Abstract
Objectives
Minimally invasive robotic assistance is being increasingly utilized to treat larger complex renal masses. We report on the technical feasibility and renal functional and oncological outcomes with minimum 1 year follow up of robot-assisted laparoscopic partial nephrectomy (RALPN) for tumors greater than 4 cm.
Methods and Materials
The urologic oncology database was queried to identify patients treated with RALPN for tumors greater than 4 cm and a minimum follow up of 12 months. We identified 19 RALPN on 17 patients treated between June 2007 and July 2009. Two patients underwent staged bilateral RALPN. Demographic, operative, and pathologic data were collected. Renal function was assessed by serum creatinine levels, estimated glomerular filtration rate and nuclear renal scans assessed at baseline, 3 and 12 months post-operatively. All tumors were assigned R.E.N.A.L. nephrometry scores (www.nephrometry.com).
Results
The median nephrometry score for the largest tumor from each kidney was 9 (range 6–11) while the median size was 5 cm (range 4.1–15). Three of 19 cases (16%) required intraoperative conversion to open partial nephrectomy. No renal units were lost. There were no statistically significant differences between preoperative and postoperative creatinine and eGFR. A statistically significant decline of ipsilateral renal scan function (49% vs. 46.5%, p=0.006) was observed at three months and at twelve months postoperatively (49% vs. 45.5%, p=0.014). No patients had evidence of recurrence or metastatic disease at a median follow up of 22 months (range 12–36).
Conclusions
RALPN is feasible for renal tumors greater than 4 cm with moderate or high nephrometry scores. Although there was a modest decline in renal function of the operated unit, RALPN may afford the ability resect challenging tumors requiring complex renal reconstruction. The renal functional and oncological outcomes are promising at a median follow up of 22 months, but longer follow up is required.
Introduction
Open partial nephrectomy (OPN) has become the gold standard for the treatment of clinical stage T1 renal tumors.(1) The reason for this acceptance stems from two observations. First, the oncologic outcomes for treatment of T1a and T1b lesions compare equally when treated with radical or partial nephrectomy.(2–9) Second, recent data has demonstrated the advantage of NSS for the avoidance of renal insufficiency as well as providing a survival advantage over radical nephrectomy for T1 renal tumors.(10)
While laparoscopic partial nephrectomy (LPN) was initially utilized for small, exophytic and easily accessible renal tumors, the growing experience in laparoscopy and the adoption of robotic assistance has allowed for the treatment of larger, hilar lesions as well as multifocal tumors in the same setting.(11–13) Regardless of the surgical technique, the goals of renal surgery for T1 lesions remain the same: oncologic efficacy, renal preservation and early convalescence.
Established advantages of robotic surgery over traditional laparoscopy include wristed articulation and the facilitation of intracorporeal suturing while maintaining the benefits of minimally invasive surgery such as pneumoperitoneum, decreased post operative pain, and early convalescence.(14) While the robotic approach is seen as an “enhancement” of traditional laparoscopy in renal surgery and may shorten the learning curve for those who do not have much laparoscopic experience, robotic renal surgery may incur a higher operating cost compared to traditional laparoscopy. Nevertheless, as we gain experience managing more challenging renal tumors utilizing robotic assistance, we wished to evaluate our initial experience with robot assisted partial nephrectomy for renal cortical tumors larger than 4 cm. In this study we report on feasibility and renal functional as well as early oncologic outcomes of this approach.
Materials and Methods
We have queried the Urologic Oncology database to identify patients treated with RALPN for tumors greater than 4 cm and a minimum follow up of 12 months. We identified a total of 19 RALPN performed on 17 patients. Two patients underwent staged bilateral RALPN. All patients were evaluated on a protocol approved by the institutional review board.
Data acquisition and patient evaluation
Data were obtained from review of operative and pathology reports, hospital admission, discharge and anesthesia records. The operative time was obtained from the anesthesia records from the time in the operating room to the time out of the operating room. At the time of surgery, no patients had pre-operative or intra-operative suspicion of locally advanced or metastatic disease. Operative data were reported on patients in whom RALPN was successfully completed. Tumor size was assessed radiographically. The tumors were assigned a nephrometry score based on the R.E.N.A.L. tumor classification system.(15) Tumors with a score of 4–6 were considered low complexity, 7–9 moderate complexity and 10–12 high complexity. This system allows for a more accurate and reproducible standardization of tumor size, location and depth.
Preoperative evaluation for all patients undergoing RALPN included abdominal and chest imaging, routine laboratory analysis and a nuclear renal scan. All studies were repeated at 3 months and 12 months post-operatively and later at the discretion of the treating team. Functional outcomes were assessed using nuclear renogram, serum creatinine, and eGFR calculated by the MDRD equation.(16)
Surgical technique
Cystoscopy and ureteral catheter placement were performed in all patients in the anticipation of collecting system entry to allow for intraoperative injection of methylene blue for help in identification of the collecting system injury requiring repair. The ureteral catheter was removed at the end of each case. A standard 3-arm Da Vinci® Surgical System with a transperitoneal approach was performed in 15 of 16 renal units (94%) that underwent successful RALPN and a retroperitoneal approach was performed in one. Patient positioning and port placement for RALPN were performed as previously described by our institution.(11) Laparoscopic ultrasound was used in each case to delineate the number of tumors, as well as location and depth and proximity to collecting system and vessels. Five of 16 renal units (31%) had more than 1 tumor; in these cases, surgical extirpation was begun with the smaller, exophytic tumors without hilar clamping and progressed to the larger, endophytic tumors requiring hilar clamping in most cases, as described previously in our experience of RALPN for multifocal renal masses.(11)
Ten of 16 renal units (63%) in patients with hereditary or multifocal lesions were resected by enucleation, a technique previously described for the management of hereditary renal tumors at the National Cancer Institute.(17) This enucleation technique is now utilized open, lapraroscopically, or robotically. Briefly, the tumor pseudocapsule was identified and a plane was developed between the tumor and the renal parenchyma. The tumor was inspected intracorporeally to ensure an intact pseudocapsule. Hilar occlusion was performed with laparoscopic bulldogs or laparoscopic Satinsky clamp only when bleeding from resection became excessive or obscured visualization. The remaining 6 of 16 renal units (37%) underwent resection with a margin of normal parenchyma. In these cases, after hilar occlusion, the tumor was resected using cold resection with robotic scissors. The tumor pseudocapsule was not visualized and a margin of normal renal parenchyma was left circumferentially surrounding the tumor.
After tumor resection, the ureteral catheter was injected with dilute methylene blue to allow precise visualization of any collecting system injury. If present, it was closed separately with running 3-0 polyglactin suture, and the renal base was over sewn with a 3-0 polyglactin suture for hemostasis. Renorrhaphy was performed using interrupted capsular 2-0 polyglactin sutures over hemostatic sealant and Surgicell® bolsters. Following renorrhaphy, the bulldog or Satinsky clamps were removed and the defect inspected for hemostasis. A 15 mm round Jackson-Pratt drain was placed to closed bulb suction at the completion of all cases.
Results
Table 1 lists clinical characteristics of patients in our cohort. There was an equal gender distribution and the median BMI was 29.8 (range 19.8–40.4). Eleven of 19 RALPN (58%) were performed on patients with known hereditary syndromes, predisposing them to the risk of bilateral, multifocal renal tumors. The remaining RALPN were performed on patients who had either a solitary solid or multiple solid tumors with unknown genetic causes. The median largest tumor size was 5.0 cm (range 4.1–15). The median R.E.N.A.L. nephrometry score of the largest tumor was 9 (range 6–11) with 12 (63%) located anteriorly and 7 (37%) posteriorly; 6 (31.5%) of these tumors had a hilar designation and 17 (89.5%) were greater than 50% endophytic. The histology of the largest tumors is also shown in Table 1.
Table 1.
Patient and Tumor Characteristics
| Operation No./Patient No. | 19/17 | ||
| Staged bilateral procedures | 2 (10.5%) | ||
| Median age, yrs (range) | 47 (26 to 76) | ||
| Median BMI (range) | 29.8 (19.8 to 40.4) | ||
| Male (%) | 9 (53) | ||
| Surgery on the right, No. (%) | 11 (58) | ||
| Hereditary disease, No. (%) | 11 (58) | ||
| VHL | 9 (47) | ||
| BHD | 1 (5) | ||
| TS | 1 (5) | ||
| Median follow-up, mo (range) | 22 (12 to 36) | ||
| Median tumors per patient, # (range) | 1 (1 to 4) | ||
| Median largest tumor (range) | 5.0 (4.1 to 15) | ||
| Nephrometry scores | 1 | 2 | 3 |
| R (size criteria) | 0 | 15 (79%) | 4 (21%) |
| E (endophytic/exophytic) | 2 (10.5%) | 15 (79%) | 2 (10.5%) |
| N (nearness to sinus or calyx) | 2 (10.5%) | 9 (42%) | 8 (47.5%) |
| A (anterior or posterior) | A = 12 (63%) | P = 7 (37%) | |
| L (location relative to polar line) | 5 (26%) | 4 (21%) | 10 (53%) |
| Hilar designation | 6 (31.5%) | ||
| Total nephrometry score | |||
| 4 | 0 | ||
| 5 | 0 | ||
| 6 | 1 (5.3%) | ||
| 7 | 2 (10.5%) | ||
| 8 | 2 (10.5%) | ||
| 9 | 8 (42.1%) | ||
| 10 | 4 (21%) | ||
| 11 | 2 (10.6%) | ||
| 12 | 0 | ||
| Median nephrometry score of largest tumor | 9 | ||
| Largest tumor histology, # (%) | |||
| Clear cell | 11 (58) | ||
| Papillary type 1 | 4 (21) | ||
| Chromophobe | 2 (11) | ||
| Oncocytoma | 1 (5) | ||
| Angiomyolipoma | 1 (5) | ||
Three RALPN (16%) required conversion to open partial nephrectomy. Two of these cases were converted due to excessive bleeding early in cases planned for resection of multiple masses, and the third was due to intraoperative ultrasound identification of an additional endophytic posterior lesion not appreciated on preoperative imaging that would have potentially subjected the patient to prolonged renal warm ischemia. In the cases converted to open technique, the tumors were successfully resected without the loss of a renal unit. Of cases completed with robotic assistance five of 16 renal units (31%) had more than one solid tumor removed. A total of 25 solid renal masses were resected in 16 surgeries completed with robotic assistance.
Table 2 indicates perioperative outcomes for the 16 successfully completed partial nephrectomies using robotic assistance. Median operative time was 390 minutes (range 220–535). Fifteen of 16 RALPN (94%) required hilar clamping with a median ischemia time of 35.6 minutes (range 17–61). Collecting system entry with subsequent closure was performed in 8 (50%) RALPN. No patient required intraoperative blood transfusion. One patient developed a urine leak and ureteropelvic junction obstruction requiring post operative stenting. We suspect that the case of UPJ obstruction is likely a result of repeat ureteral and hilar dissection of the previously operated kidney. Of the patients who underwent resection with margins, no positive surgical margins were identified on final pathology. No patients had evidence of local recurrence or metastatic disease at median follow-up of 22 months (range 12–36).
Table 2.
Perioperative data (n=16)*
| Median operative time, min (range) | 390 (220 to 535) |
| Median warm ischemia time, min (range) | 36 (17 to 61) |
| Median estimated blood loss, mL (range) | 500 (100 to 1600) |
| Hilar clamping, # (%) | 15 (94) |
| Collecting system entry/closure, # (%) | 8 (50) |
| Intraoperative transfusion, # (%) | 0 (0) |
| Complications (%) | 1 (6) |
Data reported on successful RALPN
Table 3 indicates renal function outcomes. At 3 and 12 months postoperatively, there was no statistical decrease in serum creatinine or eGFR. There was, however, a statistically significant decline in median ipsilateral renal scan function of 2.5% and 3.5% at 3 months and 12 months respectively.
Table 3.
Renal Function Outcomes (n=16)*
| Median Renal Function Outcomes | Pre-op | Post-op 3 mo. | P Value | Post-op 12 mo. | P Value |
|---|---|---|---|---|---|
| Serum Creatinine, mg/dL | 0.82 | 0.87 | 0.109 | 0.88 | 0.200 |
| eGFR, ml/min | 107.8 | 102.2 | 0.452 | 101.7 | 0.605 |
| Ipsilateral renal scan function, % | 49.0 | 46.5 | 0.006 | 45.5 | 0.014 |
Data reported on successful RALPN
Dicussion
As the application of partial nephrectomy continues to expand, the vast majority of patients presenting with an incidental renal mass may eventually be offered some form of nephron sparing surgery. In an effort to preserve renal preservation while maintaining the established advantages of minimally invasive surgery, urological surgeons have expanded the role of minimally invasive surgery, applying it toward more complex renal tumors. Robotic surgery and specifically RALPN may be partly responsible for facilitating this transition. RALPN has now been shown to be effective for the treatment of large, multiple, and hilar renal masses.(11–13) Recent AUA guidelines have recommended strong consideration of partial nephrectomy for all T1 renal masses, regardless of overall renal function or the status of the contralateral kidney.(1) The aim of our study was to evaluate the feasibility, renal functional and early oncologic outcomes of robot assisted partial nephrectomy for renal masses greater than 4 cm. Furthermore, by assigning R.E.N.A.L nephrometry scores, we attempted to provide another measure of standardization that can be used, in addition to size, for future comparison.
We chose to report our data using the nephrometry score because currently, urologists lack an accepted standard method for reporting anatomical elements of renal tumors. In 2009 Kutikov et al. described R.E.N.A.L nephrometry, as a novel system to quantify renal tumor size, location, and depth with the aim of providing a reproducible classification system of renal masses.(15) By having the ability to place a renal tumor in its anatomical context the urological surgeon may be better able to decide which surgical intervention and operative approach is most appropriate for the particular patient. In addition, the system may offer a more sophisticated and consistent way to compare results between institutions, a recurring drawback of a large number of published series.
The median R.E.N.A.L nephrometry score for the largest lesion in the present series was 9 (range 6–11), underlying the complexity of the masses removed. Examples of tumors resected using robotic assistance are displayed in Figure 1. Once again, as demonstrated by these images, historical reporting of only tumor size may underestimate the complexity of partial nephrectomy for a given tumor. All lesions shown in Figure 1 are classified as T1b, but their RENAL scores are quite different. By applying measurements of location, depth, and proximity to vital surrounding structures the nephrometry score may generate a better estimation of the surgical pitfalls that may lie ahead, regardless of technique.
Figure 1.
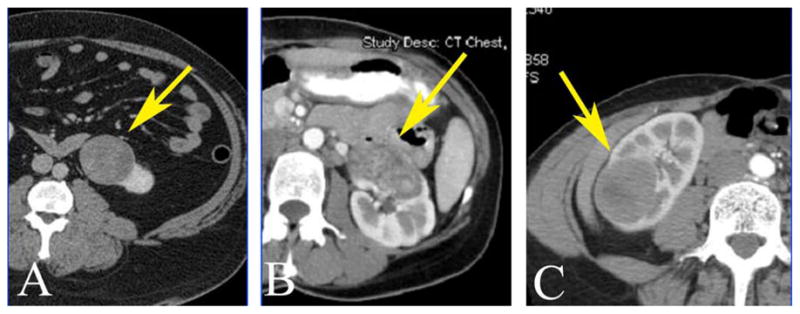
Computerized tomography demonstrating variability of anatomic location and surgical complexity of various T1b renal mass and their R.E.N.A.L nephrometry score. A, renal mass (arrow) with nephrometry score 6a; B, renal mass (arrow) with nephrometry score 11a,h; C, renal mass (arrow) nephrometry score 9p
Feasibility of performing robot assisted partial nephrectomy for the T1b or greater renal mass has been described recently.(12) In the LPN literature, Simmons et al. reported on 425 consecutive LPN by a single surgeon, 58 of which were for tumors greater than 4 cm.(18) In this subset they reported an average tumor size of 6 cm, warm ischemia times of 38 minutes and an EBL of 284 cc. Notably, as tumors became larger and more endophytic, warm ischemia times and blood loss also increased. Our cohort had similar results to both the robotic and laparoscopic series described in terms of ischemia time, complication rates, and preservation of renal function. A greater percentage of tumors removed in our study (14 of 16, 88%) were malignant by comparison. Although R.E.N.A.L nephrometry scores were not applied to these past experiences, our higher blood loss and overall operative times may be explained by the overall complexity of the RALPNs performed in this cohort. Additionally, our series represents our early experience with resecting >T1b lesions and our operative times have continued to shorten. While operative time would likely be shorter if performed open, we have demonstrated that RALPN for these tumors is safe as there was only one Clavien grade 3 complication in this series.
Initial concerns with minimally invasive techniques for complex partial nephrectomy rest on the foundation that prolonged warm ischemia may adversely affect renal function. Historical duration of safe warm ischemia was thought to be 30 minutes; however, recent reports have suggested that extended warm ischemia, up to 55 minutes in one study, does not influence long term renal function. (19–21) Of note, one of our patients had a warm ischemia of 61 minutes. After resection of a 6 cm lesion, a second tumor was identified adjacent to the resection site and the tumor was extirpated with robotic assistance. This was one of the earlier cases in the present series, and we feel that better judgment could have been exercised in selecting this case early in our experience with RALPN. Fortunately, the patient was 30 years old and did not experience a decline in her eGFR or a split function on postoperative renal nuclear scan. Nevertheless, we do believe that every attempt should be made for maximal renal preservation and minimizing warm ischemia time. With recent evidence suggesting that safe warm ischemia time should be no longer than 20 minutes, we have modified our technique in our most recent cases by attempting to resect tumors either without hilar clamping or utilizing “early unclamping” as described by Nguyen et al.(11;22;23)
When investigating the preservation of renal function after RALPN, it is important to consider the factors that may have influenced our findings. Although there was a significant decrease from baseline, the 2.5% and 3.5% change on ipsilateral renal scan at 3 and 12 months post-operatively in our series was lower than several published studies on partial nephrectomy.(23–25) This finding may be explained by the younger age of our cohort as well as the technique employed for surgical extirpation. Wedge resection of a renal mass may be expected to affect a larger percentage of ipsilateral renal function when compared to enucleative resection, since functioning nephrons are incorporated into the resection margins. In our series, ten of the 16 (63%) successfully completed RALPN utilized enucleation techniques while 6 (37%) were performed with a surgical margin pathologically confirmed to be completely negative. There was no statistically significant difference between the enucleation and wedge resection with regards to WIT (33.9 min vs. 37.8 min, p=0.28) or the change in ipsilateral renal scan (−2% vs. −6%, p=0.34), respectively. These similarities may attest to an equivalence of technique or alternatively may be explained by the complexity of the mass. Additionally, the nephrometry score was not different between the tumors resected with enucleation versus resection with margins (9.5 vs 9, p=0.48), respectively. This observation may support the use of the nephrometry score as determinant of anticipated complexity of the case and a potential surrogate predictor of the ischemia time.
The risk of operative complications and conversion to open surgery are important considerations for any minimally invasive procedure, particularly when exploring new surgical techniques. In Gill’s study of 58 T1B laparoscopic partial nephrectomies, the complication and a conversion rate were 7% of 2%, respectively.(18) Historically, complication rates for other complex RALPN series have ranged from 0% to 26.6% with conversion rates ranging from 0% to 11%.(11–13) In our series, there was only one (6.2%) Clavien grade 3 complication (ureteral stent placement) compared to three (20%) Clavien grade 3 or greater complications reported by Patel et al in the only other published series of RALPN for tumors greater than 4 cm.(12) Notably, in the series of Patel et al, only 15.4 % of tumors were greater than 50% endophytic, compared to 89.5% in the present series. Our 16% conversion rate, which may be higher by comparison, may be attributable to our high median nephrometry score (9.0), large median tumor size (5.0 cm), and multifocality (42%). With our increased experience and improved patient selection we hope to see the number of conversions decrease.
Of importance, no RALPN in this series (including conversions) resulted in the loss of a renal unit. This further underscores that the goal of renal preservation was not compromised in our early experience. The acceptance of open conversion as a change of technique rather than a complication or technical failure is of paramount importance when attempting to perform increasingly complex minimally invasive surgeries. The preservation of the renal unit and application of sound oncologic principles should remain the primary goals of nephron sparing surgery. In the present cohort, all patients retained their kidneys and no one had evidence of local recurrence or metastatic disease at median follow-up of 22 months.
This study has inherent limitations. It is a retrospective review and the sample size is small. This series represents a group of highly selected patients who were offered RALPN and factors such as tumor size, location, renal function, and history of prior surgeries were considered prior to offering RALPN. While the nephrometry score was reported for all lesions, the R.E.N.A.L nephrometry scoring system has not yet been validated with regards to perioperative, functional or oncologic outcomes but is rather used to communicate the degree of difficulty of tumor resection in this cohort and for future comparisons. Regardless of these limitations, this study is the largest series to date that evaluates the feasibility as well as outcomes of RALPN for tumors greater than 4 cm with high nephrometry score and with the longest follow up reported in the literature. Our results will hopefully add to the growing body of literature on the role of robotic assisted nephron-sparing surgery for challenging renal masses and provide useful information to physicians to help with preoperative counseling.
Conclusion
Performing RALPN for renal tumors greater than 4 cm with moderate or high nephrometry scores appears safe and feasible. Carefully selected patients may undergo safe resection of tumors with excellent preservation of renal function and encouraging oncologic outcomes at a median follow up of 22 months. Longer follow up is needed to assess long-term outcomes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Campbell SC, Novick AC, Belldegrun A, Blute ML, Chow GK, Derweesh IH, Faraday MM, Kaouk JH, Leveillee RJ, Matin SF, Russo P, Uzzo RG. Guideline for management of the clinical T1 renal mass. J Urol. 2009 Oct;182(4):1271–9. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Antonelli A, Cozzoli A, Nicolai M, Zani D, Zanotelli T, Perucchini L, Cunico SC, Simeone C. Nephron-sparing surgery versus radical nephrectomy in the treatment of intracapsular renal cell carcinoma up to 7 cm. Eur Urol. 2008 Apr;53(4):803–9. doi: 10.1016/j.eururo.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Becker F, Siemer S, Hack M, Humke U, Ziegler M, Stockle M. Excellent long-term cancer control with elective nephron-sparing surgery for selected renal cell carcinomas measuring more than 4 cm. Eur Urol. 2006 Jun;49(6):1058–63. doi: 10.1016/j.eururo.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Crispen PL, Boorjian SA, Lohse CM, Sebo TS, Cheville JC, Blute ML, Leibovich BC. Outcomes following partial nephrectomy by tumor size. J Urol. 2008 Nov;180(5):1912–7. doi: 10.1016/j.juro.2008.07.047. [DOI] [PubMed] [Google Scholar]
- 5.Hafez KS, Fergany AF, Novick AC. Nephron sparing surgery for localized renal cell carcinoma: impact of tumor size on patient survival, tumor recurrence and TNM staging. J Urol. 1999 Dec;162(6):1930–3. doi: 10.1016/S0022-5347(05)68071-8. [DOI] [PubMed] [Google Scholar]
- 6.Leibovich BC, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Nephron sparing surgery for appropriately selected renal cell carcinoma between 4 and 7 cm results in outcome similar to radical nephrectomy. J Urol. 2004 Mar;171(3):1066–70. doi: 10.1097/01.ju.0000113274.40885.db. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell RE, Gilbert SM, Murphy AM, Olsson CA, Benson MC, McKiernan JM. Partial nephrectomy and radical nephrectomy offer similar cancer outcomes in renal cortical tumors 4 cm or larger. Urology. 2006 Feb;67(2):260–4. doi: 10.1016/j.urology.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 8.Pahernik S, Roos F, Rohrig B, Wiesner C, Thuroff JW. Elective nephron sparing surgery for renal cell carcinoma larger than 4 cm. J Urol. 2008 Jan;179(1):71–4. doi: 10.1016/j.juro.2007.08.165. [DOI] [PubMed] [Google Scholar]
- 9.Patard JJ, Shvarts O, Lam JS, Pantuck AJ, Kim HL, Ficarra V, Cindolo L, Han KR, De La Taille A, Tostain J, Artibani W, Abbou CC, Lobel B, Chopin DK, Figlin RA, Mulders PF, Belldegrun AS. Safety and efficacy of partial nephrectomy for all T1 tumors based on an international multicenter experience. J Urol. 2004 Jun;171(6 Pt 1):2181–5. doi: 10.1097/01.ju.0000124846.37299.5e. quiz. [DOI] [PubMed] [Google Scholar]
- 10.Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj GV, Scardino PT, Russo P. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006 Sep;7(9):735–40. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boris R, Proano M, Linehan WM, Pinto PA, Bratslavsky G. Initial Experience With Robot Assisted Partial Nephrectomy for Multiple Renal Masses. J Urol. 2009 Aug;182(4):1280–86. doi: 10.1016/j.juro.2009.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel MN, Krane LS, Bhandari A, Laungani RG, Shrivastava A, Siddiqui SA, Menon M, Rogers CG. Robotic Partial Nephrectomy for Renal Tumors Larger Than 4 cm. Eur Urol. 2009 Nov;52(2):310–16. doi: 10.1016/j.eururo.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 13.Rogers CG, Metwalli A, Blatt AM, Bratslavsky G, Menon M, Linehan WM, Pinto PA. Robotic partial nephrectomy for renal hilar tumors: a multi-institutional analysis. J Urol. 2008 Dec;180(6):2353–6. doi: 10.1016/j.juro.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guru KA, Hussain A, Chandrasekhar R, Piacente P, Hussain A, Chandrasekhar R, Piacente P, Bienko M, Glasgow M, Underwood W, Wilding G, Mohler JL, Menon M, Peabody JO. Current status of robot-assisted surgery in urology: a multi-national survey of 297 urologic surgeons. Can J Urol. 2009 Aug;16(4):4736–41. [PubMed] [Google Scholar]
- 15.Kutikov A, Uzzo RG. The RENAL nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009 Sep;182(3):844–53. doi: 10.1016/j.juro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation, Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999 Mar 16;130(6):461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 17.Herring JC, Enquist EG, Chernoff A, Linehan WM, Choyke PL, Walther MM. Parenchymal sparing surgery in patients with hereditary renal cell carcinoma: 10-year experience. J Urol. 2001 Mar;165(3):777–81. [PubMed] [Google Scholar]
- 18.Simmons MN, Weight CJ, Gill IS. Laparoscopic radical versus partial nephrectomy for tumors >4 cm: intermediate-term oncologic and functional outcomes. Urology. 2009 May;73(5):1077–82. doi: 10.1016/j.urology.2008.11.059. [DOI] [PubMed] [Google Scholar]
- 19.Bhayani SB, Rha KH, Pinto PA, Ong AM, Allaf ME, Trock BJ, Jarrett TW, Kavoussi LR. Laparoscopic partial nephrectomy: effect of warm ischemia on serum creatinine. J Urol. 2004 Oct;172(4 Pt 1):1264–6. doi: 10.1097/01.ju.0000138187.56050.20. [DOI] [PubMed] [Google Scholar]
- 20.Kane CJ, Mitchell JA, Meng MV, Anast J, Carroll PR, Stoller ML. Laparoscopic partial nephrectomy with temporary arterial occlusion: description of technique and renal functional outcomes. Urology. 2004 Feb;63(2):241–6. doi: 10.1016/j.urology.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 21.Porpiglia F, Renard J, Billia M, Musso F, Volpe A, Burruni R, Terrone C, Colla L, Piccoli G, Podio V, Scarpa RM. Is renal warm ischemia over 30 minutes during laparoscopic partial nephrectomy possible? One-year results of a prospective study. Eur Urol. 2007 Oct;52(4):1170–8. doi: 10.1016/j.eururo.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen MM, Gill IS. Halving ischemia time during laparoscopic partial nephrectomy. J Urol. 2008 Feb;179(2):627–32. doi: 10.1016/j.juro.2007.09.086. [DOI] [PubMed] [Google Scholar]
- 23.Thompson RH, Frank I, Lohse CM, Saad IR, Fergany A, Zincke H, Leibovich BC, Blute ML, Novick AC. The impact of ischemia time during open nephron sparing surgery on solitary kidneys: a multi-institutional study. J Urol. 2007 Feb;177(2):471–6. doi: 10.1016/j.juro.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 24.Desai MM, Gill IS, Ramani AP, Spaliviero M, Rybicki L, Kaouk JH. The impact of warm ischaemia on renal function after laparoscopic partial nephrectomy. BJU Int. 2005 Feb;95(3):377–83. doi: 10.1111/j.1464-410X.2005.05304.x. [DOI] [PubMed] [Google Scholar]
- 25.Kaul S, Laungani R, Sarle R, Stricker H, Peabody J, Littleton R, Menon M. da Vinci-assisted robotic partial nephrectomy: technique and results at a mean of 15 months of follow-up. Eur Urol. 2007 Jan;51(1):186–91. doi: 10.1016/j.eururo.2006.06.002. [DOI] [PubMed] [Google Scholar]


