Abstract
Objectives
Magnetic resonance imaging (MRI) studies report hippocampal (HC) volume reductions in depression. Despite observations in the HC of functional heterogeneity and ovarian steroid influence, few studies report regional volume alterations or control for menstrual cycle phase. Using in vitro methods, we recently observed reduced anterior HC volume in antidepressant-naïve, ovarian-intact, behaviorally depressed adult female monkeys. The purpose of this study was to confirm these findings in vivo and examine whether lack of ovarian steroids affects the relationship between depression and HC volume.
Methods
MRI was used to measure whole, anterior, and posterior HC volumes in a matched sample of antidepressant-naïve, surgically-postmenopausal, adult female cynomolgus macaques characterized for behavioral depression (n=6 depressed, 6 nondepressed). High resolution structural MRIs were acquired, and HC regions of interest were manually segmented. HC volumes were normalized to whole brain volumes prior to analysis.
Results
Similar to the previous in vitro study, HC volume measured in vivo was associated with depression. In contrast to the previous study of ovarian-intact females, whole, anterior and posterior volumes of both left and right HC were significantly smaller in depressed compared to nondepressed surgically postmenopausal females.
Conclusions
These findings confirm and extend previous observations of smaller HC volumes in behaviorally depressed female monkeys, and suggest a possible role for ovarian steroids in HC protection in depression. Further studies of the potential modulating effects of ovarian function on the relationship between depression and HC volume are warranted.
Keywords: Macaca fascicularis, female, MRI, postmenopausal, posterior hippocampus, estrogen
Introduction
The structure and function of the hippocampus (HC) are implicated in the pathophysiology of depression. Numerous magnetic resonance imaging (MRI) studies support an association between depression and morphometric alterations in the HC.1 Despite the observation that depression is nearly twice as prevalent in women as in men,2 few studies of depression and HC volume focus specifically on depressed females. Clinical studies are thus complicated by heterogeneity in subject characteristics that may differentially affect HC volume, including gender and menstrual cycle phase.
An association between mood-related or depressive behavior and HC structure is supported by animal studies. However, animal models of depression typically utilize only male rodents in stress paradigms despite evidence of ovarian steroid regulation of stress response circuitry and sex differences in behavioral and neurobiological responses to stress in humans and rodents.3-4 Furthermore, hippocampal dendritic spine density varies over the estrus cycle,5 estradiol increases dendritic spines and synaptic protein expression in female rodents and non-human primates,6-8 and estradiol protects against neuronal loss in the HC of chronically-stressed female rats.9 Thus, estrogen-influenced structural alterations in the HC, particularly in response to stress, may be central to the neurobiology of depression in females. While preclinical stress models are informative, the degree to which female human depression is reflected in male animal responses to stress remains to be determined. We have developed an adult female cynomolgus macaque (Macaca fascicularis) model of depression in which the animals display behavioral and physiological characteristics like those of depressed humans.10-12 Cynomolgus macaques have menstrual cycles that parallel those of women in length and hormonal fluctuations, and the macaque HC more accurately reflects the nuclear organization and connectivity patterns of the human HC than does the rat.13 As such, this model is advantageous for furthering our understanding of the neurobiology of female depression.
The HC is functionally heterogeneous as the anterior HC plays a larger role in mood and emotion-related functioning than does the posterior HC.14 Most human volumetric studies do not detail separate investigations of the anterior and posterior HC or control for hormone levels in female subjects, even though volume alterations in the anterior HC are associated with menstrual cycle phase in women.15 We recently reported a reduction in anterior HC volume measured post mortem in depressed compared to nondepressed adult female cynomolgus monkeys controlled for menstrual cycle phase and with no prior antidepressant exposure.16 To our knowledge, no comparison of HC volumes exists between depressed and nondepressed postmenopausal human or non-human primates. To confirm these findings in vivo and examine whether a lack of ovarian steroids affects the relationship between depression and HC volume, the present study investigated whole, anterior and posterior HC volumes in vivo in antidepressant-naïve, surgically-postmenopausal animals using MRI. Given the positive influence of estrogen on HC structure mentioned above and our previous post mortem findings, we hypothesized that HC volume would be smaller in depressed compared to nondepressed surgically-postmenopausal monkeys, with the largest reduction in the anterior HC.
Methods and Materials
Subjects
Twenty-three adult female cynomolgus monkeys (M. fascicularis) were obtained from Primate Products (Woodside, CA) and housed in small social groups of five animals each for 25 months. All animals were ovariectomized at three months, and cycling characteristics were measured for the following six months to determine successful removal of ovarian material. Throughout the experiment, the animals were fed a diet based on the typical North American diet. All procedures in this study were conducted in compliance with State and Federal laws, standards of the US Department of Health and Human Services, and guidelines established by the Wake Forest University Animal Care and Use Committee.
Study Design and Treatment
The study followed a randomized, placebo-controlled, crossover trial design in which all animals received each of the following four treatments (expressed as equivalent hormone doses for women): (1) placebo; (2) micronized 17β-estradiol (E2), 1 mg/day (Estrace; Mylan Pharmaceuticals, Morgantown, WV, and Bristol-Myers Squibb, New York, NY); (3) micronized E2, 1 mg/day (Estrace) and micronized progesterone (P4), 200 mg/day (Prometrium; Solvay Pharmaceuticals, Marietta, GA); and (4) micronized E2, 1 mg/day (Estrace) and medroxyprogesterone acetate (MPA), 2.5 mg/day (Provera; Barr Laboratories, Pomona, NY).17 Absolute doses were 0.05, 11.1, and 0.139 mg/kg body weight for E2, P4, and MPA, respectively. Doses were administered based on individual body weights, while dose scaling to human equivalents was based on caloric intake rather than body weight to correct for species differences in metabolism.18 Dose equivalents represent clinically-approved, commonly prescribed amounts for postmenopausal women. Treatments were given daily for two months, followed by a one-month washout period during which all animals were given placebo doses. The last hormone regimen phase was followed by a three-month washout prior to acquisition of MR images. Behavior observations were completed during the last three weeks of each two month treatment phase, and at the end of the three-month washout just prior to acquisition of MR images.
Behavior
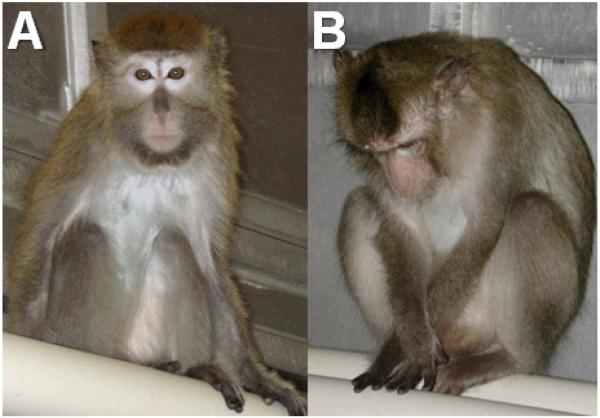
Social status was determined based on the outcomes of agonistic interactions,10-12 and was stable across time as in previous studies.19 Using the focal animal technique,20 behavior was recorded for 15 min three days/week per animal, during a three-week period every three months. Depressive behavior was defined as a slumped or collapsed body posture with open eyes, accompanied by a lack of responsiveness to environmental stimuli to which other animals are attending, and has been described in detail elsewhere (Figure 1).10-12 Behavioral depression was not induced by an experimental manipulation; captive cynomolgus macaques may spontaneously exhibit depressive behavior.
Figure 1.
A comparison between behaviorally depressed and nondepressed monkeys. The nondepressed monkey (A) appears alert and attentive, whereas the monkey displaying depressed behavior (B) appears inattentive, with a slumped body posture and open eyes pointed downward.
The effects of treatment on behavior have been described in detail.21 Briefly, the effects of treatment on behavior were analyzed using a linear mixed effects model approach with subject as a random factor to account for the intraclass correlations incurred from the design. The effects of treatment, social status (dominant, subordinate), and treatment by social status interaction were analyzed adjusting for the potential phase effect. Carryover effects were also examined in all analyses and found to be nonsignificant.
Importantly for the study reported here, there were no significant main or interaction effects of hormone regimen on depressive behavior. Thus, the percent time spent in the depressed posture was averaged over the entire study. As in previous studies, the distribution of time spent depressed was skewed with seven of the 23 monkeys falling above the mean in depressive behavior. The seven that fell above the mean in depressive behavior spent an average of 12.10 (range: 6.34-23.10) percent of their time in the depressed posture, whereas those that fell below the mean (n=16) spent an average of 2.79 (range: 0-5.29) percent time in the depressed posture.
Observations of depressive behavior were repeated following the three month washout, just prior to scanning. Six animals fell above the mean in depressive behavior during the treatment phase of the study and also just prior to scanning; these were chosen for scanning to represent the “depressed” group. Thus, the depressed monkeys in this study were currently depressed and had a prior history of depressive behavior. These six depressed animals were carefully matched with six nondepressed animals (those that exhibited below average depressive behavior during the treatment phase of the study and no depressive behavior just prior to scanning) on social status and body weight. Just prior to scanning, the depressed animals (n=6) spent an average of 4.68 (range: 1.00-10.55) percent of their time in the depressed posture, whereas nondepressed animals (n=6) spent 0 percent of their time in the depressed posture.
MR Image Acquisition and Analysis
MRI images were acquired in anesthetized monkeys (Ketamine HCl/Xylazine: 7.0/0.6 mg/kg. i.m.) on a 3-Tesla MRI scanner with Twin speed gradient coil using a single channel human quadrature knee coil (GE Healthcare, Milwaukee, WI). The knee coil was used for neuroimaging of the nonhuman primate because its smaller form factor results in a better signal-to-noise ratio per unit time compared to the human quadrature head coil. Proton density (PD) and T2-weighted (T2) images were acquired with a dual-echo fast-spin echo-pulse sequence (TE1/TE2 = 13.6 msec/81.6 msec, TR = 9.1 sec, echo-train length = 8, receiver bandwidth = 31.2 kHz, voxel size 0.5 × 0.5 × 1.0 mm, field of view = 12 × 12 × 6 cm). Two PD/T2 images were acquired sequentially and averaged offline to improve the signal-to-noise ratio of the final reconstructed image. Total acquisition time for both sets of PD/T2 images was 20 minutes. The PD and T2 images were co-registered with SPM (Statistical Parametric Mapping, University College London, London, UK) prior to averaging with in-house software (Matlab, Mathworks, Natick MA). HC regions of interest were manually segmented on PD images using MRIcro (Chris Rorden, Georgia Institute of Technology, Atlanta, GA). HC boundaries were adapted from Machado et al.22 and Willard et al.16 The anterior HC was delineated from the posterior HC by the last coronal slice in which the uncus was present (Figure 2A-C).
Figure 2.
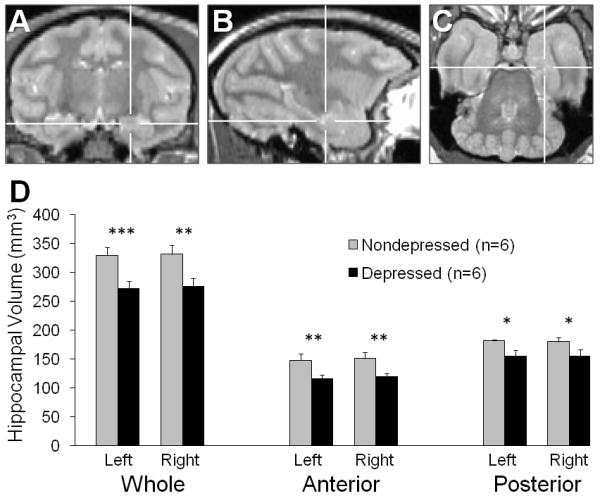
Hippocampal (HC) volume in behaviorally depressed monkeys. (A – C) The anterior HC was delineated from the posterior HC by the presence of the uncus. The cross-hairs indicate the same position in all three proton-density images. (A) Coronal: The last slice in which the uncus is present marks the caudal boundary of the anterior HC. (B) Sagittal: The anterior HC appears to the right of the vertical cross-hairs. (C) The anterior – posterior boundary of the HC as viewed in the axial plane. (D) Bilateral whole, anterior and posterior HC volumes were all smaller in depressed compared to nondepressed animals. *p < 0.05; **p ≤ 0.01; ***p ≤ 0.001.
Statistical Analysis
To correct for intersubject variation in head size, HC volume was divided by whole brain volume prior to analysis. T-tests for independent groups were used to verify that the depressed and nondepressed animals did not differ in social status and body weight. Each region (whole, anterior, posterior) of the left and right hippocampus was assessed separately using t-tests for independent groups. For the left and right hippocampi, three a priori comparisons were made. A two-tailed significance level of 0.05 was used for all comparisons.
Results
There were no differences between depressed and nondepressed animals in age (t(10) = 1.14, p = 0.28), body weight (t(10) = 0.06, p = 0.95), or social status (t(10) = 0.75, p = 0.47) as expected. Whole hippocampal volumes (Figure 2D) were compared between the depressed and nondepressed group on the left and right sides and found to be smaller in the depressed animals, bilaterally: left whole HC (t(10) = 4.34, p = 0.001), right whole HC (t(10) = 3.22, p = 0.009) . On average, the whole hippocampi of depressed monkeys was 20% smaller than their nondepressed counterparts. To test for heterogeneity along the anterior-posterior axis, each side was divided into anterior and posterior regions (Figure 2D). Volumes were significantly reduced in depressed compared to nondepressed animals in both the anterior and posterior regions, bilaterally: left anterior HC (t(10) = 3.36, p = 0.007), left posterior HC (t(10) = 3.11, p = 0.01), right anterior HC (t(10) = 3.17, p = 0.01), and right posterior HC (t(10) = 2.34, p = 0.04). Thus, no effects of laterality were observed. Averaged over left and right, the anterior hippocampi were 24% smaller, and the posterior hippocampi were 17% smaller in the depressed monkeys compared to their nondepressed counterparts. There was no difference in whole brain volume between groups (t(10) = 1.32, p = 0.22).
Discussion
This is the first study investigating whole, anterior and posterior HC volumes in depressed and nondepressed female primates with no prior antidepressant exposure and no endogenous ovarian steroid production. The results indicate global reductions in HC volume, thus supporting an association between HC morphometric alterations and depressive behavior in adult female cynomolgus macaques. The observation of reduced bilateral whole HC volume in depressed compared to nondepressed adult female monkeys is consistent with the results of a recent meta-analysis confirming significant bilateral reductions in age and sex-matched depressed humans.1 The observation of smaller HC volume in depressed monkeys is compelling because many of the characteristics that differentially affect HC volume in human studies are controlled in this primate model. The animals were all surgically-postmenopausal, lived in the same housing conditions, consumed the same diet, and were not exposed to antidepressant pharmacotherapy, recreational drugs, or alcohol. Depressive behavior was objectively documented throughout the course of two years prior to MRI, and depressed and nondepressed monkeys were matched on social status and body weight.
The observation of decreased anterior HC volume in antidepressant-naïve depressed compared to nondepressed female monkeys validates our previous in vitro findings in the same model.16 Similarly, Ballmaier et al.23 observed reduced whole and anterior HC volumes in a population of unmedicated late-life depressed patients with an average age of approximately 71 years, 74% of which were women and presumably postmenopausal, compared to age-matched controls. Bearden et al.24 also reported reduced volume in subregions of the anterior HC in unmedicated depressed adults averaging 39 years of age, 77% of which were women. In contrast, Neumeister et al.25 observed reduced posterior but not anterior HC volume in unmedicated depressed patients in remission, 77% of which were women who averaged approximately 40 years of age. Although these studies used mostly depressed women, the majority of subjects had prior antidepressant exposure, one was of elderly postmenopausal women and none of the other studies controlled for menstrual cycle phase or mention menopausal status. The variability in the results of these studies is currently unexplained. The reduced whole, anterior, and posterior HC volumes reported in depressed, ovariectomized female monkeys herein reflect an antidepressant-naïve state in which hormone production had ceased for over two years. We observed only anterior reductions in volume in our previous post mortem histological study of intact antidepressant-naïve females that lived under conditions very similar to those of the monkeys in the study reported here. It is possible that endogenous female hormones are somewhat protective of HC volume reduction in depression, particularly in the posterior HC; and that the magnitude and localization of HC volume reductions in human beings may be influenced by antidepressant exposure, ovarian steroid fluctuations, or both.
The potential for ovarian steroid modulation of the depression-HC volume relationship is important because of the functional differentiation of the HC along the anterior – posterior axis. While the posterior HC is central to memory functioning26 and memory disturbances in depression are well-documented, the anterior HC maintains close connectivity to primary brain areas in depression neurocircuitry and is implicated in mood and emotion-related functioning.27 The present study is limited in that the sample size is relatively small, and MRI comparison to females with intact ovarian function was not possible. The differences in the relationship between depression and posterior HC volumes reported for surgically postmenopausal female cynomolgus monkeys in the present in vivo study versus monkeys with intact ovarian function in the previous post mortem study suggest that further studies of the potential modulating effects of ovarian function on the relationship between depression and HC volume are warranted.
Conclusions
In this first ever investigation of whole, anterior and posterior HC volume in antidepressant naïve and surgically postmenopausal depressed and nondepressed adult female monkeys, an association between depression and global HC volume reductions was observed. Consistent with the results of numerous human studies,1 reduced bilateral whole HC volume in depressed compared to nondepressed adult female monkeys is reported herein. The observation of decreased anterior HC volume in antidepressant-naïve depressed compared to nondepressed female monkeys validates our previous in vitro findings in the same model.16 Given that we observed posterior HC reductions in the present study of ovariectomized animals, but not in our previous post mortem study of intact females, it is possible that endogenous female hormones might protect against HC volume reduction in depression, particularly in the posterior HC. Future studies using this adult female monkey model of depression in which characteristics that differentially affect HC volume in human studies are controlled, including antidepressant exposure and menstrual cycle phase, are warranted to evaluate the potential modulating effect of ovarian steroids on hippocampal volume in depression.
Summary.
This study represents the first ever in vivo investigation of hippocampal volume in antidepressant naïve and surgically postmenopausal adult female cynomolgus macaques characterized for behavioral depression. Whole, anterior and posterior volumes of both left and right hippocampus were found to be significantly smaller in depressed compared to nondepressed monkeys.
Acknowledgements
The authors would like to thank Jean Gardin, Chuck Boyd, Matt Dwyer, Dewayne Cairnes, Nancy Buchheimer and Stephanie Rideout for technical assistance, and Dr. Thomas B. Clarkson for advice in planning the experiment.
This work was supported by grants from the Martin & Sharleen Cohen Foundation for Biomedical Research (to CAS and JMC) and AA016748 (JBD).
Footnotes
Ms. Willard and co-authors reported no financial interests or potential conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Stephanie L. Willard, Neuroscience Graduate Program, Wake Forest University Graduate School of Arts & Sciences, Winston-Salem, NC
James B. Daunais, Department of Physiology & Pharmacology, Wake Forest University School of Medicine, Winston-Salem, NC
J. Mark Cline, Department of Pathology, Wake Forest University School of Medicine, Winston-Salem, NC.
Carol A. Shively, Department of Pathology, Wake Forest University School of Medicine, Winston-Salem, NC
REFERENCES
- 1.Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Pol HE Hulshoff, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30:3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- 3.Ter Horst GJ, Wichmann R, Gerrits M, Westenbroek C, Lin Y. Sex differences in stress responses: focus on ovarian hormones. Physiol Behav. 2009;97:239–249. doi: 10.1016/j.physbeh.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J Neurosci. 2010;30:431–438. doi: 10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- 6.Choi JM, Romeo RD, Brake WG, Bethea CL, Rosenwaks Z, McEwen BS. Estradiol increases pre- and post-synaptic proteins in the CA1 region of the hippocampus in female rhesus macaques (Macaca mulatta) Endocrinology. 2003;144(11):4734–8. doi: 10.1210/en.2003-0216. [DOI] [PubMed] [Google Scholar]
- 7.Hao J, Janssen WG, Tang Y, Roberts JA, McKay H, Lasley B, et al. Estrogen increases the number of spinophilin-immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. J Comp Neurol. 2003;465(4):540–50. doi: 10.1002/cne.10837. [DOI] [PubMed] [Google Scholar]
- 8.Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrin. 2008;29(2):219–37. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takuma K, Matsuo A, Himeno Y, Hoshina Y, Ohno Y, Funatsu Y, et al. 17beta-estradiol attenuates hippocampal neuronal loss and cognitive dysfunction induced by chronic restraint stress in ovariectomized rats. Neuroscience. 2007;146(1):60–68. doi: 10.1016/j.neuroscience.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Shively CA, Laber-Laird K, Anton RF. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biol Psychiatry. 1997;41:871–882. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- 11.Shively CA, Williams JK, Laber-Laird K, Anton RF. Depression and coronary artery atherosclerosis and reactivity in female cynomolgus monkeys. Psychosom Med. 2002;64:699–706. doi: 10.1097/01.psy.0000021951.59258.c7. [DOI] [PubMed] [Google Scholar]
- 12.Shively CA, Register TC, Friedman DP, Morgan TM, Thompson J, Lanier T. Social stress-associated depression in adult female cynomolgus monkeys (Macaca fascicularis) Biol Psychol. 2005;69:67–84. doi: 10.1016/j.biopsycho.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Amaral D, Lavenex P. Hippocampal neuroanatomy. In: Anderson P, Morris R, Amaral D, Bliss T, O’Keefe J, editors. The Hippocampus Book. Oxford UP; New York: 2007. pp. 37–114. [Google Scholar]
- 14.Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, et al. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Protopopescu X, Butler T, Pan H, Root J, Altemus M, Polanecsky M, et al. Hippocampal structural changes across the menstrual cycle. Hippocampus. 2008;18:985–988. doi: 10.1002/hipo.20468. [DOI] [PubMed] [Google Scholar]
- 16.Willard SL, Friedman DP, Henkel CK, Shively CA. Anterior hippocampal volume is reduced in behaviorally depressed female cynomolgus macaques. Psychoneuroendocrinology. 2009;34:1469–1475. doi: 10.1016/j.psyneuen.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wood CE, Register TC, Cline JM. Transcriptional profiles of progestogen effects in the postmenopausal breast. Breast Cancer Res Treat. 2009;114:233–42. doi: 10.1007/s10549-008-0003-8. [DOI] [PubMed] [Google Scholar]
- 18.Schneider K, Oltmanns J, Hassauer M. Allometric principles for interspecies extrapolation in toxicological risk assessment - empirical investigations. Regul Toxicol Pharmacol. 2004;39:334–347. doi: 10.1016/j.yrtph.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Shively CA, Kaplan JR. Stability of social status rankings of female cynomolgus monkeys, of varying reproductive condition, in different social groups. Am J Primatol. 1991;23:239–245. doi: 10.1002/ajp.1350230404. [DOI] [PubMed] [Google Scholar]
- 20.Altmann J. Observational study of behavior: sampling methods. Behaviour. 1974;48:1–41. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- 21.Shively CA, Wood CE, Register TC, Willard SL, Lees CJ, Chen H, et al. Hormone therapy effects on social behavior and activity levels of surgically postmenopausal cynomolgus monkeys. Psychoneuroendocrinology. 2007;32:981–990. doi: 10.1016/j.psyneuen.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Machado CJ, Snyder AZ, Cherry SR, Lavenex P, Amaral DG. Effects of neonatal amygdala or hippocampus lesions on resting brain metabolism in the macaque monkey: a microPET imaging study. Neuroimage. 2008;39:832–846. doi: 10.1016/j.neuroimage.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ballmaier M, Narr KL, Toga AW, Elderkin-Thompson V, Thompson PM, Hamilton L, et al. Hippocampal morphology and distinguishing late-onset from early-onset elderly depression. Am J Psychiatry. 2008;165:229–237. doi: 10.1176/appi.ajp.2007.07030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bearden CE, Thompson PM, Avedissian C, Klunder AD, Nicoletti M, Dierschke N, et al. Altered hippocampal morphology in unmedicated patients with major depressive illness. ASN Neuro. 2009;1:265–273. doi: 10.1042/AN20090026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neumeister A, Wood S, Bonne O, Nugent AC, Luckenbaugh DA, Young T, et al. Reduced hippocampal volume in unmedicated, remitted patients with major depression versus control subjects. Biol Psychiatry. 2005;57:935–937. doi: 10.1016/j.biopsych.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 26.Moser MB, Moser EI. Functional Differentiation in the Hippocampus. Hippocampus. 1998;8:608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 27.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]