Abstract
Background
Although most melanomas on the distal lower extremity drain exclusively to inguinal lymph nodes, a small percentage (<5%) drain to interval nodes in the popliteal basin. We investigated a possible relationship between tumor-draining popliteal and inguinal nodes in patients with lower-extremity melanoma.
Study Design
We queried our melanoma database to identify patients who underwent sentinel node biopsy (SNB) for an infrapopliteal melanoma. Patterns of nodal drainage and nodal metastasis were analyzed.
Results
Of 461 patients who underwent SNB for a primary infrapopliteal melanoma, 15 (3.2%) had drainage to the popliteal basin. Thirteen melanomas were on the posterior leg and foot, and 2 were on the anterior lower leg. Mean Breslow thickness was 2.4 mm. All 15 patients with popliteal drainage also had inguinal drainage and therefore underwent concurrent inguinal and popliteal SNB. The average number of popliteal sentinel nodes was 1.4 (range, 1 – 3). Eight patients (53%) had a tumor-positive popliteal sentinel node, and 6 of the 8 underwent completion popliteal lymphadenectomy. Four of the 8 patients (50%) also had tumor-positive inguinal sentinel nodes; all underwent complete inguinal lymphadenectomy. We also identified 9 additional patients who underwent SNB for locoregional recurrent melanomas of the infrapopliteal leg. Three (33%) of these patients had concurrent inguinal and popliteal SNB with one isolated tumor-positive popliteal node found.
Conclusions
In our series, a high percentage of popliteal sentinel lymph nodes contained metastases, and these patients frequently also had inguinal metastases. In our patients, all inguinal metastases were associated with concomitant popliteal metastases. Although it is anatomically separate, the inguinal basin appears to be a functional extension of the popliteal basin.
INTRODUCTION
A combination of preoperative lymphoscintigraphy, intraoperative lymphatic mapping and sentinel lymph node biopsy (SNB) for cutaneous melanoma, initially described by Morton et al in 1992, 1 identifies the first site(s) of regional lymphatic drainage and those lymph nodes most likely to contain occult metastasis.2 Although lymphatic drainage of cutaneous melanoma usually targets cervical, axillary or inguinal basins, 3 approximately 5–8% of melanomas drain initially to unexpected areas such as the intermuscular triangular space of the back, epitrochlear region, or the popliteal fossa. 2, 4, 5 Nodes in these sites have been described as interval, in-transit, ectopic, or intercalated.4, 6, 7 Because rates of tumor involvement are similar for these nodes and nodes in more common drainage basins, their tumor status is important for accurate staging and treatment of melanoma.2, 7–9
The literature concerning popliteal SLNs in cutaneous melanoma of the lower extremity is limited. Concomitant popliteal and inguinal lymphatic drainage has been documented 10 but there is no consensus on its biology or clinical significance. To identify a possible a relationship between tumor-draining popliteal and inguinal nodes in patients with lower-extremity melanoma, we determined the frequency of lymphatic drainage to popliteal SLNs, clinical features of patients with a popliteal SLN, patterns of nodal involvement in the popliteal and inguinal basins, and outcomes of patients with popliteal SLN metastases.
METHODS
The melanoma database at the John Wayne Cancer Institute (JWCI) contains >15,000 records spanning the years 1971 to 2010. We queried this database to identify patients who underwent SNB for a primary or recurrent infrapopliteal melanoma. At JWCI, we routinely perform SNB for cutaneous primary melanomas with high-risk characteristics and for locoregional recurrent melanoma. Recurrent lesions eligible for SNB are isolated dermal or subcutaneous metastases that recur within 2 cm of the previous wide excision or beyond 2 cm but along the lymphatic drainage pathway of the initial melanoma 11, 12, in patients with no evidence of regional adenopathy or distant metastases.11 The SNB data for patients with primary melanomas and those with locoregional melanomas are presented separately in the results as they are distinctly different disease processes.
Our technique of preoperative SNB has previously been described.1 Briefly, 0.5–1.0 mCi of ultrafiltered technetium-99m (Tc 99m) sulfur colloid is injected intradermally at the primary site. A rectangular gamma camera equipped with a high-resolution collimator is used to perform immediate dynamic imaging to follow the course of the lymphatic collecting vessels. Static images with the patient in the supine position and upright with 90° flexed knee lateral view are obtained after delays of 10 minutes and 2 hours. Delayed image acquisition is performed at both the popliteal and inguinal basins in all melanomas of the lower leg. We define SLNs as nodes directly receiving lymphatic channels and retaining tracer on delayed images. We routinely use the hand-held gamma probe (Neoprobe; Neoprobe Corporation, Dublin, Ohio) to screen the inguinal and popliteal basins intraoperatively. Intraoperative intradermal injection of 1–3 mL of 1% isosulfan blue dye (Lymphazurin; Tyco International, Norwalk, CT) in four quadrants at the site of the primary tumor is used at the beginning of the surgical procedure. SNB is performed prior to wide excision of the primary site. All blue nodes and all nodes with a level of radioactivity higher than background are removed. The inguinal and popliteal basins are treated as separate basins in regards to radioactivity counts.
All removed nodes undergo permanent histologic analysis with hematoxylin-eosin (H&E) staining at multiple levels, followed by immunohistochemistry (IHC) for melanoma-specific markers. A tumor-positive SLN contains either histologic evidence of malignancy or IHC-detected foci of malignant cells. Complete dissection is performed in any nodal basin that contains a tumor-positive SLN. We treated the popliteal basin and inguinal basins separately in our study. A tumor positive popliteal SLN with a negative inguinal SLN would receive a popliteal completion dissection alone. If the inguinal SLN was also positive, an inguinal completion dissection was also performed. Nonsentinel nodes removed during complete dissection are evaluated by means of routine H&E staining without serial sectioning or IHC.
Data regarding patient demographics, primary tumor site, lymphoscintigraphic mapping, intraoperative findings, pathologic analysis, and additional surgery were retrieved for analysis. Recurrence data was gathered from database and chart review. Patients were grouped by drainage basin (popliteal and/or inguinal) for comparison. Student t-test or Chi-squared analysis was used to compare patient and tumor factors between those with popliteal SLN and those with inguinal-only SLN. P-value <0.05 was considered significant. The study was approved by the institutional review board of our institution.
Results: SNB for Primary Infrapopliteal Melanoma
During the study period, 461 patients underwent SNB for a primary melanoma located below the knee. Fifteen (3.2%) patients had drainage to the popliteal basin and underwent popliteal SNB (Table 1); 446 (96.8%) patients showed no uptake in the popliteal space and underwent inguinal SNB only. Patients in the popliteal group were younger than those in the inguinal-only group with mean age of 39 and 52 years, respectively (p=0.005). Popliteal and inguinal-only groups were found to have no statistically significant difference with respect to sex (73% and 65% female, p=0.5), mean Breslow thickness (2.4 and 1.8 mm, p=0.3), Clark level IV/V (62% and 58%, p=0.07), and ulceration (8% and 21%, p=0.3). The lack of significant differences may be due to a small number of patients in the popliteal SLN group, however, and comparison of the groups as matched samples should be cautioned against.
Table 1.
Characteristics and Recurrence Data of 15 Patients whose Primary Infrapopliteal Melanoma Drained to a Popliteal Sentinel Lymph Node
| Age at treatment of primary melanoma, y | Sex | Breslow depth, mm | Clark level | Ulceration | Popliteal SLN | Inguinal SLN | DFI to 1st recurrence, mo | First recurrence, stage | Total length, follow-up, mo | Status at last follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| 51 | Female | 1.0 | IV | No | + | − | 56 | IV | 64 | Died |
| 43 | Male | 0.3 | II | No | − | − | N/A | N/A | 112 | NED |
| 44 | Male | 1.8 | IV | No | + | + | 7 | III/Pelvic nodal | 98 | NED |
| 25 | Female | 0.9 | IV | No | − | − | N/A | N/A | 105 | NED |
| 58 | Female | 2.5 | N/A | N/A | + | − | 14 | III/In-transit | 103 | Died |
| 47 | Female | 2.2 | N/A | N/A | + | + | 21 | III/In-transit | 57 | Died |
| 44 | Female | 1.7 | II | No | − | − | N/A | N/A | 81 | NED |
| 27 | Female | 1.0 | IV | No | − | − | N/A | N/A | 32 | NED |
| 19 | Female | 2.5 | IV | No | + | + | N/A | N/A | 55 | NED |
| 32 | Female | 0.5 | IV | No | − | − | N/A | N/A | 45 | NED |
| 35 | Male | 4.2 | III | Yes | + | − | 61 | III/Inguinal Nodal | 107 | NED |
| 59 | Male | N/A | N/A | N/A | − | − | 13 | III/In-transit | 25 | Died |
| 38 | Female | 0.9 | III | No | − | − | N/A | N/A | 32 | NED |
| 55 | Female | 1.5 | IV | No | + | + | N/A | N/A | 6 | NED |
| 25 | Female | 9.0 | V | No | + | − | 54 | III/In-transit and inguinal nodal | 66 | AWD – In-transit, inguinal nodal, pulmonary, adrenal |
DFI, disease free interval; N/A, not available; +, tumor-positive node; -, tumor-negative node; N/A, not available; NED, no evidence of disease; AWD, alive with disease.
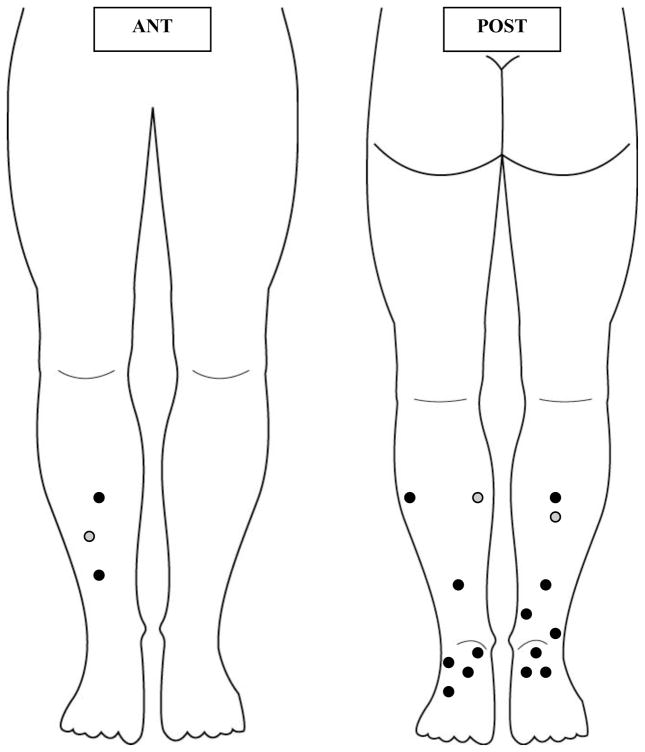
Of the 15 primary melanomas draining to a popliteal SLN, 13 (87%) were located on the posterior leg and foot and 2 (13%) were located on the anterior lower leg (Figure 1). Of the 446 patients with inguinal drainage only, 265 (59%) had lesions on the posterior leg and foot and 181 (41%) had lesions on the anterior leg.
Figure 1.
Locations of primary (black dots) and recurrent (gray dots) infrapopliteal melanomas that drained to a popliteal sentinel lymph node. Most of the primary lesions were on the posterior leg and foot. ANT, anterior; POST, posterior.
All 15 patients with popliteal drainage also had inguinal drainage and all underwent concurrent inguinal and popliteal SNB. In the eleven patients with available data, all popliteal SLNs were blue and radioactive. The average number of popliteal SLNs in the 15 patients was 1.4 (range, 1 – 3). The average number of inguinal SLNs in the 15 patients was 1.7 (range, 1–5).
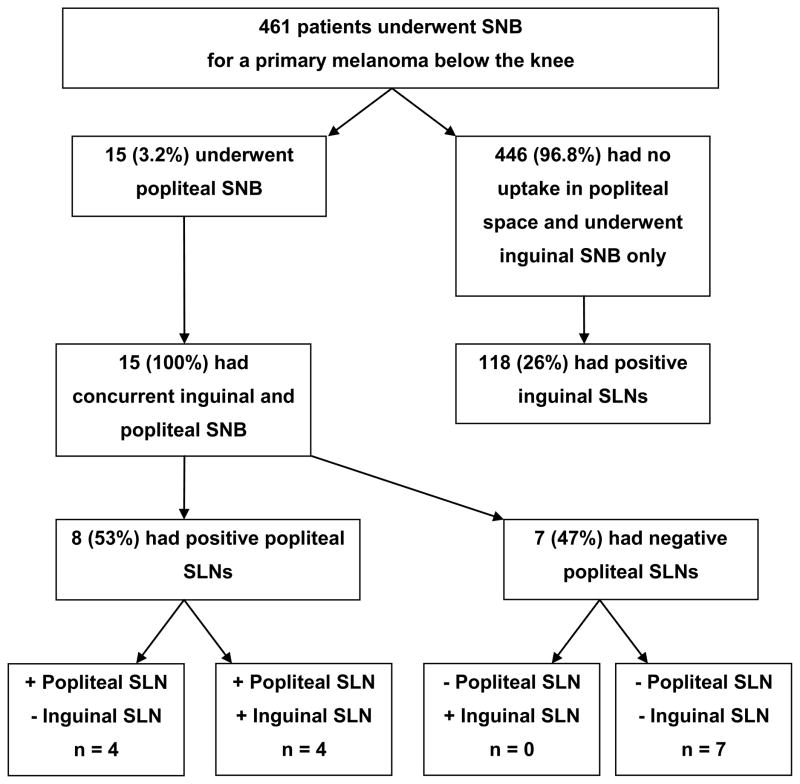
Figure 2 shows the outcome of SNB for the 461 patients with primary melanomas located below the knee. Of 15 patients who underwent popliteal SNB, 8 (53%) had metastatic disease in the popliteal SLN. Of 446 patients who underwent inguinal-only SNB, 118 (26%) had inguinal metastases. Of the 8 patients with positive popliteal SLNs, 4 (50%) also had a positive inguinal SLN. No patient had a positive inguinal SLN with a negative popliteal SLN. Thus, in 50% of patients with popliteal drainage and nodal involvement, the only site of metastatic disease was an interval popliteal lymph node (Figure 2).
Figure 2.
Outline of study findings for primary melanomas. SNB, sentinel node biopsy; SLN, sentinel lymph node.
Six of the 8 patients with tumor-positive popliteal SLNs underwent completion popliteal lymphadenectomy. Of the remaining two patients, one was lost to follow-up and one chose to start systemic treatment with interferon without further surgery and has not had a popliteal recurrence with over 100 months of follow-up. Complete popliteal dissection yielded a mean of 0.3 lymph nodes. Importantly, in 4 of 6 (67%) cases no additional nodes were found in the popliteal basin. Two patients each had one nonsentinel popliteal node removed during popliteal dissection. No nonsentinel popliteal nodes were positive. All four of the patients with positive inguinal SLNs underwent complete inguinal dissection; the average number of nonsentinel nodes removed was 8 (range 3–14). No additional nodes removed during complete inguinal dissection were positive.
Table 1 also shows recurrence data for the 15 patients whose primary infrapopliteal melanoma drained to a popliteal SLN. At an average follow-up of 64 months, 4 (27%) patients had died of melanoma-specific causes, 1 (7%) was alive with disease, and 10 (67%) were without clinical evidence of disease. Of the 6 stage III recurrences, 5 were in patients who had undergone complete popliteal dissection for a tumor-positive popliteal SLN (62% stage III recurrence rate in popliteal node-positive patients). Interestingly, 2 of the popliteal node-positive patients had nodal recurrences in the groin after disease-free intervals of 54 and 61 months. Because inguinal SLNs were tumor negative at the time of initial treatment, neither patient had undergone complete inguinal dissection.
Of 446 patients with inguinal-only drainage, only 1 patient (0.22%) developed recurrence in the popliteal space (DFI of 34 months), signifying an extremely low false-negative rate for popliteal SNB at our institution. Of 118 patients who had inguinal-only drainage and positive inguinal nodes, 50 (42%) had stage III recurrences. Overall, more popliteal node-positive patients developed locoregional recurrences (62% vs. 42%).
Results: SNB for Recurrent Infrapopliteal Melanoma
Nine patients in our melanoma database were identified who underwent SNB for locoregional recurrent melanoma located below the knee. Six (67%) patients had drainage exclusively to the inguinal basin, and 3 (33%) patients had concurrent drainage to popliteal and deep inguinal basins from the recurrent lesion. All three patients with dual-basin drainage had previously undergone ipsilateral superficial inguinal complete lymphadenectomy, with a disease-free interval of 3, 8, and 32 years, respectively. None of the three had previously undergone popliteal SNB.
Of the 3 patients undergoing popliteal SNB, 1 (33%) had a tumor-positive popliteal SLN. He underwent complete dissection of the popliteal space, which yielded 3 tumor-negative nonsentinel nodes. In these three patients, all SLNs identified during inguinal SNB were deep inguinal/external iliac nodes; none contained metastases. Of the 6 patients who underwent inguinal SNB only, 3 (50%) had tumor-positive SLNs. By comparison, a previous study by our group demonstrated that 47% of locoregional recurrent melanomas will have positive nodal disease.11
DISCUSSION
Because the popliteal lymph nodes lie under the thick popliteal fascia and because <5% of melanomas in the lower extremity drain to the popliteal space, popliteal lymphadenopathy is rare.3, 13, 14 Occult popliteal metastasis also is relatively uncommon and could be missed for several reasons. First, a surgical approach to the popliteal space requires repositioning the patient in a prone position, which may discourage some surgeons from seeking lymph nodes in this area.8 Second, operative experience with popliteal dissection is limited.5, 15, 16 Finally, the potential for permanent damage to the tibial and peroneal nerves might discourage an aggressive approach to this location.5, 14
In the lower extremity, lymphatics from the skin of the medial foot and ankle follow the greater saphenous vein and are thought to drain primarily to the inguinal lymph nodes, whereas lymphatics from the lateral foot and ankle follow the lesser saphenous vein and can drain into the popliteal space.7, 8, 14, 17 Although studies have shown that any skin site below the knee can drain to the popliteal fossa,14, 15, 17–20 the majority of our popliteal SNB patients had primaries located on the posterior leg (Figure 1) consistent with the classic description of posterior leg/foot drainage to the popliteal basin. Primary melanomas with drainage to the inguinal nodes were evenly distributed between the anterior and posterior leg. Popliteal SLNs should be carefully sought out in primary melanoma located on the posterior lower leg and foot.
Concurrent drainage to popliteal and inguinal basins occurred in all of our cases and in almost all of 83 cases of popliteal lymphoscintigraphic drainage reported by the Sydney Melanoma Unit.20 Concurrent drainage could reflect two separate lymphatic channels from a lower-extremity site, or it could be the result of a single channel that drains first to interval nodes in the popliteal space and then to second-tier SLNs in the inguinal basin. In either case, SLNs should be removed from both the popliteal and inguinal basins, and a complete lymphadenectomy should be undertaken in any basin that contains a tumor-positive SLN.12, 19 However, because the popliteal space contains no more than 2–7 nodes, 13, 14, 16–18 complete popliteal lymphadenectomy may not yield additional lymphatic tissue (67% of cases in our study).
Although rare, drainage to interval nodes may have significant implications for treatment and prognosis. A positive SLN, regardless of its site, increases the risk of recurrence and death due to melanoma.3, 8 Most series have found that 10–50% of interval nodes are positive, similar to rates for more classic lymphatic basins (Table 2).2, 8, 21–24 However, in our study, although demographic and tumor characteristics were similar between patients with popliteal and inguinal SLNs, corresponding rates of node-positive disease were not: 53% and 26%, respectively. We also found that the interval node was the only positive lymph node in 50% of patients with nodal metastasis in the popliteal space. By comparison, McMasters et al reported that an interval node was the only positive lymph node in 85% (11 of 13) of patients with metastatic interval nodes at any site.6 Finally, 63% of our patients with tumor-positive popliteal SLNs developed locoregional recurrence, as compared with 42% of patients whose SLN metastases were confined to the inguinal basin. Perhaps the presence of a positive interval node is a marker of more advanced intra-lymphatic disease causing altered drainage patterns and ultimately an increased risk of locoregional recurrence.
Table 2.
Earlier Studies of Popliteal Sentinel Lymph Nodes (Only with 2 or More Patients)
| First author, year | Lower extremity, n | With drainage to popliteal nodes, n (%) | Undergoing excision of popliteal SLN, n (%) | Popliteal SLN positive for metastatic disease, n (%) |
|---|---|---|---|---|
| Thompson,18 2000 | 236 | 17(6.9) | 6(2.5) | 1(16) |
| McMasters,6 20026 | 457 | 8(1.8) | 8(1.8) | 2 (25) |
| Hatta et al. 200521 | 9 | 3(33) | 2(22) | 1(50) |
| Roozendaal, 22 2001 | 153 | 3(2.0) | 3(2.0) | N/A |
| Lieber, 25 1998 | 10 | 2(20) | 2(20) | 1(10) |
| Uren, 9 2003 | 481 | 38(7.9) | N/A | N/A |
| Leong, 26 1999 | 57 | 2(3.5) | 2(3.5) | N/A |
| Sumner3 2002 | 300 | 7(2.3) | 7(2.3) | N/A |
| Menes, 8 2004 | 106 | 10(9.4) | 8(7.5) | 1(12.5) |
| Vidal-Sicart, 24 2004 | 188 | 9(4.7) | 9(4.7) | 2(22) |
| Kretschmer, 17 2010 | 166 | 16(10) | 6(3.6) | 3(50) |
| Current study | 461 (Primary) | 15(3.2) | 15(3.2) | 8(53) |
N/A, not available; SLN, sentinel lymph node.
Among the nine patients who underwent SNB for locoregional recurrent melanomas of the infrapopliteal leg, the incidence of popliteal drainage was 33%, higher than that for patients with primary lesions. Perhaps this can be explained by alternate lymphatic drainage caused by previous inguinal lymphadenectomy in each of these patients. Overall, the incidence of node-positive disease from locoregional recurrences was 44%, consistent with our previously reported node-positive rate of 47% for satellite and in-transit disease.11
Most of the literature on popliteal SLNs consists of case reports. Among the studies with more than two cases (Table 2), comparisons are difficult because popliteal drainage was inconsistently reported as a percentage of all lower-limb or below-knee melanomas, and a percentage of nodal mapping or nodal excision. Also, technical aspects of lymphatic mapping vary. For example, the Sydney Melanoma Unit reported that the rate of popliteal SLNs identified during lymphoscintigraphy was 2.3% without a 10-minute acquisition during delayed imaging versus 16% with a 10-minute acquisition during delayed imaging. 8, 23 Another variable is the radioactive colloid agent.9 A smaller particle such as 99mTc-labeled antimony trisulfide colloid may have advantages for interval node detection.2, 6, 21 During the dynamic phase of lymphoscintigraphy, smaller particles may show more rapid uptake into lymphatic channels and help identify interval or ectopic lymph nodes.24 Misinterpretation of lymphoscintigraphy could also lead to variation in interval node identification. Interval lymph nodes must be differentiated from lymphatic lakes or lymphangiomas.24 Lymphatic lakes are focal dilations of lymphatics seen as areas of increased tracer retention during the dynamic or early postinjection phase of lymphoscintigraphy. The activity rapidly passes through the lymphatic lake without retention, however, so it is not visible on delayed scans, differentiating it from a true ectopic or interval node.9
The surgical treatment of the next-echelon basin in patients with interval sentinel nodes is an area of debate.3, 17 Some institutions recommend full dissection of the next proximal standard regional lymph node basin draining the primary site, regardless of lymphoscintigraphic findings.3 This approach is based largely on anecdotal experience, but Roozendaal et al. reported that 1 of 4 patients had occult metastases in the traditional basin located upstream of a tumor-positive interval lymph node.22 Similarly, our study found a 50% rate of inguinal involvement in patients with popliteal involvement. Additionally, of the four patients who had positive popliteal nodes with negative inguinal SLN, two had delayed recurrences in the inguinal basin. If these inguinal recurrences could have been avoided with an elective lymph node dissection at the time of initial treatment, then 6 of 8 (75%) patients with popliteal metastasis had oncologic reason for removal of inguinal nodes. The identification of tumor metastasis in a popliteal SLN should increase the clinical suspicious of concurrent inguinal disease and prompt consideration of dissection of that basin as well. If a complete inguinal lymphadenectomy is not performed in these patients, close clinical follow-up of the inguinal lymph nodes is warranted.
There are situations involving interval popliteal nodes that our study cannot address. What if the intraoperative hand-held gamma probe identifies elevated radioactivity in the popliteal basin of a patient whose preoperative lymphoscintigram showed no popliteal drainage? A report from M.D. Anderson Cancer Center found that 71% of patients with interval nodes were identified only with the hand-held gamma probe and were not seen on the preoperative lymphoscintigram.3 It is essential for the melanoma surgeon to combine the use of quality preoperative lymphoscintigraphy and a thorough intraoperative evaluation with the gamma probe of both the classic nodal basins and potential sites of unusual drainage.
In our series, the incidence of popliteal drainage was 3.2% for patients with primary melanomas located below the knee. The incidence of metastases in the popliteal nodes was high, perhaps related to the younger age in these patients or to other adverse patient/tumor factors which we were unable to identify due to the small sample size. Popliteal drainage was invariably accompanied by inguinal drainage, popliteal metastasis was often accompanied by inguinal metastasis, and no patient had inguinal metastases without concomitant popliteal metastases. This pattern suggests that the popliteal nodal basin can occasionally be the first drainage site for lower-extremity melanoma, particularly lesions on the posterior lower leg or foot. If positive inguinal nodes are identified in a patient with popliteal drainage, then there is an extremely high chance that metastatic disease exists within the popliteal basin and should be aggressively treated. Although it is anatomically separate, the inguinal basin appears to be a functional extension of the popliteal basin.
Acknowledgments
Critical editing assistance of the manuscript by Gwen Berry.
Supported by grants P01 CA29605 and P01 CA12582 from the National Cancer Institute and by funding from the Melanoma Research Alliance (Washington, DC), Dr Miriam & Sheldon G Adelson Medical Research Foundation (Boston, MA), the Lincy Foundation (Beverly Hills, CA), the Amyx Foundation, Inc. (Boise, ID), Alan and Brenda Borstein (Los Angeles, CA), Mr and Mrs Louis Johnson (Stanfield, AZ), Heather and Jim Murren (Las Vegas, NV), the Wayne and Gladys Valley Foundation (Oakland, CA), the Lance Armstrong Foundation (Austin, TX), the Samueli Foundation (Corona del Mar, CA), the John Wayne Cancer Foundation (Newport Beach, CA), and the Wrather Family Foundation (Los Alamos, CA). Dr Steen is the Carolyn Dirks Fellow (Los Angeles, CA). The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Cancer Institute or the National Institutes of Health.
Footnotes
Presented at the Western Surgical Association 118th Scientific Session, Chicago, IL, November 2010, and presented in part at the 2010 International Sentinel Node Society Meeting, Yokohama, Japan, November 2010.
Disclosure Information: Nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–399. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 2.Vidal-Sicart S, Pons F, Puig S, et al. Identification of the sentinel lymph node in patients with malignant melanoma: what are the reasons for mistakes? Eur J Nucl Med Mol Imaging. 2003;30:362–366. doi: 10.1007/s00259-002-1051-7. [DOI] [PubMed] [Google Scholar]
- 3.Sumner WE, 3rd, Ross MI, Mansfield PF, et al. Implications of lymphatic drainage to unusual sentinel lymph node sites in patients with primary cutaneous melanoma. Cancer. 2002;95:354–360. doi: 10.1002/cncr.10664. [DOI] [PubMed] [Google Scholar]
- 4.Statius Muller MG, Hennipman FA, van Leeuwen PA, et al. Unpredictability of lymphatic drainage patterns in melanoma patients. Eur J Nucl Med Mol Imaging. 2002;29:255–1. doi: 10.1007/s00259-001-0670-8. [DOI] [PubMed] [Google Scholar]
- 5.Barrasa Shaw A, Sancho Merle F, Fuster Diana C, et al. Popliteal lymphadenectomy on sentinel lymph node melanoma metastasis. Clin Transl Oncol. 2006;8:218–220. doi: 10.1007/s12094-006-0014-z. [DOI] [PubMed] [Google Scholar]
- 6.McMasters KM, Chao C, Wong SL, et al. Interval sentinel lymph nodes in melanoma. Arch Surg. 2002;137:543–547. doi: 10.1001/archsurg.137.5.543. discussion 547–549. [DOI] [PubMed] [Google Scholar]
- 7.Aydin MA, Okudan B, Nasir S, et al. Lymphoscintigraphic drainage of acral limb skin to interval sentinel lymph nodes in healthy subjects. J Surg Oncol. 2006;93:286–293. doi: 10.1002/jso.20385. [DOI] [PubMed] [Google Scholar]
- 8.Menes TS, Schachter J, Steinmetz AP, et al. Lymphatic drainage to the popliteal basin in distal lower extremity malignant melanoma. Arch Surg. 2004;139:1002–1006. doi: 10.1001/archsurg.139.9.1002. [DOI] [PubMed] [Google Scholar]
- 9.Uren RF, Howman-Giles R, Thompson JF. Patterns of lymphatic drainage from the skin in patients with melanoma. J Nucl Med. 2003;44:570–582. [PubMed] [Google Scholar]
- 10.Thelmo MC, Morita ET, Treseler PA, et al. Micrometastasis to in-transit lymph nodes from extremity and truncal malignant melanoma. Ann Surg Oncol. 2001;8:444–448. doi: 10.1007/s10434-001-0444-3. [DOI] [PubMed] [Google Scholar]
- 11.Yao KA, Hsueh EC, Essner R, et al. Is sentinel lymph node mapping indicated for isolated local and in-transit recurrent melanoma? Ann Surg. 2003;238:743–747. doi: 10.1097/01.sla.0000094440.50547.1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355:1307–1317. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 13.Sholar A, Martin RC, 2nd, McMasters KM. Popliteal lymph node dissection. Ann Surg Oncol. 2005;12:189–193. doi: 10.1245/ASO.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Marone U, Caraco C, Chiofalo MG, et al. Resection in the popliteal fossa for metastatic melanoma. World J Surg Oncol. 2007;5:8. doi: 10.1186/1477-7819-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Georgeu G, El-Muttardi N, Mercer D. Malignant melanoma metastasis to the sentinel node in the popliteal fossa. Br J Plast Surg. 2002;55:443–445. doi: 10.1054/bjps.2002.3872. [DOI] [PubMed] [Google Scholar]
- 16.Karakousis CP. The technique of popliteal lymph node dissection. Surg Gynecol Obstet. 1980;151:420–423. [PubMed] [Google Scholar]
- 17.Kretschmer L, Sahlmann CO, Bardzik P, et al. The popliteal fossa - a problem zone for sentinel lymphonodectomy. J Dtsch Dermatol Ges. doi: 10.1111/j.1610-0387.2010.07536.x. [DOI] [PubMed] [Google Scholar]
- 18.Thompson JF, Hunt JA, Culjak G, et al. Popliteal lymph node metastasis from primary cutaneous melanoma. Eur J Surg Oncol. 2000;26:172–176. doi: 10.1053/ejso.1999.0765. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds HM, Dunbar PR, Uren RF, et al. Three-dimensional visualisation of lymphatic drainage patterns in patients with cutaneous melanoma. Lancet Oncol. 2007;8:806–812. doi: 10.1016/S1470-2045(07)70176-6. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds HM, Walker CG, Dunbar PR, et al. Functional anatomy of the lymphatics draining the skin: a detailed statistical analysis. J Anat. 2010;216:344–355. doi: 10.1111/j.1469-7580.2009.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatta N, Morita R, Yamada M, et al. Implications of popliteal lymph node detected by sentinel lymph node biopsy. Dermatol Surg. 2005;31:327–330. doi: 10.1111/j.1524-4725.2005.31083. [DOI] [PubMed] [Google Scholar]
- 22.Roozendaal GK, de Vries JD, van Poll D, et al. Sentinel nodes outside lymph node basins in patients with melanoma. Br J Surg. 2001;88:305–308. doi: 10.1046/j.1365-2168.2001.01676.x. [DOI] [PubMed] [Google Scholar]
- 23.Uren RF, Howman-Giles R, Thompson JF, et al. Interval nodes: the forgotten sentinel nodes in patients with melanoma. Arch Surg. 2000;135:1168–1172. doi: 10.1001/archsurg.135.10.1168. [DOI] [PubMed] [Google Scholar]
- 24.Vidal-Sicart S, Pons F, Fuertes S, et al. Is the identification of in-transit sentinel lymph nodes in malignant melanoma patients really necessary? Eur J Nucl Med Mol Imaging. 2004;31:945–949. doi: 10.1007/s00259-004-1485-1. [DOI] [PubMed] [Google Scholar]
- 25.Lieber KA, Standiford SB, Kuvshinoff BW, Ota DM. Surgical management of aberrant sentinel lymph node drainage in cutaneous melanoma. Surgery. 1998;124:757–761. doi: 10.1067/msy.1998.90943. discussion 761–762. [DOI] [PubMed] [Google Scholar]
- 26.Leong SP, Achtem TA, Habib FA, et al. Discordancy between clinical predictions vs lymphoscintigraphic and intraoperative mapping of sentinel lymph node drainage of primary melanoma. Arch Dermatol. 1999;135:1472–1476. doi: 10.1001/archderm.135.12.1472. [DOI] [PubMed] [Google Scholar]