Abstract
Scutellaria baicalensis (SB) and SB-derived polyphenols possess anti-proliferative activities in several cancers, including pancreatic cancer (PaCa). However, the precise molecular mechanisms have not been fully defined. SB extract and SB-derived polyphenols (wogonin, baicalin, and baicalein) were used to determine their anti-proliferative mechanisms. Baicalein significantly inhibited the proliferation of PaCa cell lines in a dose-dependent manner, whereas wogonin and baicalin exhibited a much less robust effect. Treatment with baicalein induced apoptosis with release of cytochrome c from mitochondria, and activation of caspase-3 and -7 and PARP. The general caspase inhibitor zVAD-fmk reversed baicalein-induced apoptosis, indicating a caspase-dependent mechanism. Baicalein decreased expression of Mcl-1, an anti-apoptotic member of the Bcl-2 protein family, presumably through a transcriptional mechanism. Genetic knockdown of Mcl-1 resulted in marked induction of apoptosis. The effect of baicalein on apoptosis was significantly attenuated by Mcl-1 over-expression, suggesting a critical role of Mcl-1 in this process. Our results provide evidence that baicalein induces apoptosis in pancreatic cancer cells through down-regulation of the anti-apoptotic Mcl-1 protein.
Keywords: Mcl-1, baicalein, apoptosis, pancreatic cancer, Bcl-2 family proteins
1. Introduction
Pancreatic cancer (PaCa) is the fourth leading cause of cancer-related death in the United States. An estimated 21,370 men and 21,770 women will be diagnosed with pancreatic cancer in the year 2010 in the United States, and the majority of these patients will die within 6 months [1]. Gemcitabine has been considered the standard chemotherapeutic agent in the treatment of PaCa. However, studies using gemcitabine either as monotherapy or as combination therapy with cytotoxic or molecular targeted agents revealed only a marginal overall survival benefit [2]. One reason for the dismal prognosis of patients with pancreatic cancers and the inefficiency of most chemo- and radio-therapeutic regimens is the strong resistance of PaCa cells to cell death. The resistance to apoptotic cell death is often caused by the up-regulation of anti-apoptotic molecules of the Bcl-2 protein family. The identification of operative ant-iapoptotic mechanisms and the development of potent and non-toxic strategies to overcome the anti-apoptotic mechanisms in PaCa cells are of utmost importance to improve the outcome of PaCa patients.
The Bcl-2 protein family consists of anti-apoptotic proteins (Bcl-2, Bcl-xL, Bcl-w, and Mcl-1) and pro-apoptotic proteins that include multi-domain molecules Bak and Bax, and the BH3-only proteins Bad, Bid, Bim, Puma, and Noxa [3]. Mcl-1 is expressed in a wide variety of cell types, and its expression is highly induced by survival and differentiation signals such as cytokines and growth factors [4]. While Mcl-1 is only weakly expressed in the exocrine pancreas [5], in PaCa Mcl-1 is over-expressed in 90% of invasive ductal adenocarcinomas [6]. Several reports describe that Mcl-1 expression is correlated with cancer cell resistance to chemotherapy and genetic inhibition of Mcl-1 induces apoptosis and enhances drug- and radiation-mediated apoptosis in several types of cancer, including PaCa [7–10].
Scutellaria Baicalensis (SB) is a member of the Lamiaceae or mint family and is known as Chinese skullcap (or Huang Qin) and as Japanese Ogon. SB is a widely used herb in traditional Chinese and Japanese medicine with anti-inflammatory properties [11]. The root is rich in flavonoids with over 50 different compounds currently identified. It has been reported that SB or SB-derived polyphenols, particularly baicalein and wogonin, have potent anti-tumor activity in several cancers [12–16]. Baicalein was recently reported to reduce Bcl-2 and Mcl-1 protein expression in PaCa cells [15], but the precise molecular mechanism and contribution of Mcl-1 to PaCa cell survival in general and to the effect of baicalein was not investigated.
In this study, we provide evidence that baicalein induces apoptosis by reducing the expression of the pro-survival Mcl-1, at least partially via a transcriptional mechanism. Furthermore, our data highlight the importance of Mcl-1 as a crucial mediator of PaCa cell resistance to cell death.
2. Materials and methods
2.1. Reagents
Antibodies against caspase-3 (total and cleaved), caspase-7 (total and cleaved), PARP (total and cleaved), Bcl-2, Bcl-xL, Mcl-1, Bad, Bim, Bid, PUMA, Bax, cytochrome c, cytochrome c oxidase subunit IV (cox-IV), and GAPDH were purchased from Cell Signaling Technology (Danvers, MS). Bak antibodies were obtained from Abcam (Cambridge, MA). The pan-caspase inhibitor zVAD-fmk was from Calbiochem (Gibbstown, NJ). The proteasome inhibitor MG132 was from Assay Designs (Ann Arber, MI). SB extract (powder) was from CORTEX Scientific Botanicals (Ojai, CA). Baicalin, baicalein, and wogonin were purchased from ChromaDex (Irvine, CA). Other reagents were obtained from common commercial sources.
2.2. Cell culture
The human PaCa cell lines, BxPC-3, HPAF-II, Capan-2, AsPc-1, MIA PaCa-2, and Panc-1 were obtained from the American Type Culture Collection (ATCC, Rockville, MD) and cultured as described previously [17].
2.3. Preparation of SB extract and SB-derived polyphenols
The SB powder was dissolved in serum-free medium as described previously [12]. Baicalin, baicalein, and wogonin were dissolved in DMSO (Sigma) to produce 50 mM stock solutions that were aliquoted and stored at −20 °C prior to use. The stock solutions were diluted with culture media to the indicated concentrations (final DMSO concentration 0.1%).
2.4. Cell proliferation
To examine the effect of SB and SB-derived polyphenols on cell proliferation, we used 3-(4,5-dimethlthiazol-2-yl)-2,5-diphenyltetrazolim bromide (MTT) assay and 5-bromo-2’-deoxy-uridine (BrdU) Labeling and Detection Kit III (Roche Applied Science, Mannheim, Germany). Cells (1 × 104/well) were seeded into 96-well tissue culture plates and incubated at 37 °C overnight. Medium was changed to serum-free condition and incubated for another 4 h. Cells were subsequently treated with SB or SB-derived polyphenols for 48 h in serum-free medium. Absorbance was measured according to the manufacturer’s instructions.
2.5. DNA fragmentation
DNA fragmentation was measured as described previously [18]. Briefly, cells were seeded into 6-well culture plates and incubated at 37 °C overnight. Medium was changed to serum-free condition and incubated for another 4 h. Cells were then treated with baicalein for 24 h. DNA fragmentation was analyzed by the Cell Death Detection ELISAPlus kit (Roche) according to the manufacturer’s instructions. The extent of apoptosis was presented as an enrichment factor (mU of the treated cells / mU of the non-treated cells).
2.6. Hoechst 33258 staining
To assess changes in nuclear morphology during apoptosis, staining using fluorescent Hoechst 33258 (Invitrogen, Carlsbad, CA) was performed. Cells were seeded into a Lab-Tek®II Chamber Slide™ System (Nalgene Nunc International, Naperville, IL). Baicalein was added to the medium and incubated for 24 h. Cells were fixed with 4% paraformaldehyde and stained with Hoechst 33258. Slides were mounted with VECTASHIELD (Vector Laboratories Inc., Burlingame, CA), and were observed under a fluorescence microscope (Nikon, Eclipse 90i, Tokyo, Japan). Apoptotic nuclei were identified by morphologic changes such as chromatin condensation and nuclear fragmentation. Total nuclei and apoptotic nuclei were counted respectively by NIS-Elements AR software (Nikon). This method allows the assessment of apoptosis through identification of nuclear changes and avoids possible artifacts in membrane morphology caused by cell detachment, which is detected by Annexin/PI and flow cytometry.
2.7. Cell lysates
Total cell lysates were collected as described previously [19]. Protein concentration was measured using the BCA Protein Assay Kit (Pierce, Rockford, IL). For preparation of mitochondrial and cytosolic fractions, cells were re-suspended in a lysis buffer as described previously [20], allowed to swell for 30 min at 4 °C, and then disrupted by 80 strokes with a Dounce homogenizer. Homogenates were centrifuged at 1,000 ×g for 5 min to pellet nuclei and cell debris. Supernatants were centrifuged at 16,000 ×g for 30 min, and the cytosolic fractions (supernatants) were collected. Pellets (heavy membranes enriched with mitochondria) were lysed in RIPA buffer. To determine the quality of cytosolic and mitochondrial separation, both fractions were assessed by immunoblotting for the mitochondrial marker cox-IV.
2.8. Western blot analysis
Western Blot was performed as described previously [21]. Protein-antibody complexes were visualized with the SuperSignal West Pico or SuperSignal West Femto Chemiluminescent Substrate (Pierce).
2.9. Caspase 3 and 7 activity
Caspase-3 and -7 activity was measured by the caspase-Glo™ 3/7 assay (Promega, Madison, WI). Cells (1×104/well) were seeded into 96-well tissue culture plates and incubated overnight at 37 °C. Cells were treated with the indicated doses of baicalein for 24 h. 100 µl of the caspase-Glo™ 3/7 reagent was added to each well and incubated at room temperature for 30 min, and the luminescence in each well was measured using a luminometer.
2.10. Immunoprecipitation
Cells were lysed in NP-40 buffer (150 mM sodium chloride, 1% NP-40, 50 mM Tris-HCl, protease inhibitor cocktail) for 30 min on ice and clarified by centrifugation at 16,000 ×g for 15 min at 4°C. 500 µg of protein was subjected to overnight immunoprecipitation with specific antibodies at 4°C using Catch and Release Reversible Immunoprecipitation kit (Millipore, Billerica, MA). Unbound proteins were washed and bound proteins on the column were eluted, after which samples were loaded for Western blot.
2.11. RNA interference
siGENOME SMARTpool against Bcl-2, Bcl-xL, Mcl-1, and Bad as well as siGENOME Non-Targeting Pool (#2) were purchased from Dharmacon (Lafayette, CO). Cells were seeded into 6-well tissue culture plates and incubated overnight at 37 °C without antibiotics. The following day, cells were transfected with specific siRNA using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions. Five hours after transfection, medium was changed to serum free DMEM or RPMI medium and incubated for another 24 h.
2.12. RNA isolation and quantitative real-time RT-PCR
Total RNA was extracted as described previously [22]. RNA (1 µg) was reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad). Real time RT-PCR was performed on an iQ5 Real-Time PCR Detection System (Bio-Rad, Hercules, CA) using iQ™ SYBR Green Supermix (Bio-Rad) and specific intron-spanning primers. Primer pairs were as follows: Mcl-1 (accession number: NM_021960) forward: TTAAACAAAGAGGCTGGGATG (exon 2–3) and reverse: ACCAGCTCCTACTCCAGCAA (exon 3), GAPDH (accession number: NP_002037) forward: GACATCAAGAAGGTGGTGAAGC (exon 8) and reverse: GTCCACACCCTGTTGCTGTAG (exon 9). Amplification of unspecific products was excluded by melt curve analysis and agarose gel electrophoresis of PCR products. Gene expression was normalized to GAPDH as a housekeeping gene and calculated using the ΔΔCT method.
2.13. Plasmid vector transfection
BxPC-3 cells were seeded into 6-well tissue culture plates and incubated overnight at 37 °C without antibiotics. The following day, cells were transfected with plasmid vector encoding human Mcl-1 [23] (pCDNA3.1-hMcl-1, Addgene) or control vector (pCDNA3.1, Invitrogen) using Lipofectamine 2000 (Invitrogen) according to manufacturer’s instructions. Five hours after transfection, medium was changed to 10% serum DMEM or RPMI medium and incubated for another 48 h. Stable lines were selected by adding G418 (Sigma) into the medium.
2.14. Statistical analysis
Data are presented as means ± SD, and statistical comparisons were made using the Student’s t test for paired observations. Comparisons of more than two groups were made by a one-way ANOVA with post hoc Holm-Sidak analysis for pairwise comparisons and comparisons versus control. An alpha value of 0.05 was used to determine significant differences. All statistics were done in SigmaStat 3.1 (Systat Software, Inc.).
3. Results
3.1. SB and SB-derived polyphenols reduce proliferation of pancreatic cancer cells
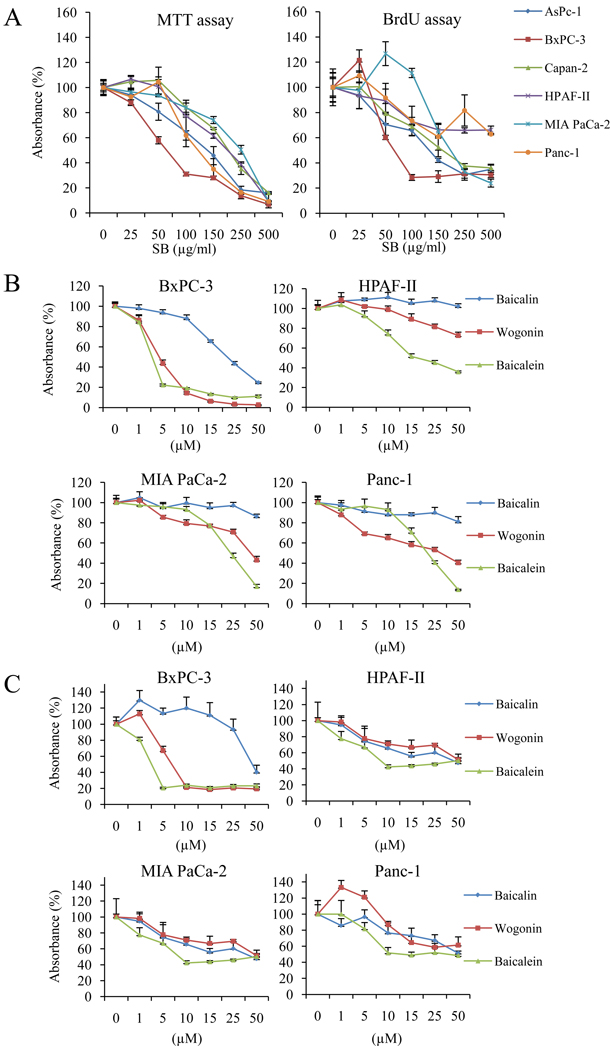
To confirm whether SB and SB-derived polyphenols can inhibit cell growth in PaCa cells, six PaCa cell lines were exposed to whole SB extract and SB-derived polyphenols for 48 h and cell growth was measured by MTT and BrdU assay. Whole SB extract reduced proliferation of all six PaCa cell lines (with varying degrees of differentiation) in a dose-dependent manner in both assays (Fig. 1A). We then compared the growth inhibitory effects of three individual SB-derived polyphenols (wogonin, baicalin, and baicalein) in four PaCa cell lines (ranging from undifferentiated to moderately/well differentiated cell lines). In both MTT and BrdU assays, baicalein displayed the most robust growth inhibitory effect compared to wogonin and baicalin (Fig. 1B and C). This effect was most prominent in BxPC-3 cells.
Figure 1.
The effect of SB and SB-derived polyphenols on cell proliferation. A, six human pancreatic cancer cell lines were treated with the indicated concentrations of SB extract for 48 h and cell proliferation was measured by MTT assay (left) and BrdU assay (right). Results are presented as mean ± SD (bars). Four human pancreatic cancer cells were then exposed to three purified polyphenols (baicalin, baicalein, and wogonin) for 48 h and cell proliferation was measured by MTT assay (B) and BrdU assay (C).
3.2. Baicalein induces apoptosis in pancreatic cancer cells
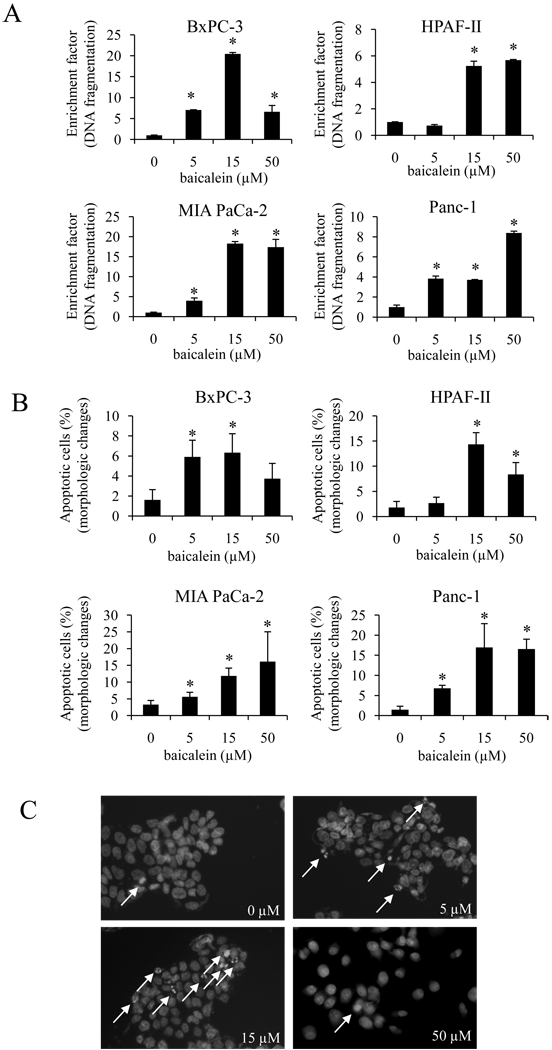
To elucidate the mechanism, by which baicalein reduces PaCa cell growth, we determined whether the decrease in cell growth by baicalein was mediated by the induction of apoptotic cell death. We first measured DNA fragmentation, a hallmark of apoptosis. As shown in Fig. 2A, baicalein induced DNA fragmentation in a dose-dependent manner in all four cell lines. Induction of apoptosis was further confirmed by Hoechst 33258 staining. Baicalein significantly and dose-dependently led to morphological changes in cell nuclei (chromatin condensation and nuclear fragmentation) consistent with DNA fragmentation and apoptosis (Fig. 2B and C).
Figure 2.
The effect of baicalein on cell apoptosis. A, cells were seeded in 6-well plates and treated with baicalein for 24 h, after which DNA fragmentation was measured. Increase in apoptosis (DNA fragmentation) is presented as enrichment factor. *p<0.05. B, changes in nuclear morphology were measured by Hoechst 33258 staining as described in materials and methods. *p<0.05. C, representative images of Hoechst 33258 staining in BxPC-3 cells. Arrowhead show apoptotic cells as identified by morphologic changes such as chromatin condensation and nuclear fragmentation.
3.3. Baicalein induces apoptosis through a caspase-dependent pathway
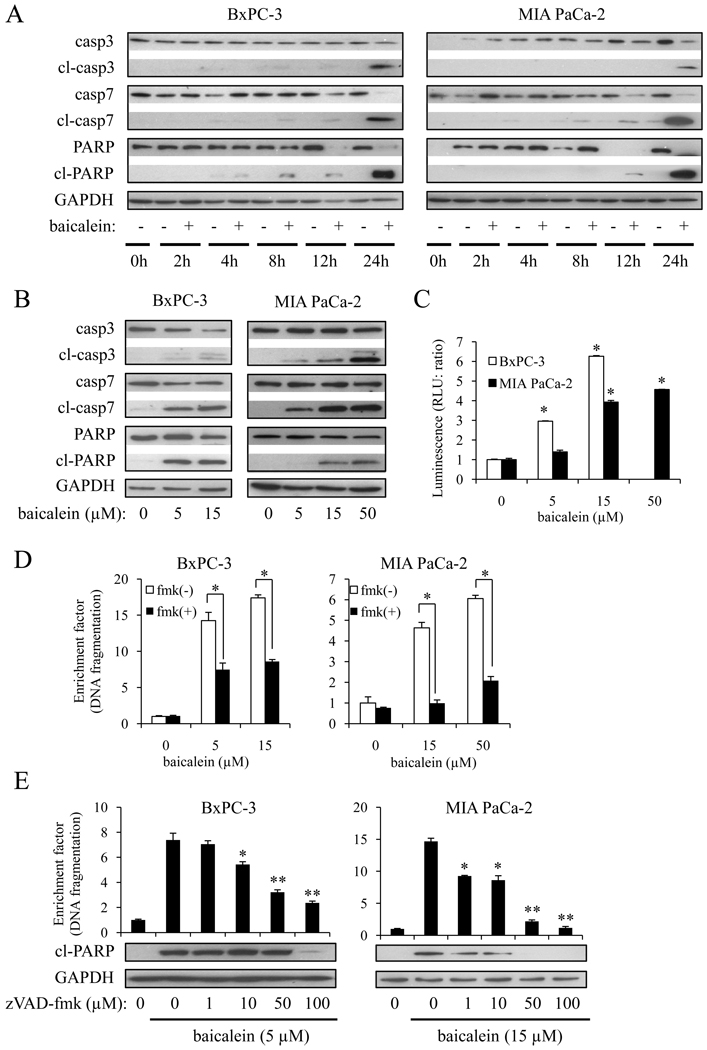
Having demonstrated that baicalein induced apoptosis, we next investigated whether this effect was mediated by activation of caspases. Exposure of BxPC-3 or MIA PaCa-2 cells to baicalein resulted in a time-dependent cleavage of caspase-3, -7, and PARP (Fig. 3A). These events were readily observed after 8–12 h of treatment and peaked at 24 h. Baicalein also dose-dependently induced cleavage of caspase-3, -7, and PARP (Fig. 3B). We further confirmed caspase-3/7 activity by ELISA (Fig. 3C), which was consistent with the Western blot results. Furthermore, the pan-caspase inhibitor zVAD-fmk reversed baicalein-induced DNA fragmentation and cleavage of PARP (Fig. 3D and E) in a dose-dependent manner, indicating that the induction of apoptosis by baicalein in PaCa cells proceeds through the activation of caspases.
Figure 3.
Baicalein induced apoptosis through a caspase-dependent mechanism. A, cells were treated with 5 µM (BxPC-3) or 15 µM (MIA PaCa-2) of baicalein for the indicated times. The expression of (cleaved) caspase-3, -7, and PARP were measured by Western blot. B, cells were treated with baicalein for 24 h and the expression of (cleaved) caspase-3, -7, and PARP were measured. C, caspase-3 and -7 activity was measured by caspase-Glo™ 3/7 assay. Results are presented as means ± SD (bars). *p<0.01. D, cells were pre-treated with the pan-caspase inhibitor zVAD-fmk (100 µM) for 1 h followed by the indicated concentrations of baicalein for another 24 h. The extent of apoptosis was quantified by Cell Death ELISA and presented as enrichment factor (DNA fragmentation). *p<0.005 vs. cells in the absence of zVAD-fmk. E, cells were pre-treated with the indicated concentrations of zVAD-fmk for 1 h followed by 5 µM (BxPC-3) or 15 µM (MIA PaCa-2) of baicalein for another 24 h. The extent of apoptosis as presented as enrichment factor (DNA fragmentation; upper panel) and the cleavage of PARP (lower panel) were quantified. *p<0.05, **p<0.01 vs. cells treated with baicalein in the absence of zVAD-fmk.
3.4. Baicalein reduces the expression of the anti-apoptotic Bcl-2 family member Bcl-2 and Mcl-1
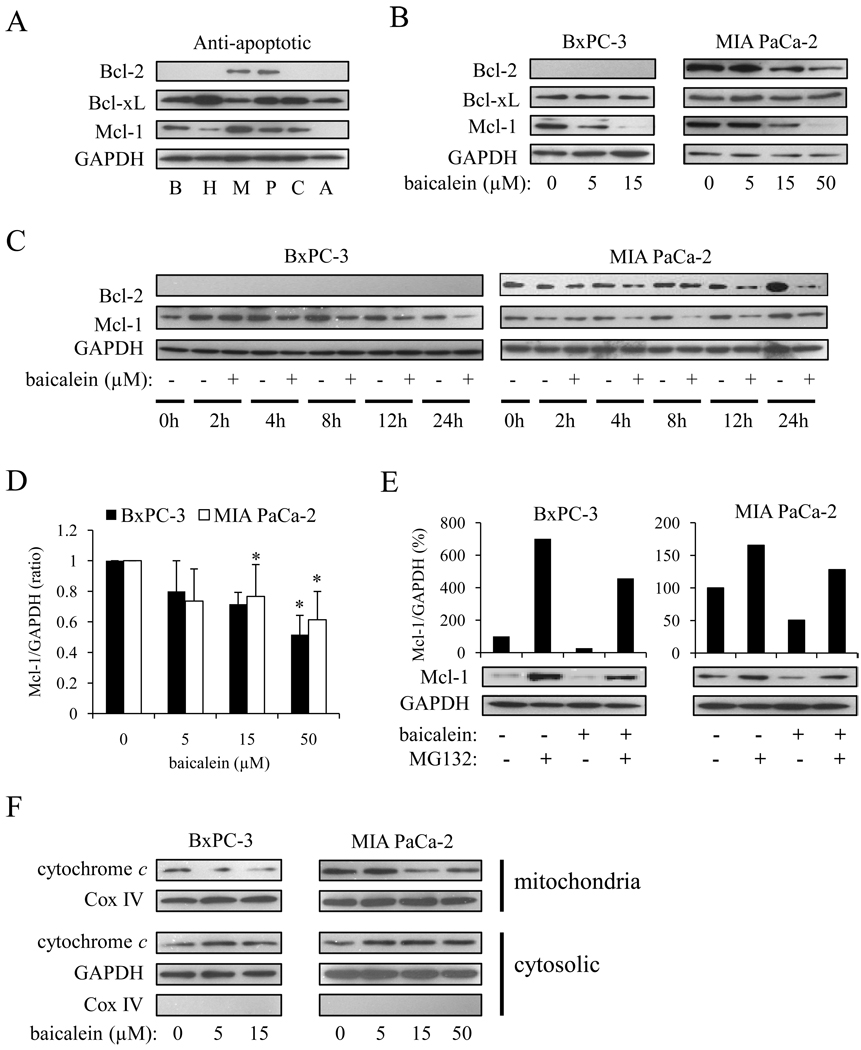
Since the anti-apoptotic Bcl-2 family proteins play a major role in the protection against DNA damage-induced apoptosis [23], we sought to determine the endogenous expression of anti-apoptotic Bcl-2 family proteins, as well as the effect of baicalein on anti-apoptotic Bcl-2 family proteins. Mcl-1 was detected in BxPC-3, MIA PaCa-2, HPAF-II, Capan-2, and Panc-1 cells, but was not detected in AsPc-1 cells (Fig. 4A). Bcl-2 was expressed in MIA PaCa-2 and Panc-1 cells, but virtually undetectable in other cell lines. Bcl-xL was expressed in all six cell lines.
Figure 4.
Baicalein modulated expression of a subset of anti-apoptotic Bcl-2 family proteins and induced cytochrome c release from mitochondria. A, endogenous expression of anti-apoptotic Bcl-2 family proteins in BxPC-3 (B), HPAF-II (H), MIA PaCa-2 (M), Panc-1 (P), Capan-2 (C), and AsPc-1 (A) cells. B, cells were treated with baicalein for 24 h and changes in anti-apoptotic Bcl-2 family protein expression were measured. C, cells were treated with 5 µM (BxPC-3) or 15 µM (MIA PaCa-2) baicalein for the indicated times and the expression of Mcl-1 and Bcl-2 were measured. D, cells were treated with baicalein for 2 h, after which total RNA was isolated and Mcl-1 mRNA was quantified by real time RT-PCR. Values represent the means ± SD for three independent experiments performed in triplicate and are expressed as a fold increase relative to the non-treated cells. *p<0.01. E, cells were pre-treated with the proteasome inhibitor MG132 (0.2 µM) for 1 h, followed by 5 µM (BxPC-3) or 15 µM (MIA PaCa-2) baicalein for another 6 h. Mcl-1 protein expression was measured by Western blot (lower panel) and the signal density measured by Image J software and was expressed as Mcl-1/GAPDH (%) (upper panel). F, cells were treated with the indicated concentrations of baicalein for 12 h, after which mitochondrial and cytosolic fraction were separated as described in materials and methods. Levels of cytochrome c in each subfraction were measured by Western Blot. Cox IV and GAPDH served as loading controls for mitochondrial and cytosolic fraction, respectively.
Baicalein significantly reduced the expression of Bcl-2 and Mcl-1 in a dose-dependent manner, but had no effect on Bcl-xL expression (Fig. 4B). The doses of baicalein needed to reduce Mcl-1 and Bcl-2 expression closely matched those sufficient to induce apoptosis. To determine the kinetics of baicalein, we measured the time-dependent decrease in Mcl-1 and Bcl-2 expression. The decrease in Mcl-1 expression in BxPC-3 and MIA PaCa-2 cells was detected after 4 h of baicalein treatment, whereas the reduction of Bcl-2 in MIA PaCa-2 cells was only detectable after 12 h of treatment (Fig. 4C). This is consistent with previous studies showing that Mcl-1 mRNA and protein exhibit a short half-life and the expression levels depend on the balance of de novo synthesis and degradation [25, 26].
It has been reported that Mcl-1 expression is regulated at the transcriptional level by a variety of transcriptional factors [27, 28]. In addition, post-translational regulation of Mcl-1 expression, e.g. through proteosomal degradation, has been documented [29]. To investigate the transcriptional regulation of Mcl-1, we performed real-time RT-PCR. Baicalein treatment dose-dependently reduced Mcl-1 mRNA levels (Fig. 4D). In addition, the proteosome inhibitor MG132 (0.2 µM for 1 h) increased Mcl-1 protein expression, suggesting a post-transcriptional regulation of Mcl-1 expression through proteosomal degradation. Importantly, baicalein was capable of reducing Mcl-1 protein expression in MG132-treated cells (Fig. 4E). Collectively, these results strongly suggest that the reduction of Mcl-1 expression by baicalein involves at least a transcriptional mechanism. In addition, baicalein induced cytochrome c release from mitochondria after 12 h treatment (Fig. 4F).
3.5. Apoptosis-inducing effect of baicalein is mediated by Mcl-1
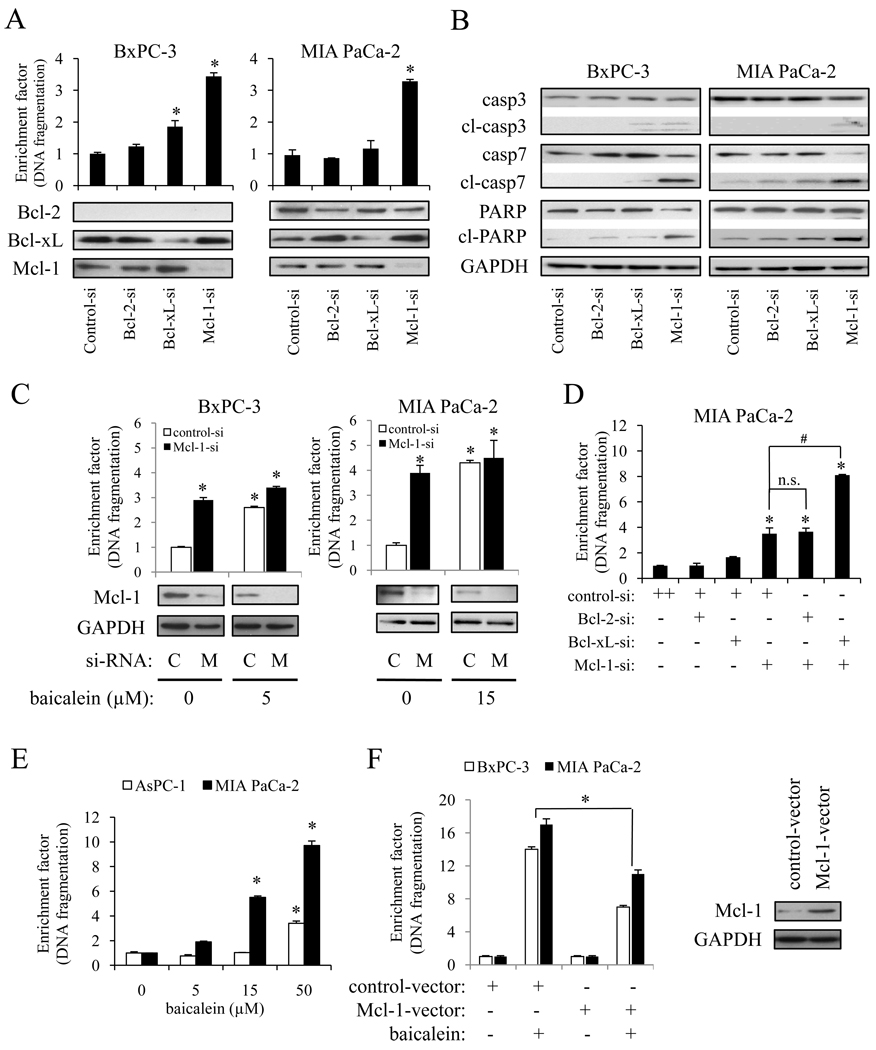
Since we observed a close correlation between baicalein-induced apoptosis and decrease in Mcl-1 expression, we sought to determine the role of Mcl-1 in PaCa cell survival and baicalein-induced apoptosis in more detail. As shown in Fig. 5A, knock-down of Mcl-1 expression by siRNA significantly induced apoptosis, whereas Bcl-2 or Bcl-xL knock-down had a much weaker effect on apoptosis. In addition, Mcl-1 knock-down strongly induced cleavage of caspase-3, -7 as well as PARP, while Bcl-2 or Bcl-xL knock-down showed no or only a slight effect on caspase and PARP cleavage (Fig. 5B).
Figure 5.
Role of Mcl-1 in baicalein-induced apoptosis. Cells were transiently transfected with specific siRNA against Bcl-2, Bcl-xL, Mcl-1, or control siRNA for 24 h. Anti-apoptotic Bcl-2 family protein expression (A, lower panel) and DNA fragmentation (A, upper panel), as well as expression of total and cleaved caspases (B) were measured by Western blot and Cell Death ELISA. C, cells were transiently transfected with siRNA against Mcl-1 (M) or control siRNA (C) for 24 h and then treated with the indicated concentration of baicalein for another 24 h. The extent of apoptosis (upper panel) and Mcl-1 protein expression (lower panel) were measured. *p<0.01 vs. control si-RNA and baicalein 0 µM. D, MIA PaCa-2 cells were transiently transfected with siRNA against Mcl-1, Bcl-2, Bcl-xL alone or in combination for 24 h and the extent of apoptosis was measured and presented as enrichment factor (DNA fragmentation). *p<0.01 vs. control-si alone; #p<0.01; n.s.=not significant. E, AsPC-1 and MIA PaCa-2 cells were dose-dependently treated with baicalein for 24 h, after which DNA fragmentation was measured. *p<0.05 vs. baicalein 0 µM. F, BxPC-3 and MIA PaCa-2 cells were transfected with Mcl-1 or control DNA for 24 h, after which DNA fragmentation was measured (left panel). Right panel illustrates successful overexpression of Mcl-1 in BxPC-3 cells. *p <0.05
Next, we investigated whether the down-regulation of Mcl-1 is the principal mechanism of baicalein-induced apoptosis. Cells were treated for 24 h with the indicated concentrations of baicalein 24 h after siRNA transfection. Baicalein induced apoptosis 2.6-fold in BxPC-3 cells transfected with control siRNA, while in BxPC-3 cells transfected with Mcl-1 siRNA, baicalein induced apoptosis only by 1.2-fold (p<0.01). Similarly, in MIA PaCa-2 cells, baicalein induced apoptosis in control siRNA and Mcl-1 siRNA transfected cells by 4.7 and 1.3-fold (p<0.01), respectively (Fig. 5C). These data clearly suggest that the apoptosis-inducing effect of baicalein is mediated by Mcl-1.
Since siRNA-mediated knock-down of Bcl-2 did not induce apoptosis, but baicalein reduced Bcl-2 as well as Mcl-1in MIA PaCa-2 cells, we measured the effect of simultaneous knock-down of Mcl-1 and Bcl-2 in MIA PaCa-2 cells. However, there was no additional effect compared to Mcl-1 knock-down alone (Fig. 5D). These results suggest that Bcl-2 down-regulation by baicalein did not play an important role on baicalein-induced PaCa apoptosis. In addition, double knock-down of Mcl-1 and Bcl-xL showed a more robust induction of apoptosis than Mcl-1 knock-down alone, while single knock-down of Bcl-xL did not significantly induced apoptotic cell death in MIA PaCa-2 cells (Fig. 5D).
Since the expression of Mcl-1 in AsPC-1 cells was not detected, we used AsPC-1 cells to further confirm the importance of Mcl-1 in protection against baicalein-induced apoptosis. As shown in Fig. 5E, the apoptosis-inducing effect of baicalein in AsPC-1 cells was much less robust than in Mcl-1 positive MIA PaCa-2 cells. Finally, we generated stable, Mcl-1 over-expressing BxPC-3 and MIA PaCa-2 cells as described in materials and methods. In control-vector transfected BxPC-3 and MIA PaCa-2 cells, baicalein induced DNA fragmentation by 13.5±0.3 and 17.1±0.2, respectively. On the other hand, baicalein induced DNA fragmentation in Mcl-1 over-expressing BxPC-3 and MIA PaCa-2 cells significantly less robust by 7.6±0.1 and 10.8±0.3, respectively (Fig. 5F). Taken together, these results further emphasize the critical role of Mcl-1 on baicalein-induced apoptosis in PaCa.
3.6. The effect of baicalein on pro-apoptotic Bcl-2 proteins
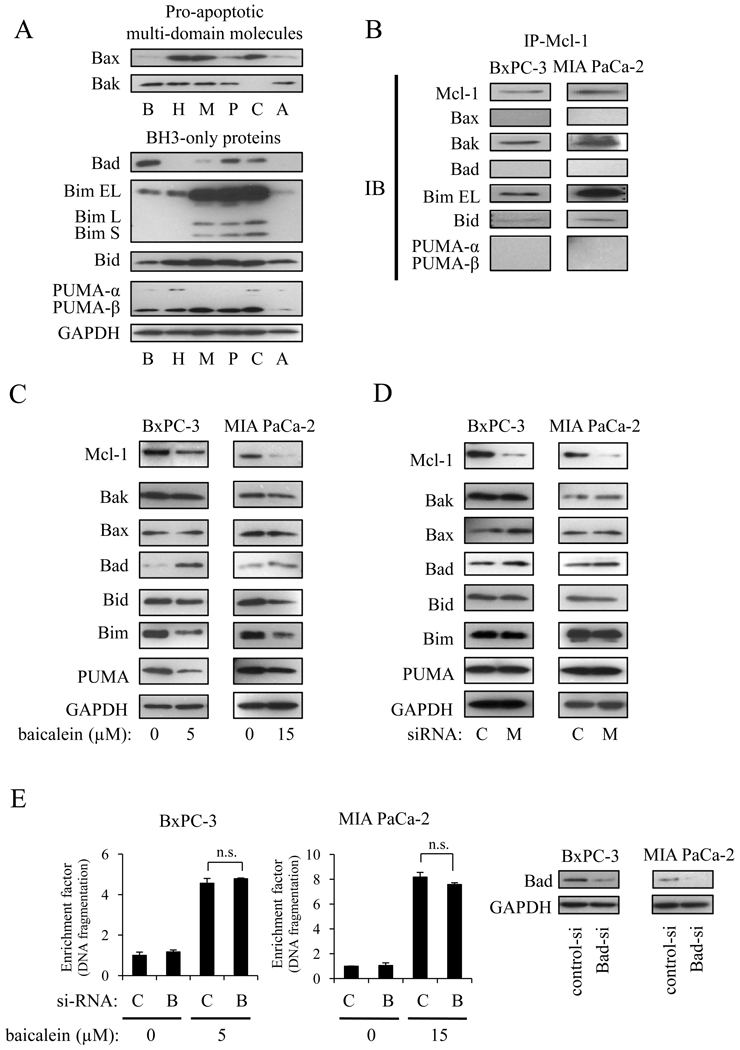
Since it has been reported that the pro-apoptotic Bcl-2 family proteins control cell death cooperatively with anti-apoptotic Bcl-2 family proteins, we next measured which pro-apoptotic Bcl-2 family proteins may play a role on baicalein-induced apoptosis. In this context, we first measured the endogenous expression of pro-apoptotic Bcl-2 family proteins. The expression of pro-apoptotic Bcl-2 family proteins varied between the cell lines and was in no correlation with baicalein sensitivity (Fig. 6A). Immunoprecipitation showed that Mcl-1 endogenously binds to Bim, Bak, and Bid, whereas no binding with Bax, Bad, and PUMA was detected (Fig. 6B). Next, we measured the effect of baicalein on the expression of pro-apoptotic Bcl-2 family proteins. Baicalein clearly reduced Bim and PUMA expression, whereas it induced Bad expression. Increased Bad expression by baicalein was thereby associated with increased Bad phosphorylation (Ser112), which is important for translocation of Bad from the mitochondria to the cytosol (not shown). No changes of Bak, Bax, and Bid expressions were observed (Fig. 6C). To confirm whether the effect of baicalein on pro-apoptotic protein expression correlated with Mcl-1 down-regulation, cells were transfected with Mcl-1 siRNA, after which changes in pro-apoptotic proteins were measured. Of note, Mcl-1 knock-down did not change expression of any pro-apoptotic proteins (Fig. 6D), suggesting that the effect of baicalein on pro-apoptotic proteins did not correlate with Mcl-1down-regulation. Finally, Bad knock-down by siRNA had no effect on baicalein-induced apoptosis (Fig. 6E). This together with the increased phosphorylation of Bad by baicalein (thereby counteracting the pro-apoptotic function of Bad) strongly indicated that the pro-apoptotic effect of baicalein did not involve Bad.
Figure 6.
Changes in expression of pro-apoptotic Bcl-2 family proteins by baicalein. A, endogenous expression of pro-apoptotic multi-domain and BH3-only proteins in BxPC-3 (B), HPAF-II (H), MIA PaCa-2 (M), Panc-1 (P), Capan-2 (C), and AsPc-1 (A) cells. B, endogenous binding between Mcl-1 and Bcl-2 family proteins were measured by immunoprecipitation assay. C, cells were treated with the indicated concentrations of baicalein for 24 h and changes in the expression of pro-apoptotic Bcl-2 family proteins were measured. D, cells were transiently transfected with Mcl-1 (M) or control (C) siRNA for 24 h, after which changes in the expression of pro-apoptotic Bcl-2 family proteins were measured. E, cells were transiently transfected with control siRNA (C) or Bad siRNA (B) for 24 h and then treated with the indicated concentration of baicalein for another 24 h. The extent of apoptosis (left panels) and Bad protein expression (right panels) were measured by Cell Death ELISA and Western Blot. n.s.=not significant
4. Discussion
Previous reports have demonstrated that SB and SB-derived polyphenols reduce the growth of several cancer cell lines in vitro [12–16], but their anti-proliferative effect in PaCa cells is less explored. The results of this study showed that SB extract and all SB-derived polyphenols reduced the proliferation of PaCa cell lines in a dose-dependent manner. While baicalin (10% of raw material, ref. 4) had only a minimal effect on PaCa growth, its aglycone baicalein (5% of raw material) had the most potent growth inhibitory effect, consistent with a previous report in multiple myeloma cell lines [16]. In general, the growth-inhibitory effect was more clearly seen in the BrdU assay, which detects changes in DNA synthesis, compared to the MTT assay, which measures cell viability. Although baicalein was most effective in Kras wildtype BxPC-3 cells, it is important to note that it also inhibited growth of Kras mutated pancreatic cancer cells at concentrations that can be achieved in vivo in mice fed a diet supplemented with 1% SB extract for 8 weeks (manuscript submitted). Furthermore, Bonham et al. reported that most of the activity seen with the SB extract could be attributed to baicalein in prostate cancer cells [14]. Based on these reports and our study, we decided to investigate the mechanisms of baicalein-induced growth inhibition in PaCa cells.
Anti-apoptotic Bcl-2 family proteins play an important role in response to a variety of death stimuli. Mcl-1 is one of the anti-apoptotic Bcl-2 family proteins, and its over-expression has been reported in a variety of hematopoietic, lymphoid, and some solid tumors including PaCa [6, 28]. In these cancers, Mcl-1 plays a key role in the resistance to conventional chemotherapy. Importantly, some papers showed that Bcl-2 or Bcl-xL knock-down by siRNA is not enough to promote apoptosis, but Mcl-1 knock-down alone can clearly induce apoptosis in melanoma and chronic lymphocytic leukemia cells [30, 31]. From these reports, Mcl-1 is considered to be a promising therapeutic target in several cancers, but the importance of Mcl-1 in PaCa is unknown. In this context, we first confirmed the expression of anti-apoptotic Bcl-2 family proteins in six PaCa cell lines ranging from undifferentiated to well differentiated cell lines. Mcl-1 was expressed in five PaCa cell lines, but Bcl-2 was detected in only MIA PaCa-2 and Panc-1 cells. Miyamoto et al. reported that Bcl-2 protein was strongly expressed in MIA PaCa-2 and Panc-1 cells [6]. Others showed that Bcl-2 protein was strongly expressed in BxPC-3 cells, but weakly expressed in Panc-1 cells [32]. The exact reasons for this discrepancy are unclear, but different culture conditions, cell passages, and different antibodies may be potential causes. We carefully explored which anti-apoptotic Bcl-2 family proteins play a critical role on PaCa apoptosis by siRNA-mediated knock-down of Bcl-2, Bcl-xL, and Mcl-1. We confirmed a marked induction of apoptosis following knock-down of Mcl-1, whereas Bcl-2 and Bcl-xL knock-down resulted only in minimal induction of apoptosis. However, double knock-down of Mcl-1 and Bcl-xL in MIA PaCa-2 cells had a significantly more robust effect on apoptosis than Mcl-1 knock-down alone. Among the anti-apoptotic Bcl-2 family proteins, Mcl-1 seems to play the most critical pro-survival role in pancreatic cancer cells (as evident by the single knock-down of each protein). However, once Mcl-1 is knocked-down, additional reduction of other anti-apoptotic Bcl-2 family members, such as Bcl-xL, greatly elevates the induction of cell death. To our knowledge, this is the first report comparing the effect of three anti-apoptotic Bcl-2 family proteins in PaCa.
In this study, we demonstrated that baicalein treatment significantly induced apoptosis through a caspase-dependent mechanism. We observed a close correlation between baicalein-induced apoptosis and decrease in Mcl-1 expression, and over-expression of Mcl-1 confers resistance against baicalein treatment. It is of note that baicalein did not change expression of Bcl-xL proteins in any pancreatic cancer cell line. In addition, genetic knock-down of Mcl-1 by siRNA significantly abrogated the apoptosis-inducing effect of baicalein, suggesting that the pro-apoptotic effect of baicalein was mediated through reduction of Mcl-1. However, baicalein still had an additional (albeit significantly less robust) effect on apoptosis in cells, in which Mcl-1 was knocked-down. The reasons for this phenomenon may include the lack of complete knock-down of Mcl-1 by siRNA and consequently an additional effect of baicalein on the remaining Mcl-1 expression, or alternative pathways (independent of Mcl-1) of apoptosis induction by baicalein.
BH3-only proteins are classified in activator BH3-only protein and sensitizer BH3-only protein [3]. Activator BH3-only proteins can directly activate pro-apoptotic multi-domain molecules Bak/Bax, but the effect of sensitizer BH3-only protein is indirect; sensitizer BH3-only proteins lower the threshold of apoptosis by occupying the binding pocket of anti-apoptotic proteins, such as Bcl-xL or Bcl-2 [3]. In this report, baicalein reduced Bim and PUMA, and induced sensitizer BH3-only protein Bad. However Bad knock-down by siRNA did not reverse baicalein-induced apoptosis, indicating that Bad is not involved in baicalein-induced apoptosis. In addition to the increased expression of Bad, baicalein also increased phosphorylation of Bad on Ser112, which is important for translocation of Bad from the mitochondrial membrane to the cytosol, thereby inactivating the pro-apoptotic function of Bad. Again, this finding suggests that the pro-apoptotic effects of baicalein in pancreatic cancer cells do not involve activation of Bad. We observed a strong binding between Mcl-1 and Bak in PaCa cells. Bak has been reported to play a critical role on UV- or chemotherapeutic agent-induced apoptosis. To clarify the involvement of Bak in this setting, further studies are necessary to delineate the role of Bak in baicalein-induced apoptosis in PaCa cells. Our results suggest the following possible scenario: In untreated pancreatic cancer cells, Mcl-1 exerts a strong survival function by binding to pro-apoptotic Bcl-2 molecules, i.e. Bak. Baicalein leads to reduced expression on Mcl-1, thereby possibly freeing pro-apoptotic Bcl-2 proteins, i.e. Bak, from their suppression by Mcl-1.
Mcl-1 is considered as a promising therapeutic target because of its short half-life and multiple mechanisms regulating its expression. So far, some therapeutic approaches have been reported to abrogate the anti-apoptotic function of Mcl-1. It is reported that some BH3 mimetic, such as obatoclax, can bind to and disable Bcl-2 family proteins, including Mcl-1 [33]. Recently, the multi-kinase inhibitor sorafenib has been reported to down-regulate Mcl-1 protein expression through a translational mechanism [34]. Furthermore, certain natural products, such as piceatannol, have been shown to reduce Mcl-1 protein expression [35]. Based on our data, we propose that baicalein may be a therapeutic tool targeting Mcl-1. Importantly, SB and SB-derived polyphenols are known to have almost no or very minimal toxicity in vitro and in vivo [11], rendering them intriguing drug candidates.
In summary, we demonstrated that the pro-apoptotic effect of baicalein in PaCa cells is mediated through reducing the expression of the pro-survival protein Mcl-1, at least partially via a transcriptional mechanism. Mcl-1 is highly expressed in PaCa cell lines and down-regulation of Mcl-1 by siRNA showed a strong pro-apoptotic effect. These data suggest that baicalein may represent a promising therapeutic agent in PaCa and may have a beneficial value as a sensitizing agent for standard chemotherapeutic drugs by targeting a major survival molecule.
Acknowledgments
Financial support: This study was supported by the National Institutes of Health (P01AT003960, R01CA122042)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010, CA. Cancer J. Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Hochster HS, Haller DG, de Gramont A, Berlin JD, Philip PA, Moore MJ, Ajani JA. Consensus report of the international society of gastrointestinal oncology on therapeutic progress in advanced pancreatic cancer. Cancer. 2006;107:676–685. doi: 10.1002/cncr.22036. [DOI] [PubMed] [Google Scholar]
- 3.Danial NN. Clin. BCL-2 family proteins: critical checkpoints of apoptotic cell death. Cancer Res. 2007;13:7254–7263. doi: 10.1158/1078-0432.CCR-07-1598. [DOI] [PubMed] [Google Scholar]
- 4.Craig RW. MCL1 provides a window on the role of the BCL2 family in cell proliferation, differentiation and tumorigenesis. Leukemia. 2002;16:444–454. doi: 10.1038/sj.leu.2402416. [DOI] [PubMed] [Google Scholar]
- 5.Krajewski S, Bodrug S, Krajewska M, Shabaik A, Gascoyne R, Berean K, Reed JC. Immunohistochemical analysis of Mcl-1 protein in human tissues. Differential regulation of Mcl-1 and Bcl-2 protein production suggests a unique role for Mcl-1 in control of programmed cell death in vivo. Am J Pathol. 1995;146:1309–1319. [PMC free article] [PubMed] [Google Scholar]
- 6.Miyamoto Y, Hosotani R, Wada M, Lee JU, Koshiba T, Fujimoto K, Tsuji S, Nakajima S, Doi R, Kato M, Shimada Y, Imamura M. Immunohistochemical analysis of Bcl-2, Bax, Bcl-X, 23 and Mcl-1 expression in pancreatic cancers. Oncology. 1999;56:73–82. doi: 10.1159/000011933. [DOI] [PubMed] [Google Scholar]
- 7.Chen W, Bai L, Wang X, Xu S, Belinsky SA, Lin Y. Acquired activation of the Akt/cyclooxygenase-2/Mcl-1 pathway renders lung cancer cells resistant to apoptosis. Mol. Pharmacol. 2010;77:416–423. doi: 10.1124/mol.109.061226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei SH, Dong K, Lin F, Wang X, Li B, Shen JJ, Zhang Q, Wang R, Zhang HZ. Inducing apoptosis and enhancing chemosensitivity to gemcitabine via RNA interference targeting Mcl-1 gene in pancreatic carcinoma cell. Cancer Chemother. Pharmacol. 2008;62:1055–1064. doi: 10.1007/s00280-008-0697-7. [DOI] [PubMed] [Google Scholar]
- 9.Schulze-Bergkamen H, Fleischer B, Schuchmann M, Weber A, Weinmann A, Krammer PH, Galle PR. Suppression of Mcl-1 via RNA interference sensitizes human hepatocellular carcinoma cells towards apoptosis induction. BMC Cancer. 2006;6:232. doi: 10.1186/1471-2407-6-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guoan X, Hanning W, Kaiyun C, Hao L. Adenovirus-mediated siRNA targeting Mcl-1 gene increases radiosensitivity of pancreatic carcinoma cells in vitro and in vivo. Surgery. 147:553–561. doi: 10.1016/j.surg.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 11.Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009;35:57–68. doi: 10.1016/j.ctrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Zhang DY, Wu J, Ye F, Xue L, Jiang S, Yi J, Zhang W, Wei H, Sung M, Wang W, Li X. Inhibition of cancer cell proliferation and prostaglandin E2 synthesis by Scutellaria baicalensis. Cancer Res. 2003;63:4037–4043. [PubMed] [Google Scholar]
- 13.Tong WG, Ding XZ, Adrian TE. The mechanisms of lipoxygenase inhibitor-induced apoptosis in human breast cancer cells. Biochem. Biophys. Res. Commun. 2002;296:942–948. doi: 10.1016/s0006-291x(02)02014-4. [DOI] [PubMed] [Google Scholar]
- 14.Bonham M, Posakony J, Coleman I, Montgomery B, Simon J, Nelson PS. Characterization of chemical constituents in Scutellaria baicalensis with antiandrogenic and growth-inhibitory activities toward prostate carcinoma. Clin. Cancer Res. 2005;11:3905–3914. doi: 10.1158/1078-0432.CCR-04-1974. [DOI] [PubMed] [Google Scholar]
- 15.Tong WG, Ding XZ, Witt RC, Adrian TE. Lipoxygenase inhibitors attenuate growth of human pancreatic cancer xenografts and induce apoptosis through the mitochondrial pathway. Mol. Cancer Ther. 2002;1:929–935. [PubMed] [Google Scholar]
- 16.Ma Z, Otsuyama K, Liu S, Abroun S, Ishikawa H, Tsuyama N, Obata M, Li FJ, Zheng X, Maki Y, Miyamoto K, Kawano MM. Baicalein, a component of Scutellaria radix from Huang-Lian-Jie-Du-Tang (HLJDT), leads to suppression of proliferation and induction of apoptosis in human myeloma cells. Blood. 2005;105:3312–3318. doi: 10.1182/blood-2004-10-3915. [DOI] [PubMed] [Google Scholar]
- 17.Eibl G, Takata Y, Boros LG, Liu J, Okada Y, Reber HA, Hines OJ. Growth stimulation of COX-2-negative pancreatic cancer by a selective COX-2 inhibitor. Cancer Res. 2005;65:982–990. [PubMed] [Google Scholar]
- 18.Eibl G, Reber HA, Wente MN, Hines OJ. The selective cyclooxygenase-2 inhibitor nimesulide induces apoptosis in pancreatic cancer cells independent of COX-2. Pancreas. 2003;26:33–41. doi: 10.1097/00006676-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Angst E, Reber HA, Hines OJ, Eibl G. Mononuclear cell-derived interleukin-1 beta confers chemoresistance in pancreatic cancer cells by upregulation of cyclooxygenase-2. Surgery. 2008;144:57–65. doi: 10.1016/j.surg.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohno I, Eibl G, Odinokova I, Edderkaoui M, Damoiseaux RD, Yazbec M, Abrol R, Goddard WA, 3rd, Yokosuka O, Pandol SJ, Gukovskaya AS. Rottlerin stimulates apoptosis in pancreatic cancer cells through interactions with proteins of the Bcl-2 family. Am. J. Physiol. Gastrointest. Liver Physiol. 298:G63–G73. doi: 10.1152/ajpgi.00257.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Funahashi H, Satake M, Hasan S, Sawai H, Newman RA, Reber HA, Hines OJ, Eibl G. Opposing effects of n-6 and n-3 polyunsaturated fatty acids on pancreatic cancer growth. Pancreas. 2008;36:353–362. doi: 10.1097/MPA.0b013e31815ccc44. [DOI] [PubMed] [Google Scholar]
- 22.Angst E, Dawson DW, Nguyen A, Park J, Go VL, Reber HA, Hines OJ, Eibl G. Epigenetic regulation affects N-myc downstream-regulated gene 1 expression indirectly in pancreatic cancer cells. Pancreas. 39:675–679. doi: 10.1097/MPA.0b013e3181c8b476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morel C, Carlson SM, White FM, Davis RJ. Mcl-1 integrates the opposing actions of signaling pathways that mediate survival and apoptosis. Mol. Cell Biol. 2009;29:3845–3852. doi: 10.1128/MCB.00279-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunelle JK, Letai A. Control of mitochondrial apoptosis by the Bcl-2 family. J. Cell Sci. 2009;122:437–441. doi: 10.1242/jcs.031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moulding DA, Akgul C, Derouet M, White MR, Edwards SW. BCL-2 family expression in human neutrophils during delayed and accelerated apoptosis. J. Leukoc. Biol. 2001;70:783–792. [PubMed] [Google Scholar]
- 26.Akgul C, Moulding DA, White MR, Edwards SW. In vivo localisation and stability of human Mcl-1 using green fluorescent protein (GFP) fusion proteins. FEBS Lett. 2000;478:72–76. doi: 10.1016/s0014-5793(00)01809-3. [DOI] [PubMed] [Google Scholar]
- 27.Kim SH, Ricci MS, El-Deiry WS. Mcl-1: a gateway to TRAIL sensitization. Cancer Res. 2008;68:2062–2064. doi: 10.1158/0008-5472.CAN-07-6278. [DOI] [PubMed] [Google Scholar]
- 28.Akgul C. Cell Mol. Mcl-1 is a potential therapeutic target in multiple types of cancer. Life Sci. 2009;66:1326–1336. doi: 10.1007/s00018-008-8637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Derouet M, Thomas L, Cross A, Moots RJ, Edwards SW. Granulocyte macrophage colony-stimulating factor signaling and proteasome inhibition delay neutrophil apoptosis by increasing the stability of Mcl-1. J. Biol. Chem. 2004;279:26915–26921. doi: 10.1074/jbc.M313875200. [DOI] [PubMed] [Google Scholar]
- 30.Chetoui N, Sylla K, Gagnon-Houde JV, Alcaide-Loridan C, Charron D, Al-Daccak R, Aoudjit F. Down-regulation of mcl-1 by small interfering RNA sensitizes resistant melanoma cells to fas-mediated apoptosis. Mol Cancer Res. 2008;6:42–52. doi: 10.1158/1541-7786.MCR-07-0080. [DOI] [PubMed] [Google Scholar]
- 31.Longo PG, Laurenti L, Gobessi S, Sica S, Leone G, Efremov DG. The Akt/Mcl-1 pathway plays a prominent role in mediating antiapoptotic signals downstream of the B-cell receptor in chronic lymphocytic leukemia B cells. Blood. 2008;111:846–855. doi: 10.1182/blood-2007-05-089037. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, Song W, Aboukameel A, Mohammad M, Wang G, Banerjee S, Kong D, Wang S, Sarkar FH, Mohammad RM. TW-37, a small-molecule inhibitor of Bcl-2, inhibits cell growth and invasion in pancreatic cancer. Int. J. Cancer. 2008;123:958–966. doi: 10.1002/ijc.23610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Huang S, Okumura K, Sinicrope FA. BH3 mimetic obatoclax enhances TRAIL-mediated apoptosis in human pancreatic cancer cells. Clin. Cancer Res. 2009;15:150–159. doi: 10.1158/1078-0432.CCR-08-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahmani M, Davis EM, Bauer C, Dent P, Grant S. Apoptosis induced by the kinase inhibitor BAY 43-9006 in human leukemia cells involves down-regulation of Mcl-1 through inhibition of translation. J. Biol. Chem. 2005;280:35217–35227. doi: 10.1074/jbc.M506551200. [DOI] [PubMed] [Google Scholar]
- 35.Kim EJ, Park H, Park SY, Jun JG, Park JH. The grape component piceatannol induces apoptosis in DU145 human prostate cancer cells via the activation of extrinsic and intrinsic pathways. J. Med. Food. 2009;12:943–951. doi: 10.1089/jmf.2008.1341. [DOI] [PubMed] [Google Scholar]