Abstract
Background
N-3 fatty acids are associated with favorable, and obesity with unfavorable, concentrations of chronic disease risk biomarkers.
Objective
We examined whether high eicosapentaenoic (EPA) and docosahexaenoic (DHA) acid intakes, measured as percentages of total red blood cell (RBC) fatty acids, modify associations of obesity with chronic disease risk biomarkers.
Methods
In a cross-sectional study of 330 Yup'ik Eskimos, generalized additive models (GAM) and linear and quadratic regression models were used to examine associations of BMI with biomarkers across RBC EPA and DHA categories.
Results
Median (5th–95th percentile) RBC EPA and DHA were 2.6% (0.5–5.9%) and 7.3% (3.3–8.9%), respectively. In regression models, associations of BMI with triglycerides, glucose, insulin, C-reactive protein (CRP) and leptin differed significantly by RBC EPA and DHA. The GAM confirmed regression results for triglycerides and CRP: At low RBC EPA and RBC DHA, the predicted increases in triglycerides and CRP concentrations associated with a BMI increase from 25 to 35 were 99.5±45.3 mg/dl (106%) and 137.8±71.0 mg/dl (156%), respectively, for triglycerides and 1.2±0.7 mg/l (61%) and 0.8±1.0 mg/l (35%), respectively, for CRP. At high RBC EPA and RBC DHA, these predicted increases were 13.9±8.1 mg/dl (23%) and 12.0±12.3 mg/dl (18%), respectively, for triglycerides and 0.5±0.5 mg/l (50%) and −0.5±0.6 mg/l (−34%), respectively, for CRP.
Conclusions
In this population, high RBC EPA and DHA were associated with attenuated dyslipidemia and low-grade systemic inflammation among overweight and obese persons. This may help inform recommendations for n-3 fatty acid intakes in the reduction of obesity-related disease risk.
Keywords: EPA, DHA, generalized additive models, Yup'ik Eskimos, triglycerides, C-reactive protein
Introduction
The very long chain n-3 polyunsaturated fatty acids eicosapentaenoic acid (EPA; 20:5(n-3)) and docosahexaenoic acid (DHA; 22:6(n-3)), found primarily in fish and marine mammals, are associated with reduced risks of cardiovascular disease (CVD) (Kris-Etherton et al., 2002) and possibly diabetes (Nettleton and Katz, 2005). EPA and DHA have important biological functions including increased membrane fluidity, which affects nutrient transport and signal transduction (Alexander, 1998), eicosanoid metabolism (Fritsche, 2006) and gene expression and transcription (Sampath and Ntambi, 2005). In particular, high EPA and DHA intakes are associated with reduced concentrations of triglycerides (Block et al., 2008; Bonaa et al., 1992; Dewailly et al., 2001a; Dewailly et al., 2001b; Dewailly et al., 2002; Ferrucci et al., 2006; He et al., 2008; Motoyama et al., 2009; Okuda et al., 2005), C-reactive protein (CRP) (Farzaneh-Far et al., 2008; Klein-Platat et al., 2005; Niu et al., 2006) and proinflammatory cytokines, and increased concentrations of high-density lipoprotein (HDL) (Block et al., 2008; Dewailly et al., 2001a; Dewailly et al., 2001b; Dewailly et al., 2002; He et al., 2008; Motoyama et al., 2009; Okuda et al., 2005) and anti-inflammatory cytokines (Fritsche, 2006). Most of these biomarkers are also associated, albeit in the opposite direction, with obesity (Pi-Sunyer, 2002), which raises the question of whether the associations of obesity with chronic disease risk biomarkers could be modified by high EPA and DHA intakes.
To address this question, we analyzed data from Yup'ik Eskimos living in the Yukon Kuskokwim Delta region in Southwest Alaska, a population with mean (±SD) EPA and DHA intakes >20 times the current mean intakes of the general US population (4.1±0.5 g/d versus 0.05 g/d in men and 2.8±0.3 g/d versus 0.09 g/d in women) (Bersamin et al., 2008; Johnson et al., 2009) and obesity prevalence similar to that of the overall US population (Mohatt et al., 2007). Results of this study may provide evidence useful to developing recommendations for EPA and DHA intakes in the reduction of obesity-related disease risk.
Materials and methods
Participant recruitment and procedures
Data are from the Center for Alaska Native Health Research study, a cross-sectional community-based participatory study of obesity and disease risk in Yup'ik Eskimos. Study protocols were approved by the Institutional Review Boards of the University of Alaska, the National and Alaska Area Indian Health Service and the Yukon Kuskokwim Health Corporation Human Studies Committee.
Participant recruitment and procedures are described elsewhere (Boyer et al., 2005; Mohatt et al., 2007). In brief, between 2003 and 2006, 1003 participants aged ≥14 y and residing in 10 Southwest Alaskan communities were enrolled. Extensive socio-demographic and health-related data were collected through in-person interviews and self-administered questionnaires. Diet was assessed using a 24-h dietary recall and a 3-d food record. Height, weight, percent body fat (through bioelectrical impedance) and blood pressure were measured. Physical activity was measured using pedometers (counts/h). Blood samples collected in the field were separated into serum, plasma and packed RBC, frozen and stored locally at −20°C for up to 6 d and transferred to a central location and stored at −80°C.
Study sample
Blood samples in this study were from a subset of 497 participants selected from 7 out of the 10 participating communities. From 4 communities, we selected a random sample of 84 participants balanced across age strata (14–19, 20–49 and ≥50 y). The remaining 3 communities had <84 participants and thus all participants were included. We excluded 92 participants aged ≤18 y, 28 with CRP>1 mg/dl (acute inflammation), 20 with missing data on biochemical measurements, 10 with BMI<20 and 17 with BMI>40, leaving 330 for the present analyses.
Biochemical measurements
Disease risk measures examined in this study included: systolic blood pressure (SBP); diastolic blood pressure (DBP); triglycerides; low-density lipoprotein (LDL); HDL; total cholesterol; apolipoprotein A1 (apoA1); glucose; insulin; homeostasis model assessment of insulin resistance (HOMA-IR) index; insulin-like growth factor-I (IGF1); insulin-like growth factor binding protein-3 (IGFBP3); CRP; interleukin-6 (IL6); soluble tumor necrosis factor receptor-2 (sTNFR2); leptin; and adiponectin. Triglycerides, HDL, total cholesterol and apoA1 were measured with the Poly-Chem System Chemistry Analyzer (Polymedco Inc., Cortlandt Manor, NY). Intra- and inter-assay coefficients of variation (CV) were, respectively: 3.2% and 4.1% for triglycerides; 3.6% and 3.9% for HDL; 1.8% and 6.2% for total cholesterol; and 2.7% and 4.5% for apoA1. LDL was calculated as: total cholesterol−HDL−(triglycerides/5). Leptin, total adiponectin and fasting insulin were assayed using human-specific radioimmunoassay kits (Linco Research Inc., St. Charles, MO). Intra- and inter-assay CV were, respectively: 6.6% and 12.0% for leptin; 5.1% and 9.1% for adiponectin; and 5.8% and 10.2% for insulin. Fasting blood glucose was measured with a Cholestech LDX analyzer (Hayward, CA). Insulin resistance was assessed using the HOMA-IR index: (fasting insulin (μU/ml)×fasting glucose (mg/dl))/405 (Matthews et al., 1985). CRP was measured with an Immulite Analyzer and high sensitivity CRP reagents (Diagnostic Products Corporation, Los Angeles, CA). Manufacturer's intra-and inter-assay CV were 2.8% and 3.3%, respectively. IGF1, IGFBP3, IL6 and sTNFR2 were assayed using ELISA kits (Biosource, Carlsbad, CA and Diagnostic Systems Laboratories Inc, Webster, TX). Intra- and inter-assay CV were, respectively: 6.5% and 5.4% for IGF1; 8.8% and 10.0% for IGFBP3; 6.4% and 7.8% for IL6; and 4.2% and 3.3% for sTNFR2.
RBC fatty acid measurements
RBC fatty acids were analyzed as previously described (O'Brien et al., 2009). Briefly, fatty acids were extracted from washed RBC with 2-propanol and chloroform (Rose and Oklander, 1965). Fatty acids were converted to fatty acid methyl esters (FAME) by direct transesterification (Lepage and Roy, 1986). FAME were recovered in hexane, dried under nitrogen (40°C) and re-dissolved in hexane for gas chromatography analysis. The FAME of individual fatty acids were separated on a gas chromatograph (Model 5890B, Hewlett-Packard (HP), Agilent, Santa Clara, CA) equipped with a flame ionization detector, automatic sampler (HP 7673), electronic pressure programming (HP) and Chemstation software (HP). Quantitative precision and identification were evaluated using model mixtures of known FAME and an established control pool. The inter-assay CV was 2.7% for EPA and 2.0% for DHA. RBC EPA and DHA composition is reported as a weight percentage of total RBC fatty acids.
Statistical analyses
Triglycerides, HOMA-IR index, CRP, IL6, sTNFR2 and leptin concentrations were log-transformed. IL6 values (n=90) below the limit of detection (0.02 pg/ml) were replaced with (Hornung and Reed, 1990). Outliers for triglycerides (n=1), glucose (n=1), insulin (n=1), adiponectin (n=1), apoA1 (n=2) and sTNFR2 (n=3) were excluded because they were >4 SD above the mean and were physiologically unreasonable.
Participants’ demographic and health-related characteristics are given for the entire sample and stratified by BMI (kg/m2): <25 (normal-weight), 25–30 (overweight) and ≥30 (obese). Values are presented as means (±SD) or proportions, which were compared across BMI categories using ANOVA F-test and χ2 test, respectively.
Age- and sex-adjusted means of biomarkers stratified by BMI were computed using least square means. Adjusted means are presented with SE and log-transformed means are back-transformed for ease of interpretation and given with 95% CI. Tests for significant linear trends across BMI categories were based on age- and sex-controlled linear regression models.
We used three approaches to describe the associations of BMI with disease biomarkers at different RBC EPA and DHA levels. First, we used linear regression to test whether the BMI-biomarker associations differed significantly across the following RBC EPA and DHA categories: <25th percentile, 25th–75th percentiles and >75th percentile (<1.5%, 1.5–3.9% and >3.9% for EPA; <5.8%, 5.8–8.2% and >8.2% for DHA). Because we could not assume linear associations of BMI with disease biomarkers, we examined both linear and quadratic models in which the F-test for the interaction of BMI with RBC EPA and DHA categories was used to determine statistical significance. We report the linear models for all biomarkers and the quadratic models only when statistically significant. Second, we used non-parametric generalized additive models (GAM) (Wood, 2006) that included a two-dimensional thin-plate regression spline over BMI and RBC EPA and DHA to graphically display the BMI-biomarker associations across RBC EPA and DHA. GAM enable the fitted associations to take their natural shapes by relaxing assumptions about the form of the functional associations. To illustrate typical BMI-biomarker associations for the three RBC EPA and DHA categories, we present the estimated two-dimensional covariate-adjusted GAM at the 10th, 50th and 90th percentiles of RBC EPA (1%, 3% and 5%) and DHA (4%, 7% and 9%). Third, based on GAM, we predicted mean concentrations (±SE) of biomarkers at the 10th and 90th percentiles of RBC EPA and DHA for BMI 25, 30 and 35 using simple case resampling over 1000 bootstrap replicates. All models were adjusted for age (continuous), sex and current smoking (yes/no). Models for IGF1 were additionally adjusted for IGFBP3. We also considered whether control for RBC n-6 fatty acids, dietary macronutrient intake and physical activity would modify interactions between BMI and RBC EPA and DHA, because these can affect disease biomarkers. However, control for these variables did not affect results and these are therefore not included in final models. Alcohol consumption is prohibited in the participating Yup'ik communities and was therefore not considered as a potential covariate.
We report results for EPA and DHA separately for two reasons. First, DHA intake is very high in this population and while RBC EPA increases linearly in response to dietary intake, RBC DHA plateaus at ≈9% of total fatty acids (O'Brien et al., 2009), suggesting that RBC DHA does not reflect variability at high intake (Arterburn et al., 2006; Cao et al., 2006). Second, previous studies reported differential associations of EPA and DHA with disease biomarkers (Egert et al., 2009; Leigh-Firbank et al., 2002; Mori et al., 2000). We also present results for the n-3 index (RBC EPA+DHA) (Harris, 2009) as supplementary material.
Statistical analyses were performed using Stata/SE 11.0 (StataCorp LP, College Station, TX) and the R package mgcv version 1.4-0 (R-Project, 2009).
Results
Overall, the median age was 45.5 y, 57% were women and 71% were overweight or obese. RBC EPA and DHA ranged from 0.2% to 9.6% and 1.6% to 10.3%, respectively, with medians (5th–95th percentile) of 2.6% (0.5–5.9%) and 7.3% (3.3–8.9%). Women were more likely to be overweight and obese than men and smokers were less likely to be obese than non-smokers. There was a significant trend for increased BMI with increasing age. Dietary energy, total fat, carbohydrate, protein, EPA and DHA intakes, the means of RBC EPA and DHA and n-3 index and the proportions of persons in each RBC EPA and DHA and n-3 index category did not differ significantly across BMI groups (Table 1).
Table 1.
Demographic, dietary and health-related characteristics of all study participants and stratified by BMIa
| Allb | BMIc(kg/m2) |
P-trendd | |||
|---|---|---|---|---|---|
| <25 | 25 – 30 | ≥30 | |||
| n | 330 | 96 | 123 | 111 | |
| Sex | |||||
| Women | 187 (57) | 44 (23) | 64 (34) | 79 (42) | <0.01 |
| Men | 143 (43) | 52 (36) | 59 (41) | 32 (22) | |
| Age (years) | 44.8 ± 15.4 | 41.9 ± 15.4 | 44.8 ± 15.5 | 47.3 ± 15.0 | 0.04 |
| 18–29 | 65 (20) | 25 (38) | 26 (40) | 14 (21) | 0.11 |
| 30–54 | 169 (51) | 49 (29) | 58 (34) | 62 (37) | |
| ≥55 | 96 (29) | 22 (23) | 39 (41) | 35 (36) | |
| BMI (kg/m2) | 28.1 ± 4.8 | 22.7 ± 1.2 | 27.3 ± 1.4 | 33.6 ± 2.8 | <0.001 |
| Body fat (%) | 30.9 ± 9.5 | 22.1 ± 6.3 | 29.8 ± 6.2 | 39.7 ± 6.7 | <0.001 |
| Current smokers | 92 (28) | 40 (43) | 25 (27) | 27 (29) | <0.01 |
| Energy intake (kcal/d) | 1953 ± 878 | 2013 ± 903 | 2000 ± 948 | 1839 ± 752 | 0.32 |
| Total fat | |||||
| (g/d) | 87 ± 55 | 89 ± 58 | 89 ± 61 | 83 ± 45 | 0.65 |
| (% energy) | 38 ± 11 | 37 ± 12 | 38 ± 10 | 39 ± 11 | 0.63 |
| Carbohydrate | |||||
| (g/d) | 208 ± 114 | 223 ± 116 | 207 ± 120 | 196 ± 103 | 0.27 |
| (% energy) | 44 ± 14 | 46 ± 13 | 43 ± 14 | 43 ± 14 | 0.27 |
| Protein | |||||
| (g/d) | 87 ± 47 | 83 ± 35 | 95 ± 61 | 82 ± 35 | 0.09 |
| (% energy) | 19 ± 7 | 18 ± 6 | 20 ± 8 | 19 ± 8 | 0.13 |
| Dietary EPA (g/d) | 1.6 ± 2.2 | 1.3 ± 1.9 | 1.9 ± 2.7 | 1.6 ± 2.0 | 0.16 |
| Dietary DHA, (g/d) | 1.9 ± 2.7 | 1.6 ± 2.2 | 2.2 ± 3.2 | 1.8 ± 2.2 | 0.24 |
| RBC EPAe (% of total fatty acids) | 2.8 ± 1.8 | 2.5 ± 1.5 | 2.9 ± 2.0 | 3.0 ± 1.8 | 0.09 |
| <1.5 | 87 (26) | 26 (30) | 33 (38) | 28 (32) | 0.17 |
| 1.5–3.9 | 161 (49) | 54 (33) | 59 (37) | 48 (30) | |
| >3.9 | 82 (25) | 16 (19) | 31 (38) | 35 (43) | |
| RBC DHAe (% of total fatty acids) | 6.8 ± 1.8 | 6.6 ± 1.7 | 6.8 ± 1.8 | 7.0 ± 1.8 | 0.17 |
| <5.8 | 84 (25) | 29 (34) | 30 (36) | 25 (30) | 0.25 |
| 5.8–8.2 | 168 (51) | 51 (30) | 64 (38) | 53 (32) | |
| >8.2 | 78 (24) | 16 (20) | 29 (37) | 33 (42) | |
| N-3 indexe, f (% of total fatty acids) | 9.6 ± 3.4 | 9.0 ± 3.0 | 9.8 ± 3.5 | 10.0 ± 3.4 | 0.11 |
| <7.0 | 83 (25) | 28 (34) | 30 (36) | 25 (30) | 0.25 |
| 7.0-12.1 | 164 (50) | 51 (31) | 62 (38) | 51 (31) | |
| >12.1 | 83 (25) | 17 (20) | 31 (37) | 35 (42) | |
Values are means ± s.d. or n (%). Percentages may not sum to 100 due to rounding.
Percentages are column percents.
Percentages are row percents
Based on tests for trend across BMI using F tests from ANOVA for continuous variables and χ2 tests for categorical variables.
Categorized as <25th percentile, 25th to 75th percentiles and >75th percentile.
RBC EPA + DHA
BMI was positively associated with higher SBP, DBP, triglycerides, glucose, insulin, HOMA-IR index, CRP and leptin, and negatively associated with HDL, apoA1 and adiponectin. Mean LDL, total cholesterol, IGF1, IL6 and sTNFR2 concentrations did not differ significantly by BMI (Table 2).
Table 2.
Age- and sex-adjusted means of biomarkers of chronic disease risk for all study participants and stratified by BMIa
| All | BMI (kg/m2) |
P-trendb | |||
|---|---|---|---|---|---|
| <25 | 25 – 30 | ≥30 | |||
| n | 330 | 96 | 123 | 111 | |
| SBP (mm Hg) | 124.1 ± 0.8 | 121.6 ± 1.5 | 123.4 ± 1.3 | 127.1 ± 1.4 | 0.009 |
| DBP (mm Hg) | 72.7 ± 0.5 | 68.8 ± 0.9 | 71.7 ± 0.8 | 77.0 ± 0.9 | <0.001 |
| Triglyceridesc, d (mg/dl) | 80.5 (76.9–84.3) | 66.0 (60.9–71.6) | 77.1 (71.9–82.7) | 99.9 (92.7–107.7) | <0.001 |
| Total cholesterole (mg/dl) | 223.0 ± 2.3 | 223.5 ± 4.3 | 228.5 ± 3.7 | 216.6 ± 4.0 | 0.23 |
| LDL cholesterole (mg/dl) | 142.2 ± 2.0 | 138.7 ± 3.8 | 147.0 ± 3.3 | 139.9 ± 3.6 | 0.87 |
| HDL cholesterole (mg/dl) | 62.7 ± 0.9 | 71.0 ± 1.5 | 64.7 ± 1.3 | 53.4 ± 1.4 | <0.001 |
| ApoAl (g/l) | 1.74 ± 0.01 | 1.83 ± 0.02 | 1.77 ± 0.02 | 1.64 ± 0.02 | <0.001 |
| Glucosef (mg/dl) | 95.1 ± 0.6 | 91.4 ± 1.0 | 94.5 ± 0.9 | 99.0 ± 1.0 | <0.001 |
| Insuling (μU/ml) | 15.0 ± 0.4 | 12.0 ± 0.6 | 13.9 ± 0.5 | 18.7 ± 0.6 | <0.001 |
| HOMA-IRc | 3.2 (3.1–3.4) | 2.5 (2.3–2.7) | 3.1 (2.8–3.3) | 4.2 (3.9–4.6) | <0.001 |
| IGFl (μg/l) | 266.6 ± 4.7 | 267.1 ± 8.8 | 265.6 ± 7.7 | 267.4 ± 8.2 | 0.98 |
| IGFBP3 (μg/l) | 4391 ± 51 | 4240 ± 93 | 4227 ± 81 | 4699 ± 86 | <0.001 |
| CRPc (mg/l) | 0.9 (0.8–1.1) | 0.7 (0.5–0.9) | 0.9 (0.7–1.0) | 1.3 (1.1–1.6) | <0.001 |
| IL6c (ng/l) | 0.08 (0.07–0.09) | 0.07 (0.06–0.10) | 0.07 (0.06–0.09) | 0.10 (0.08–0.12) | 0.17 |
| sTNFR2c (ng/l) | 1995 (1869–2129) | 1963 (1734–2223) | 1974 (1773–2198) | 2045 (1824–2292) | 0.64 |
| Leptinc (μg/l) | 8.0 (7.4–8.6) | 4.1 (3.7–4.5) | 8.0 (7.3–8.7) | 13.9 (12.7–15.3) | <0.001 |
| Adiponectin (mg/l) | 8.8 ± 0.2 | 11.3 ± 0.4 | 9.0 ± 0.3 | 6.4 ± 0.4 | <0.001 |
Values are age- and sex-adjusted means ± SE or geometric means (95% CI) for log-transformed variables.
P values for trend across BMI categories based on linear regression models adjusted for age and sex.
Log-transformed values were used in the least square means analysis; adjusted means and 95% CIs were back-transformed.
To convert triglycerides from mg/dl to mmol/l, multiply by 0.0113.
To convert cholesterol (total, HDL and LDL) from mg/dl to mmol/l, multiply by 0.0259.
To convert glucose from mg/dl to mmol/l, multiply by 0.0555.
To convert insulin from μU/ml to pmol/l, multiply by 6.945.
Associations of BMI with disease biomarkers across RBC EPA and DHA
Based on either the linear or quadratic models (models 1 or 2 in Table 3, respectively), the associations of BMI with triglycerides, CRP, glucose and insulin differed significantly by both RBC EPA and DHA; associations with leptin differed only by RBC DHA. To best display these associations, Figure 1 and Supplementary Figure 1 show the GAM for the BMI-biomarker associations plotted at the 10th, 50th and 90th percentiles of RBC EPA and DHA. We also inspected the GAM for the other biomarkers and found that they were consistent with the lack of statistical significance in linear models. The linear but not quadratic models best approximated the GAM presented in Figure 1 and Supplementary Figure 1, and therefore only the linear models are described below. Similar results were obtained when using the n-3 index in the regression models (Supplementary Table) and GAMs (Supplementary Figure 2).
Table 3.
Associations of BMI with biomarkers of chronic disease risk by EPA and DHA, as percentages of total fatty acids in RBCa
| RBC EPAb (% of total fatty acids) |
RBC DHAb (% of total fatty acids) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1.5 (n = 87) | 1.5–3.9 (n = 161) | >3.9 (n = 82) | Pc | <5.8 (n = 84) | 5.8 – 8.2 (n = 168) | >8.2 (n = 78) | Pc | |||||||
| β ± SE | P | β ± SE | P | β ± SE | P | β ± SE | P | β ± SE | P | β ± SE | P | |||
| SBP (mm Hg) | 0.3 ± 0.3 | 0.34 | 0.5 ± 0.2 | 0.03 | 0.6 ± 0.3 | 0.07 | 0.83 | 0.4 ± 0.4 | 0.29 | 0.6 ± 0.2 | 0.01 | 0.4 ± 0.3 | 0.19 | 0.87 |
| DBP (mm Hg) | 1.0 ± 0.2 | <0.001 | 0.5 ± 0.1 | <0.001 | 0.8 ± 0.2 | <0.001 | 0.23 | 0.9 ± 0.2 | <0.001 | 0.6 ± 0.1 | <0.001 | 0.7 ± 0.2 | 0.001 | 0.50 |
| Triglyceridesd, e (mg/dl) | ||||||||||||||
| Model 1 | 0.06 ± 0.01 | <0.001 | 0.04 ± 0.01 | <0.001 | 0.02 ± 0.01 | 0.02 | 0.01 | 0.06 ± 0.01 | <0.001 | 0.04 ± 0.01 | <0.001 | 0.02 ± 0.01 | 0.01 | 0.03 |
| Model 2 | ||||||||||||||
| Linear | –0.31 ± 0.11 | 0.005 | 0.17 ± 0.07 | 0.01 | –0.01 ± 0.09 | 0.93 | <0.001 | –0.32 ± 0.12 | 0.008 | 0.07 ± 0.07 | 0.30 | 0.12 ± 0.09 | 0.19 | <0.001 |
| Quadratic | 0.006 ± 0.002 | 0.001 | –0.002 ± 0.001 | 0.05 | 0.0005 ± 0.001 | 0.75 | 0.006 ± 0.002 | 0.002 | –0.001 ± 0.001 | 0.62 | –0.002 ± 0.001 | 0.28 | ||
| Total cholesterolf (mg/dl) | 0.6 ± 1.0 | 0.50 | –0.7 ± 0.7 | 0.32 | –2.1 ± 0.9 | 0.02 | 0.12 | 0.5 ± 1.0 | 0.63 | –0.7 ± 0.7 | 0.28 | –1.8 ± 0.9 | 0.06 | 0.26 |
| LDL cholesterolf (mg/dl) | –0.2 ± 0.9 | 0.82 | 0.1 ± 0.6 | 0.84 | –0.6 ± 0.8 | 0.48 | 0.78 | –0.2 ± 0.9 | 0.79 | 0.1 ± 0.6 | 0.91 | –0.4 ± 0.8 | 0.60 | 0.87 |
| HDL cholesterolf (mg/dl) | –1.2 ± 0.3 | 0.001 | –1.4 ± 0.2 | <0.001 | –1.8 ± 0.3 | <0.001 | 0.46 | –1.4 ± 0.4 | <0.001 | –1.4 ± 0.2 | <0.001 | –1.7 ± 0.3 | <0.001 | 0.78 |
| ApoAl (g/l) | –0.02 ± 0.005 | <0.001 | –0.02 ± 0.003 | <0.001 | –0.02 ± 0.004 | <0.001 | 0.99 | –0.02 ± 0.005 | <0.001 | –0.02 ± 0.003 | <0.001 | –0.01 ± 0.005 | 0.003 | 0.64 |
| Glucoseg (mg/dl) | 0.7 ± 0.2 | 0.003 | 0.4 ± 0.2 | 0.02 | 1.1 ± 0.2 | <0.001 | 0.03 | 0.6 ± 0.2 | 0.01 | 0.5 ± 0.2 | 0.005 | 1.2 ± 0.2 | <0.001 | 0.03 |
| Insulin11 (μU/ml) | ||||||||||||||
| Model 1 | 0.8 ± 0.1 | <0.001 | 0.6 ± 0.1 | <0.001 | 0.6 ± 0.1 | <0.001 | 0.42 | 0.8 ± 0.1 | <0.001 | 0.6 ± 0.1 | <0.001 | 0.6 ± 0.1 | <0.001 | 0.47 |
| Model 2 | ||||||||||||||
| Linear | –6.5 ± 1.7 | <0.001 | 0.9 ± 1.1 | 0.41 | 1.5 ± 1.4 | 0.30 | 0.001 | –5.5 ± 1.8 | 0.003 | –0.2 ± 1.1 | 0.88 | 2.3 ± 1.4 | 0.11 | 0.01 |
| Quadratic | 0.12 ± 0.03 | <0.001 | –0.006 ± 0.019 | 0.76 | –0.01 ± 0.02 | 0.53 | 0.11 ± 0.03 | 0.001 | 0.01 ± 0.02 | 0.49 | –0.03 ± 0.02 | 0.25 | ||
| HOMA-IRd | 0.05 ± 0.01 | <0.001 | 0.04 ± 0.01 | <0.001 | 0.05 ± 0.01 | <0.001 | 0.83 | 0.05 ± 0.01 | <0.001 | 0.04 ± 0.01 | <0.001 | 0.05 ± 0.01 | <0.001 | 0.71 |
| IGF1i (μg/l) | –4.9 ± 1.7 | 0.004 | –1.6 ± 1.2 | 0.18 | –2.6 ± 1.6 | 0.11 | 0.28 | –3.3 ± 1.8 | 0.07 | –2.6 ± 1.2 | 0.03 | –1.4 ± 1.6 | 0.39 | 0.72 |
| IGFBP3 (μg/l) CRPd (mg/l) | 33.9 ± 21.7 | 0.12 | 42.3 ± 15.2 | 0.006 | 57.1 ± 20.8 | 0.006 | 0.73 | 47.5 ± 22.8 | 0.04 | 45.8 ± 14.8 | 0.002 | 39.5 ± 20.9 | 0.06 | 0.96 |
| Model 1 | 0.08 ± 0.03 | 0.002 | 0.05 ± 0.02 | 0.02 | 0.05 ± 0.03 | 0.05 | 0.49 | 0.06 ± 0.03 | 0.02 | 0.07 ± 0.02 | <0.001 | 0.02 ± 0.03 | 0.37 | 0.38 |
| Model 2 | ||||||||||||||
| Linear | –0.76 ± 0.33 | 0.02 | 0.04 ± 0.20 | 0.84 | 0.51 ± 0.27 | 0.06 | 0.03 | –0.75 ± 0.35 | 0.03 | 0.02 ± 0.20 | 0.93 | 0.35 ± 0.27 | 0.20 | 0.07 |
| Quadratic | 0.01 ± 0.01 | 0.01 | 0.0001 ± 0.003 | 0.98 | –0.008 ± 0.004 | 0.09 | 0.01 ± 0.01 | 0.02 | 0.001 ± 0.003 | 0.80 | –0.005 ± 0.004 | 0.23 | ||
| IL6d, ng/l | 0.06 ± 0.03 | 0.06 | 0.02 ± 0.02 | 0.31 | 0.01 ± 0.03 | 0.82 | 0.46 | 0.06 ± 0.03 | 0.05 | 0.003 ± 0.02 | 0.87 | 0.04 ± 0.03 | 0.13 | 0.23 |
| sTNFR2d (ng/l) | –0.001 ± 0.015 | 0.97 | 0.004 ± 0.01 | 0.67 | 0.007 ± 0.014 | 0.61 | 0.93 | 0.001 ± 0.015 | 0.94 | –0.002 ± 0.01 | 0.86 | 0.023 ± 0.014 | 0.10 | 0.33 |
| Leptind (μg/l) | 0.11 ± 0.01 | <0.001 | 0.11 ± 0.01 | <0.001 | 0.08 ± 0.01 | <0.001 | 0.19 | 0.12 ± 0.01 | <0.001 | 0.11 ± 0.01 | <0.001 | 0.08 ± 0.01 | <0.001 | 0.03 |
| Adiponectin (mg/l) | –0.36 ± 0.09 | <0.001 | –0.45 ± 0.06 | <0.001 | –0.35 ± 0.09 | <0.001 | 0.59 | –0.34 ± 0.10 | 0.001 | –0.39 ± 0.06 | <0.001 | –0.45 ± 0.09 | <0.001 | 0.71 |
Values are β coefficients ± SE and corresponding P values from linear (Model 1) and quadratic (Model 2) regression models controlling for age (continuous), sex and current smoking status (yes, no). Quadratic models are reported only when statistically significant.
Categories are <25th percentile, 25th to 75th percentiles and >75th percentile.
P values for interaction are based on the likelihood ratio F-test test comparing linear regression models with and without interaction of BMI (continuous) with RBC EPA and DHA (categorical).
Log-transformed values were used for the regression analysis.
To convert triglycerides from mg/dl to mmol/l, multiply by 0.0113.
To convert cholesterol (total, HDL and LDL) from mg/dl to mmol/l, multiply by 0.0259.
To convert glucose from mg/dl to mmol/l, multiply by 0.0555.
To convert insulin from μU/ml to pmol/l, multiply by 6.945.
Regression model additionally adjusted for IGFBP3.
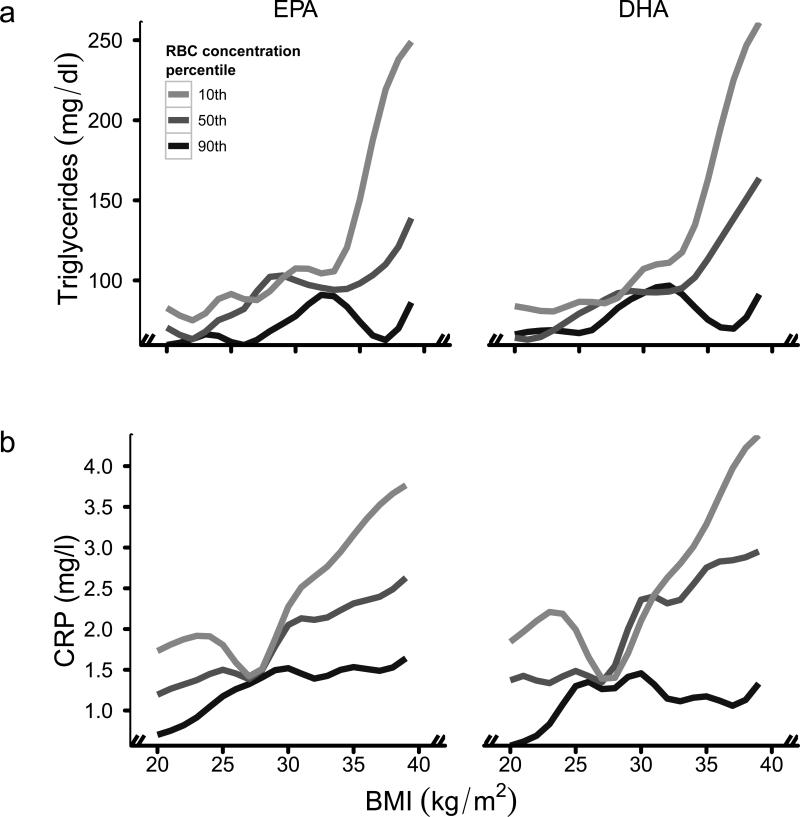
Figure 1.
Generalized Additive Models (GAM) of the associations of BMI with triglycerides (a) and CRP (b) by eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), as percentages of total fatty acids in RBC. The GAM include a bivariate smooth surface over EPA or DHA and BMI (all continuous). The estimated GAM were evaluated at mean values of age, gender and current smoking status and at the 10th (light grey line), 50th (dark grey line) and 90th (black line) percentiles of RBC EPA (1%, 3% and 5% of total fatty acids) and DHA (4%, 7% and 9% of total fatty acids). To convert triglycerides from mg/dl to mmol/l, multiply by 0.0113.
Triglycerides
In linear regression models, the positive associations of BMI with triglycerides were attenuated with increasing RBC EPA and DHA concentrations (Table 3). The GAM showed strong positive associations of BMI with triglycerides at low RBC EPA and DHA concentrations, which became weaker at moderate concentrations and flat at high concentrations (Figure 1a). At low RBC EPA and DHA, the predicted increases in triglyceride concentrations associated with a BMI increase from 25 to 35 were 99.5±45.3 mg/dl (106%) and 137.8±71.0 mg/dl (156%), respectively. At high RBC EPA and DHA, these predicted increases were 13.9±8.1 mg/dl (23%) and 12.0±12.3 mg/dl (18%), respectively.
C-reactive protein
In linear regression models, the positive association of BMI with CRP was modestly, but not significantly, attenuated in the moderate and high RBC EPA categories and the high RBC DHA category (Table 3). The GAM showed clear differential associations of BMI with CRP by RBC EPA and DHA at BMI>28; strong positive associations of BMI with CRP among persons with low RBC EPA and DHA were weak at moderate concentrations and flat at high concentrations (Figure 1b). At low RBC EPA and DHA, the predicted increases in CRP concentrations associated with a BMI increase from 30 to 35 were 0.8±0.7 mg/l (32%) and 1.0±0.8 mg/l (45%), respectively. At high RBC EPA and DHA, these predicted increases were 0.1±0.6 mg/l (6%) and −0.5±0.6 mg/l (−32%), respectively. Similarly, an increase in BMI from 25 to 35 at low versus high RBC EPA and DHA was associated with similar, albeit less significant, differences in the predicted increases in CRP concentrations. These were 1.2±0.7 mg/l (61%) and 0.8±1.0 mg/l (35%) at low RBC EPA and DHA, respectively, and 0.5±0.5 mg/l (50%) and −0.5±0.6 mg/l (−34%) at high RBC EPA and DHA, respectively.
Glucose and insulin
In linear regression models, there were significant positive associations of BMI with glucose in the highest RBC EPA and DHA categories, which were significantly and similarly attenuated in the moderate and low RBC EPA and DHA categories (Table 3). For insulin, there were significant positive associations with BMI in the lowest category of RBC EPA and DHA, which were slightly, but not significantly, attenuated in the moderate and high RBC EPA and DHA categories (Table 3). The GAM for glucose and insulin (Supplementary Figure 1) were complex but overall showed no differential associations with BMI by RBC EPA and DHA.
Leptin
The positive association of BMI with leptin was significantly weaker in the high compared to the moderate and low categories of RBC DHA (Table 3). However, GAM showed no differential association by RBC DHA (Supplementary Figure 1).
Discussion
In this population-based sample of Yup'ik Eskimos, the strong positive associations of obesity with triglycerides and CRP concentrations were substantially attenuated in adults with high RBC EPA and DHA. There were suggestive findings for glucose, insulin and leptin in linear regression models, but GAM provided little support for differential associations of BMI with these biomarkers at different RBC EPA and DHA levels. We judge the GAM to better represent associations of obesity with disease biomarkers (discussed below) and therefore consider the limited findings for glucose, insulin and leptin to be uninformative. There were no differential associations of BMI with all other biomarkers by RBC EPA and DHA levels.
Most studies on the associations of EPA and DHA with chronic disease biomarkers were conducted in populations with relatively low and narrow range of EPA and DHA intakes and none of these studies examined the interactions of EPA and DHA with obesity. However, a number of published studies indirectly support our findings. First, obesity is strongly associated with elevated triglycerides (Lamon-Fava et al., 1996). Second, most observational studies (Blocket al., 2008; Bonaa et al., 1992; Ferrucci et al., 2006; He et al., 2008; Motoyama et al., 2009), including those on populations with chronic, high EPA and DHA intakes (Dewailly et al., 2001b; Dewailly et al., 2002), and supplementation studies (Damsgaard et al., 2008; Kelley et al., 2007; Leigh-Firbank et al., 2002; Maki et al., 2005; Mori et al., 2000; Schwellenbach et al., 2006; Woodman et al., 2002) reported decreased triglycerides concentrations with increasing EPA and DHA intakes.
Similarly, obesity is strongly and positively associated with CRP (Visser et al., 1999). Furthermore, most observational studies reported inverse associations of EPA and DHA intakes with CRP (Fernandez-Real et al., 2003; Klein-Platat et al., 2005; Lopez-Garcia et al., 2004; Niuet al., 2006; Pischon et al., 2003). The single study that addressed whether these associations differed by BMI found a stronger association of EPA with CRP among overweight versus normal-weight adolescents (Klein-Platat et al., 2005), a finding consistent with ours. Most EPA and DHA supplementation studies found no effect on CRP (Damsgaard et al., 2008; Fujioka et al., 2006; Madsen et al., 2003; Yusof et al., 2008). These studies did not examine the interaction of obesity with EPA and DHA. The effect may be specific to a BMI subgroup and therefore could not be detected in the overall sample. Also, associations of EPA and DHA with CRP may be nonlinear, as previously shown (Makhoul et al., 2010), and therefore could not be detected using conventional linear regression models.
Our findings may have important clinical relevance for the prevention of some obesity-related diseases. Obesity prevalence in the US (Ogden et al., 2006) and worldwide (James et al., 2001) has been increasing over the past decades, with subsequent increases in rates of diabetes and other obesity-associated diseases (Pi-Sunyer, 2002). It is likely that these associations are partly mediated by the positive associations of obesity with triglycerides and CRP, two biomarkers that strongly and independently predict risks of CVD (Nordestgaard et al., 2007; Pai et al., 2004) and possibly diabetes (Hu et al., 2009; Pradhan et al., 2001; Tirosh et al., 2008). Chronic, high EPA and DHA intakes, similar to those of Yup'ik Eskimos, could at least partly ameliorate the obesity-associated disease risks.
Indeed, observations in Yup'ik Eskimos suggest that EPA and DHA intakes may lower their risk for diabetes. Yup'ik Eskimos have levels of obesity similar to those observed in the general US population and yet their prevalence of type 2 diabetes is significantly lower (3.3% (Mohatt et al., 2007) versus 7.7% in NHANES 2005–2006 (Cowie et al., 2009)). We recognize that genetic, lifestyle and dietary factors may account for this difference; yet it is consistent with our findings that chronic, high EPA and DHA consumption in Yup'ik Eskimos may protect overweight and obese persons against obesity-associated dyslipidemia and low-grade systemic inflammation and as a result may partially explain the low prevalence of diabetes in this population.
This study has several strengths. First, our study population was uniquely suitable for examining associations among BMI, disease biomarkers and RBC EPA and DHA: Yup'ik Eskimos have a broad range of EPA and DHA intakes (Bersamin et al., 2008) and also a broad BMI distribution (Mohatt et al., 2007). Second, we used RBC EPA and DHA concentrations, in preference to diet assessment, because they provide unbiased dietary intake measures and they better reflect biologically relevant exposure. Third, our analytical approach of using both GAM and linear regression was unique: linear models provide straightforward inference about the dependence of BMI-biomarker associations on EPA and DHA, while GAM allow for a more data-driven quantification of these associations based on a more flexible set of model assumptions (Wood, 2006).
This study also has limitations. First, although RBC EPA and DHA were previously found to be significantly associated with HDL, LDL and total cholesterol (Makhoul et al., 2010), we did not detect statistically significant differences in the associations of BMI with these biomarkers at different RBC EPA and DHA levels probably due to limited sample size in the RBC EPA and DHA categories. Second, this study was cross-sectional and associations observed may not be causal. Third, although we adjusted for several confounders, we are aware that unmeasured factors could affect RBC fatty acids composition (such as fatty acid desaturase gene polymorphisms) (Simopoulos, 2010), weight and disease biomarkers. Finally, our findings require replication in other populations.
In conclusion, high EPA and DHA intakes may attenuate the associations of obesity with dyslipidemia and low-grade systemic inflammation, two strong risk factors for CVD and diabetes. While these findings need to be replicated, they motivate experimental research on the uses of high-dose n-3 fatty acid supplementation for the attenuation of obesity-related disease risk.
Supplementary Material
Acknowledgements
We thank all study participants and their communities and the CANHR research team that made this study and manuscript possible. We thank Irena King and her staff at the Fred Hutchinson Cancer Research Center, Seattle, Washington, for the red blood cell fatty acid analyses. We thank Mario Kratz for his thoughtful input.
This project has been funded by a National Institute of Health (NIH) grant P20 RR016430, a Centers for Biomedical Research Excellence grant from the National Center for Research Resources (NCRR), and NIH grant R01 DK074842 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR, NIDDK, NIH or the National Science Foundation.
Footnotes
Supplementary information is available at The European Journal of Clinical Nutrition's website
Conflict of Interest
The authors declare no conflict of interest.
References
- Alexander J. Immunonutrition: the role of omega-3 fatty acids. Nutrition. 1998;14:627–633. doi: 10.1016/s0899-9007(98)00004-5. [DOI] [PubMed] [Google Scholar]
- Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83:1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- Bersamin A, Luick BR, King IB, Stern JS, Zidenberg-Cherr S. Westernizing Diets Influence Fat Intake, Red Blood Cell Fatty Acid Composition, and Health in Remote Alaskan Native Communities in the Center for Alaska Native Health Study. J Am Diet Assoc. 2008;108:266–273. doi: 10.1016/j.jada.2007.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block RC, Harris WS, Reid KJ, Sands SA, Spertus JA. EPA and DHA in blood cell membranes from acute coronary syndrome patients and controls. Atherosclerosis. 2008;197:821–828. doi: 10.1016/j.atherosclerosis.2007.07.042. [DOI] [PubMed] [Google Scholar]
- Bonaa KH, Bjerve KS, Nordoy A. Habitual fish consumption, plasma phospholipid fatty acids, and serum lipids: the Tromso study. Am J Clin Nutr. 1992;55:1126–1134. doi: 10.1093/ajcn/55.6.1126. [DOI] [PubMed] [Google Scholar]
- Boyer BB, Mohatt GV, Lardon C, Plaetke R, Luick BR, Hutchison SH, et al. Building a community-based participatory research center to investigate obesity and diabetes in Alaska Natives. Int J Circumpolar Health. 2005;64:281–290. doi: 10.3402/ijch.v64i3.18002. [DOI] [PubMed] [Google Scholar]
- Cao J, Schwichtenberg KA, Hanson NQ, Tsai MY. Incorporation and clearance of omega-3 fatty acids in erythrocyte membranes and plasma phospholipids. Clin Chem. 2006;52:2265–2272. doi: 10.1373/clinchem.2006.072322. [DOI] [PubMed] [Google Scholar]
- Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, et al. Full accounting of diabetes and pre-diabetes in the US population in 1988-1994 and 2005-2006. Diabetes Care. 2009;32:287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsgaard CT, Frøkier H, Andersen AD, Lauritzen L. Fish oil in combination with high or low intakes of linoleic acid lowers plasma triacylglycerols but does not affect other cardiovascular risk markers in healthy men. J Nutr. 2008;138:1061–1066. doi: 10.1093/jn/138.6.1061. [DOI] [PubMed] [Google Scholar]
- Dewailly E, Blanchet C, Gingras S, Lemieux S, Sauve L, Bergeron J, et al. Relations between n-3 fatty acid status and cardiovascular disease risk factors among Quebecers. Am J Clin Nutr. 2001a;74:603–611. doi: 10.1093/ajcn/74.5.603. [DOI] [PubMed] [Google Scholar]
- Dewailly E, Blanchet C, Lemieux S, Sauve L, Gingras S, Ayotte P, et al. n-3 Fatty acids and cardiovascular disease risk factors among the Inuit of Nunavik. Am J Clin Nutr. 2001b;74:464–473. doi: 10.1093/ajcn/74.4.464. [DOI] [PubMed] [Google Scholar]
- Dewailly E, Blanchet C, Gingras S, Lemieux S, Holub BJ. Cardiovascular disease risk factors and n-3 fatty acid status in the adult population of James Bay Cree. Am J Clin Nutr. 2002;76:85–92. doi: 10.1093/ajcn/76.1.85. [DOI] [PubMed] [Google Scholar]
- Egert S, Kannenberg F, Somoza V, Erbersdobler HF, Wahrburg U. Dietary α-linolenic acid, EPA, and DHA have differential effects on LDL fatty acid composition but similar effects on serum lipid profiles in normolipidemic humans. J Nutr. 2009;139:861–868. doi: 10.3945/jn.108.103861. [DOI] [PubMed] [Google Scholar]
- Farzaneh-Far R, Harris WS, Garg S, Na B, Whooley MA. Inverse association of erythrocyte n-3 fatty acid levels with inflammatory biomarkers in patients with stable coronary artery disease: The Heart and Soul Study. Atherosclerosis. 2008;205:538–543. doi: 10.1016/j.atherosclerosis.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Real JM, Broch M, Vendrell J, Ricart W. Insulin resistance, inflammation, and serum fatty acid composition. Diabetes Care. 2003;26:1362–1368. doi: 10.2337/diacare.26.5.1362. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, et al. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab. 2006;91:439–446. doi: 10.1210/jc.2005-1303. [DOI] [PubMed] [Google Scholar]
- Fritsche K. Fatty acids as modulators of the immune response. Ann Rev Nutr. 2006;26:45–73. doi: 10.1146/annurev.nutr.25.050304.092610. [DOI] [PubMed] [Google Scholar]
- Fujioka S, Hamazaki K, Itomura M, Huan M, Nishizawa H, Sawazaki S, et al. The effects of eicosapentaenoic acid-fortified food on inflammatory markers in healthy subjects--A randomized, placebo-controlled, double-blind study. J Nutr Sci Vitaminol. 2006;52:261–265. doi: 10.3177/jnsv.52.261. [DOI] [PubMed] [Google Scholar]
- Harris WS. The omega-3 index: From biomarker to risk marker to risk factor. Current Atherosclerosis Reports. 2009;11:411–417. doi: 10.1007/s11883-009-0062-2. [DOI] [PubMed] [Google Scholar]
- He K, Liu K, Daviglus ML, Mayer-Davis E, Jenny NS, Jiang R, et al. Intakes of long-chain n-3 polyunsaturated fatty acids and fish in relation to measurements of subclinical atherosclerosis. Am J Clin Nutr. 2008;88:1111–1118. doi: 10.1093/ajcn/88.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- Hu G, Jousilahti P, Tuomilehto J, Antikainen R, Sundvall J, Salomaa V. Association of serum C-reactive protein level with sex-specific type 2 diabetes risk: a prospective Finnish study. J Clin Endocrinol Metab. 2009;94:2099–2105. doi: 10.1210/jc.2008-2260. [DOI] [PubMed] [Google Scholar]
- James PT, Leach R, Kalamara E, Shayeghi M. The worldwide obesity epidemic. Obesity. 2001;9:228S–233S. doi: 10.1038/oby.2001.123. [DOI] [PubMed] [Google Scholar]
- Johnson JS, Nobmann ED, Asay E, Lanier AP. Dietary intake of Alaska native people in two regions and implications for health: The Alaska Native Dietary and Subsistence Food Assessment Project. Int J Circumpolar Health. 2009;68:109–122. doi: 10.3402/ijch.v68i2.18320. [DOI] [PubMed] [Google Scholar]
- Kelley DS, Siegel D, Vemuri M, Mackey BE. Docosahexaenoic acid supplementation improves fasting and postprandial lipid profiles in hypertriglyceridemic men. Am J Clin Nutr. 2007;86:324–333. doi: 10.1093/ajcn/86.2.324. [DOI] [PubMed] [Google Scholar]
- Klein-Platat C, Drai J, Oujaa M, Schlienger JL, Simon C. Plasma fatty acid composition is associated with the metabolic syndrome and low-grade inflammation in overweight adolescents. Am J Clin Nutr. 2005;82:1178–1184. doi: 10.1093/ajcn/82.6.1178. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- Lamon-Fava S, Wilson PWF, Schaefer EJ. Impact of body mass index on coronary heart disease risk factors in men and women: the Framingham Offspring Study. Arterioscler Thromb Vasc Biol. 1996;16:1509–1515. doi: 10.1161/01.atv.16.12.1509. [DOI] [PubMed] [Google Scholar]
- Leigh-Firbank EC, Minihane AM, Leake DS, Wright JW, Murphy MC, Griffin BA, et al. Eicosapentaenoic acid and docosahexaenoic acid from fish oils: differential associations with lipid responses. Br J Nutr. 2002;87:435–445. doi: 10.1079/BJNBJN2002556. [DOI] [PubMed] [Google Scholar]
- Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res. 1986;27:114–120. [PubMed] [Google Scholar]
- Lopez-Garcia E, Schulze MB, Manson JAE, Meigs JB, Albert CM, Rifai N, et al. Consumption of (n-3) fatty acids is related to plasma biomarkers of inflammation and endothelial activation in women. J Nutr. 2004;134:1806–1811. doi: 10.1093/jn/134.7.1806. [DOI] [PubMed] [Google Scholar]
- Madsen T, Christensen JH, Blom M, Schmidt EB. The effect of dietary n-3 fatty acids on serum concentrations of C-reactive protein: a dose–response study. Br J Nutr. 2003;89:517–522. doi: 10.1079/BJN2002815. [DOI] [PubMed] [Google Scholar]
- Makhoul Z, Kristal AR, Gulati R, Luick B, Bersamin A, Boyer B, et al. Associations of very high intakes of eicosapentaenoic and docosahexaenoic acids with biomarkers of chronic disease risk among Yup'ik Eskimos. Am J Clin Nutr. 2010;91:777–785. doi: 10.3945/ajcn.2009.28820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki KC, Van Elswyk ME, McCarthy D, Hess SP, Veith PE, Bell M, et al. Lipid responses to a dietary docosahexaenoic acid supplement in men and women with below average levels of high density lipoprotein cholesterol. J Am Coll Nutr. 2005;24:189–199. doi: 10.1080/07315724.2005.10719465. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Mohatt GV, Plaetke R, Klejka J, Luick B, Lardon C, Bersamin A, et al. The Center for Alaska Native Health Research study: a community-based participatory research study of obesity and chronic disease-related protective and risk factors. Int J Circumpolar Health. 2007;66:8–18. doi: 10.3402/ijch.v66i1.18219. [DOI] [PubMed] [Google Scholar]
- Mori TA, Burke V, Puddey IB, Watts GF, O'Neal DN, Best JD, et al. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am J Clin Nutr. 2000;71:1085–1094. doi: 10.1093/ajcn/71.5.1085. [DOI] [PubMed] [Google Scholar]
- Motoyama KR, Curb JD, Kadowaki T, El-Saed A, Abbott RD, Okamura T, et al. Association of serum n-6 and n-3 polyunsaturated fatty acids with lipids in 3 populations of middle-aged men. Am J Clin Nutr. 2009;90:49–55. doi: 10.3945/ajcn.2008.26761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettleton JA, Katz R. N-3 long-chain polyunsaturated fatty acids in type 2 diabetes: a review. J Am Diet Assoc. 2005;105:428–440. doi: 10.1016/j.jada.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Niu K, Hozawa A, Kuriyama S, Ohmori-Matsuda K, Shimazu T, Nakaya N, et al. Dietary long-chain n-3 fatty acids of marine origin and serum C-reactive protein concentrations are associated in a population with a diet rich in marine products. Am J Clin Nutr. 2006;84:223–229. doi: 10.1093/ajcn/84.1.223. [DOI] [PubMed] [Google Scholar]
- Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- O'Brien DM, Kristal AR, Jeannet MA, Wilkinson MJ, Bersamin A, Luick B. Red blood cell δ 15N: a novel biomarker of dietary eicosapentaenoic acid and docosahexaenoic acid intake. Am J Clin Nutr. 2009;89:913–919. doi: 10.3945/ajcn.2008.27054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Okuda N, Ueshima H, Okayama A, Saitoh S, Nakagawa H, Rodriguez BL, et al. Relation of long chain n-3 polyunsaturated fatty acid intake to serum high density lipoprotein cholesterol among Japanese men in Japan and Japanese–American men in Hawaii: The INTERLIPID Study. Atherosclerosis. 2005;178:371–379. doi: 10.1016/j.atherosclerosis.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai JK, Pischon T, Ma J, Manson JAE, Hankinson SE, Joshipura K, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer FX. The obesity epidemic: pathophysiology and consequences of obesity. Obese Res. 2002;10:97S–104S. doi: 10.1038/oby.2002.202. [DOI] [PubMed] [Google Scholar]
- Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Willett WC, Rimm EB. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation. 2003;108:155–160. doi: 10.1161/01.CIR.0000079224.46084.C2. [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Manson JAE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- R-Project . R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2009. [Google Scholar]
- Rose HG, Oklander M. Improved procedure for the extraction of lipids from human erythrocytes. J Lipid Res. 1965;6:428–431. [PubMed] [Google Scholar]
- Sampath H, Ntambi JM. Polyunsaturated fatty acid regulation of genes of lipid metabolism. Ann Rev Nutr. 2005;25:317–340. doi: 10.1146/annurev.nutr.25.051804.101917. [DOI] [PubMed] [Google Scholar]
- Schwellenbach LJ, Olson KL, McConnell KJ, Stolcpart RS, Nash JD, Merenich JA. The triglyceride-lowering effects of a modest dose of docosahexaenoic acid alone versus in combination with low dose eicosapentaenoic acid in patients with coronary artery disease and elevated triglycerides. J Am Coll Nutr. 2006;25:480–485. doi: 10.1080/07315724.2006.10719562. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. Genetic variants in the metabolism of omega-6 and omega-3 fatty acids: their role in the determination of nutritional requirements and chronic disease risk. Exp Biol Med. 2010;235:785–795. doi: 10.1258/ebm.2010.009298. [DOI] [PubMed] [Google Scholar]
- Tirosh A, Shai I, Bitzur R, Kochba I, Tekes-Manova D, Israeli E, et al. Changes in triglyceride levels over time and risk of type 2 diabetes in young men. Diabetes Care. 2008;31:2032–2037. doi: 10.2337/dc08-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- Wood SN. Generalized additive models: an introduction with R. CRC Press; 2006. [Google Scholar]
- Woodman RJ, Mori TA, Burke V, Puddey IB, Watts GF, Beilin LJ. Effects of purified eicosapentaenoic and docosahexaenoic acids on glycemic control, blood pressure, and serum lipids in type 2 diabetic patients with treated hypertension. Am J Clin Nutr. 2002;76:1007–1015. doi: 10.1093/ajcn/76.5.1007. [DOI] [PubMed] [Google Scholar]
- Yusof HM, Miles EA, Calder P. Influence of very long-chain n-3 fatty acids on plasma markers of inflammation in middle-aged men. Prostaglandins Leukot Essent Fatty Acids. 2008;78:219–228. doi: 10.1016/j.plefa.2008.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.