Abstract
Background
It is increasingly evident that there is a close connection between the generation of cutaneous inflammatory cytokines and elevated neuropeptide signaling in complex regional pain syndrome (CRPS) patients. Previously we observed in the rat tibia fracture model of CRPS that activation of caspase-1 containing NALP1 inflammasomes was required for interleukin (IL)-1β production in keratinocytes, and that administration of an IL-1 receptor antagonist (anakinra) reduced the fracture-induced hindpaw mechanical allodynia. We therefore hypothesized that neuropeptides lead to nociceptive sensitization through activation of the skin’s innate immune system by enhancing inflammasome expression and caspase-1 activity.
Methods
We determined whether the neuropeptides substance P (SP) and calcitonin gene-related peptide (CGRP) require IL-1β in order to support nociceptive sensitization when injected into mouse hindpaw skin by testing mechanical allodynia. We then investigated whether these neuropeptides could stimulate production of IL-1β in a keratinocyte cell line (REKs), and could increase the expression of inflammasome component proteins including NALP1 and caspase-1. Finally, we determined if neuropeptide-stimulated IL-1β production required activation of caspase-1 and cathepsin B.
Results
Intraplantar injections of SP and CGRP lead to allodynia in mouse hindpaws but CGRP was approximately 10-fold less potent in causing this response. Moreover, systemic administration of the IL-1 receptor (IL-1R) antagonist anakinra prevented sensitization after neuropeptide injection. Also, mouse skin keratinocytes express IL-1R which is up-regulated after local neuropeptide application. In vitro data demonstrated that both SP and CGRP increased IL-1β gene and protein expression in REKs in a dose-dependent manner. Furthermore, SP time- and dose-dependently up-regulated NALP1 and caspase-1 mRNA and protein levels in REKs. In contrast, CGRP time- and dose-dependently enhanced NALP1 and caspase-1 mRNA levels without causing a significant change in NALP1 or caspase-1 protein expression in REKs. Inhibition of caspase-1 activity using the selective inhibitor Ac-YVAD-CHO reduced SP and, less effectively, CGRP induced increases in IL-1β production in REK cells. The selective cathepsin B inhibitor CA-74Me inhibited neuropeptide induced IL-1β production in REKs as well.
Conclusions
Collectively, these results demonstrate that neuropeptides induce nociceptive sensitization by enhancing IL-1 β production in keratinocytes. Neuropeptides rely on both caspase-1 and cathepsin B for this enhanced production. Neuro-cutaneous signaling involving neuropeptide activation of the innate immunity may contribute to pain in CRPS patients.
Introduction
Peripheral mechanisms supporting chronic pain remain enigmatic. While we have an advanced understanding of neuroplastic events in spinal cord and brain tissue which accompany and support many types of chronic pain, peripheral mechanisms have not enjoyed the same degree of attention. Studies on complex regional pain syndrome (CRPS) indicate that this condition requires the generation of inflammatory mediators such as cytokines and the activation of neuropeptide expressing small afferent nerve fibers in the joints and skin (1,2). Peripheral neuropeptide release is required for cytokine production and subsequent nociceptive sensitization after incision as well (3). Thus, interest in the mechanisms linking peripheral neural activity to inflammation and sensitization has increased as our understanding of neuroinflammation has grown.
Focusing on CRPS, it appears that dysregulated innate immunity characterizes this syndrome. For example, measurements of the levels of cytokines in venous blood, as well as the analysis of fluid from suction blisters created on the skin of CRPS-affected extremities showed elevated levels of several cytokines (4,5). Likewise, elevated levels of both substance P (SP) and calcitonin gene-related peptide (CGRP) have been found in venous blood from the extremities of CRPS patients (5,6). A connection between the release of neuropeptides and CRPS has been suggested by clinical studies demonstrating abnormal plasma extravasation in the skin of CRPS patients after cutaneous SP administration (7). Also, observations of abnormal responses to cutaneous capsaicin administration causing afferent fiber neuropeptide release in a human model of CRPS highlight this connection (8).
In a recently developed rat tibia fracture/cast immobilization model of CRPS, the human findings were greatly extended. Using this model it was observed that SP signaling through its cognate neurokinin 1 receptor was required for the nociceptive sensitization characteristic of CRPS and that both SP and CGRP were up-regulated in primary afferent neurons (9,10). Similarly, the continuous administration of the interleukin (IL)-1 receptor blocker anakinra reduced both the nociceptive sensitization in these animals, and evidence of peripheral inflammation (11). Caspase-1 enzyme cleaves pro-IL-1β to its mature form IL-1β, which is required for nociceptive sensitization (12). It is also the active enzymatic component of the NALP1 inflammasome. This structure consists of three proteins, NALP1, an adaptor known as apoptosis-associated speck-like protein containing CARD, and caspase-1 (13). Additionally, a recent report showed that NALP1 inflammasomes containing caspase-1 are activated in keratinocytes after fracture and immobilization of rat hindlimbs (12). Lastly, cathepsin B contributes to the maturation of pro-IL-1β by mechanisms including direct cleavage of pro-IL-1β, and by participating in the activation of inflammasomes including the NALP1 inflammasome (14–17).
In this report we attempt to address directly the question of whether the neuropeptides SP and CGRP require the production of IL-1β in order to support nociceptive sensitization when injected into rodent hindpaw skin. In complementary experiments we sought to determine whether these neuropeptides could stimulate the production of IL-1β in a keratinocyte cell line, whether any such IL-1β production required caspase-1 or cathepsin B activation, and whether these changes were accompanied by the increased expression of components of the NALP1 inflammasome.
Materials and methods
Animals
These experiments were approved by our Institutional Animal Care and Use Committee. All animals were treated in accordance with the guideline of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and followed the guidelines of the International Association for the Study of Pain. Eight to ten week old male C57BL/6 mice were used in all experiments. They were housed 5/cage in standard isolator cages maintained at 22 ± 1°C with 14-h light and 10-h dark cycles. The animals were allowed free access to food and drinking water.
Drug treatments
The C57BL/6 mice were treated once with IL-1β receptor antagonist anakinra (Amgen, Thousand Oaks, CA) at a dose of 120 mg/kg i.p. or saline 1h before the SP or CGRP administration. The anakinra dose was based on previous studies (12,18). Intraplantar injection of saline, SP (300 ng and 1000 ng, 15 μl of saline), or CGRP (3 μg and 10 μg, 15 μl of saline) into the hindpaw were made before nociceptive testing.
Hindpaw nociception
The hindpaw mechanical allodynia was measured using nylon von Frey filaments according to the “up-down” algorithm described by Chaplan et al (19) as we have used previously(20–22). Briefly, mice were placed in clear plastic cylinder (10 cm in diameter and 40 cm in height) on a wire mesh platform and allowed to acclimate for 15 minutes. Fibers of sequentially increasing stiffness (0.2–2.0 g; seven fibers) were applied to the center of the hindpaw plantar skin just distal to the first set of foot pads. Four fibers were applied after the first one causing a withdrawal response in order to estimate withdraw threshold.
Cell Culture
The epidermal keratinocyte (REK) cell line was generously provided by Dr. Howard Baden (Massachussetts General Hospital, Boston, MA) and grown as described previously. (23–25) In brief, cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, CA) supplemented with 10% fetal bovine serum (FBS), 1% penicillin-streptomycin, 0.4 μg/ml hydrocortisone, and 0.75 mM aminoguanidine. On reaching approximately 90% confluence, the cells were starved with the above culture medium supplemented with only 1% FBS overnight. Cells were then washed twice with fresh DMEM before addition of SP (Sigma Chemical Co, S. Louis, MO), CGRP (Sigma) or saline vehicle. At least five culture dishes were used for each time point, treatment condition and experiment.
Quantitative real-time PCR
Total RNA from the REKs was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA). The cDNA (20 μL final volume) was synthesized from 1 μg RNA using iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA). Real-time polymerase chain reactions (PCRs) were conducted using the SYBR Green PCR master mix (Applied Biosystems, Foster City, CA). Real-time PCR amplification of IL-1R, NAPL1, caspase-1, and 18S was performed on the ABI 7900HT sequencing detection system. To validate the primer sets used, dissociation curves were performed to document single product formation and agarose gel analysis was conducted to confirm the size. The data were analyzed by the comparative CT (cycle threshold) method as described in the manufacturer’s manual. All results were confirmed by repeating the experiment 3 times.
IL-1β ELISA
On reaching confluence, cells were treated with neuropeptides SP and CGRP (Final concentrations up to 10−7 M) with/out addition of selective caspase-1 inhibitor, Ac-YVAD-CHO (Cayman Chemical, Ann Arbor, MI) or a selective cathepsin B inhibitor, CA-74Me (EMD Biosciences, USA) for 24 hours. The cell culture supernatant and cell lysate were then collected at the indicated time. The IL-1β protein level in the undiluted cell culture supernatant was measured by a R&D System EIA kit (Minneapolis, MN) according to the manufacturer’s protocol. Protein content in the culture medium was determined using a Coomassie Blue Protein Assay Kit (Pierce, Rockford, IL). The results of this assay were confirmed by repeating the experiment twice.
Western blot
Cultured rat keratinocytes were harvested and homogenized in Tris buffer pH 6.8 with freshly added protease inhibitors, β-mercaptoethanol, and glycerol. The homogenate was centrifuged at 13,000 g for 15 min at 4°C. Equal amounts of protein were size fractionated by 10% SDS-PAGE gel electrophoresis and transferred onto a polyvinylidene difluorided membrane, which were then blocked overnight and incubated with primary rabbit anti NAPL1 (1:400, Cell Signalling Technology, Danvers, MA) or rabbit anti-caspase-1 antibody (1:200, BioVision Research, Mountain View, CA). After washing, the blots were incubated with horseradish peroxidase conjugated anti-rabbit antibody (Santa Cruz Biotechnology, Santa Cruz, CA) (diluted in 1:5000) for 1h at room temperature. The membrane was then washed again and exposed to film after chemiluminescence reagent treatment with the ECL plus (Amersham, Piscataway, NJ). Bands were quantified using densitometry of digitalized images. Each blot was then stripped and reprobed with anti-β actin antibodies, thus allowing normalization of expression among samples. The results of this assay were confirmed by repeating the experiment 3 times.
Immunohistochemical Analysis
The immunohistochemical analysis of mouse paw skin was done according to previously published methods (20,26). The primary antibodies used were: interleukin-1 (IL-R1) receptor 1:50 (Abcam Inc., Cambridge, MA) and keratin 1:50 (Thermo Scientific Inc., Waltham, MA). Fluorescein-conjugated secondary antibodies against the primary antibodies were applied in 1:150 concentrations (Jackson ImmunoResearch Laboratories, West Grove, PA & Vector Laboratories Inc, Burlingame, CA). Confocal laser-scanning microscopy was performed using a Zeiss LSM 510 and LSM 510 META Laser Scanning Microscopes (Thornwood, NY). Control experiments included incubation of slices in primary and secondary antibody-free solutions, both of which led to low-intensity nonspecific staining patterns in preliminary experiments.
Statistical analysis
To compare the time course effects of treatments and individual groups for the behavior studies testing mechanical allodynia, two-way ANOVA (time, treatment) with repeated measures for time was performed with Bonferroni tests for multiple comparisons. Other data were analyzed by two-way analysis of variance (ANOVA) followed by post hoc Bonferroni multiple comparisons tests. All data are presented as means ± SE and differences are considered significant at a P value less than 0.05 (Prism 4.0, GraphPad Software, San Diego, CA).
Results
Neuropeptides SP and CGRP cause nociceptive sensitization dependent on IL-1β signaling
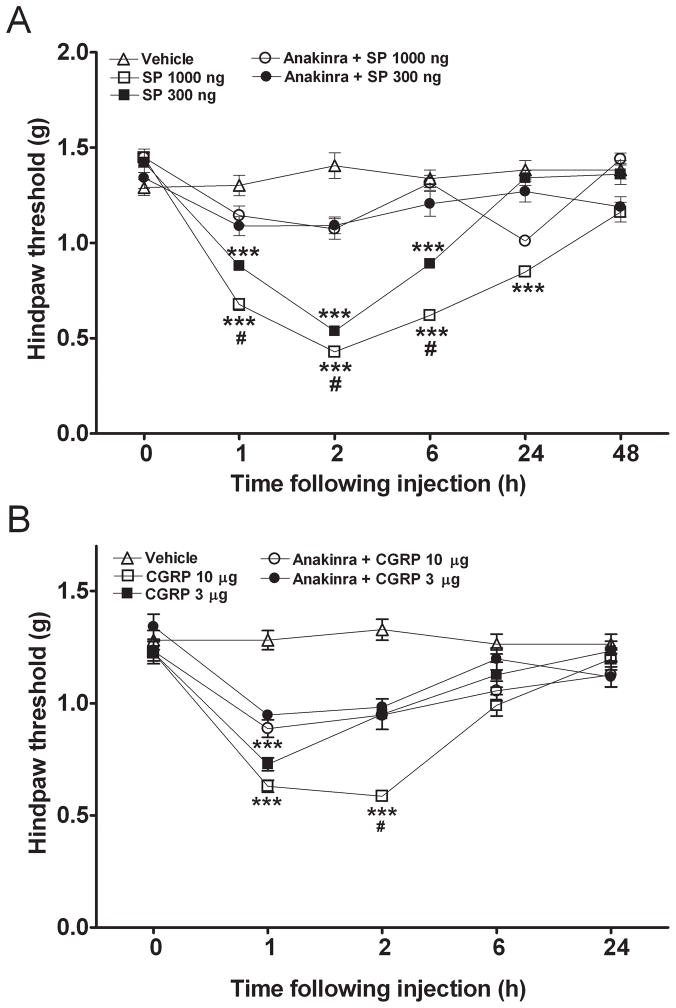
The first hypothesis addressed was that neuropeptides released from primary afferent neurons lead to nociceptive sensitization, and that this sensitization relies upon IL-1β signaling. We selected a measurement approach using von Frey filaments application to hindpaw skin because it was the function of skin and superficial tissues in support of nociception, which was of interest. Figure 1 shows that subcutaneous injections of SP and CGRP cause allodynia in the hindpaws of mice, though the response was delayed by several hours from the time of injection. CGRP was approximately 10-fold less potent in causing this response, and the response was more transient. Recombinant IL-1 receptor antagonist (anakinra) was systemically administered before local application of neuropeptides in additional experiments. For SP, anakinra readily prevented sensitization caused by relatively low doses of the neuropeptide (Fig. 1A). Anakinra also blocked CGRP-induced sensitization even at a high (10 μg) dose (Fig. 1B).
Figure 1.
Interleukin (IL)-1 β receptor antagonist (IL-1ra, anakinra, 120 mg/kg, i.p.) prevents the mechanical sensitization induced by intraplantar injection of various doses of substance P (SP) or calcitonin-gene related peptide (CGRP). A) SP (300 ng and 1000 ng) caused significant mechanical allodynia lasting between 1 and 6 hours; whereas the anakinra preinjected mice displayed reduced hindpaw mechanical allodynia caused by SP. B) CGRP (3 μg and 10 μg) caused significant mechanical allodynia between 1 and 2 hours; whereas the anakinra preinjected mice displayed reduced hindpaw mechanical allodynia induced by CGRP. Values are means ± SE. N=6 mice for all experimental groups. * p<0.05, ** p<0.01, *** p<0.001 for differences relative to baseline; # p<0.05 for differences between the non-anakinra and anakinra pretreated mice that were injected with the same dose of SP or CGRP.
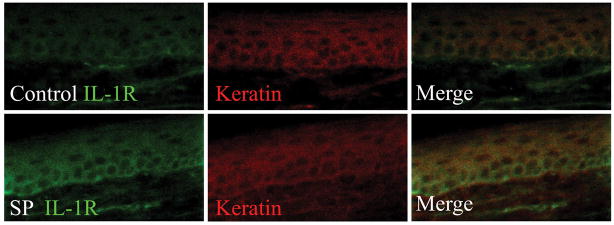
Next, determination of IL-1 receptor (IL-1R) presence and expression in the hindpaw skin was performed. Figure 2A demonstrates that the epidermal keratinocytes express IL-1R before and after neuropeptide SP application. Additionally, there is an increased IL-1R1 mRNA expression in the skin 2 hours after local SP application versus controls (Fig. 2B).
Figure 2.
Neuropeptide mediated up-regulation of epidermal interleukin (IL)-1 receptor (IL-1R) expression. A) Immunohistochemistry of IL-1R and keratin before and after (2h) Substance P (SP) injection of hindpaw skin. B) The IL-1 receptor mRNA expression in the hindpaw skin 2 hours after administration of SP 1000 ng/15 μl and vehicle.
Neuropeptides SP and CGRP dose- and time-dependently increase IL-1β mRNA and protein levels in cell cultures
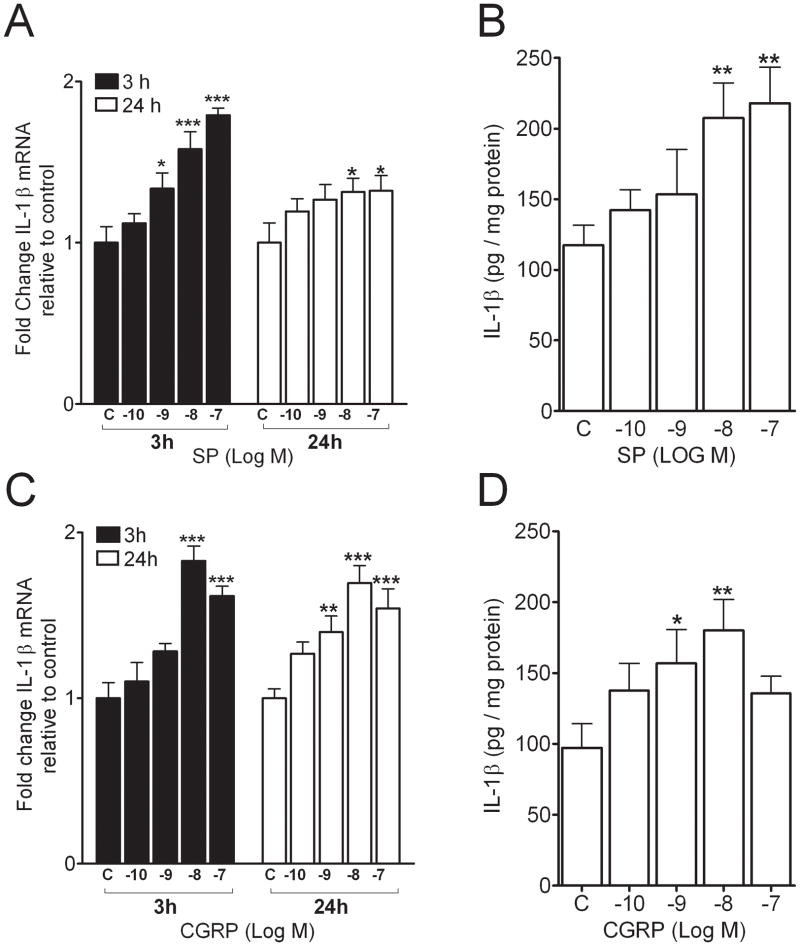
The second hypothesis addressed was that neuropeptide stimulation of IL-1β production could be recapitulated in a commonly used rodent keratinocyte cell line, REK. Cultures of these cells were exposed to 10−10 to 10−7 M concentrations of the neuropeptides for 3 or 24 hours separately. Enhanced levels of both IL-1β mRNA and protein were observed in these cultures, and these effects were dose-dependent (Figure 3). For both neuropeptides maximal responses occurred at approximately 10−8 neuropeptide concentration.
Figure 3.
Neuropeptides Substance P (SP) and calcitonin-gene related peptide (CGRP) time- and dose-dependently increased the interleukin (IL)-1β mRNA level as measured by real-time polymerase chain reaction (PCR) and protein level as measured by ELISA (Fig. 2A, B, C, and D). Values are means ± SE. * p<0.05, ** p<0.01, *** p<0.001 vs. the vehicle-treated control cells (C).
The neuropeptides SP and CGRP dose- and time-dependently enhance NALP1 mRNA and protein levels
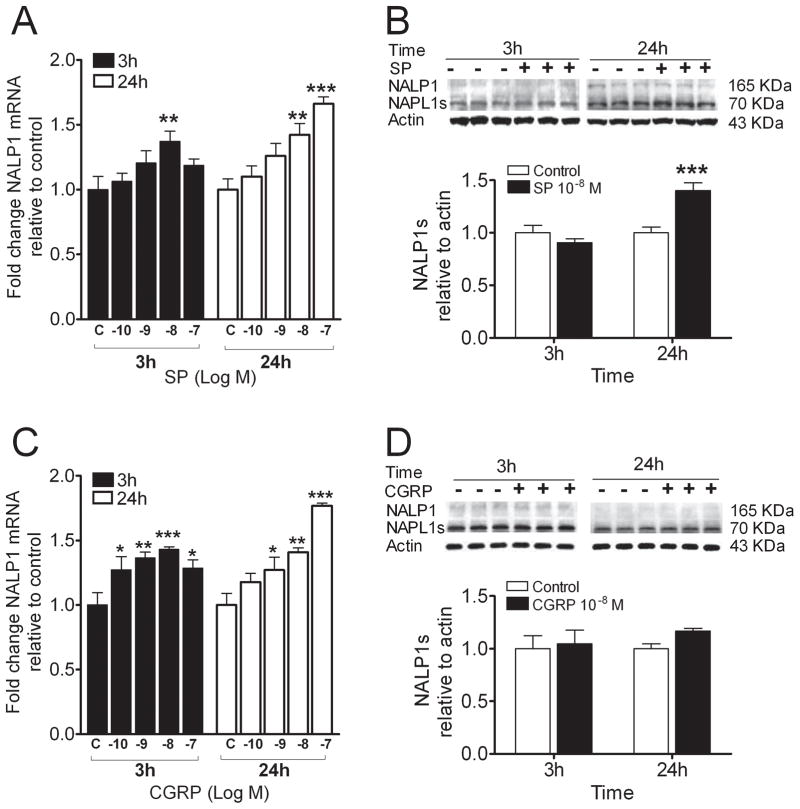
Because epidermal NALP-1 protein is up-regulated in the rat fracture model of CRPS, a model which depends on intact SP signaling (12), we hypothesized that exposure of REKs to neuropeptides would lead to an up-regulation in expression of NALP1. Figure 4 shows that SP up-regulates both NALP1 mRNA and protein, with the increase in protein concentration seen at 24 hours after exposure to SP. Specifically, the short form of NALP1, which is capable of assembling with other proteins to form the NALP1 inflammasome, was increased (27). The neuropeptide CGRP enhanced NALP1 mRNA levels without causing an increase in NALP1 protein in the cultures.
Figure 4.
Neuropeptides induced gene and protein expression of inflammasome component, NALP1 in the REK cultures. A) Substance P (SP) stimulated NAPL1 gene expression in a dose-dependent manner at 3 and 24 h posttreatment, respectively. B) Western blot band appearances (upper panel) and scanned densities presented as a bar graph (lower panel) for the full length NAPL1 and the short form NALP1 (NALP1s) in cultures treated with and without SP. C) calcitonin-gene related peptide (CGRP) dose-dependently stimulated NAPL1 gene expression at 3 and 24 h posttreatment, separately. D) Western blot band appearances (upper panel) and scanned densities presented as a bar graph (lower panel) for the full length NAPL1 and NALP1s in cultures treated with and without CGRP. Values are means ± SE. * p<0.05, ** p<0.01, *** p<0.001 vs. the vehicle-treated control cells (C).
Neuropeptides SP and CGRP dose- and time-dependently enhance caspase-1 mRNA and protein levels
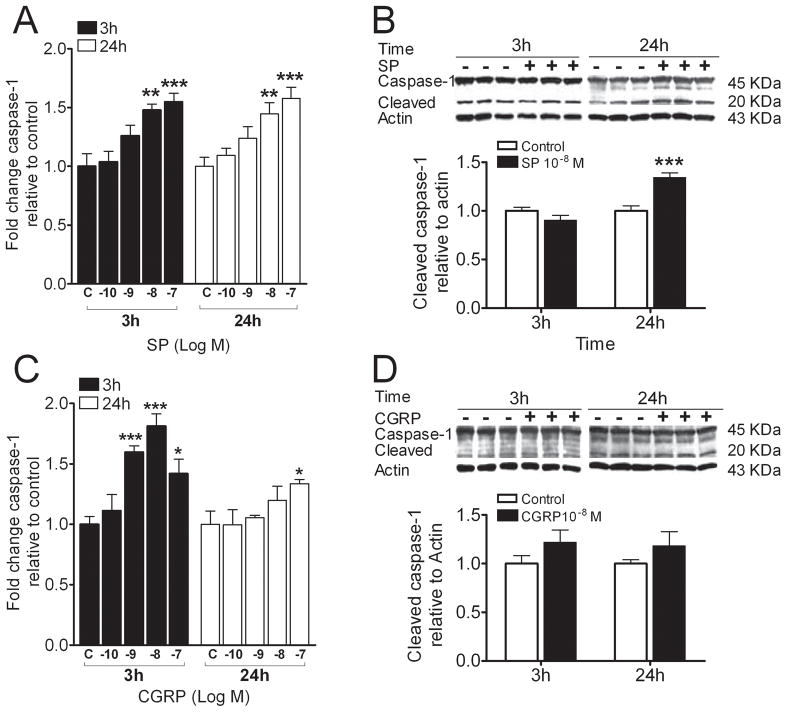
As neuropeptides enhance IL-1β levels and also NALP1 protein is up-regulated by SP, we measured changes in caspase-1 levels 3 and 24 hours after beginning SP or CGRP treatment. Similar to the results for NALP1, SP and CGRP enhanced caspase-1 mRNA levels, but only SP (10−8 M) at 24 hours caused a statistically significant increase in caspase-1 protein levels (Figure 5).
Figure 5.
Neuropeptides induced gene and protein expression of inflammasome component, caspase-1 in REK cells. A) Substance P (SP) stimulated caspase-1 gene expression in a dose-dependent manner at 3 and 24 h post-treatment, respectively. B) Western blot band appearances (upper panel) and scanned densities presented as a bar graph (lower panel) for the full length and the cleaved caspase-1 in cultures treated with and without SP. C) calcitonin-gene related peptide (CGRP) dose-dependently stimulated caspase-1 gene expression was mainly observed at 3h posttreatment. D) Western blot band appearances (upper panel) and scanned densities presented as a bar graph (lower panel) for the full length and the cleaved caspase-1 for cultures treated with and without CGRP. Values are means ± SE. * p<0.05, ** p<0.01, *** p<0.001 vs. the vehicle-treated control cells (C).
The activation of caspase-1 is required for neuropeptide enhancement of IL-1β production
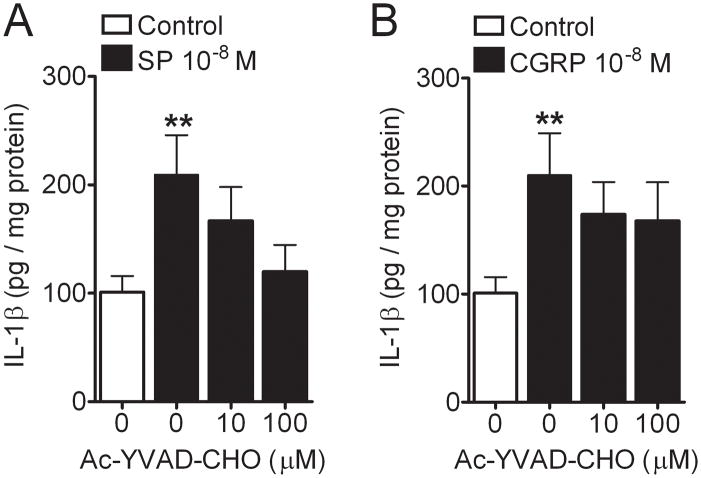
To determine whether neuropeptide-stimulated IL-1β production in REK cells was caspase-1-dependent, we incubated separate REK cultures with each neuropeptide at concentrations that had near-maximal efficacy, and with two doses of Ac-YVAD-CHO which is a selective caspase-1 inhibitor (28). Figure 6 displays the data showing that caspase-1 activity is in fact required for SP-induced IL-1β production. The effect of Ac-YVAD-CHO on CGRP-induced IL-1β production was less prominent at the highest dose of inhibitor used.
Figure 6.
Caspase-1 inhibitor, Ac-YVAD-CHO inhibited the Substance P (SP) (10−8 M), not calcitonin-gene related peptide (CGRP) (10−8 M), induced interleukin (IL)-1β protein production (A and B) in the REK cultures. Values are means ± SE. ** p<0.01 vs. the control cells. # p<0.05 vs. the cultures treated with SP or CGRP.
Cathepsin B contributes to neuropeptide stimulated IL-1β production
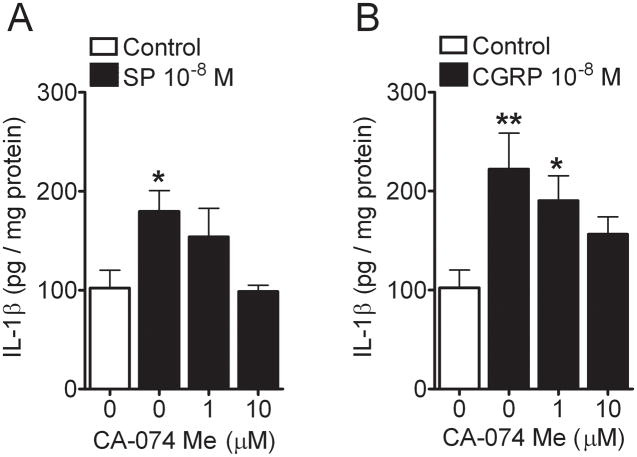
We used CA-74Me, which is a selective cathepsin B inhibitor, in the REK culture system along with SP and CGRP to determine the contribution of this pathway in the production of IL-1β (29). Figure 7 shows the dose-dependent effect of the cathepsin B inhibitor in reducing the neuropeptide-stimulated cytokine production.
Figure 7.
Cathepsin B inhibitor, CA-74Me dose-dependently inhibited the Substance P (SP) (10−8 M) and calcitonin-gene related peptide (CGRP) (10−8 M) induced interleukin (IL)-1β protein production (A and B) in the REK cultures, respectively. Values are means ± SE. * p<0.05, ** p<0.01 vs. the vehicle-treated control cells.
Discussion
In attempting to better understand the mechanisms supporting CRPS, attention has recently shifted to the periphery, particularly to the question of how the peripheral nervous system interacts with somatic tissues. In this series of studies we examined the link between two specific neuropeptides expressed and released by primary afferent nerve fibers and keratinocytes, cells producing cytokines such as IL-1β supporting pain and neurogenic inflammation in CRPS. Our principal findings were, 1) that SP and CGRP could by themselves cause nociceptive sensitization which relied upon IL-1β signaling, 2) that SP and CGRP stimulate the production of IL-1β in a keratinocyte cell line via caspase-1 dependent and possibly independent mechanisms, and 3) that key components of the NALP1 inflammasome were up-regulated after exposure of cultures to SP, but not CGRP to a measurable extent. Taken as a group, our results suggest that neuro-cutaneous signaling mediated by the activation of the innate system of immunity by neuropeptides may contribute to the pain experienced by CRPS sufferers.
Because earlier studies demonstrated that keratinocytes might be an important target for neuropeptides in stimulating cytokine production, we then applied SP and CGRP directly to REK cultures and demonstrated an enhanced production of IL-1β at both the mRNA and protein levels with protein changes lagging behind those of IL-1β mRNA. Keratinocytes express receptors for SP (neurokinin 1) and CGRP (CRLR, RAMP1 and RAMP2)(10,30–32), and previous reports have provided data demonstrating that epidermal and other types of epithelial cells produce IL-1β in response to neuropeptide stimulation (33). Moreover, we had previously demonstrated that in vivo direct application of SP to mouse hindpaw skin results in an increased production of IL-1β (3). The keratinocyte culture data taken together with the skin IL-1β production after SP stimulation support the notion of epidermal keratinocytes participating in the neurogenic inflammation process. The presence of IL-1R on skin epidermal cells and the present finding of IL-1R antagonist anakinra blocking local SP-induced skin sensitization further support this notion. Additional studies may be required to determine the extent of involvement of other cell types (neutrophil, macrophage, etc.) in neuropeptide-mediated skin sensitization; nevertheless, the present findings implicate keratinocytes as notable participants in neuropeptide-induced pain. Further experiments using the selective caspase-1 inhibitor Ac-YVAD-CHO demonstrated that SP and CGRP stimulated IL-1β production was dependent on the activation of caspase-1. Caspase-1 has a well established ability to convert pro-IL-1β to its active form (34,35). We pursued further pharmacological experiments using a cathepsin B inhibitor since this enzyme can regulate inflammasome-associated caspase-1 activity (15). Moreover, cathepsin B is produced and secreted by keratinocytes (36). The selective cathepsin B inhibitor CA-74Me potently reduced neuropeptide-stimulated IL-1β production. On the other hand, Ac-YVAD-CHO was somewhat less effective against CGRP stimulated IL-1β production despite high concentrations of the inhibitor being used. This suggests a caspase-1-independent IL-1β production mechanism might be present. Examples of such independent mechanisms include those involving cathepsin B, separate from its indirect effects on caspase-1 activity, and direct effects of matrix metalloproteinase to cleave pro-IL-1β to its active mature form (37,38). Additional data from our experiments demonstrating a lack of effect at the protein expression level for NALP1 and caspase-1 when CGRP was added to the cultures are also consistent with a possible inflammasome-independent pathway for CGRP-stimulated IL-1β production.
Much recent attention has been focused on the biology of inflammasomes, multiprotein complexes which are part of the innate system of immunity (39). Though present in many cell types, inflammasomes have been studied in some detail in epithelial cells. Dysfunction of these structures leads to a number of diseases including Muckle Wells syndrome, vitiligo and other conditions characterized by dysregulated immunity (40,41). There are at least three distinct inflammasomes including the NALP1, NALP3 and IPAF varieties, though the molecular biology of NALP proteins in humans and other species suggests many more types of NALP inflammasomes may form (42). Our work focused on the NALP1 inflammasome because it was implicated in supporting nociceptive sensitization and the generation of IL-1β in the rat tibial fracture model of CRPS (12). To this point relatively few specific molecules were known to activate the NALP1 inflammasome, though muramyl dipeptide (MDP) and anthrax toxin promote the NALP1-mediated caspase1 activation (43). For at least one other inflammasome, the NALP3 inflammasome, a cell surface receptor (P2X7) causes activation of the inflammasome and caspase activity (44). Therefore, while it is not clear precisely how caspase-1 is being activated in either CRPS or in our keratinocyte model system, it does appear that receptor-mediated activation of the NALP1 inflammasome by neuropeptides is a plausible hypothesis.
Our observations support and are consistent with other observations concerning the roles for IL-1β in pain. This cytokine is found in increased abundance in the skin of animals (12,27,45) and humans (46,47) experiencing pain from several different etiologies including those involving inflammation. The cytokine IL-1β causes the sensitization of skin and other tissues by several mechanisms. The first mechanism is via direct interaction of the cytokine with IL-1 receptors expressed on neurons. Observations from Binshtok et al. indicate that certain populations of nociceptive neurons respond to IL-1β application in p38-dependent fashion by enhancing tetrodotoxin-resistant sodium ion currents and therefore excitability (48). The second general peripheral pathway of IL-1β participation in nociception is by enhancing the production of other nociceptive mediators including prostaglandin E2, tumor necrosis factor α, IL-6 and nerve growth factor (49). Treatment of animals having had tibia fracture and cast immobilization with IL-1R antagonist anakinra reduces not only the CRPS-like nociceptive sensitization, but also levels of the pro-nociceptive mediators IL-6, tumor necrosis factor α and nerve growth factor in hindpaw skin (12). Finally, IL-1β may have a feedback input on peripheral afferent neurons to augment their effects by increasing the production and release of SP (50).
Though our results indicate that neuropeptides can stimulate the production of IL-1β possibly via a NALP1 inflammasome-dependent pathway, it is unclear how the signaling promoting this up-regulation takes place. There is little information at present to help identify possible mechanisms given the relative lack of study of NALP1 inflammasome activation through cell surface neurotransmitter receptors. Whatever that mechanism of activation, the linking of the peripheral nervous system with the innate system of immunity represents an important advancement in our understanding of neurogenic inflammation, and neuro-somatic communication in general. Eventually it may be possible to control pain and other problematic consequences of inflammation by selectively regulating such signaling pathways.
Acknowledgments
Funding: NIH/NIGMS award GM079126 to JDCVA Merit Review award to WSK.
Information for LWW regarding depositing manuscript into PubMed Central: This research was funded by National Institutes of Health grant number GM079126.
Footnotes
The authors declare no conflicts of interest.
Reprints will not be available from the authors.
DISCLOSURES
Name: Xiaoyou Shi, MD
Contribution: This author helped conduct the study and analyze the data.
Attestation: Xiaoyou Shi has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Liping Wang, MD
Contribution: This author helped conduct the study and write the manuscript.
Attestation: Liping Wang has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Xiangqi Li, MD
Contribution: This author helped conduct the study and analyze the data.
Attestation: Xiangqi Li has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Peyman Sahbaie, MD
Contribution: This author helped design the study, conduct the study, and analyze the data.
Attestation: Peyman Sahbaie has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Wade S. Kingery, MD
Contribution: This author helped design the study and write the manuscript.
Attestation: Wade S. Kingery has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: J. David Clark, MD, PhD
Contribution: This author helped design the study, analyze the data, and write the manuscript.
Attestation: J. David Clark has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Contributor Information
Xiaoyou Shi, Veterans Affairs Palo Alto Health Care System, Palo Alto, CA and Stanford University, Stanford, CA.
Liping Wang, Veterans Affairs Palo Alto Health Care System, Palo Alto, CA.
Xiangqi Li, Veterans Affairs Palo Alto Health Care System, Palo Alto, CA and Stanford University, Stanford, CA.
Peyman Sahbaie, Veterans Affairs Palo Alto Health Care System, Palo Alto, CA and Stanford University, Stanford, CA.
Wade S. Kingery, Veterans Affairs Palo Alto Health Care System, Palo Alto, CA.
J. David Clark, Veterans Affairs Palo Alto Health Care System, Palo Alto, CA and Stanford University, Stanford, CA.
References
- 1.Birklein F, Schmelz M. Neuropeptides, neurogenic inflammation and complex regional pain syndrome (CRPS) Neurosci Lett. 2008;437:199–202. doi: 10.1016/j.neulet.2008.03.081. [DOI] [PubMed] [Google Scholar]
- 2.McDougall JJ. Arthritis and pain. Neurogenic origin of joint pain. Arthritis Res Ther. 2006;8:220. doi: 10.1186/ar2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sahbaie P, Shi X, Guo TZ, Qiao Y, Yeomans DC, Kingery WS, Clark JD. Role of substance P signaling in enhanced nociceptive sensitization and local cytokine production after incision. Pain. 2009;145:341–9. doi: 10.1016/j.pain.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huygen FJ, De Bruijn AG, De Bruin MT, Groeneweg JG, Klein J, Zijlstra FJ. Evidence for local inflammation in complex regional pain syndrome type 1. Mediators Inflamm. 2002;11:47–51. doi: 10.1080/09629350210307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schinkel C, Gaertner A, Zaspel J, Zedler S, Faist E, Schuermann M. Inflammatory mediators are altered in the acute phase of posttraumatic complex regional pain syndrome. Clin J Pain. 2006;22:235–9. doi: 10.1097/01.ajp.0000169669.70523.f0. [DOI] [PubMed] [Google Scholar]
- 6.Schinkel C, Scherens A, Koller M, Roellecke G, Muhr G, Maier C. Systemic inflammatory mediators in post-traumatic complex regional pain syndrome (CRPS I) -longitudinal investigations and differences to control groups. Eur J Med Res. 2009;14:130–5. doi: 10.1186/2047-783X-14-3-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leis S, Weber M, Schmelz M, Birklein F. Facilitated neurogenic inflammation in unaffected limbs of patients with complex regional pain syndrome. Neurosci Lett. 2004;359:163–6. doi: 10.1016/j.neulet.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 8.Terkelsen AJ, Bach FW, Jensen TS. Experimental forearm immobilization in humans reduces capsaicin-induced pain and flare. Brain Res. 2009;1263:43–9. doi: 10.1016/j.brainres.2009.01.054. [DOI] [PubMed] [Google Scholar]
- 9.Guo TZ, Offley SC, Boyd EA, Jacobs CR, Kingery WS. Substance P signaling contributes to the vascular and nociceptive abnormalities observed in a tibial fracture rat model of complex regional pain syndrome type I. Pain. 2004;108:95–107. doi: 10.1016/j.pain.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Wei T, Li WW, Guo TZ, Zhao R, Wang L, Clark DJ, Oaklander AL, Schmelz M, Kingery WS. Post-junctional facilitation of Substance P signaling in a tibia fracture rat model of complex regional pain syndrome type I. Pain. 2009;144:278–86. doi: 10.1016/j.pain.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li WW, Sabsovich I, Guo TZ, Zhao R, Kingery WS, Clark JD. The role of enhanced cutaneous IL-1beta signaling in a rat tibia fracture model of complex regional pain syndrome. Pain. 2009;144:303–13. doi: 10.1016/j.pain.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li WW, Guo TZ, Liang D, Shi X, Wei T, Kingery WS, Clark JD. The NALP1 inflammasome controls cytokine production and nociception in a rat fracture model of complex regional pain syndrome. Pain. 2009;147:277–86. doi: 10.1016/j.pain.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tschopp J, Martinon F, Burns K. NALPs: a novel protein family involved in inflammation. Nat Rev Mol Cell Biol. 2003;4:95–104. doi: 10.1038/nrm1019. [DOI] [PubMed] [Google Scholar]
- 14.Hentze H, Lin XY, Choi MS, Porter AG. Critical role for cathepsin B in mediating caspase-1-dependent interleukin-18 maturation and caspase-1-independent necrosis triggered by the microbial toxin nigericin. Cell Death Differ. 2003;10:956–68. doi: 10.1038/sj.cdd.4401264. [DOI] [PubMed] [Google Scholar]
- 15.Newman ZL, Leppla SH, Moayeri M. CA-074Me protection against anthrax lethal toxin. Infect Immun. 2009;77:4327–36. doi: 10.1128/IAI.00730-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terada K, Yamada J, Hayashi Y, Wu Z, Uchiyama Y, Peters C, Nakanishi H. Involvement of cathepsin B in the processing and secretion of interleukin-1beta in chromogranin A-stimulated microglia. Glia. 58:114–24. doi: 10.1002/glia.20906. [DOI] [PubMed] [Google Scholar]
- 17.Vancompernolle K, Van Herreweghe F, Pynaert G, Van de Craen M, De Vos K, Totty N, Sterling A, Fiers W, Vandenabeele P, Grooten J. Atractyloside-induced release of cathepsin B, a protease with caspase-processing activity. FEBS Lett. 1998;438:150–8. doi: 10.1016/s0014-5793(98)01275-7. [DOI] [PubMed] [Google Scholar]
- 18.Wolf G, Livshits D, Beilin B, Yirmiya R, Shavit Y. Interleukin-1 signaling is required for induction and maintenance of postoperative incisional pain: genetic and pharmacological studies in mice. Brain Behav Immun. 2008;22:1072–7. doi: 10.1016/j.bbi.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 20.Clark JD, Qiao Y, Li X, Shi X, Angst MS, Yeomans DC. Blockade of the complement C5a receptor reduces incisional allodynia, edema, and cytokine expression. Anesthesiology. 2006;104:1274–82. doi: 10.1097/00000542-200606000-00024. [DOI] [PubMed] [Google Scholar]
- 21.Liang DY, Li X, Clark JD. Formalin-induced spinal cord calcium/calmodulin-dependent protein kinase II alpha expression is modulated by heme oxygenase in mice. Neurosci Lett. 2004;360:61–4. doi: 10.1016/j.neulet.2004.02.050. [DOI] [PubMed] [Google Scholar]
- 22.Liang D, Li X, Lighthall G, Clark JD. Heme oxygenase type 2 modulates behavioral and molecular changes during chronic exposure to morphine. Neuroscience. 2003;121:999–1005. doi: 10.1016/s0306-4522(03)00483-4. [DOI] [PubMed] [Google Scholar]
- 23.Baden HP, Kubilus J. The growth and differentiation of cultured newborn rat keratinocytes. J Invest Dermatol. 1983;80:124–30. doi: 10.1111/1523-1747.ep12532899. [DOI] [PubMed] [Google Scholar]
- 24.Haydock PV, Blomquist C, Brumbaugh S, Dale BA, Holbrook KA, Fleckman P. Antisense profilaggrin RNA delays and decreases profilaggrin expression and alters in vitro differentiation of rat epidermal keratinocytes. J Invest Dermatol. 1993;101:118–26. doi: 10.1111/1523-1747.ep12363609. [DOI] [PubMed] [Google Scholar]
- 25.Kuechle MK, Thulin CD, Presland RB, Dale BA. Profilaggrin requires both linker and filaggrin peptide sequences to form granules: implications for profilaggrin processing in vivo. J Invest Dermatol. 1999;112:843–52. doi: 10.1046/j.1523-1747.1999.00599.x. [DOI] [PubMed] [Google Scholar]
- 26.Liang DY, Li X, Li WW, Fiorino D, Qiao Y, Sahbaie P, Yeomans DC, Clark JD. Caspase-1 modulates incisional sensitization and inflammation. Anesthesiology. 113:945–56. doi: 10.1097/ALN.0b013e3181ee2f17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F, van Bruggen R, Tschopp J. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem. 2007;55:443–52. doi: 10.1369/jhc.6A7101.2006. [DOI] [PubMed] [Google Scholar]
- 28.Thornberry NA, Peterson EP, Zhao JJ, Howard AD, Griffin PR, Chapman KT. Inactivation of interleukin-1 beta converting enzyme by peptide (acyloxy)methyl ketones. Biochemistry. 1994;33:3934–40. doi: 10.1021/bi00179a020. [DOI] [PubMed] [Google Scholar]
- 29.Buttle DJ, Saklatvala J, Tamai M, Barrett AJ. Inhibition of interleukin 1-stimulated cartilage proteoglycan degradation by a lipophilic inactivator of cysteine endopeptidases. Biochem J. 1992;281 ( Pt 1):175–7. doi: 10.1042/bj2810175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albertin G, Carraro G, Parnigotto PP, Conconi MT, Ziolkowska A, Malendowicz LK, Nussdorfer GG. Human skin keratinocytes and fibroblasts express adrenomedullin and its receptors, and adrenomedullin enhances their growth in vitro by stimulating proliferation and inhibiting apoptosis. Int J Mol Med. 2003;11:635–9. [PubMed] [Google Scholar]
- 31.Yu XJ, Li CY, Wang KY, Dai HY. Calcitonin gene-related peptide regulates the expression of vascular endothelial growth factor in human HaCaT keratinocytes by activation of ERK1/2 MAPK. Regul Pept. 2006;137:134–9. doi: 10.1016/j.regpep.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Staniek V, Doutremepuich J, Schmitt D, Claudy A, Misery L. Expression of substance P receptors in normal and psoriatic skin. Pathobiology. 1999;67:51–4. doi: 10.1159/000028051. [DOI] [PubMed] [Google Scholar]
- 33.Dallos A, Kiss M, Polyanka H, Dobozy A, Kemeny L, Husz S. Effects of the neuropeptides substance P, calcitonin gene-related peptide, vasoactive intestinal polypeptide and galanin on the production of nerve growth factor and inflammatory cytokines in cultured human keratinocytes. Neuropeptides. 2006;40:251–63. doi: 10.1016/j.npep.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Yu HB, Finlay BB. The caspase-1 inflammasome: a pilot of innate immune responses. Cell Host Microbe. 2008;4:198–208. doi: 10.1016/j.chom.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–7. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katz AB, Taichman LB. A partial catalog of proteins secreted by epidermal keratinocytes in culture. J Invest Dermatol. 1999;112:818–21. doi: 10.1046/j.1523-1747.1999.00572.x. [DOI] [PubMed] [Google Scholar]
- 37.Terada K, Yamada J, Hayashi Y, Wu Z, Uchiyama Y, Peters C, Nakanishi H. Involvement of cathepsin B in the processing and secretion of interleukin-1beta in chromogranin A-stimulated microglia. Glia. 2010;58:114–24. doi: 10.1002/glia.20906. [DOI] [PubMed] [Google Scholar]
- 38.Russo R, Siviglia E, Gliozzi M, Amantea D, Paoletti A, Berliocchi L, Bagetta G, Corasaniti MT. Evidence implicating matrix metalloproteinases in the mechanism underlying accumulation of IL-1beta and neuronal apoptosis in the neocortex of HIV/gp120-exposed rats. Int Rev Neurobiol. 2007;82:407–21. doi: 10.1016/S0074-7742(07)82023-X. [DOI] [PubMed] [Google Scholar]
- 39.Lamkanfi M, Dixit VM. Inflammasomes: guardians of cytosolic sanctity. Immunol Rev. 2009;227:95–105. doi: 10.1111/j.1600-065X.2008.00730.x. [DOI] [PubMed] [Google Scholar]
- 40.Jin Y, Mailloux CM, Gowan K, Riccardi SL, LaBerge G, Bennett DC, Fain PR, Spritz RA. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med. 2007;356:1216–25. doi: 10.1056/NEJMoa061592. [DOI] [PubMed] [Google Scholar]
- 41.Church LD, Cook GP, McDermott MF. Primer: inflammasomes and interleukin 1beta in inflammatory disorders. Nat Clin Pract Rheumatol. 2008;4:34–42. doi: 10.1038/ncprheum0681. [DOI] [PubMed] [Google Scholar]
- 42.Huang S, Yuan S, Guo L, Yu Y, Li J, Wu T, Liu T, Yang M, Wu K, Liu H, Ge J, Yu Y, Huang H, Dong M, Yu C, Chen S, Xu A. Genomic analysis of the immune gene repertoire of amphioxus reveals extraordinary innate complexity and diversity. Genome Res. 2008;18:1112–26. doi: 10.1101/gr.069674.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, Bailly-Maitre B, Volkmann N, Hanein D, Rouiller I, Reed JC. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell. 2007;25:713–24. doi: 10.1016/j.molcel.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 44.Di Virgilio F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci. 2007;28:465–72. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Loram LC, Themistocleous AC, Fick LG, Kamerman PR. The time course of inflammatory cytokine secretion in a rat model of postoperative pain does not coincide with the onset of mechanical hyperalgesia. Can J Physiol Pharmacol. 2007;85:613–20. doi: 10.1139/y07-054. [DOI] [PubMed] [Google Scholar]
- 46.Salemi S, Rethage J, Wollina U, Michel BA, Gay RE, Gay S, Sprott H. Detection of interleukin 1beta (IL-1beta), IL-6, and tumor necrosis factor-alpha in skin of patients with fibromyalgia. J Rheumatol. 2003;30:146–50. [PubMed] [Google Scholar]
- 47.Angst MS, Clark JD, Carvalho B, Tingle M, Schmelz M, Yeomans DC. Cytokine profile in human skin in response to experimental inflammation, noxious stimulation, and administration of a COX-inhibitor: a microdialysis study. Pain. 2008;139:15–27. doi: 10.1016/j.pain.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 48.Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji RR, Bean BP, Woolf CJ, Samad TA. Nociceptors are interleukin-1beta sensors. J Neurosci. 2008;28:14062–73. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ren K, Torres R. Role of interleukin-1beta during pain and inflammation. Brain Res Rev. 2009;60:57–64. doi: 10.1016/j.brainresrev.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skoff AM, Zhao C, Adler JE. Interleukin-1alpha regulates substance P expression and release in adult sensory neurons. Exp Neurol. 2009;217:395–400. doi: 10.1016/j.expneurol.2009.03.022. [DOI] [PubMed] [Google Scholar]