Abstract
Pdx1 is a homeobox transcription factor required for the embryonic development of the pancreas. Pdx1 expression has been previously identified in pancreatic ductal adenocarcinomas and endocrine neoplasms. This study characterizes Pdx1 protein expression in pancreatic precursor lesions and neoplasms, including pancreatic intraepithelial neoplasia (PanIN, n=32), intraductal papillary mucinous neoplasm (IPMN, n=88), mucinous cystic neoplasm (MCN, n=3), acinar cell carcinoma (ACC, n=8), pancreatic endocrine neoplasm (PEN, n=44), pancreatoblastoma (PB, n=1), solid pseudopapillary neoplasm (SPN, n=8), invasive ductal adenocarcinoma (n=67) and non-dysplastic ductal epithelium. A mouse monoclonal antibody for Pdx1 was used to examine archived surgical pathology cases and tissue microarrays containing >655 tissue cores from over 250 pancreatic specimens. Immunohistochemical labeling for Pdx1 was performed using standard methods, and scored for percentage and intensity of nuclear labeling. Among non-neoplastic pancreatic tissues, Pdx1 nuclear protein was expressed in islet cells, cells of the centroacinar cell compartment, and non-neoplastic ductal epithelium. No expression of Pdx1 was seen in non-neoplastic acinar cells. Among pancreatic neoplasms, Pdx1 consistently labeled >50% of the tumor cells in 87.5% of ACC cases and 38.6% of PEN cases. Pdx1 expression was variable in invasive ductal adenocarcinoma and precursor lesions of ductal adenocarcinomas (PanIN, IPMN and MCN). A single case of PB was examined and it showed Pdx1 in the acinar component, but no expression in squamoid nests. SPNs did not express Pdx1. This study demonstrates Pdx1expression in precursor lesions of ductal adenocarcinomas, ductal adenocarcinomas, PEN, ACC and a case of PB. In the immunohistochemical evaluation of neoplasms of the pancreas, Pdx1 expression is not a finding specific to pancreatic endocrine neoplasms and ductal adenocarcinomas, but also occurs in precursor lesions (PanIN, IPMN, MCN) and other neoplasms of the pancreas.
Keywords: Pdx1, Pancreas, Adenocarcinoma, Acinar cell carcinoma, Pancreatoblastoma
Introduction
Pdx1 (pancreatic and duodenal homeobox 1) is a critical transcription factor in the embryologic development of the pancreas.1 Pdx1 is important for both the embryologic development of the pancreas as well as the maintenance of mature pancreatic islets. Homozygous deletion of Pdx1 in the mouse results in pancreatic agenesis;2 similarly a patient with pancreatic agenesis was studied and found to have single nucleotide deletion in the Pdx1 coding region.3
The mechanisms and patterns of pancreatic Pdx1 expression in injury and carcinogenesis have been elucidated over the last decade. Initially, much of the work focused on the induction of Pdx1 expression in animal models of injury. One of the initial studies observed induction of Pdx1 in a rat model of pancreatectomy induced pancreas regeneration.4 This study demonstrated a positive correlation between ductal proliferation following pancreatectomy and Pdx1 protein levels. In addition to studies of the regulation Pdx1 expression, the Pdx1 promoter has been used as a tool for pancreatic specific expression in transgenic mice.5–8
Because of Pdx1’s key role in the embryologic development of the pancreas and its induction in injury and regeneration, the expression of Pdx1 has been examined in pancreatic cancer cell lines and human tumors.9–11 Studies of human pancreatic carcinomas have found Pdx1 to be expressed in approximately 40% of pancreatic ductal carcinomas with minimal expression in the pancreatic exocrine tissue adjacent to tumor. Pdx1 expression in pancreatic carcinomas has been shown to positively correlate with lymph node metastasis and increased mortality. In these previous studies, pancreatic cancer metastases were found to retain Pdx1 protein expression; indeed, liver metastases had higher expression compared to the primary tumors. Taken together, the prior studies of Pdx1 expression in pancreatic cancers suggest that Pdx1 expression in pancreatic ductal carcinomas is a poor prognostic factor. There have been several studies that examined Pdx1 expression in non-ductal carcinomas, including pancreatic endocrine neoplasms,12–13 as well as solid pseudopapillary neoplasms.14 The present study is an investigation of Pdx1 expression in precursor lesions of pancreatic ductal adenocarcinomas as well as pancreatic neoplasms that have not been previously characterized.
MATERIALS AND METHODS
This study was performed at the Johns Hopkins University and approved by the Johns Hopkins University Institutional Review Board.
Tissue specimens
As previously described, tissue microarrays were constructed from surgical pathology samples in the archives of the Departments of Pathology at the Johns Hopkins Hospital and Memorial Sloan-Kettering Cancer Center.15 The archived samples consisted of formalin-fixed, paraffin-embedded tissue blocks. Representative paraffin tissue cores were punched by a manual tissue punch/arrayer (Beecher Instruments, Silver Spring, MD). Four cores with a 1.4 mm diameter were extracted from each case and were transferred into recipient TMA blocks. Cases with loss of either lesion or matched normal cores were excluded from this study. After excluding these cases, we evaluated Pdx1 nuclear labeling in 12 tissue microarrays containing >655 tissue cores from over 250 specimens. These tissue specimens included non-neoplastic normal tissue controls as well as the following lesions: pancreatic intraepithelial neoplasia (PanIN), intraductal papillary mucinous neoplasm (IPMN), mucinous cystic neoplasm (MCN), acinar cell carcinomas (ACC), well-differentiated pancreatic endocrine neoplasms (PEN), solid pseudopapillary neoplasms (SPN), invasive ductal adenocarcinoma and non-dysplastic ductal epithelium. A single pancreatoblastoma (PB) case was evaluated from a standard section of a paraffin block. In the case of IPMN, subtype analysis based on intestinal, pancreatobiliary or gastric type was not performed.
A subset of the tissue microarray cases were from 67 patients with pancreatic ductal adenocarcinomas who had pancreatic surgery (Whipple procedure, distal pancreatectomy, or total pancreatectomy) at the Johns Hopkins Hospital and had survival information previously established.16 Of the 67 patients, 37 were men, and 30 were women. They had a mean age of 69.0 years at the time of surgery (range, 39–90 years).
Several non-neoplastic extra-pancreatic tissues with no pathologic changes were also examined for Pdx1 nuclear labeling including 15 stomach, 8 duodenum, 10 small intestines (jejunum), 4 large intestines, 3 gall bladder, 25 liver, 11 prostate, 23 kidney, 11 ovary, 4 skin, 11 spleen, 7 thyroid, 8 lung, 10 breast, 18 brain (cerebellum), 10 tonsils, and 3 placenta.
Immunohistochemistry
Pdx1 mouse monoclonal antibody that recognizes the C-terminus of Pdx1 (amino acids 91 to 283) (MAB2419, clone 267712; R&D Systems, Minneapolis, MN) was used for immunohistochemistry. In brief, formalin-fixed paraffin embedded tissue sections were stained on a Bond-Leica autostainer (Leica Microsystems, Bannockburn, IL). All tissue sections were baked for 20 minutes at 65°C prior to loading on the autostainer. Heat induced epitope retrieval was performed by high pH (8.0) buffer. This was followed by a 15 minute room temperature incubation with the primary antibody at 1:100 and 1:50 dilutions for TMA and standard paraffin tissue sections, respectively. The reaction was developed with the biotin-free Bond Polymer Refine Detection system (Leica Microsystems, Bannockburn, IL). A secondary anti-mouse antibody conjugated to 3,3′-diaminobenzidine label was used as a chromogenic reporter molecule. Negative controls included non-pancreatic tissue as well as normal saline substitution for primary antibody.
Scoring
Immunohistochemical labeling for Pdx1 on TMA were scored by two pathologists (JYP, SMH). Each tissue type was scored for the percentage of positive nuclear labeling (0=0%, 1=1–25%, 2=26–50%, 3=51–75%, 4=76–100%). In addition, a composite score was created by multiplying the percentage score by an intensity of nuclear labeling (0, 1+, 2+). The controls for scoring were the nuclei of Pdx1-labeled islet cells (e.g., islet cells have a percentage score of “4” multiplied by an intensity score of “2” for the maximum composite score of “8”).
Statistical Analysis
ANOVA and Duncan test was performed for comparison of the composite immunohistochemistry scores. Overall patients’ survival was defined as the time from surgical resection of PDAs to the death of the patients or the last follow-up of the patients. Survival rates were calculated using the Kaplan-Meier method and statistical significance was evaluated using the log-rank test. p-values less than 0.05 were considered significant.
RESULTS
The tissue from TMAs available for evaluation included 250 unique tumors and precursor lesions including: 32 PanIN, 88 IPMN, 3 MCN, 8 ACC, 44 PEN, 8 SPN, and 67 invasive ductal adenocarcinomas (Table 1). There were an additional 73 TMA cases of non-dysplastic ductal epithelia from pancreatic cancer resection cases. A single pancreatoblastoma case was evaluated by immunohistochemistry separate from the TMA cases.
Table 1.
Pdx1 Immunohistochemitry.
| Tissue Type | Percentage positive cells | Composite Score* |
||||
|---|---|---|---|---|---|---|
| 0% | 1–25% | 26–50% | 51–75% | 76–100% | ||
| Normal pancreatic ductal epithelium (n=73) |
17 | 5 | 10 | 7 | 34 | 2.79±1.79 |
| PanIN | 2.44±2.12 | |||||
| PanIN1 (n=14) | 4 | 0 | 5 | 2 | 3 | |
| PanIN2 (n=16) | 2 | 3 | 3 | 1 | 7 | |
| PanIN3 (n=2) | 2 | 0 | 0 | 0 | 0 | |
| IPMN | 1.72±1.96 | |||||
| IPMN, mild dysplasia (n=41) |
24 | 0 | 2 | 2 | 13 | |
| IPMN, moderate dysplasia (n=27) |
14 | 0 | 3 | 4 | 6 | |
| IPMN, severe dysplasia (n=20) |
10 | 2 | 2 | 3 | 3 | |
| MCN (n=3) | 1 | 0 | 0 | 1 | 1 | 4.11±3.68 |
| Ductal adenocarcinoma (n=67) | 40 | 9 | 9 | 5 | 4 | 0.99±1.63 |
| Pancreatic endocrine neoplasm (n=44) |
21 | 2 | 4 | 3 | 14 | 1.87±2.19 |
| Acinar cell carcinoma (n=8) | 0 | 0 | 1 | 1 | 6 | 4.58±3.62 |
| Solid Pseudopapillary (n=8) | 7 | 1 | 0 | 0 | 0 | 0.40±0.12 |
Composite score represents a percentage score (0% =0; 1% to 25% = 1; 26% to 50% =2; 51% to 75% = 3; 76% to 100% =4) multiplied by a score for intensity ± 1 S.D.
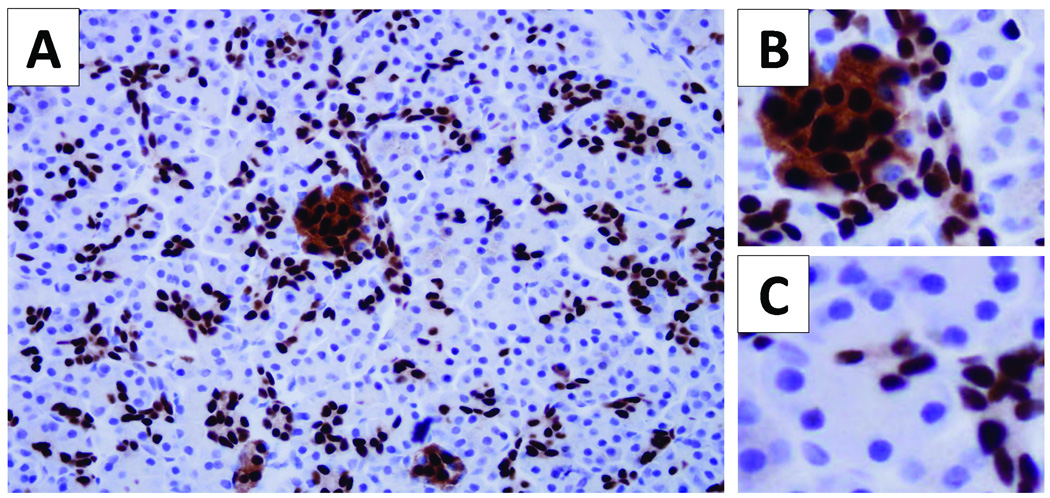
Pdx1 nuclear labeling by immunohistochemistry was consistently present in the non-neoplastic islet cells and cells of the centroacinar cell compartment (Figure 1A and 1B). No acinar cell labeling was identified (Figure 1C). Outside of the centroacinar cell compartment, Weak and variable Pdx1 nuclear labeling was identified in both intralobular and interlobular ducts. Non-dysplastic duct epithelia in 56% of cases had Pdx1 nuclear labeling in greater than 50% of the cells examined (Table 1).
Figure 1.
Pdx1 expression pattern in normal pancreas. Pdx1 nuclear labeling occurs in cells of the islets of Langerhans, small ductules and cells within the centroacinar compartment (A). Islet cells have intense labeling of the nuclei with moderate labeling of the cytoplasm. The centroacinar cell compartment lies between the acinar cells and the small ductules. Acinar cells do not express Pdx1 (C).
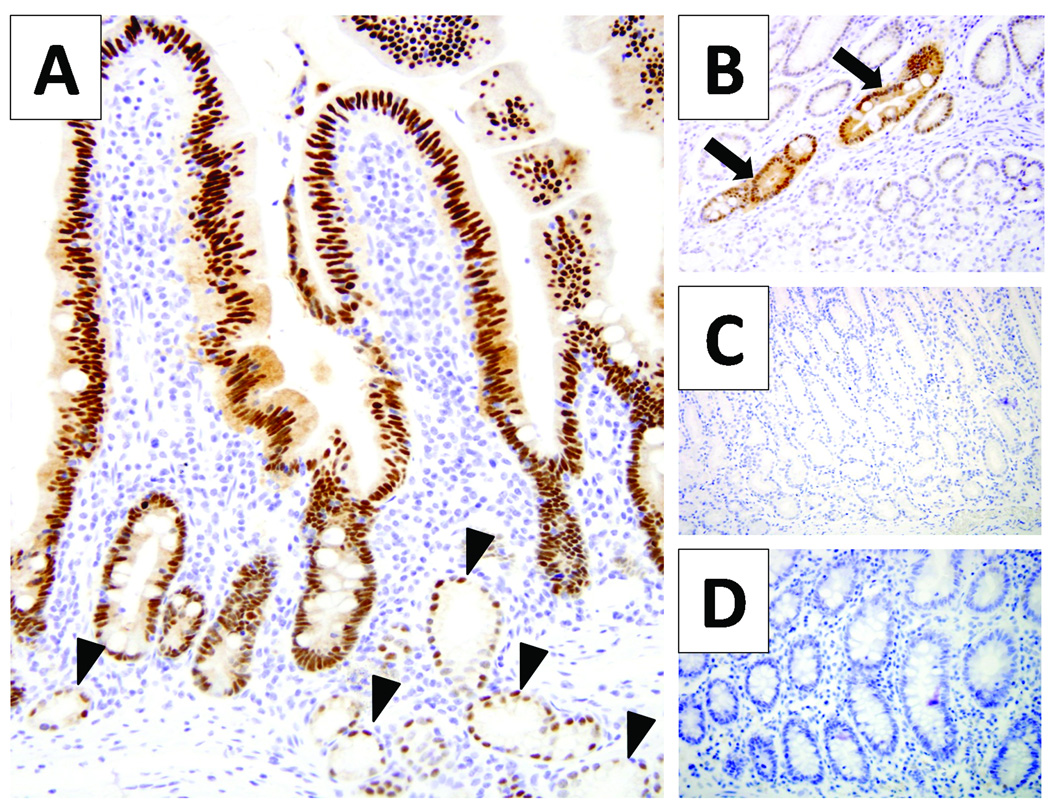
In non-pancreatic tissues, consistent labeling of epithelium of the duodenum was observed (Figure 2A). In addition to labeling the nuclei of cells from the base of the crypts to the villous tips, focal Pdx1 nuclear labeling was seen in Brunner’s glands. In the limited number of gastric mucosal specimens examined, no Pdx1 labeling was seen in the parietal, chief, neuroendocrine or mucous necks cells (Figure 2B and 2C); however, a focus of intestinal metaplasia in oxyntic mucosa had uniform Pdx1 labeling (Figure 2B). Weak Pdx1 nuclear labeling was seen in the basal layer of the skin. No Pdx1 labeling was seen in colonic mucosa, gallbladder, liver, prostate, kidney, ovary, spleen, thyroid, lung, breast, cerebellum, tonsils, and placenta (data not shown).
Figure 2.
Pdx1 expression in non-pancreatic tissues. The duodenum has nuclear pdx1 labeling from the base of the crypt to top of the villous tip; in addition, the Brunner’s glands (arrow heads) have occasional nuclear Pdx1 labeling (A). Oxyntic mucosa from the body of the stomach does not have pdx1 labeling, but glands of intestinal metaplasia (arrows) have nuclear pdx1 labeling (B). No labeling of pdx1 is seen in the antral mucosa of the stomach (C) or in colonic mucosa (D).
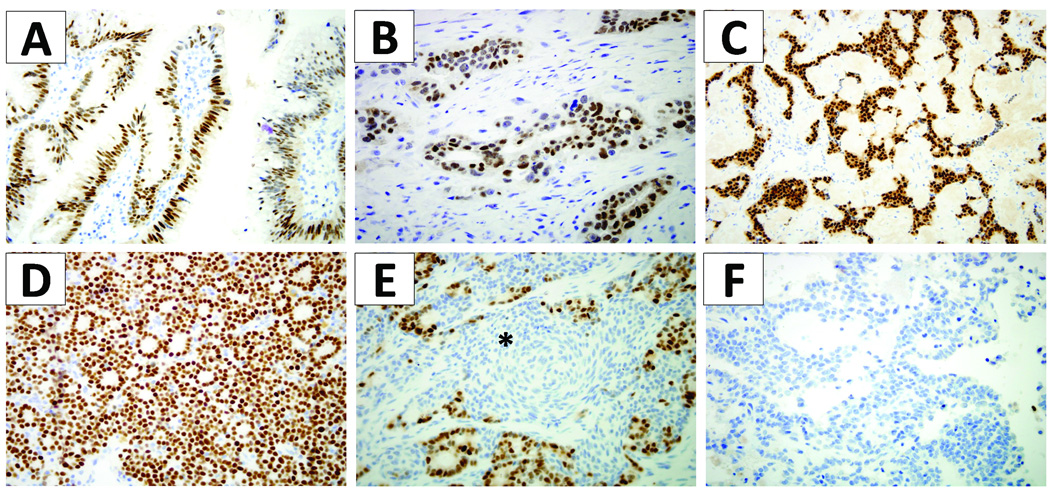
Frequent and sometimes strong labeling of the precursor lesions of pancreatic ductal adenocarcinoma was observed. In PanIN lesions, 40.6% of cases had Pdx1 nuclear labeling in >50% of the cells examined. For IPMNs, 35.2% of cases had Pdx1 nuclear labeling in >50% of the cells examined (Figure 3A). There was no significance difference according to degree of dysplasia of the IPMN. Similarly, for MCNs, two of the three cases had Pdx1 nuclear labeling in greater than 50% of the cells.
Figure 3.
Pdx1 expression in precursor lesions and neoplasms of the pancreas. Intraductal papillary mucinous neoplasms had frequent nuclear labeling (A). Ductal adenocarcinomas had variable nuclear labeling, but intensely labeled in some cases (B). Well-differentiated endocrine neoplasms of the pancreas had frequent strong nuclear labeling (C). Acinar cell carcinomas had uniform intense labeling (D). Pancreatoblastomas had strong nuclear labeling, but only in the acinar component; the squamoid nests (*) were negative for pdx1 labeling (E). No pdx1 labeling was seen in solid pseudopapillary neoplasms (F).
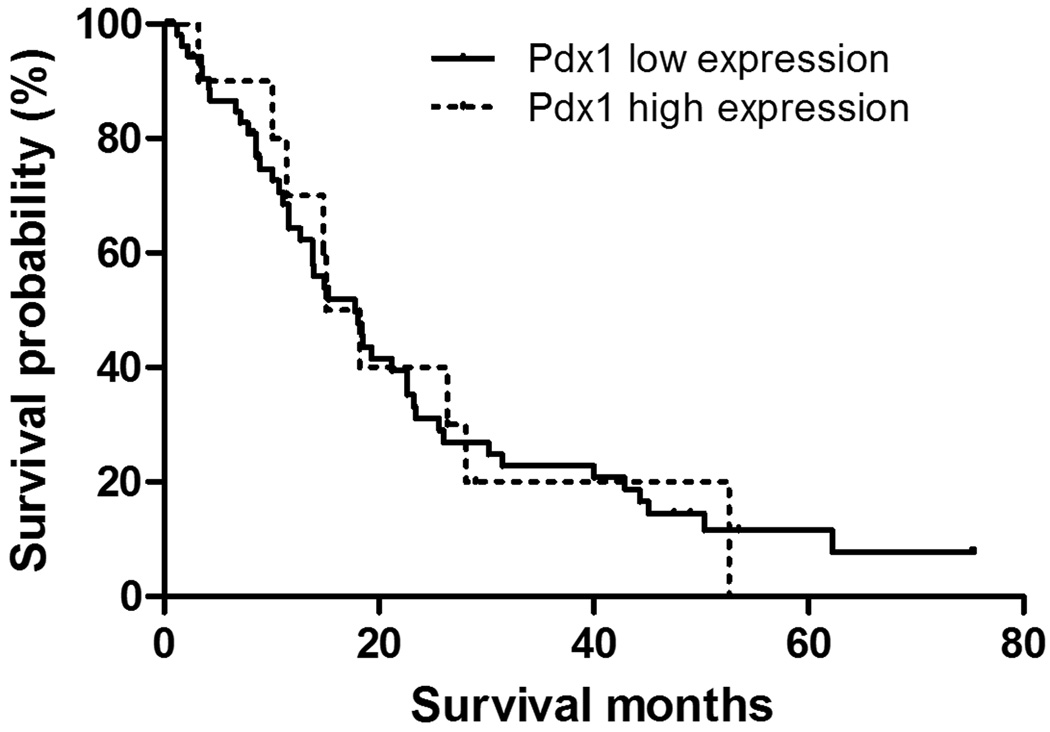
Invasive pancreatic ductal adenocarcinomas were more variable in their nuclear Pdx1 labeling; the majority had no Pdx1 labeling. However, there was a subset of carcinomas with strong nuclear labeling in the majority of cells examined (Figure 3B). Nine of sixty-seven cases (13.4% of all ductal adenocarcinomas) had >50% of cells labeled. The median survival time for the patients with Pdx1 higher (score ≥4) and lower (score<4) expression was 17.8 and 15.1 months, respectively. However, this was not a significant survival difference (p=0.95, Table 2, Figure 4).
Table 2.
Median survival stratified by Pdx1 composite immunohistochemistry score.
| Pdx1 expression (composite score) | No. of cases | Median survival time (months) | 95% CI | P value* |
|---|---|---|---|---|
| Pdx1 high (≥4) | 54 | 17.8 | 11.8–23.9 | 0.95 |
| Pdx1 low (<4) | 13 | 15.1 | 9.8–20.4 |
<0.05 is significant.
Figure 4.
Kaplan-Meier Plot of Survival based on Pdx1 expression. The median survival based on the composite score of Pdx1 expression was 17.8 for high expression and 15.1 for low expression; this did not reach statistical significance (p=0.95)
The non-ductal neoplasms had varying levels of Pdx1 expression as measured by Pdx1 nuclear labeling. Well-differentiated pancreatic endocrine neoplasms had strong Pdx1 expression in a subset. No Pdx1 labeling was seen in 47.7% of cases, but 38.6% of cases (17 of 44) had consistent nuclear Pdx1 labeling in >50% of the tumor cells (Table 1; Figure 3C). For AACs, 87.5% cases (6 of 8) had nuclear labeling in >50% of the tumors cells; furthermore, ACCs had the highest composite score which was significantly higher than all other ductal or non-ductal neoplasm groups analyzed (mean composite score, 4.58; p<0.0001, ANOVA ; p=0.05, post-hoc Duncan test). All cases of acinar cell carcinoma had strong Pdx1 expression in >25% of cells; most cases had a strong and diffuse pattern of expression (Figure 3D). Interestingly, the single pancreatoblastoma case examined exhibited strong nuclear Pdx1 labeling only in its acinar component (Figure 3E). The squamoid nests in the pancreatoblastoma did not label with Pdx1. All SPNs were negative for nuclear and cytoplasmic labeling with Pdx1 (Figure 3F).
DISCUSSION
We report that Pdx1protein expression in human pancreatic ductal epithelium is not lost after embryologic development, and indeed expression occurs in non-neoplastic cells of the centroacinar cell compartment in addition to islet cells. Furthermore, Pdx1 expression occurs in multiple types of pancreatic neoplasms that express different lines of epithelial differentiation: ductal adenocarcinomas, endocrine neoplasms, acinar cell carcinomas, and pancreatoblastoma. The pattern of Pdx1 expression in precursor lesions and neoplasms of the pancreas suggests that Pdx1 may have a wider role in pancreatic tumorigenesis. In practical terms, detection of Pdx1 expression in a pancreatic neoplasm is not specific for ductal adenocarcinomas or endocrine neoplasms.
Regarding the correlation of Pdx1 expression with survival of patients with pancreatic ductal adenocarcinomas, the current study showed a lack of statistically significant difference in median survival based on Pdx1 expression. In a previous study of thirty-five patients with pancreatic ductal adenocarcinomas, the expression of Pdx1 was correlated with a worse survival compared to cases without Pdx1 expression, 14.0 versus 21.4 months, respectively.10 It is important to note that there are significant experimental differences in the antibody and immunohistochemical assay method used in the previous and current study. Although the current study did not reach statistically significant difference in survival, Pdx1 expression had a trend towards worse survival. A limitation of the present study was the examination of a small percentage of the tumor volume limited to a tissue microarray core. Further studies that evaluate Pdx1 expression based on more extensive sampling of the tumor may be informative in determining the relationship of Pdx1 expression with survival in pancreatic ductal adenocarcinomas.
The current study demonstrated that Pdx1 was strongly expressed (>50% of nuclei) in 38.6% of well-differentiated endocrine neoplasms. This is similar to the findings of a previous study of Pdx1 expression in pancreatic endocrine neoplasms.12 This previous study not only examined Pdx1 in pancreatic endocrine neoplasms, but also compared the expression pattern in gastrointestinal carcinoid and pulmonary carcinoid tumors. These investigators found that 28.2% (11 of 39) of pancreatic endocrine neoplasms expressed Pdx1. In addition to expression in endocrine neoplasms primary to the pancreas, Pdx1 was found to be expressed in well-differentiated neuroendocrine neoplasms of the stomach and duodenum. Indeed, these investigators demonstrated that positive Pdx1 immunohistochemistry may have diagnostic utility in determining that the site of origin of metastatic well differentiated neuroendocrine neoplasms is the pancreas or foregut.
The focus of the current study was not to examine Pdx1 expression in non-pancreatic tissue; however, the pattern of Pdx1 seen in the duodenum and stomach in the current study confirms previous reports. Pdx1 expression was not seen in the oxyntic or antral mucosa, but was identified in intestinal metaplasia. Previous studies have identified no expression of Pdx1 in normal gastric fundus, but variable levels of expression in gastric antrum.17–19 The lack of antral expression in the present study may reflect sampling as the previous studies were more extensive in their characterization of Pdx1 expression in the stomach. The finding of Pdx1 expression in intestinal metaplasia as well as gastric adenocarcinomas has been previously identified.17–18 In addition, Pdx1 has been previously identified in Menetrier’s Disease19 as well as various serrated lesions of the colon (hyperplastic polyps, sessile serrated adenomas and traditional serrated adenomas).20
The present study identified that Pdx1 was strongly expressed in precursor lesions of pancreatic adenocarcinomas (PanIN, IPMN, and MCN); however, there is no correlation between the degree of dysplasia and the intensity of labeling. Furthermore, non-neoplastic ductules not directly associated with tumors frequently labeled with Pdx1. This study did not specifically examine the differences in Pdx1 expression based on the location of non-neoplastic ductules. Based on previously published experimental studies in animals, Pdx1 may be re-expressed in pancreatic ducts that are exposed to pancreatitis.4 Thus, the current finding of extensive Pdx1 expression in the neoplastic and non-neoplastic ducts may be the result of chronic damage or obstruction. The non-neoplastic pancreas ducts examined in this study were uninvolved by tumor, but were from pancreas resected for tumor and may not be representative of truly normal pancreas. Future examination of human pancreas that has no association with tumor or chronic damage will be informative to the state of Pdx1 expression in normal pancreas.
In summary, the present study confirms the previous findings of Pdx1 expression in pancreatic ductal carcinoma and endocrine neoplasms. The novel findings in this study are that Pdx1 is also expressed in non-neoplastic ducts, precursors of ductal carcinoma (PanIN, IPMN, MCN) and non-ductal neoplasms (ACC, PB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ashizawa S, Brunicardi FC, Wang XP. PDX-1 and the pancreas. Pancreas. 2004;28:109–120. doi: 10.1097/00006676-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Jonsson J, Carlsson L, Edlund T, et al. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- 3.Stoffers DA, Zinkin NT, Stanojevic V, et al. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet. 1997;15:106–110. doi: 10.1038/ng0197-106. [DOI] [PubMed] [Google Scholar]
- 4.Sharma A, Zangen DH, Reitz P, et al. The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes. 1999;48:507–513. doi: 10.2337/diabetes.48.3.507. [DOI] [PubMed] [Google Scholar]
- 5.Du YC, Klimstra DS, Varmus H. Activation of PyMT in beta cells induces irreversible hyperplasia, but oncogene-dependent acinar cell carcinomas when activated in pancreatic progenitors. PLoS One. 2009;4:e6932. doi: 10.1371/journal.pone.0006932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gidekel Friedlander SY, Chu GC, Snyder EL, et al. Context-dependent transformation of adult pancreatic cells by oncogenic K-Ras. Cancer Cell. 2009;16:379–389. doi: 10.1016/j.ccr.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 8.Habbe N, Shi G, Meguid RA, et al. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc Natl Acad Sci U S A. 2008;105:18913–18918. doi: 10.1073/pnas.0810097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tirone TA, Wang XP, Templeton NS, et al. Cell-specific cytotoxicity of human pancreatic adenocarcinoma cells using rat insulin promoter thymidine kinase-directed gene therapy. World J Surg. 2004;28:826–833. doi: 10.1007/s00268-004-7291-x. [DOI] [PubMed] [Google Scholar]
- 10.Koizumi M, Doi R, Toyoda E, et al. Increased PDX-1 expression is associated with outcome in patients with pancreatic cancer. Surgery. 2003;134:260–266. doi: 10.1067/msy.2003.231. [DOI] [PubMed] [Google Scholar]
- 11.Liu T, Gou SM, Wang CY, et al. Pancreas duodenal homeobox-1 expression and significance in pancreatic cancer. World J Gastroenterol. 2007;13:2615–2618. doi: 10.3748/wjg.v13.i18.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srivastava A, Hornick JL. Immunohistochemical staining for CDX-2, PDX-1, NESP-55, and TTF-1 can help distinguish gastrointestinal carcinoid tumors from pancreatic endocrine and pulmonary carcinoid tumors. Am J Surg Pathol. 2009;33:626–632. doi: 10.1097/PAS.0b013e31818d7d8b. [DOI] [PubMed] [Google Scholar]
- 13.van Eeden S, de Leng WW, Offerhaus GJ, et al. Ductuloinsular tumors of the pancreas: endocrine tumors with entrapped nonneoplastic ductules. Am J Surg Pathol. 2004;28:813–820. doi: 10.1097/01.pas.0000112546.57641.c7. [DOI] [PubMed] [Google Scholar]
- 14.Galmiche L, Sarnacki S, Verkarre V, et al. Transcription factors involved in pancreas development are expressed in paediatric solid pseudopapillary tumours. Histopathology. 2008;53:318–324. doi: 10.1111/j.1365-2559.2008.03108.x. [DOI] [PubMed] [Google Scholar]
- 15.Maitra A, Adsay NV, Argani P, et al. Multicomponent analysis of the pancreatic adenocarcinoma progression model using a pancreatic intraepithelial neoplasia tissue microarray. Mod Pathol. 2003;16:902–912. doi: 10.1097/01.MP.0000086072.56290.FB. [DOI] [PubMed] [Google Scholar]
- 16.Cao D, Zhang Q, Wu LS, et al. Prognostic significance of maspin in pancreatic ductal adenocarcinoma: tissue microarray analysis of 223 surgically resected cases. Mod Pathol. 2007;20:570–578. doi: 10.1038/modpathol.3800772. [DOI] [PubMed] [Google Scholar]
- 17.Sakai H, Eishi Y, Li XL, et al. PDX1 homeobox protein expression in pseudopyloric glands and gastric carcinomas. Gut. 2004;53:323–330. doi: 10.1136/gut.2003.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leys CM, Nomura S, Rudzinski E, et al. Expression of Pdx-1 in human gastric metaplasia and gastric adenocarcinoma. Hum Pathol. 2006;37:1162–1168. doi: 10.1016/j.humpath.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Nomura S, Settle SH, Leys CM, et al. Evidence for repatterning of the gastric fundic epithelium associated with Menetrier's disease and TGFalpha overexpression. Gastroenterology. 2005;128:1292–1305. doi: 10.1053/j.gastro.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Tsukamoto T, Mizoshita T, Tatematsu M. Gastric-and-intestinal mixed-type intestinal metaplasia: aberrant expression of transcription factors and stem cell intestinalization. Gastric Cancer. 2009;9:156–166. doi: 10.1007/s10120-006-0375-6. [DOI] [PubMed] [Google Scholar]