiazinane heterocycle has no obvious explanation. We speculated that replacing the N4-Mbs group with an acyl substituent might mitigate the decomposition of these heterocycles by influencing the ring conformation. Such an alteration would present new challenges for substrate synthesis and would likely affect the oxidative cyclization reaction; however, the ease of removing the N4-acyl group as compared to the Mbs-would be advantageous for the overall utility of the method. Accordingly, N-Boc and N-Troc substrates were targeted with these considerations in mind.
Figure 2.
Unusual base-promoted decomposition of Mbs-substituted oxathiadiazinanes. Mbs = p-methoxybenzenesulfonyl.
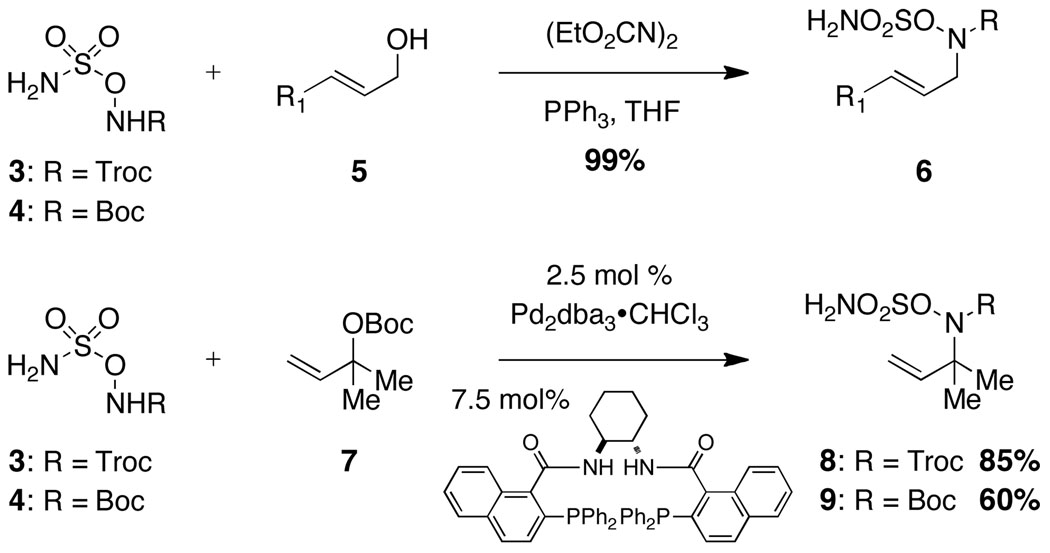
Synthesis of TrocNHOSO2NH2 3 and BocNHOSO2NH2 4 is easily accomplished in two steps from hydroxylamine. Both reagents are crystalline and can be prepared on >10 gram scale without recourse to chromatography. These sulfamate derivatives engage in either Mitsunobu or asymmetric π-allyl Pd-coupling reactions with alcohol or carbonate starting materials, respectively. The Troc-reagent 3 in particular operates with superior performance in both types of displacement reactions when compared to either 4 or the Mbs-variant. As shown in Figure 3, Pd-catalyzed π-allylation of 3 using carbonate 7 gives the α,α-disubstituted sulfamate in high yield and with excellent regiocontrol (>20:1 branched/linear product). The analogous reaction utilizing 4 proceeds in modest yields while the same reaction, when performed with MbsNHOSO2NH2, fails to give any allylated product. In light of these findings, most of our subsequent investigations have been conducted with the N-Troc sulfamate reagent 4. The identification of this sulfamate derivative should markedly facilitate access to a range of substituted oxathiadiazinane heterocycles and thus 1,2- and 1,3-diamine products.
Figure 3.
N-Carbamoyl sulfamate reagents perform optimally in allylation reactions.
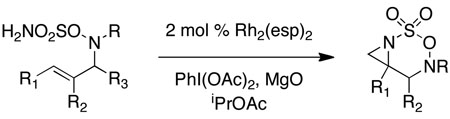
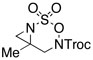
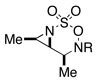
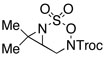
Intramolecular aziridination reactions with both Boc-and Troc-derived sulfamates are highlighted in Table 1. In general, cyclizations performed optimally using a dinuclear tetracarboxylate rhodium catalyst (2 mol %), PhI(OAc)2, and MgO.11 Oxidations occur smoothly in isopropyl acetate, which proves more effective as a solvent than others typically employed for Rh-catalyzed amination (e.g., CH2Cl2, benzene).12 Olefinic substitution does not have a large influence on the reaction as terminal (entries 1 and 2), di-substituted (entries 3 and 4), and tri-substituted (entry 5) alkenes are all converted in high yields to the corresponding bicyclic aziridines. While product yields of N-Boc substrates were slightly depressed, those of N-Troc substrates were excellent and comparable to those obtained using the analogous N-Mbs substrates.1,3b
Table 1.
Rh-catalyzed intramolecular aziridination.
 | |||||
|---|---|---|---|---|---|
| entrya | substrateb | product | yield | ||
| 1 | 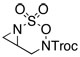 |
64c | |||
| 2 | 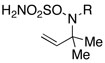 |
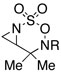 |
R = Troc | 98 | |
| R = Boc | 78 | ||||
| 3 | 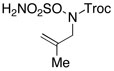 |
 |
94 | ||
| 4d | 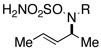 |
 |
R = Troc | 86 | 3:1 dr |
| R = Boc | 82 | 3:1 dr | |||
| R = Mbs | 93 | 13:1 dr | |||
| 5 | 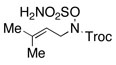 |
 |
82 | ||
| 6 | 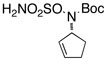 |
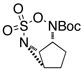 |
65 | ||
| 7 | 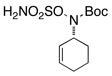 |
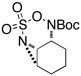 |
51 | ||
Reactions conducted in iPrOAc using 2 mol % Rh2(esp)2, 2.3 equiv of MgO and 1.1 equiv of PhI(OAc)2.
Substrates were prepared using either Mitsunobu or π-allyl Pd chemistry in yields ranging from 60–99%. See supporting information for details.
Isolated yield of this aziridine was reduced due to difficulties associated with its purification. See supporting information for details.
Product diastereomeric ratios determined by 1H NMR integration.
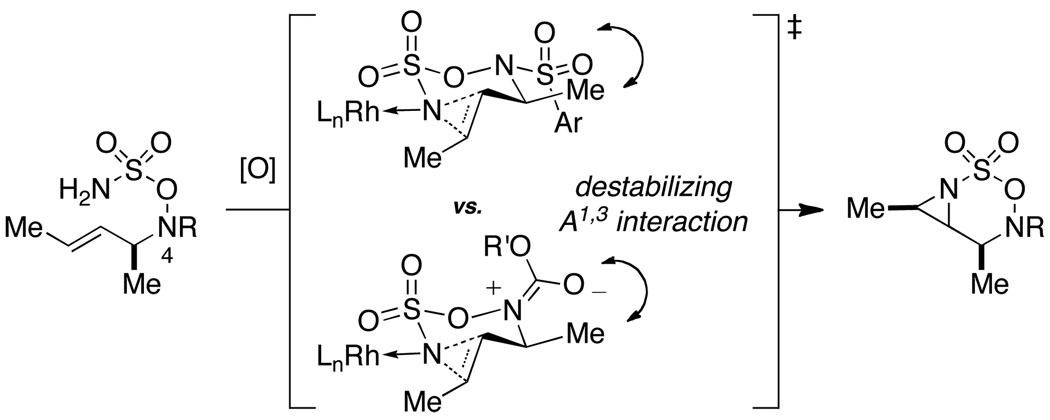
As noted in Table 1, modest to high levels of product diastereocontrol are observed for acyclic and cyclic alkene starting materials, respectively (entries 4, 6, 7). For acyclic structures (entry 4), stereoselectivity is clearly influenced by the choice of the N4-substituent group. The sense of induction in these products may be rationalized through a chair-like transition state model (Figure 4). Introduction of a destabilizing A1,3-type interaction in both the N-Boc and N-Troc sulfamate derivatives could account for the reduced degree of product stereocontrol in comparison to the N-sulfonyl compound.13 With cyclic olefins, cis-fused tricyclic structures are formed exclusively regardless of the N-blocking group.2
Figure 4.
Diastereoselectivity in aziridination reactions influenced by nitrogen protecting group (R = Troc, Boc, or Mbs; R’ = CH2CCl3, tBu).
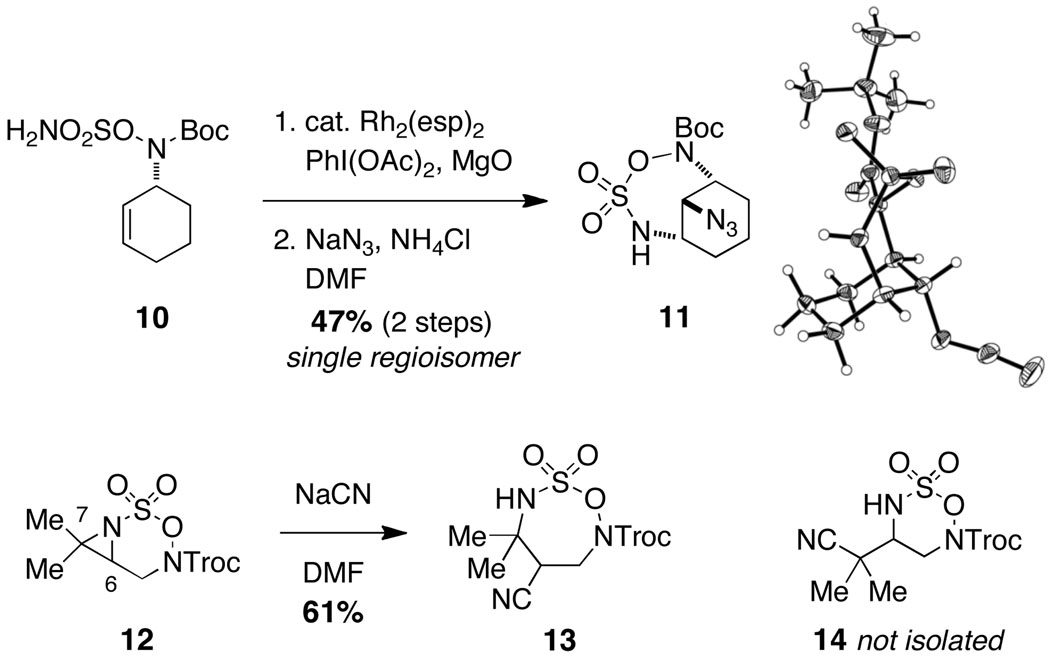
Oxathiadiazinane-derived aziridines undergo facile ring opening with a range of nucleophiles. In agreement with our earlier observation, tricyclic structures like the one depicted in entry 7 (Table 1) react to form bridged 7-membered ring products. One of these derivatives, 11, has been crystallographically characterized (Figure 5).14 Interestingly, the cyclohexane unit in 11 adopts an almost perfect chair-like configuration despite having all three substituent groups positioned axially. The preferential formation of 11 over the corresponding fused-bicyclic [4.4.0] isomer is likely the result of stereoelectronic factors, which favor a trans-diaxial transition structure for ring opening. The predilection for the aziridine derived from 10 and related tricyclic aziridines to ring open in this manner appears to be invariant of the choice of N-substituent group (i.e., acyl or sulfonyl).
Figure 5.
Regioselective aziridine ring-opening favors seven-membered ring projects.
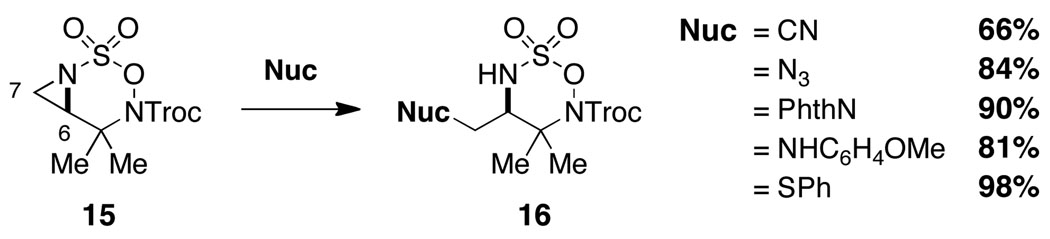
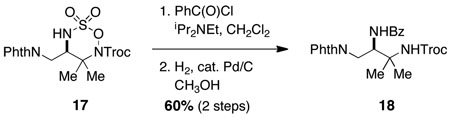
We have found that bicyclic aziridines, like their tricyclic counterparts, share a similar tendency for nucleophilic ring opening to afford 7-membered ring heterocycles; however, optimal selectivity is only achieved when steric factors reinforce this preference (Figure 5).15 Furthermore, judicious placement of substitutent groups can in select cases bias attack towards C7. As shown in Figure 6, nucleophilic displacement reactions with 15 afford the corresponding six-membered oxathiadiazinanes as the exclusive products. These N-Troc-substituted oxathiadiazinanes are stable to chromatography and are isolable, a finding that stands in stark contrast to the marked instability of analogous N-Mbs oxathiadiazinane derivatives (see Figure 2). As shown in eq 1, the N-Troc heterocycles serve as precursors to highly functionalized, differentially protected 1,2-diamines. While the assembly of structures of this type using traditional methods for amine synthesis would generally require multiple transformations, such products are now easily prepared from commercial allylic alcohols using the protocols outlined in this report.
Figure 6.
Isolable oxathiadiazinane derivatives through regioselective aziridine displacement.
We speculate that subtle conformational differences between the N-Troc 16 (see Figure 6) and N-Mbs 2 (see Figure 2) six-membered heterocycles are responsible for the striking difference in stability between these two structurally related species. Although the mechanism by which oxathiadiazinanes such as 2 undergo fragmentation to liberate MbsNH2 is still opaque, the switch from Mbs to a carbamoyl blocking group has resulted in manifold improvements in the overall aziridination process and, perhaps more importantly, has made available a potentially important class of six-membered heterocyclic structures as 1,2-diamine surrogates. These findings have motivated us to examine the use of saturated N-Troc hydroxylamine sulfamate esters as substrates for C–H amination.16
 |
(1) |
Rhodium-catalyzed alkene aziridination has given rise to a collection of unprecedented heterocyclic structures that serve as useful precursors to functionalized diamine products. In the course of developing these chemistries, we have identified an unexpected and heretofore unknown fragmentation reaction of N-sulfonyl oxathiadiazinanes. This reaction is entirely suppressed by changing the nature of the N-protecting group, a modification that enables for the first time isolation and subsequent reactions of structures such as 16. Importantly, the necessary N-Troc sulfamate starting materials can be synthesized in high yield through two different protocols, and perform exceptionally well in intramolecular Rh-catalyzed olefin aziridination. All told, our discoveries should facilitate the use of and greatly expand the application potential of this unique family of oxathiadiazinane heterocycles.
Supplementary Material
Acknowledgment
This work has been supported by the National Institutes of Health and the National Science Foundation. Partial funding for this work was also provided to J.D.B by the National Science Foundation Center for Stereoselective C–H Functionalization (CSCHF). D.E.O and S.M. gratefully acknowledge Eli Lilly and Stanford University, respectively, for fellowship support. Palladium salts were generously supplied by Johnson-Matthey. X-ray crystallographic analysis was performed by Dr. Allen Oliver; diffraction data were recorded on an instrument supported by the National Science Foundation, Major Research Instrumentation (MRI) Program under Grant No. CHE-0521569.
Footnotes
Supporting Information Available. Experimental procedures, characterization data, and X-ray crystallographic data. This material is available free of charge via the internet at http://pubs.acs.org.
Contributor Information
Barry M. Trost, Email: bmtrost@stanford.edu.
J. Du Bois, Email: jdubois@stanford.edu.
References
- 1.For general discussions on C–H and π-bond amination, see: Zalatan DN, Du Bois J. Top. Curr. Chem. 2010;292:347–378. doi: 10.1007/128_2009_19. Li Z, Capretto DA, He C. Silver in Organic Chemistry. John Wiley & Sons, Inc.; 2010. pp. 167–182. Collet F, Dodd R, Dauban PH. Chem. Comm. 2009:5061–5074. doi: 10.1039/b905820f. Davies HML, Manning JR. Nature. 2008;451:417–424. doi: 10.1038/nature06485.
- 2.Trost BM, Malhotra S, Olson DE, Maruniak A, Du Bois J. J. Am Chem. Soc. 2009;131:4190–4191. doi: 10.1021/ja809697p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.For general applications of diamines, see: Kizirian J. Chem. Rev. 2008;108:140–205. doi: 10.1021/cr040107v. Kotti SRSS, Timmons C, Li G. Chem. Biol. Drug Des. 2006;67:101–114. doi: 10.1111/j.1747-0285.2006.00347.x. de Parrodi CA, Juaristi E. Synlett. 2006;17:2699–2715.
- 4.For reviews on the synthesis of 1,2-diamines, see: Cardona F, Goti A. Nature Chem. 2009;1:269–275. doi: 10.1038/nchem.256. de Figueiredo RM. Angew. Chem. Int. Ed. 2009;48:1190–1193. Mortensen MS, O’Doherty GA. Chemtracts: Org. Chem. 2005;18:555–561. Lucet D, Le Gall T, Mioskowski C. Angew. Chem. Int. Ed. 1998;37:2580–2627. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2580::AID-ANIE2580>3.0.CO;2-L. and references therein.
- 5.For examples of polyamine-derived natural products, see: Busscher GF, Rutjes FPJT, van Delft FL. Chem. Rev. 2005;105:775–791. doi: 10.1021/cr0404085.
- 6.Differential diamine protection is possible using the diamination method of Shi, see: Du H, Zhao B, Shi Y. J. Am. Chem. Soc. 2007;129:762–763. doi: 10.1021/ja0680562. For the synthesis of differentially-protected 1,2-diamines through Rh-catalyzed C–H amination see Olson DE, Du Bois J. J. Am. Chem. Soc. 2008;130:11248–11249. doi: 10.1021/ja803344v.
- 7.We refer to these heterocycles as oxathiadiazinanes for simplicity.
- 8.Prior to our work, we are only aware of one report of these heterocycles, see Arfaei A, Smith S. J. Chem. Soc., Perkin Trans. 1984;1:1791–1794.
- 9.For the synthesis and reactivity of related aziridine-fused oxathiazinanes, see: Wehn PM, Du Bois J. Angew. Chem. 2009;121:3860–3863. Guthikonda K, Wehn PM, Caliando BJ, Du Bois J. Tetrahedron. 2006;62:11331–11342. Wehn PM, Lee J, Du Bois J. Org. Lett. 2003;5:4823–4826. doi: 10.1021/ol035776u. Duran F, Leman L, Ghini A, Burton G, Dauban P, Dodd RH. Org. Lett. 2002;4:2481–2483. doi: 10.1021/ol0200899.
- 10.Compound 2 was prepared using Rh-catalyzed C–H amination, see ref. 6.
- 11.Rh2(esp)2 is sold by Aldrich Chemical Co. as Rh2(α,α,α’,α’-tetramethyl-1,3-benzenedipropionate)2. For the initial report of this catalyst, see Espino CG, Fiori KW, Kim M, Du Bois J. J. Am. Chem. Soc. 2004;126:15378–15379. doi: 10.1021/ja0446294.
- 12.The use of i-PrOAc was prompted by an earlier report, see Chan J, Baucom KD, Murry JA. J. Am. Chem. Soc. 2007;129:14106–14107. doi: 10.1021/ja073872a.
- 13.A similar rationale was put forth to explain diastereoselectivity in analogous reactions involving sulfamides, see: Kurokawa T, Kim M, Du Bois J. Angew. Chem. Int. Ed. 2009;48:2777–2779. doi: 10.1002/anie.200806192.
- 14.Crystallographic data has been deposited at the Cambridge Crystallographic Data Centre (CCDC 809399).
- 15.Nucleophilic ring opening of bicyclic aziridines such as those featured in Figure 5 are currently limited to azide, cyanide and thiolate nucleophiles. Reactions performed with oxygen-based nucleophiles (e.g., carboxylates, alkoxides, peroxide) failed to give the desired 7-membered ring product.
- 16.Olson DE, Roberts DA, Du Bois J. manuscript in preparation. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.