Abstract
Objective
Blood pressure, urine albumin-to-creatinine ratio, and estimated glomerular filtration rate (GFR), are highly correlated conditions. The longitudinal effect of exposure to postmenopausal estrogen therapy on these traits studied together has not been reported.
Methods
A cross-sectional study of 1044 older postmenopausal community-dwelling women from the Rancho Bernardo Study (1992-1996); 443 were re-evaluated ~10 years later (2002-2005). We determined the cross-sectional and prospective association of baseline postmenopausal estrogen therapy and blood pressure, urine albumin-to-creatinine ratio, GFR, and the odds of categorical hypertension (physician diagnosis, medication, or blood pressure ≥140/≥90 mmHg), chronic kidney disease (GFR ≥60 ml/min/1.73 m2), and albuminuria (urine albumin-to-creatinine ratio ≥25 mg/g).
Results
At baseline the mean age of current estrogen users was 68.3 years, 75.4 for past users, and 74.3 for never users. In cross-sectional analyses, current users had lower diastolic blood pressure and lower odds of having chronic kidney disease, independent of covariates. After ~10-year follow-up, the mean diastolic blood pressure declined over time in current users while systolic blood pressure increased among never users. Urine albumin-to-creatinine ratio increased in never users and decreased in current users, with no differences in GFR by estrogen use.
Conclusions
In cross-sectional analyses, estrogen users had better GFR and blood pressure than nonusers, but the 10-year follow-up showed improved blood pressure and decreased urine albumin-to-creatinine ratio among current users, without differences in GFR by estrogen use. This study suggests no association of GFR with ten years of continuous estrogen use, and an inverse association with albuminuria.
Keywords: hypertension, albuminuria, chronic kidney disease, postmenopausal estrogen therapy
Introduction
Microalbuminuria, hypertension (HTN), and kidney disease are closely related conditions. Microalbuminuria often precedes HTN1 and kidney disease2 and may be a marker of increased severity of disease3. HTN can worsen kidney disease, and kidney disease can also lead to HTN4. The presence of any of these conditions also increases the risk of cardiovascular disease and mortality5, which may be further increased by the presence of more than one of these conditions6.
Despite many previous observational studies suggesting that postmenopausal estrogen (PME) may have cardio-protective effects for postmenopausal women7, large randomized controlled studies from the Women's Health Initiative (WHI)8, 9 and the Heart and Estrogen/progestin Replacement Study (HERS)10 showed an increased risk of cardiovascular disease (CVD) and stroke in women on PME. PME use in the U.S. has declined dramatically since 200211.
The long-term effects of PME on blood pressure (BP) and kidney disease are not well defined. Results of studies of estrogen's effects on BP have been mixed12. Long-term oral contraceptives are known to increase BP13, smaller, short-term trials have reported a decline in BP14, 15, while clinical trials such as the WHI 8 and the Postmenopausal Estrogen/Progestin Interventions Trial (PEPI)16 noted little effect of PME on BP.
The effect of PME on the kidney is uncertain. Prior studies are few and primarily small and of case-control design17, 18. One large population study from Alberta, Canada, reported that PME was associated with a decline in eGFR over a 2-year period19. Effects of PME on microalbuminuria, a marker of endothelial dysfunction 20, have been evaluated in only a limited number of studies17, 18, 21, 22. Previous small short clinical trials showed no effect of oral or transdermal estrogen on microalbuminuria23, 24, whereas large observational studies reported decreased microalbuminuria in PME users21, 22, 25.
The present study evaluates the cross-sectional and longitudinal association of PME with BP, kidney function, and albuminuria, as continuous and categorical outcomes. This study started in 1992 when PME use was high, and continued to 2005, after the WHI results were known.
Methods
The Rancho Bernardo Study was established in 1972; the details of the initial study have been described previously26, 27. Briefly, this cohort was based on a planned suburban development in southern California that was largely Caucasian and middle to upper middle class. 5052 persons (82%) of the target population were recruited by telephone, of which 54% were female. The research protocol for each clinic visit was approved by the institutional review board of the University of California, San Diego; all participants gave written informed consent.
Between 1992 and 1996, 1082 (> 80%) of surviving local female participants participated in a research clinic visit focused on diabetes. We excluded 31 women who were less than 50 years of age with uncertain menopausal status, and seven women who failed to answer a question about ever use of oral PME. The remaining 1044 women form the basis of the cross-sectional part of this report, of which 31.6% had never taken PME, 23.5% had taken PME previously (mean of 7.9 years), and 44.9% were taking PME at the time (mean of 16.5 years of use), as noted in Table 1.
Table 1.
Results of cross-sectional study of women at baseline visit 1992-1996 based on Never, Past or Current PME use
| Post menopausal estrogen (PME) use group | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Overall Mean±SD | Never users (330) | Past users (245) | Current users (469) | P value for trend | Age-adj P value | Multivariate-adj P value |
| Age (years) | 71.9±10.8 | 74.3±10.7 | 75.4±9.3 | 68.3±10.5*† | <0.0001 | ------------- | ------------- |
| Age started PME (years) | 50.9±9.8 | ------------- | 50.6±9.9 | 51.1±9.7 | 0.5 | <0.01 | ------------- |
| Years on PME (years) | 9.2±11.1 | ------------- | 7.9±8.2 | 16.5±11.1 | <0.0001 | <0.0001 | ------------- |
| Age at menopause | 46.4±7.4 | 47.7±6.4 | 46.7±7.2¥ | 45.2±8.2* | <0.01 | 0.02 | ------------- |
| BMI (kg/m2) | 24.8±4.2 | 25.0±4.6 | 24.8±4.0 | 24.6±4.0 | 0.5 | 0.04 | 0.1 |
| SBP (mmHg) | 137.9±22.8 | 140.2±23.0 | 140.4±22.9 | 134.9±22.4*† | <0.001 | 0.8 | 0.9 |
| DBP (mmHg) | 74.6±9.4 | 74.9±9.7 | 74.4±9.4 | 74.6±9.3 | 0.76 | 0.07 | 0.01 |
| Pulse (beats/min) | 65.6±11.1 | 66.2±10.8 | 66.4±11.8 | 64.8±11.0 | 0.09 | 0.2 | 0.06 |
| Serum creatinine (mg/dl) | 0.94±0.2 | 0.97±0.2 | 0.94±0.2¥ | 0.91±0.2* | <0.001 | 0.01 | 0.02 |
| eGFR by MDRD (ml/min/1.73m2) | 66.1±15.8 | 63.3±15.7 | 66.0±17.5¥ | 68.1±14.7* | 0.0001 | 0.02 | 0.03 |
| Ln Urine albumin: creatinine ratio (UACR) | 2.70±0.85 | 2.77±0.92 | 2.69±0.84 | 2.66±0.81 | 0.2 | 0.4 | 0.5 |
| UACR (mg/g)$ | 13.1 (8.4-21.8) | 13.7 (9.2-22.6) | 12.6 (8.0-22.3) | 13.1 (8.1-21.6) | |||
| Percentages- N (%) | |||||||
| Hysterectomy | 491 (47.0) | 96 (29.1) | 105 (42.9)¥ | 290 (61.8)*† | <0.0001 | <0.0001 | ------------- |
| Obese (BMI ≥ 30) | 106 (10.2) | 35 (10.6) | 24 (9.8) | 47 (10.0) | 0.9 | 0.5 | 0.6 |
| History of smoking (ever) | 531 (50.9) | 154 (46.7) | 129 (52.7) | 248 (52.9) | 0.2 | 0.3 | 0.3 |
| Alcohol use/drinker | 543 (52.3) | 160 (48.9) | 120 (49.2) | 263 (56.3)* | 0.07 | 0.2 | 0.3 |
| Exercise 3x/week | 712 (68.5) | 217 (66.2) | 171 (70.1) | 324 (69.4) | 0.5 | 0.5 | 0.6 |
| Diabetes | 56 (5.4) | 29 (8.8) | 15 (6.1) | 12 (2.6)*† | <0.001 | 0.04 | 0.01 |
| CKD (eGFR ≤60 ml/min/1.73m2) | 388 (37.2) | 149 (45.2) | 102 (41.6) | 137 (29.2)*† | <0.0001 | 0.02 | 0.04 |
| Albuminuria (UACR≥25 mg/g) | 207 (19.8) | 70 (21.2) | 52 (21.2) | 85 (18.1) | 0.5 | 0.9 | 0.9 |
| History of a heart attack | 42 (4.0) | 16 (4.9) | 14 (5.7) | 12 (2.6)† | 0.08 | 0.8 | |
| History of kidney stones | 37 (3.6) | 16 (4.9) | 4 (1.6)¥ | 17 (3.6) | 0.1 | 0.1 | |
| History of kidney disorder | 13 (1.3) | 4 (1.2) | 4 (1.6) | 5 (1.1) | 0.8 | 0.9 | |
| On any blood pressure medication | 296 (28.5) | 97 (29.6) | 80 (33.0) | 119 (25.5)† | 0.1 | 0.8 | |
| On beta blocker | 106 (10.2) | 38 (11.6) | 21 (8.6) | 47 (10.1) | 0.5 | 0.4 | |
| On diuretic | 203 (19.5) | 65 (19.8) | 59 (24.2) | 79 (16.9)† | 0.07 | 0.5 | |
| On CCB | 128 (12.3) | 40 (12.2) | 34 (13.9) | 54 (11.6) | 0.7 | 0.8 | |
| Using NSAIDs | 249 (23.9) | 77 (45.2) | 50 41.63 | 122 (29.2) | 0.2 | 0.3 | |
eGFR- estimated glomerular filtration rate by Modification of Diet in Renal Disease Equation. UACR- urine albumin: creatinine ratio.
age adjusted P<0.05, for Current users compared to Never users
age- adjusted P<0.05 for Current users when compared to Past PME users.
age-adjusted P<0.05 for Past users compared to Never users.
Statistics performed on natural log transformed values for normalization (see row above) but medians also presented with 25th-75th interquartile range. Multivariate adjustment includes age, weight (BMI obese, overweight, or normal weight), HTN, diabetes, hypercholesterolemia, smoking. When adjusting the variables included in the multivariate adjustment model, that particular variable is not included such as for BMI the multivariate adjustment does not include weight, for SBP then HTN is not included, etc. Bold values signify P<0.05.
At baseline, women were seen between 8 am and 11 am after an overnight fast. Information about known heart disease and angina pectoris, HTN, menopause, diabetes, life-style habits, and current and past estrogen use was obtained by a trained interviewer using standard Rancho Bernardo Study questions and the Rose questionnaire28. Women reported current (within the past 2 weeks), past, or never use of estrogen; a nurse examined pills or prescriptions brought to the clinic for validation of reported current medications, providing validation of 92.4% of reported current PME use. More than 80% of PME was oral conjugated equine estrogen 0.625 mg/day taken without a progestin. Women who reported previous use but not current use within the previous 2 weeks were classified as past users. Women who had never taken PME were classified as never users. Information about current cigarette smoking, alcohol consumption (number of drinks per day during the last 2 weeks), and physical exercise was also obtained. Height and weight were measured using a calibrated stadiometer and balance-beam scale with participants wearing light clothing and no shoes. Systolic and diastolic BP (SBP and DBP) were measured twice in seated participants after a 5-minute rest, using the HTN Detection and Follow-up Program protocol29.
Baseline laboratory tests on morning fasting blood included glucose, lipids, and serum creatinine. A single, clean-catch, untimed morning urine sample (usually second void of the day) was obtained, frozen, and shipped to the National Institutes of Health laboratory (NIH; Phoenix, Arizona) of Dr. Peter Bennett. Urine albumin was measured using the Behring Nephelometer BNA (Dade Behring GmbH, Marburg, Germany). The lower limit of detection of the assay was 6.8 mg/dL; values <6.8 mg/dL were assigned a value of 6.7 mg/dL. The interassay coefficient of variance was 4.5%. Urine creatinine was measured by the kinetic alkaline picrate method using the Ciba-Corning Express (Corning, Medfield, MA). Serum creatinine was measured using a variation of the Jaffe enzymatic method at a Smith Kline Beacham laboratory on a Hitachi 911 analyzer (Roche Diagnostics, Indianapolis, IN), with inter- and intra-assay CVs of 4.0%. Glomerular filtration rate was estimated (eGFR) using the abbreviated Modification of Diet in Renal Disease Equation30. Lipids were measured in a Lipid Research Clinic Laboratory certified by the Center for Disease Control. Total cholesterol and triglycerides were measured by enzymatic methods with an ABA-200 biochromatic analyzer (Abbott, Abbott Park, IL ), HDL cholesterol was measured by precipitation according to Lipid Research Clinic protocol, and LDL cholesterol was calculated by means of the formula of Friedewald et al31.
As a categorical variable, HTN was defined by a physician diagnosis, BP medication use, and/or measured SBP ≥140 mmHg or DBP ≥90 mmHg. Diabetes was defined by the 1997 American Diabetes Association (ADA) criteria32, fasting plasma glucose ≥126 mg/dL (7 mmol/L), physician diagnosis, or use of diabetes-specific medication. Hypercholesterolemia was defined as total cholesterol ≥200 mg/dl or use of cholesterol-lowering medication. Alcohol consumption was defined as at least one alcoholic beverage per week. Obesity was defined as body mass index (BMI) ≥30 kg/m2, overweight as BMI 25-29.9 kg/m2, and normal weight as BMI <25 kg/m2. Chronic kidney disease (CKD) included those with eGFR by abbreviated MDRD equation ≤60 ml/min/1.73m2 33. Microalbuminuria was defined by sex-specific criteria as a urine albumin-to-creatinine ratio (ACR) ≥25 mg/g, which approximates an albumin excretion rate of 30 micrograms/min34 as reported in other studies22. Macroalbuminuria was defined as ≥355 mg/g34. Because only 9 participants had macroalbuminuria, the two categories were combined into one albuminuria group (≥25 mg/g) for these analyses.
Between 2003-2005, approximately 10 years after the baseline visit, surviving participants were invited to a follow-up visit in our research clinic. Again a nurse validated pills and prescriptions, including PME use. BP was measured, and urine and plasma samples were collected using the same protocols used previously. Urine albumin and creatinine were measured in the same NIH laboratory as the prior visit using the same techniques, but serum creatinine was measured in the commercial Quest laboratory also using a modified Jaffe enzymatic method on an Olympus 5400 (Olympus Diagnostic Systems and Beckman Coulter, Brea, CA). There were 443 participants (60.4% of survivors) who returned and answered questions regarding previous and current PME use. The most common reason for non-response was death, recorded in 310 women; other non-respondents had moved away or were too busy, too ill, or institutionalized.
All analyses were performed using SAS software (version 9.2, Cary, NC). Descriptive statistics report mean±standard deviations. Most measures were normally distributed; however, urine ACR was skewed, and natural-log (ln) transformation was performed, with presentation of geometric means and geometric standard deviations as well as medians and interquartile ranges in tables. T-test and chi-squared testing were used to evaluate differences between cohorts. Analysis of covariance was performed (PROC GLM) for cross-sectional analyses of continuous outcomes and categorical outcomes by PME status. Logistic regression was used to define odds ratios (OR; 95% CI) of the categorical outcomes of HTN, CKD (eGFR≤60mL/min/1.73m2), and albuminuria (ACR ≥ 25mg/g) by PME status. Multivariate models were adjusted for age, BMI category (obese, overweight, normal weight), HTN, diabetes, hypercholesterolemia, and smoking status (ever or never). Linear mixed models with population mean specifications were utilized to assess the prospective association between PME use and body weight, BP, serum creatinine, eGFR, and ACR in order to accommodate the different time points between visits35, 36. Statistical tests were 2-tailed, with statistical significance defined as P <0.05.
Statistical power was determined by PASS software (Kaysville, UT). In the cross-sectional analysis, with 1000 participants and alpha =0.05, we had >95% power to detect < 5 ml/min/1.73 m2 difference in eGFR by MDRD with standard deviation up to 30. Similarly for the natural-log of ACR, we had >95% power to detect a difference <0.5 with standard deviation up to 1.8.
Results
Cross-sectional
The initial 1992-1996 visit included 1044 women, who are the basis for the cross-sectional analyses. Their mean age was 71.9±10.8 years (range 50-97 years), mean age at menopause was 47.2±6.5 year, and mean BMI was 24.7±4.2 (range 15.5-47.7). 54.7% met the criteria for HTN and 5.4% had diabetes by ADA criteria (fasting glucose ≥ 126 mg/dl, a physician's diagnosis, or the use of glucose-lowering medications). Overall, 330 (31.6%) participants had never used PME (never users), 245 (23.5%) reported PME use in the past (past users), and 469 (44.9%) were current PME users (current users). Albuminuria was present in 19.8% (<1% met criteria for macroalbuminuria [N=9]), and CKD based on GFR was present in 37.6%.
Table 1 shows the baseline characteristics of the cohort by PME status in age-adjusted and multivariate-adjusted models. Current users were younger than both past and never users (P<0.001) and had been taking PME for an average of 16.5 years compared with the past users who reported PME for an average of 7.9 years (age-adjusted P<0.001). Current users were also more likely to have had a hysterectomy (61.8% age-adjusted P<0.001), while never users were the least likely (29.1%).
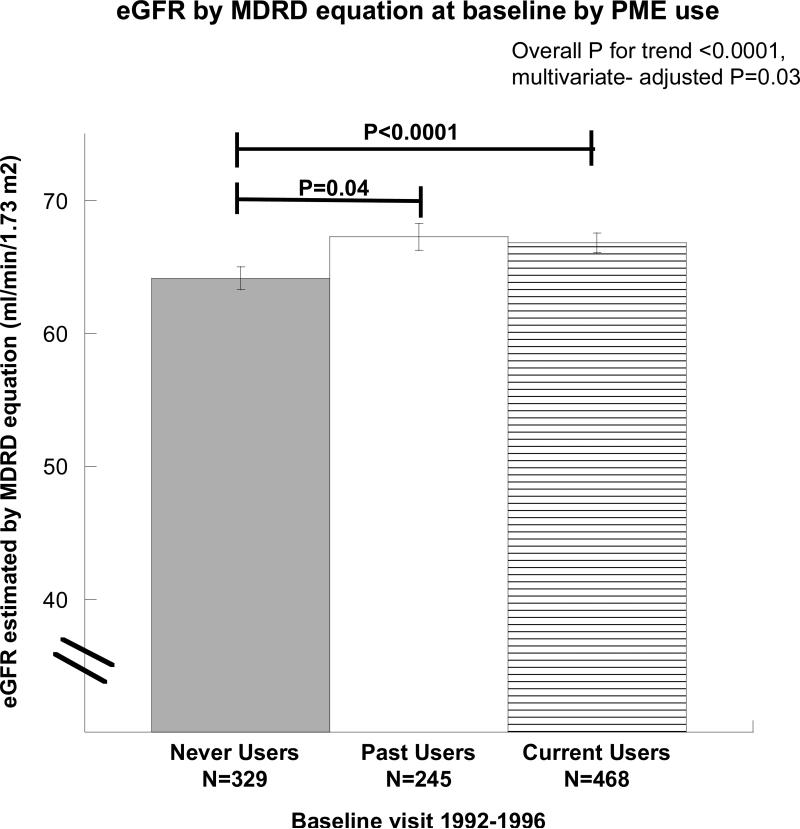
BMI was lowest in current users, intermediate in past users, and greatest in never users (age-adjusted P=0.04), but the women as a whole had a mean BMI of only 24.8 kg/m2. Serum creatinine was lowest in the current PME users (age-adjusted P=0.01, multivariate-adjusted P=0.02). eGFR based on the MDRD equation was lower in never users when compared with either current or past users, as shown in Table 1 and Figure 1 (unadjusted, P<0.0001, multivariate-adjusted P=0.03). There was no difference in ACR by PME status. DBP was highest in never users (age-adjusted P=0.07, multivariate-adjusted P=0.01), but SBP did not differ by PME status after adjustment for covariates.
Figure 1. Differences in estimated glomerular filtration rate (eGFR) by oral postmenopausal estrogen therapy (PME) use at baseline visit (1992-1996).
Both current and past users of PME had greater eGFR (as determined by the abbreviated Modification in Diet and Renal Disease (MDRD) equation) than the never users (unadjusted P<0.0001 and P=0.038 respectively). Results were unchanged after age and multivariate-adjustments.
Also shown in Table 1, both diabetes (age-adjusted P<0.01) and CKD (eGFR <60 ml/min/1.73m2) were most common in never users (age-adjusted P=0.02). HTN was less common in the current users compared with either the never users (age-adjusted P=0.04) or the past users (age-adjusted P<0.05).
Table 2 indicates the crude, age-adjusted, and multivariate-adjusted odds ratios between the PME use groups and the presence of the categorical traits CKD, albuminuria, and HTN. After adjustment for the covariates listed, current users had lower odds of CKD (OR 0.66 (95% CI 0.48-0.90)) compared with never users, but did not differ for BP or ACR.
Table 2.
Odds ratios of having chronic kidney disease, albuminuria, or hypertension based on never, past or current use of oral post menopausal estrogen (PME) use.
| Diagnosis (N) | ||||||
|---|---|---|---|---|---|---|
| Crude odds ratio | P value | Age-adj OR | P value | Multivariate-adj OR | P value | |
| (95% confidence interval) | (95% confidence interval) | (95% confidence interval) | ||||
| Chronic kidney disease (388) | <0.0001 | 0.02 | 0.03 | |||
| Never (149) | 1.00 | 1.00 | 1.00 | |||
| Past (102) | 0.87 (0.62-1.21) | 0.4 | 0.82 (0.58-1.16) | 0.3 | 0.81 (0.57-1.15) | 0.2 |
| Current (137) | 0.50 (0.37-0.67) | <0.0001 | 0.65 (0.48-0.89) | 0.01 | 0.66 (0.48-0.90) | 0.01 |
| | ||||||
| Albuminuria (207) | 0.5 | 0.9 | 0.9 | |||
| Never (70) | 1.00 | 1.00 | 1.00 | |||
| Past (52) | 1.00 (0.67-1.50) | 0.9 | 0.97 (0.64-1.46) | 0.9 | 0.99 (0.65-1.50) | 0.9 |
| Current (85) | 0.82 (0.58-1.71) | 0.9 | 1.08 (0.74-1.56) | 0.7 | 1.07 (0.73-1.56) | 0.7 |
| | ||||||
| Hypertension (571) | 0.05 | 0.5 | 0.2 | |||
| Never (191) | 1.00 | 1.00 | 1.00 | |||
| Past (143) | 1.02 (0.73-1.43) | 0.9 | 0.94 (0.66-1.34) | 0.7 | 0.98 (0.68-1.41) | 0.9 |
| Current (237) | 0.74 (0.56-.99) | 0.04 | 1.15 (0.84-1.58) | 0.4 | 1.26 (0.91-1.75) | 0.2 |
Albuminuria defined as urine albumin to creatinine ratio≥25 mg/g, hypertension (HTN) defined as doctor diagnosed, on antihypertensive medications, SBP≥140mmHg, or DBP ≥90mmHg) and Chronic kidney disease (CKD) was defined as eGFR (by Modification of Diet in Renal Disease abbreviated equation) ≤60 ml/min/1.73m2. Multivariate adjustment includes age, weight (obese, overweight, or normal), diabetes (Y/N), ever smoker (Y/N), hypercholesterolemia (Total cholesterol ≥200, or on cholesterol lowering medication, Y/N), HTN (as noted above, Y/N, not entered for HTN model). OR-odds ratio.
Prospective
The 443 participants in the prospective study (60.4% of surviving participants) who returned for a follow-up visit after a mean of 10.1±0.9 years included 87 who were never users of PME, 247 who were past users, and 109 who were current users; 99 of the 109 current users (90.8%) had used PME continuously between the baseline and follow-up visit, which was validated by pill or prescription examination at both visits. Women who attended the follow-up visit differed from those who did not; at baseline, they were younger (65.4±9.0 versus 76.6±9.5, P<0.0001), with lower serum creatinine (0.90±0.2 versus 0.96±0.2, P=0.02), higher BMI (25.1±4.1 versus 24.5±4.2, P=0.04), lower SBP (131.0±21.5 versus 143.0±22.5, P<0.001), higher DBP (75.4±8.5 versus 74.1±10.0, P=0.02), higher eGFR by MDRD equation (69.0±14.1 versus 64.0±16.7, P<0.001), and lower ACR (P<0.001) compared with those that did not return, though only ACR remained significant after age-adjustment. Women in the follow-up cohort were more likely to be current users of PME at baseline (50.9% versus 34.6% of past users followed-up and 36.1% of never users, P<0.001), but this association was not statistically significant after adjustment for baseline age (P=0.83).
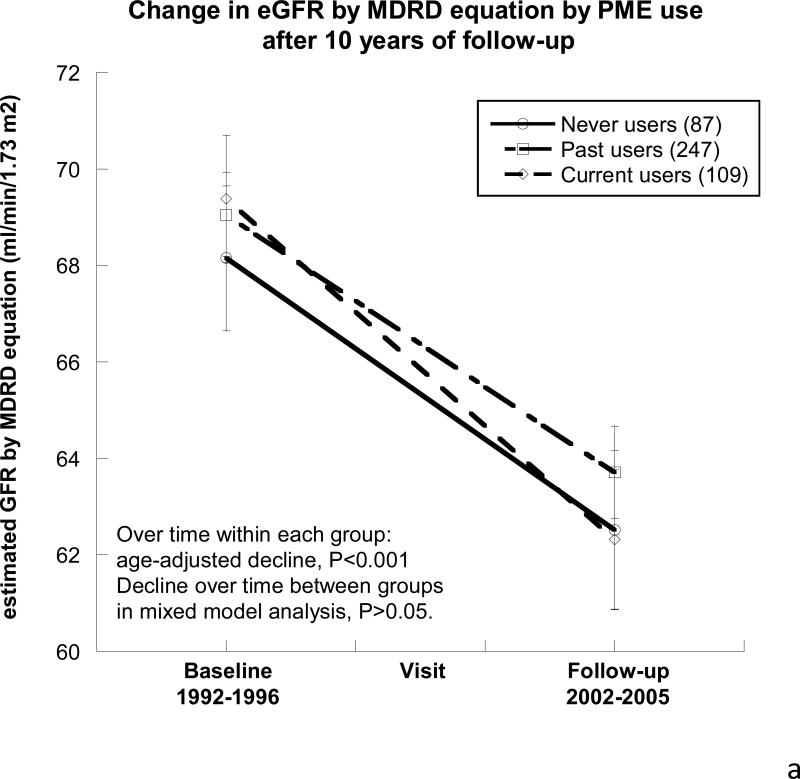
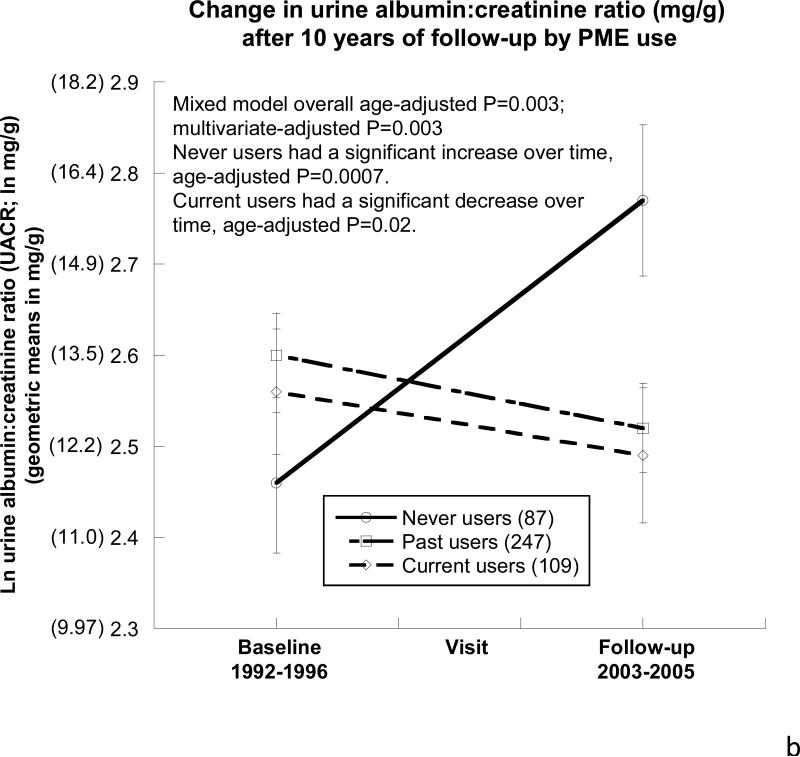
As shown in Table 3, after a mean of 10.1±0.9 years follow-up, eGFR declined over time in each of the 3 PME use groups, by a mean of 5.9±14.9 ml/min/1.73m2 over 10.1 years, which is a mean decline of 0.57±1.5 ml/min/1.73m2/year, and there was no difference by PME use (Figure 2a). As shown in Figure 2b , ACR differed over time by PME use such that never users had an age-adjusted increase in their ACR over the 10-year follow-up period (ln 0.31 mg/g; P<0.001).
Table 3.
Results of prospective study of oral postmenopausal estrogen use in women that followed up ~10 years later
| Trait | PME use group (N) | Baseline | Follow-up | Unadjusted P value | Age-adj P value | Multivariate-adj P value |
|---|---|---|---|---|---|---|
| Follow up (years) | Overall (443) | 10.06±0.9 | 0.40 | |||
| Never (87) | 10.10±0.9 | |||||
| Past (247) | 10.10±1.0 | |||||
| Current (109) | 9.56±0.8 | |||||
| | ||||||
| Age (years) | Overall | 65.4±9.0 | 76.0±9.1 | <0.0001 | <0.0001 | <0.0001 |
| Never† | 68.8±8.5 | 79.3±8.4 | ||||
| Past† | 65.0±8.8 | 75.7±8.9 | ||||
| Current† | 63.7±9.4 | 74.2±9.5 | ||||
| | ||||||
| Weight (lbs) | Overall | 144.6±24.6 | 143.2±27.6 | 0.03 | 0.03 | <0.05 |
| Never† | 141.7±22.9 | 137.2±24.5 | ||||
| Past | 144.4±23.3 | 143.5±26.7 | ||||
| Current | 147.3±29.0 | 147.4±31.0 | ||||
| | ||||||
| BMI (kg/m2) | Overall | 25.1±4.1 | 25.5±4.7 | 0.05 | 0.05 | 0.06 |
| Never | 24.7±4.1 | 24.6±4.3 | ||||
| Past† | 25.1±4.0 | 25.6±4.7 | ||||
| Current† | 25.3±4.3 | 25.9±4.7 | ||||
| | ||||||
| SBP (mmHg) | Overall | 131.0±21.5 | 131.6±20.2 | <0.05 | <0.05 | 0.05 |
| Never† | 132.3±19.9 | 137.3±20.3 | ||||
| Past | 130.1±21.5 | 130.2±19.9 | ||||
| Current | 132.0±22.6 | 130.2±19.9 | ||||
| | ||||||
| DBP (mmHg) | Overall | 75.4±8.5 | 72.2±9.7 | 0.07 | 0.07 | 0.08 |
| Never | 75.1±8.2 | 73.9±9.8 | ||||
| Past† | 75.2±8.2 | 71.8±10.3 | ||||
| Current† | 76.3±9.3 | 71.9±8.6 | ||||
| | ||||||
| MAP(mmHg) | Overall | 94.0±11.3 | 92.0±11.3 | 0.03 | 0.03 | 0.04 |
| Never | 94.2±10.4 | 95.1±11.5 | ||||
| Past† | 93.5±11.1 | 91.3±11.5 | ||||
| Current† | 94.8±12.3 | 91.3±10.1 | ||||
| | ||||||
| Serum creatinine (mg/dL) | Overall | 0.90±0.16 | 0.97±0.26 | 0.6 | 0.6 | 0.4 |
| Never† | 0.92±0.18 | 1.01±0.32 | ||||
| Past† | 0.90±0.15 | 0.96±0.25 | ||||
| Current† | 0.89±0.14 | 0.97±0.22 | ||||
| | ||||||
| eGFR by MDRD equation (ml/min/1.73 m2) | Overall | 69.0±14.1 | 63.3±16.0 | 0.5 | 0.5 | 0.4 |
| Never† | 67.0±13.8 | 60.3±15.1 | ||||
| Past† | 69.2±14.1 | 64.2±16.5 | ||||
| Current† | 70.0±14.1 | 63.5±15.2 | ||||
| | ||||||
| Natural-log UACR (mg/g) | Overall | 2.56±0.72 | 2.56±0.77 | <0.01 | <0.01 | <0.01 |
| Never† | 2.46±0.67 | 2.77±0.87 | ||||
| Past | 2.60±0.70 | 2.52±0.69 | ||||
| Current† | 2.56±0.78 | 2.49±0.84 | ||||
| | ||||||
| UACR (mg/g) | Overall | 12.4 (7.6-19.1) | 11.9 (7.3-20.3) | |||
| Median (25th-75th quartiles) | Never† | 10.8 (7.2-17.6) | 13.9 (8.3-26.3) | |||
| Past | 13.1 (7.6-20.9) | 11.2 (7.6-19.1) | ||||
| Current† | 12.2 (7.9-18.1) | 11.8 (6.1-20.3) | ||||
Means±standard deviations are reported. eGFR- estimated glomerular filtration rate by Modification of Diet in Renal Disease Equation. UACR- urine albumin: creatinine ratio (natural log due to non-normal distribution). Overall P values are determined by linear mixed models. Multivariate adjustment includes age, weight (obese, overweight, normal BMI), diabetes (Y/N), ever smoker (Y/N), hypercholesterolemia (Total cholesterol ≥200, or on cholesterol lowering medication, Y/N), HTN (as noted above, Y/N, not entered for HTN model).
age- adjusted P value <0.05 at follow-up visit as compared to baseline visit within each group (Never, Past, or Current users). Bold P value signifies P<0.05.
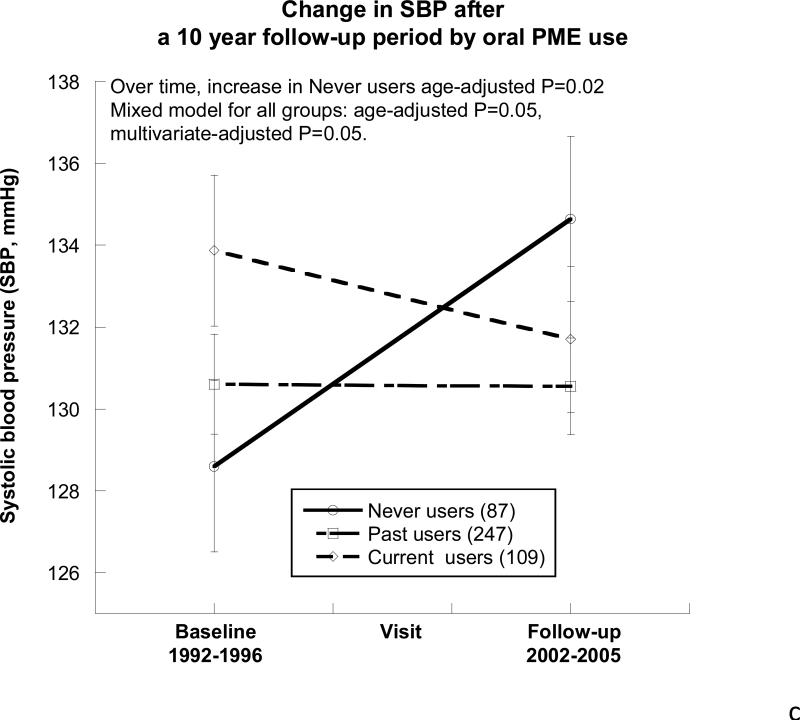
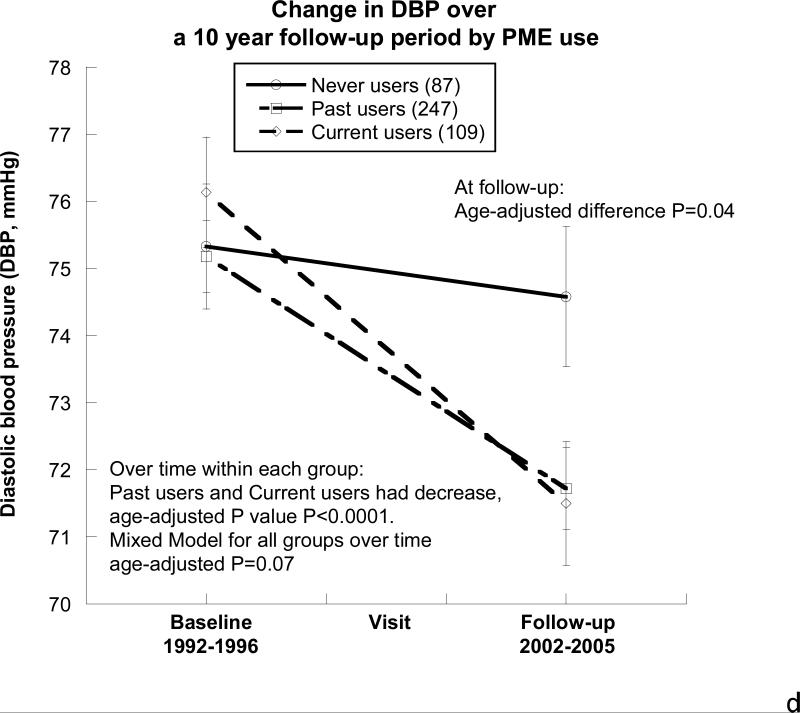
Figure 2. Changes in eGFR, urine albumin-to-creatinine ratio, and blood pressure by oral postmenopausal estrogen therapy (PME) use over the ~10-year follow-up period.
A) Estimated Glomerular Filtration Rate (eGFR by the abbreviated Modification in Diet and Renal Disease equation). All of the participants had significant declines in their eGFR over the follow-up period (P<0.001). There were no differences between the 3 groups at the baseline visit, the follow-up visit, or in their decline over time (P>0.05).
B) Urine albumin-to-creatinine ratio (ACR). Overall there was a difference in ACR by PME use (age-adjusted P=0.003). Never users had a significant increase in ACR (age-adjusted P=0.0007), whereas current users had a significant decline over time (age-adjusted P=0.02). Statistics performed on natural-log of ACR due to non-normal distribution, geometric means are presented in (parentheses).
C) Systolic blood pressure (SBP). At the baseline visit and the follow-up visit, no differences in SBP were noted by PME usage. Never users showed an increase in SBP over the follow-up (age-adjusted P value= 0.02), whereas there was no change in the past and current users. Overall, by mixed model analysis the differences between the 3 PME groups were statistically significant (age-adjusted P=0.05).
D) Diastolic blood pressure (DBP). At the baseline visit, DBP was similar in the 3 PME groups, but by the follow-up visit, never users had higher DBP than the current and past users (age-adjusted P=0.04), in part because the current and past users had significant declines in their DBP over the 10-year follow-up (age-adjusted P<0.001 for each group). The overall mixed model between the 3 PME groups had age-adjusted P=0.07.
BP also differed over time and between PME groups, as shown in Figure 2c and 2d, such that SBP did not differ in the past and current user groups, but never users exhibited an age-adjusted increase in SBP over time (mean increase 6mm Hg, P=0.02). The multivariate-adjusted difference remained (P=0.05). At follow-up, DBP was greatest in the never PME users (age-adjusted P=0.04) when compared with past and current users. Over the follow-up period, the DBP of never users showed no significant age-adjusted change (P=0.1) while past users (age-adjusted P<0.0001) and current users (age-adjusted P<0.0001) showed similar declines in their DBP, Figure 2d.
Discussion
This study demonstrates some statistically significant associations between PME use and BP, eGFR, and albuminuria. Results differed in the cross-sectional and prospective study. Cross-sectional analyses demonstrated (after adjustment for age, BMI, smoking, presence of diabetes, HTN, and hypercholesterolemia) that current users had lower BP and higher eGFR than never and past users. As in prospective studies of the elderly where participation is reduced by death and illness, the prospective cohort was younger and healthier than the original cohort. Nevertheless, we found that continuous long-term (~10 years) PME use was associated with decreased DBP and ACR over time in age-adjusted analyses. In the prospective cohort 186 women (95% of current and never users) maintained the same estrogen use status over the entire follow-up period, which is, to our knowledge, the longest confirmed continuous follow-up period of PME use and measures of kidney function and albuminuria.
In both human37 and animal studies38, estrogen has a known vasodilatory effect on vascular smooth muscle, mediated through increased bioavailability and synthesis of nitric oxide (NO) and cyclic guanine monophosphate (cGMP)38, and may reduce the breakdown of NO by superoxide free radicals39, 40. This enhances vascular oxidative stress and anti-inflammatory action resulting in vasoprotection and contributes to improvements in GFR and blood pressure41. Animal studies have also indicated a protective role for estrogen against glomerulosclerosis and albuminuria 42.
BP is a major risk factor for stroke and cardiovascular disease. Though PME did increase the risk of stroke 43, studies of PME use and BP have shown mixed results12, 15, 16. Diverse research designs, including varying types of participants (normotensive16, 44 or hypertensive45) or estrogen administration (oral16 or transdermal44 or injectable45), may contribute to inconsistent results, as well as the addition of different progestins46. Our women were primarily taking conjugated equine estrogens (CEE) identified as Premarin (>85%), without a progestin. After three years in the randomized placebo controlled PEPI trial16, there was no effect of any hormone regimen (opposed or unopposed CEE) on SBP or DBP. The PEPI participants were relatively young (45-64 years of age). Our findings that never users of PME did not have a decline in DBP yet demonstrated an increase in SBP over time, compared with both current and past users, may reflect survivor bias. Additionally, the decline in DBP may be a marker of aortic stiffness and widened pulse pressure which is not desirable as it has been shown to be another cardiovascular disease risk factor47, though our current and past PME users displayed only ~4mmHg change of their DBP over the follow-up period.
Our study adds to the mixed findings of prior prospective studies of PME and kidney function over time. In congruence with our findings, a 5-year follow-up of 491 postmenopausal women in the Insulin Resistance Atherosclerotic Study also showed no effect of PME on GFR but a decline in proteinuria22. A cross-sectional analysis from the Nurses’ Health Study found that microalbuminuria was decreased in postmenopausal women that had been using oral PME for at least 6 years21; they did not evaluate GFR. A large case-control study of over 4300 females revealed a higher creatinine clearance in postmenopausal women on PME, but this analysis was not adjusted for covariates; in contrast to our study, they also reported an increased risk of microalbuminuria after age and multivariate adjustment18. Our results also contrast to the largest observational prospective study of PME on renal function, in which Ahmed et al.19 reported on 5845 postmenopausal female patients who were at least 66 years old. In this study PME was associated with a loss of renal function in a dose dependent fashion. They showed a greater mean decline in eGFR (1.57 ml/min/1.73m2 over 2 years) than our study using the same MDRD eGFR estimating equation. However, their use of an administrative database did not allow adjustment for covariates, and albuminuria was not assessed in the study.
Two small interventional clinical trials have assessed the effects of PME on proteinuria in diabetics, and a third studied healthy females. The first had only 16 participants with both HTN and diabetes, and reported a decline in proteinuria but an increase in creatinine clearance after 14 weeks of treatment with PME 17. The other two studies indicated no change in microalbuminuria after 6 months of treatment in diabetics25 or healthy postmenopausal women23. Our study included only a small number of participants with diabetes and we did not have the power to evaluate PME in this subset of women.
Albuminuria is associated with increased risk of CVD and all-cause mortality, even in older adults 48. Though albuminuria may indicate glomerular damage in the kidney2, systemically, it is also a marker of vascular and endothelial damage and is associated with inflammation49 and the coagulation cascade. Reducing ACR, which is a marker of microvascular disease, with PME may have clinical significance. For example, in this Rancho Bernardo population, ACR has been associated with decreased cognitive function50, but whether decreasing ACR may have long-term beneficial effects in such microvascular disease needs further investigation.
The strengths of our study include the well-characterized community base with all three outcomes measured using the same methodology, which was state of the art at the time of the baseline visit. There was a high prevalence of PME use validated at both visits by examination of pills and prescriptions. Data were collected prior to 2002, before major changes in hormone use followed publicity about the increased CVD risks shown in the large HERS and WHI trials. These women probably started their estrogen before general awareness of the endometrial cancer risk associated with unopposed estrogen, and many continued with this regimen thereafter because they did not want to have intermittent bleeding and continuous combined estrogen and progestin regimens were not available. More recent studies have much lower prevalence of PME use, mostly younger and symptomatic women. Because medications were confirmed with pill bottles at both visits and in-depth interviews were performed for major CVD risk factors, fully adjusted models could be determined. In the longitudinal analysis, all but 10 of the baseline current users of PME had been on continuous PME over the entire follow-up, and all of the never users had consistently not been on PME throughout the course of their study, limiting the recall bias associated with complex medication histories in older women. To our knowledge, this is the only longitudinal study of PME use on kidney function and albuminuria that has confirmed continuous use of PME for approximately 10 years.
Some study limitations should be noted. Because of social class and ethnic homogeneity, conclusions may not be generalizable to the general population, but this reduces the healthy, wealthy woman bias that often determines who is prescribed estrogen51. Furthermore, as noted, participant bias selects against the return of those who have severe hypertension and renal disease after the baseline visit. This is an observational study, so associations, particularly the cross-sectional portion of the study, may not be causal. Cross-sectional studies have a number of limitations, most importantly the inability to establish causality, and may be influenced by confounding relationships and variables not studied. The prospective portion of the study allows us to evaluate changes over time, though residual confounding may exist. For example, there may be residual confounding by environmental variables, particularly diet and sodium intake—that may affect blood pressure--which were not evaluated here. Protein intake may also confound the relationship with ACR.
We have determined eGFR based on the abbreviated MDRD equation30, which is most accurate in persons with GFR ≤ 60 ml/min/1.73m2, tending to overestimate GFR in healthy persons52. The goal of our study was to compare changes over time and between groups, rather than determine the precise GFR for each subject. Additionally, the use of different laboratories for serum creatinine measurements, despite using similar assay techniques, may have biased our longitudinal results. The anticipated trends toward higher serum creatinine and lower eGFR over time and by age were intact. All of the samples at each visit were determined by the same lab.
Conclusion
We found that current PME users had lower BP and eGFR and decreased odds of having CKD. However, our longitudinal study of younger and likely healthier survivors over a 10-year follow-up period revealed an increase in ACR in never users of PME, but no effect on eGFR. Thus we provide evidence that long-term continuous oral PME use may be inversely associated with albuminuria but has no substantially positive or inverse association with eGFR. Differences were noted in BP trends over time, particularly in never users of PME, which should be confirmed in further longitudinal and clinical studies.
Summary.
We studied the cross-sectional and prospective association of blood pressure, urine albumin-to-creatinine ratio, and estimated glomerular filtration rate (GFR) and exposure to postmenopausal estrogen therapy. In cross-sectional analyses of 1044 women, estrogen users had better GFR and blood pressure than nonusers, but the 10-year follow-up of 443 women showed improved blood pressure and decreased urine albumin-to-creatinine ratio among current users, without differences in GFR by estrogen use.
Acknowledgments
Support for this work was provided by the National Institutes of Health/National Institute on Aging grant AG07181 and grant AG028507 and the National Institute of Diabetes and Digestive and Kidney Diseases, grant DK31801, and the Veterans Affairs Healthcare System (Career Development Award)
Footnotes
Disclosures: MF has grant support from Forest Laboratories, unrelated to this project. No other conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Forman JP, Fisher ND, Schopick EL, Curhan GC. Higher levels of albuminuria within the normal range predict incident hypertension. J Am Soc Nephrol. 2008;19:1983–8. doi: 10.1681/ASN.2008010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoy W, McDonald SP. Albuminuria: marker or target in indigenous populations. Kidney Int Suppl. 2004:S25–31. doi: 10.1111/j.1523-1755.2004.09207.x. [DOI] [PubMed] [Google Scholar]
- 3.Bigazzi R, Bianchi S, Baldari D, Campese VM. Microalbuminuria predicts cardiovascular events and renal insufficiency in patients with essential hypertension. J Hypertens. 1998;16:1325–33. doi: 10.1097/00004872-199816090-00014. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan NM. Microalbuminuria: a risk factor for vascular and renal complications of hypertension. Am J Med. 1992;92:8S–12S. [PubMed] [Google Scholar]
- 5.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–69. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 6.Foster MC, Hwang SJ, Larson MG, et al. Cross-classification of microalbuminuria and reduced glomerular filtration rate: associations between cardiovascular disease risk factors and clinical outcomes. Arch Intern Med. 2007;167:1386–92. doi: 10.1001/archinte.167.13.1386. [DOI] [PubMed] [Google Scholar]
- 7.Barrett-Connor E. Clinical review 162: cardiovascular endocrinology 3: an epidemiologist looks at hormones and heart disease in women. J Clin Endocrinol Metab. 2003;88:4031–42. doi: 10.1210/jc.2003-030876. [DOI] [PubMed] [Google Scholar]
- 8.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. Jama. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 9.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. Jama. 2004;291:1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 10.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. Jama. 1998;280:605–13. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 11.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. Jama. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 12.Mueck AO, Seeger H. Effect of hormone therapy on BP in normotensive and hypertensive postmenopausal women. Maturitas. 2004;49:189–203. doi: 10.1016/j.maturitas.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Chasan-Taber L, Willett WC, Manson JE, et al. Prospective study of oral contraceptives and hypertension among women in the United States. Circulation. 1996;94:483–9. doi: 10.1161/01.cir.94.3.483. [DOI] [PubMed] [Google Scholar]
- 14.Jespersen CM, Arnung K, Hagen C, et al. Effects of natural oestrogen therapy on blood pressure and renin-angiotensin system in normotensive and hypertensive menopausal women. J Hypertens. 1983;1:361–4. doi: 10.1097/00004872-198312000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Hassager C, Christiansen C. Blood pressure during oestrogen/progestogen substitution therapy in healthy post-menopausal women. Maturitas. 1988;9:315–23. doi: 10.1016/0378-5122(88)90097-7. [DOI] [PubMed] [Google Scholar]
- 16.Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. Jama. 1995;273:199–208. [PubMed] [Google Scholar]
- 17.Szekacs B, Vajo Z, Varbiro S, et al. Postmenopausal hormone replacement improves proteinuria and impaired creatinine clearance in type 2 diabetes mellitus and hypertension. BJOG. 2000;107:1017–21. doi: 10.1111/j.1471-0528.2000.tb10406.x. [DOI] [PubMed] [Google Scholar]
- 18.Monster TB, Janssen WM, de Jong PE, de Jong-van den Berg LT. Oral contraceptive use and hormone replacement therapy are associated with microalbuminuria. Arch Intern Med. 2001;161:2000–5. doi: 10.1001/archinte.161.16.2000. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed SB, Culleton BF, Tonelli M, et al. Oral estrogen therapy in postmenopausal women is associated with loss of kidney function. Kidney Int. 2008;74:370–6. doi: 10.1038/ki.2008.205. [DOI] [PubMed] [Google Scholar]
- 20.Remuzzi G. Abnormal protein traffic through the glomerular barrier induces proximal tubular cell dysfunction and causes renal injury. Curr Opin Nephrol Hypertens. 1995;4:339–42. doi: 10.1097/00041552-199507000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Schopick EL, Fisher ND, Lin J, Forman JP, Curhan GC. Post-menopausal hormone use and albuminuria. Nephrol Dial Transplant. 2009;24:3739–44. doi: 10.1093/ndt/gfp321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agarwal M, Selvan V, Freedman BI, Liu Y, Wagenknecht LE. The relationship between albuminuria and hormone therapy in postmenopausal women. Am J Kidney Dis. 2005;45:1019–25. doi: 10.1053/j.ajkd.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 23.Machado RB, Careta MF, Balducci GP, Araujo TS, Bernardes CR. Effects of estrogen therapy on microalbuminuria in healthy post-menopausal women. Gynecol Endocrinol. 2008;24:681–5. doi: 10.1080/09513590802444159. [DOI] [PubMed] [Google Scholar]
- 24.Fenkci S, Fenkci V, Yilmazer M, Serteser M, Koken T. Effects of short-term transdermal hormone replacement therapy on glycaemic control, lipid metabolism, C-reactive protein and proteinuria in postmenopausal women with type 2 diabetes or hypertension. Hum Reprod. 2003;18:866–70. doi: 10.1093/humrep/deg146. [DOI] [PubMed] [Google Scholar]
- 25.Manning PJ, Sutherland WH, Allum AR, de Jong SA, Jones SD. HRT does not improve urinary albumin excretion in postmenopausal diabetic women. Diabetes Res Clin Pract. 2003;60:33–9. doi: 10.1016/s0168-8227(02)00279-6. [DOI] [PubMed] [Google Scholar]
- 26.Criqui MH, Barrett-Connor E, Austin M. Differences between respondents and non-respondents in a population-based cardiovascular disease study. Am J Epidemiol. 1978;108:367–72. doi: 10.1093/oxfordjournals.aje.a112633. [DOI] [PubMed] [Google Scholar]
- 27.Barrett-Connor E. The prevalence of diabetes mellitus in an adult community as determined by history or fasting hyperglycemia. Am J Epidemiol. 1980;111:705–12. doi: 10.1093/oxfordjournals.aje.a112948. [DOI] [PubMed] [Google Scholar]
- 28.Rose GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ. 1962;27:645–58. [PMC free article] [PubMed] [Google Scholar]
- 29.The hypertension detection and follow-up program: Hypertension detection and follow-up program cooperative group. Prev Med. 1976;5:207–15. doi: 10.1016/0091-7435(76)90039-6. [DOI] [PubMed] [Google Scholar]
- 30.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 31.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 32.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 33.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 34.Warram JH, Gearin G, Laffel L, Krolewski AS. Effect of duration of type I diabetes on the prevalence of stages of diabetic nephropathy defined by urinary albumin/creatinine ratio. J Am Soc Nephrol. 1996;7:930–7. doi: 10.1681/ASN.V76930. [DOI] [PubMed] [Google Scholar]
- 35.Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. Springer; New York: 2000. [Google Scholar]
- 36.Daniels MJ, Zhao YD. Modelling the random effects covariance matrix in longitudinal data. Stat Med. 2003;22:1631–47. doi: 10.1002/sim.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanada M, Higashi Y, Nakagawa K, et al. Oral estrogen replacement therapy increases forearm reactive hyperemia accompanied by increases in serum levels of nitric oxide in postmenopausal women. Gynecol Endocrinol. 2001;15:150–7. [PubMed] [Google Scholar]
- 38.Darkow DJ, Lu L, White RE. Estrogen relaxation of coronary artery smooth muscle is mediated by nitric oxide and cGMP. Am J Physiol. 1997;272:H2765–73. doi: 10.1152/ajpheart.1997.272.6.H2765. [DOI] [PubMed] [Google Scholar]
- 39.John S, Schmieder RE. Impaired endothelial function in arterial hypertension and hypercholesterolemia: potential mechanisms and differences. J Hypertens. 2000;18:363–74. doi: 10.1097/00004872-200018040-00002. [DOI] [PubMed] [Google Scholar]
- 40.Ganz P. Vasomotor and vascular effects of hormone replacement therapy. Am J Cardiol. 2002;90:11F–6F. doi: 10.1016/s0002-9149(01)02218-4. [DOI] [PubMed] [Google Scholar]
- 41.Xing D, Nozell S, Chen YF, Hage F, Oparil S. Estrogen and mechanisms of vascular protection. Arterioscler Thromb Vasc Biol. 2009;29:289–95. doi: 10.1161/ATVBAHA.108.182279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antus B, Hamar P, Kokeny G, et al. Estradiol is nephroprotective in the rat remnant kidney. Nephrol Dial Transplant. 2003;18:54–61. doi: 10.1093/ndt/18.1.54. [DOI] [PubMed] [Google Scholar]
- 43.Wassertheil-Smoller S, Hendrix SL, Limacher M, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women's Health Initiative: a randomized trial. Jama. 2003;289:2673–84. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 44.Vongpatanasin W, Tuncel M, Mansour Y, Arbique D, Victor RG. Transdermal estrogen replacement therapy decreases sympathetic activity in postmenopausal women. Circulation. 2001;103:2903–8. doi: 10.1161/01.cir.103.24.2903. [DOI] [PubMed] [Google Scholar]
- 45.Kornhauser C, Malacara JM, Garay ME, Perez-Luque EL. The effect of hormone replacement therapy on blood pressure and cardiovascular risk factors in menopausal women with moderate hypertension. J Hum Hypertens. 1997;11:405–11. doi: 10.1038/sj.jhh.1000420. [DOI] [PubMed] [Google Scholar]
- 46.Wren BG, Routledge DA. Blood pressure changes: oestrogens in climacteric women. Med J Aust. 1981;2:528–31. doi: 10.5694/j.1326-5377.1981.tb112974.x. [DOI] [PubMed] [Google Scholar]
- 47.Smulyan H, Safar ME. The diastolic blood pressure in systolic hypertension. Ann Intern Med. 2000;132:233–7. doi: 10.7326/0003-4819-132-3-200002010-00010. [DOI] [PubMed] [Google Scholar]
- 48.Perkovic V, Verdon C, Ninomiya T, et al. The relationship between proteinuria and coronary risk: a systematic review and meta-analysis. PLoS Med. 2008;5:e207. doi: 10.1371/journal.pmed.0050207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsioufis C, Dimitriadis K, Antoniadis D, Stefanadis C, Kallikazaros I. Inter-relationships of microalbuminuria with the other surrogates of the atherosclerotic cardiovascular disease in hypertensive subjects. Am J Hypertens. 2004;17:470–6. doi: 10.1016/j.amjhyper.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 50.Jassal SK, Kritz-Silverstein D, Barrett-Connor E. A prospective study of albuminuria and cognitive function in older adults: the Rancho Bernardo study. Am J Epidemiol. 171:277–86. doi: 10.1093/aje/kwp426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barrett-Connor E. Postmenopausal estrogen and prevention bias. Ann Intern Med. 1991;115:455–6. doi: 10.7326/0003-4819-115-6-455. [DOI] [PubMed] [Google Scholar]
- 52.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141:929–37. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]