Abstract
Scleraxis (Scx) is a basic helix-loop-helix transcription factor expressed in tendon and ligament progenitor cells and the differentiated cells within these connective tissues in the axial and appendicular skeleton. Unexpectedly, we found expression of the Scx transgenic reporter mouse, Scx-GFP, in interdental cells, sensory hair cells, and cochlear supporting cells at embryonic day 18.5 (E18.5). We evaluated Scx-null mice to gain insight into the function of Scx in the inner ear. Paradoxical hearing loss was detected in Scx-nulls, with ~50% of the mutants presenting elevated auditory thresholds. However, Scx-null mice have no obvious, gross alterations in cochlear morphology or cellular patterning. Moreover, we show that the elevated auditory thresholds correlate with middle ear infection. Laser interferometric measurement of sound-induced malleal movements in the infected Scx-nulls demonstrates increased impedance of the middle ear that accounts for the hearing loss observed. The vertebrate middle ear transmits vibrations of the tympanic membrane to the cochlea. The tensor tympani and stapedius muscles insert into the malleus and stapes via distinct tendons and mediate the middle ear muscle reflex that in part protects the inner ear from noise-induced damage. Nothing, however, is known about the development and function of these tendons. Scx is expressed in tendon progenitors at E14.5 and differentiated tenocytes of the stapedius and tensor tympani tendons at E16.5–18.5. Scx-nulls have dramatically shorter stapedius and tensor tympani tendons with altered extracellular matrix consistent with abnormal differentiation in which condensed tendon progenitors are inefficiently incorporated into the elongating tendons. Scx-GFP is the first transgenic reporter that identifies middle ear tendon lineages from the time of their formation through complete tendon maturation. Scx-null is the first genetically defined mouse model for abnormal middle ear tendon differentiation. Scx mouse models will facilitate studies of tendon and muscle formation and function in the middle ear.
Keywords: middle ear muscle reflex, tendon, tenocytes, laser interferometry, otitis media
Introduction
The special sense of hearing requires functional integration of the outer, middle, and inner ears (Hudspeth 1997). Sound energy is collected and focused by the auricle and external acoustic meatus to efficiently vibrate the tympanic membrane. Movement of the tympanic membrane transmits force sequentially to the three bones of the ossicular chain: the malleus, incus, and stapes. The footplate of the stapes impinges upon the oval window of the cochlea and transmits energy to the cochlear partition that will ultimately displace sensory hair cell bundles, the first step in mechanotransduction (Gillespie and Muller 2009). The movement of the middle ear ossicles is modulated by the tensor tympani and stapedius muscles which insert via tendons into bony prominences on the malleus and stapes, respectively. The middle ear muscle reflex involves contraction of the tensor tympani and stapedius muscles to modulate mechanical impedance of the middle ear and, in part, shield the inner ear from intense, damaging sound (Mukerji et al. 2010). Progress in understanding middle ear tendon formation and function has been hampered by the absence of a suitable reporter to facilitate dynamic analyses in vivo. Indeed, middle ear tendons and their associated musculature have been largely ignored in developmental studies of the middle ear (Mallo 2001, 2003).
Scx is a basic helix-loop-helix (bHLH) transcription factor with distinct expression in a number of tissues including the musculoskeletal system, epididymi, testes, adrenal gland, kidney, lung, heart, dorsal root ganglia, and body wall (Cserjesi et al. 1995; Schweitzer et al. 2001; Pryce et al. 2007). In the axial and appendicular skeleton, the Scx-GFP transgenic reporter labels tendon and ligament progenitor cells and all differentiated cell types within these connective tissues (Pryce et al. 2007). We hypothesized that Scx-GFP would serve as a useful marker of middle ear tendon progenitors and permit analysis of middle ear tendon formation. We show that Scx-GFP is a highly specific marker for both tensor tympani and stapedius tendon progenitors, middle ear ligament progenitors, and differentiated tenocytes in the mature tendon. Contrary to expectation, Scx was also expressed in interdental cells, sensory hair cells, and supporting cells in the embryonic inner ear suggesting a unique role in cochlear formation or function.
To gain insight into the putative functions of Scx in the middle and inner ears, we analyzed the Scx-null mouse mutant that has a targeted deletion of exon 1 (Murchison et al. 2007). Scx-null mice are viable but have severe locomotor deficits, postural weakness, and inability to move the tail. In the axial and appendicular skeleton, Scx-nulls display a wide range of tendon phenotypes from incomplete differentiation and shortening of some tendons to the complete absence of other tendons (Murchison et al. 2007). The tensor tympani and stapedius tendons are dramatically shorter in Scx-nulls consistent with failed tendon progenitor incorporation into the elongating tendons. While no gross alterations in cochlear morphogenesis or patterning are present in Scx-null mice, ~50% of nulls demonstrate elevated auditory thresholds. We show that middle ear infection in Scx-nulls accounts for the elevated auditory thresholds in affected mutant mice. We discuss the role of Scx in tensor tympani and stapedius tendon formation and speculate about Scx-mediated mechanisms for the pathogenesis of otitis media in Scx-nulls. We conclude that Scx mouse models will be useful tools to advance developmental studies of middle ear tendons and associated musculature and increase our understanding of the biomechanical function of the mature middle ear.
Materials and methods
Mice
Male Scx transgenic reporter mice, Scx-GFP, were outcrossed to CD1 females to produce embryonic (timed pregnant breeding) or adult middle and inner ears for analysis. Scx−/− (Scx-null) were generated by intercrossing Scx+/− mice which also carried the Scx-GFP allele. The validity of the Scx-GFP reporter expression in the middle and inner ears was confirmed by in situ hybridization with the full-length Scx probe (Brown et al. 1999) in CD1 tissues since this probe also detects the Scx-GFP mRNA (Pryce et al. 2007). The breeding paradigm described above and all mouse procedures and animal husbandry protocols described below were approved by the Oregon Health & Science University Institutional Animal Care and Use Committee.
Auditory brainstem response testing
Auditory thresholds were determined by auditory brainstem response (ABR) testing (Mitchell et al. 1996). Adult mice (postnatal day 16–28 [P16–18]) were anesthetized using a ketamine (0.066 mg/g body weight)/xylazine (0.013 mg/g body weight) cocktail in 0.9% saline. A speaker was connected to the ear canal using a flexible tube, and three electrodes were inserted under the skin. One recording electrode was placed along the sagittal suture of the skull, and two noise canceling electrodes were placed adjacent to the ipsilateral mandible and the distal portion of the ipsilateral foreleg. Tone bursts at 4, 8, 16, and 32 kHz were presented as stimuli. The duration of each tone burst was 2 ms, and responses were recorded for 12 ms. Responses were then averaged to obtain an event related potentials at each frequency and intensity (Mitchell et al. 1996).
Laser interferometric measurement of sound-induced malleal movement
To explore the cause of the mild hearing loss revealed by the ABRs, the middle ear function was measured in living Scx-null (n = 2) and Scx-GFP (n = 1) mice. The sound-induced malleal vibrations were measured at different frequencies and sound levels using a custom-modified sensitive laser interferometer.
Animal preparation and surgical procedures were similar to those described in previous studies (Ren 2004; He et al. 2008). The initial anesthesia was induced by subcutaneous injection of ketamine (100–120 mg/kg). The animal’s head was firmly attached to a custom-made holder with x-y-z translation and rotation capabilities. A tracheotomy was performed to ensure free natural breathing. Body temperature was maintained at 38 ± 1°C with a heating blanket. The Scx-GFP and Scx-null mutants without infection have clear middle ear cavities, and the mucosa shows no congestion or inflammation. In contrast, the bony wall of the auditory bulla in the infected Scx-null mice was thickened and difficult to open surgically. The mucosa inside the bulla and on the surface of the ossicular chain was also thicker and swollen. The middle ear cavity was filled with thick yellowish fluid. Hence, for vibration measurements, the fluid above the head of the malleus was removed. Due to the partial removal of this middle ear fluid, the measured reduction in middle ear function was likely underestimated. Moreover, stapes vibration measurements could not be conducted since additional fluid would need to be removed to gain access to the stapes.
The left auditory bulla was opened surgically, and the head of the malleus was visualized through a surgical microscope. One gold-coated glass bead (~20 μm in diameter) was placed on the surface of malleus. The object beam of a modified heterodyne laser interferometer (OFV 302, Polytec, Inc., Germany) was focused on the bead on the malleus through a long-working-distance objective. The reflected light with Doppler frequency shift from the vibrating bead was collected by the same objective and sent back to the interferometer. The voltage output of a digital decoder was proportional to the vibration velocity of the bead along the optical axis. The noise floor of the measurement was smaller than a picometer (i.e., one trillionth of a meter).
A custom-written program was used to control hardware (System II, Tucker–Davis Technologies, Gainesville, FL) for signal generation and data acquisition. Tone bursts at different frequencies with 23-ms duration and 1-ms rise/fall time were generated by a D/A converter. The signals were sent to a power amplifier through a programmable attenuator and then used to drive a speaker. A sensitive microphone (10 B + Etymotic Research, Inc. Elk Grove Village, IL) was used to measure the sound pressure in the ear canal. The microphone–earphone probe was coupled into the external ear canal to form a closed sound field. The signals from the interferometer decoder were digitized with an A/D converter and averaged 10 to 40 times, depending on the signal level. The magnitude and phase of the vibrations at the stimulus frequency were obtained by use of Fourier transformation. The vibrations were measured at sound levels of 60, 70, and 80 dB SPL and at frequencies from 0.25 to 25.00 kHz in 0.25 kHz steps. The displacement of the malleus vibration (D) in nanometers was obtained from recorded vibration velocity (V) in micrometers per second according to D = V/(2πf) × 1,000, where f is frequency (Hz).
Middle ear dissection and histological preparation
Adult (P16–28) Scx-null mice were fixed by transcardial perfusion with 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS; 137 mM NaCl; 2.7 mM KCl; 9.9 mM Na2HPO4, 2 mM KH2PO4, pH = 7.4) and hemisected crania were immersion fixed overnight. The middle ears were dissected under a Leica MZFLIII stereofluorescence dissecting microscope. Skin, muscle, and connective tissue overlying the temporal bone were removed with forceps and a scalpel, and the tympanic membrane was excised with a tungsten wire hook. The middle ear cavity was further exposed by chipping away portions of the bulla with #5 Inox forceps (Fine Science Tools) filed to ~30° wedge-shaped point. Adult (P16–28) Scx-GFP mice were immersion fixed in 4% PFA overnight, and then dissected as described above. The Scx-GFP reporter is extremely intense (Pryce et al. 2007), allowing simultaneous bright field (halogen) and epifluorescence (BF/EPI) illumination to record precise anatomical localization of GFP expression in whole mount middle ears without the need to merge separate bright field and epifluorescence images. BF/EPI-illuminated Scx-null and Scx-GFP middle ears were visualized with the Leica MZFLIII stereofluorescence dissecting microscope and imaged with a Retiga 1300 CCD camera.
Plastic thin sections of the adult middle ear
The tympanic membrane was punctured to facilitate dehydration and infiltration of the middle ear. Opening the middle ear cavity secures optimal histology but, in the case of infected Scx-nulls, displaced some of the cellular infiltrate and middle ear fluid. Whole, fixed Scx-null temporal bones were dehydrated in a graded ethanol series (70%, 95%, 100% ethanol for 1 h each) and vacuum infiltrated (~25 mmHg) with 100% ethanol/unpolymerized glycomethacrylate (GMA; 1:1 by volume) overnight and 100% unpolymerized GMA overnight at 22°C. The tissue was placed in an embedding mold with polymerizing GMA and allowed to cure for 24 h. The tissue block was cut into 5 μm sections using a Sorvall JB-4 microtome and mounted on glass slides using Protocol Mounting Medium (Fisher Scientific). Sections were stained with methylene blue/basic fuchsin and visualized with a Leica DM2500 transmitted light/epifluorescence microscope and imaged with a Leica DFC 420C CCD camera.
Cryostat sections
The whole head of E14.5, E16.5, and E18.5 Scx-GFP embryos were cryoprotected in graded sucrose/PBS (10%, 20%, 30% until the tissue had sunk), embedded in Tissue Freezing Medium (Triangle Biomedical Sciences, Durham, NC) and serially sectioned at 12 μm in the coronal plane. Slides were mounted in VectaShield (Vector Laboratories, Burlingame, CA). Tissue sections were visualized using a Leica DM2500 transmitted light/epifluorescence microscope and imaged with a Leica DFC 420C CCD camera or the Olympus FV1000 laser-scanning confocal microscope.
Immunohistochemistry
Scx-GFP embryos at E18.5 were immersion fixed in 4% PFA in PBS overnight at 4°C with gentle agitation. For cryostat sections, the entire head was cryoprotected, embedded, and serially sectioned as described above. For Scx-GFP and Scx-null whole mounts, the cochlea was dissected free of the cartilaginous capsule, and the lateral wall was removed. For tensor tympani and stapedius tendon whole mounts, the malleus and stapes, respectively, were dissected from Scx-GFP and Scx-null adult mice being careful to include a portion of the inserting muscle. The arms of the malleus were trimmed to facilitate orientation for confocal microscopic analyses. All tissues were permeabilized and blocked in 0.2% saponin in blocking solution (PBS containing 1% bovine serum albumin and 3% serum from the species in which the secondary antibody was generated). Antisera against the hair cell marker, Myosin7a, and the muscle marker, Myosin 32 (Myo32), were applied in blocking solution overnight at 4°C with gentle agitation. Alexa Fluor conjugated secondary antibodies were applied in blocking solution for 2 h at 22°C. Phalloidin-Alexa Fluor conjugates were applied in PBS for 30 min at 22°C. Sectioned cochleae and whole mount cochleae were mounted in VectaShield prior to epifluorescence or confocal analysis. Antibodies, labeling reagents, and their dilutions: Myosin 7a (dilution 1/100; 25–6790, Proteus Biosciences Inc, Ramona, CA); Myo32 (dilution 1/1,000; M4276, Sigma); Alexa 568-conjugated goat anti-rabbit (dilution 1/300; A11036, Molecular Probes, Eugene, OR); Alexa 647-conjugated goat anti-rabbit (dilution 1/300;A21245, Molecular Probes); Alexa 660-conjugated phalloidin (dilution 1/150; A22285, Molecular Probes); Alexa 568-conjugated phalloidin (dilution 1/150; A12380, Molecular Probes).
In situ hybridization
CD1 embryos at E14.5, E16.5, and E18.5 were used for in situ hybridization according to protocols found on the Tabin Laboratory web pages: http://genepath.med.harvard.edu/~cepko/protocol/insituprotocol.pdf. The following probes were used: the Scx full-length probe (Brown et al. 1999) for tendon and ligament progenitors and differentiated cells within these connective tissues; the Atonal homolog 1 probe (Helms and Johnson 1998) for sensory hair cells; and the Acan probe for chondrocytes (Sandell et al. 1991).
Statistical analyses
While the Scx-GFP mice had normal ABR thresholds, ~50% of Scx-null mice had elevated thresholds. We initially conducted parametric analyses to gain insight into statistically significant relationships. Pearson correlations indicate that otitis media is a better predictor of elevated average ABR threshold (N = 9 Scx-nulls [five ears without otitis media, four with otitis media] and 10 Scx-GFP ears, r = 0.85, p < 0.0001) than is GFP or null status (10 Scx-GFP ears and 13 Scx-null ears, r = 0.58, p = 0.004). Moreover, t tests indicate that there is a more significant difference between the average ABR thresholds of animals with otitis media versus those with clear middle ears (df = 7, t = 5.114, p = 0.001) than there is between the average ABR thresholds of Scx-GFP versus Scx-null mice (df = 21, t = 3.267, p = 0.004). These statistical differences generally held true for the individual frequencies tested as well as for averaged ABR responses across frequencies. These results suggest that it is probably not GFP or null status that affects ABR threshold but rather the middle ear infection that is associated with null status. The laser interferometric analysis of Scx-null mice with otitis media was deployed to quantitatively validate this association. Hence, the average ABR thresholds are presented in Figure 2 as mean dB SPL ± standard deviation and compared by t test to identify the statistically significant relationships. In addition, the probability values of the t tests comparing average ABR thresholds of Scx-null mice with otitis media to Scx-null mice without otitis media at each frequency tested are presented in Figure 2. T test probability relationships are presented for all other comparisons, and p values are listed in the legend.
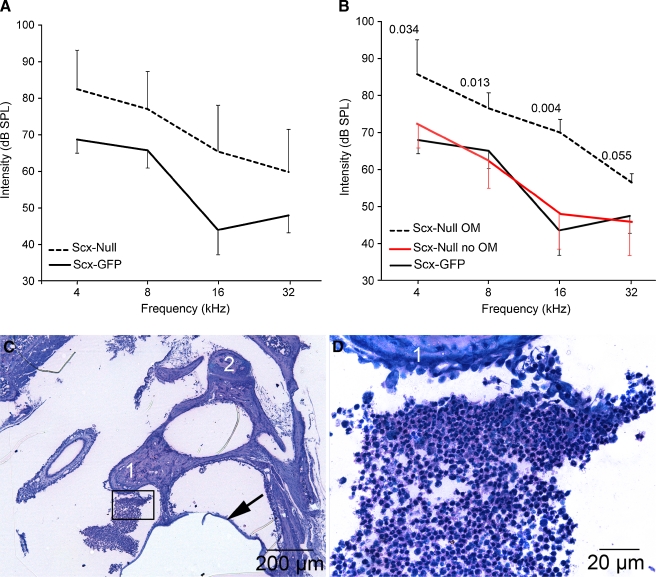
FIG. 2.
Middle ear infection correlates with elevated hearing thresholds in affected Scx-null mice. A The average auditory brainstem response thresholds in sound pressure level (dB SPL) as means ± standard deviation (SD) are plotted for Scx-null (n = 26 ears; 13 mice) and Scx-GFP (n = 20 ears; 10 mice) mice at 4, 8, 16, and 32 kHz. Auditory thresholds in Scx-null mice are statistically significantly elevated at all four frequencies tested (t > 2.02; p < 0.001). B The average auditory brainstem response thresholds (dB SPL ± SD) are plotted for Scx-null mice with otitis media (OM; dashed line, n = 4 ears from four mice) versus Scx-null mice without otitis media (red line; n = 5 ears from five mice) at 4, 8, 16, and 32 kHz. The average ABR thresholds of Scx-null mice with otitis media are statistically significantly elevated at three of the four frequencies tested (t test, p values indicated on the graph) compared to Scx-null mice without otitis media. The ABR thresholds of Scx-null mice without otitis media (B; red line) are not statistically significantly different from Scx-GFP mice (B, black line; data from A) at all frequencies tested (t > 2.07, p > 0.12). C, D Methylene blue/basic fuchsin-stained plastic thin sections of a representative Scx-null middle ear that presented with white, viscous fluid behind the tympanic membrane upon postmortem analysis. The boxed area in C is shown at higher magnification in (D). The methylene blue-stained cells between the malleus (1) and tympanic membrane (arrow) have small nuclei with scant cytoplasm consistent with infiltrating lymphocytes. Weakly methylene blue-stained acellular debris is associated with the incus (2).
Results
Scx is expressed in sensory and nonsensory cells in the organ of Corti
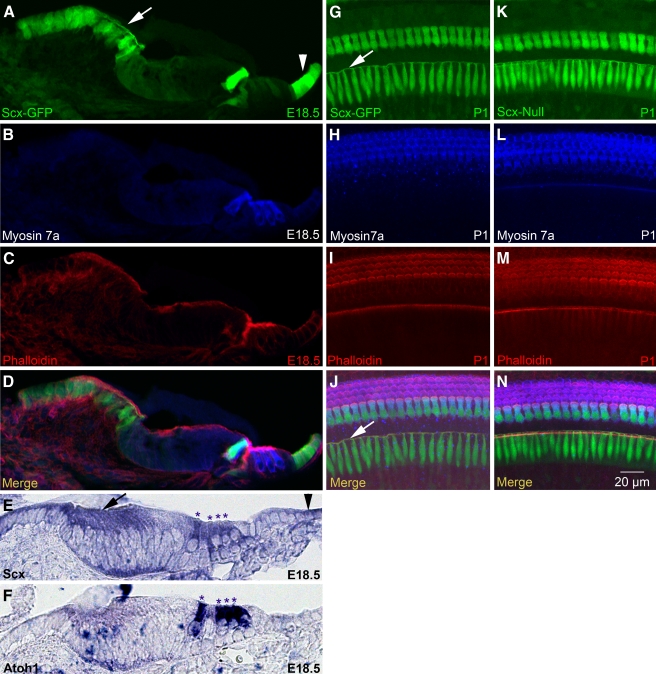
Scx is a member of the small Twist subfamily of bHLH transcription factors (Franco et al. 2010). Cell fate specification and patterning in the mammalian cochlea are influenced by the balance of bHLH transcription factor activity in otic epithelial progenitor cells (Kelley et al. 2009). We, therefore, evaluated the expression of Scx in the E18.5 mouse organ of Corti using the Scx-GFP transgenic reporter mouse in which green fluorescent protein expression is driven by regulatory sequences of the Scx gene (Pryce et al. 2007). We detected robust GFP expression in cryostat sections of the E18.5 mouse cochlea (Fig. 1A–D). GFP co-localized with myosin 7a (Myo7a) in inner hair cells and was weakly expressed in outer hair cells (Fig. 1A, B, D). GFP was also expressed in a superficial layer of cells in the spiral limbus with distinct apical associations consistent with interdental cell morphology (Fig. 1A, G, J, arrows). In addition, GFP was present in cuboidal supporting cells on the strial side of the organ of Corti consistent with Claudius cell identity (Fig. 1A, arrowhead, D). Scx-GFP expression in the E18.5 cochlea largely agrees with Scx mRNA expression, with the notable exception of outer hair cells which have weak Scx-GFP expression but robust Scx mRNA expression (Fig. 1E, F, asterisks). Confocal analysis of P1 Scx-GFP cochlear whole mounts (Fig. 1G–J) further confirmed robust Scx expression in inner hair cells (Fig. 1G–J) and interdental cells (Fig. 1G, J, arrows).
FIG. 1.
Scx is expressed in interdental cells, sensory hair cells, and Claudius cells. A–D Confocal microscopy of a cryostat section from the E18.5 Scx-GFP organ of Corti labeled with myosin 7a (B) and phalloidin (C). Panels A–C are merged in D. E, FIn situ hybridization for Scx (E) and Atoh1 (F) mRNA in cryostat sections of the E18.5 wild-type organ of Corti. Asterisks indicate apices of inner and outer hair cells. G–N Confocal microscopy of whole mount Scx-GFPG–J and Scx-nullK–N organs of Corti labeled with myosin 7a H, L and phalloidin I, M. Panels G–I are merged in J, and panels K–M are merged in N. Arrows indicate interdental cells and the arrowheads indicate Claudius cells. The cryostat section in A–D is from a more basal location in the cochlea than the sections in E and F. Scale bar in N applies to all panels.
Scx is not required for gross morphogenesis or patterning of the organ of Corti
To determine if Scx is required for cochlear development, we evaluated the Scx-null mouse bearing the Scx-GFP reporter transgene. GFP expression in the Scx-null mouse thus identifies cells that would normally express Scx, though no functional Scx gene product is produced in the mutant background. Confocal analysis of E18.5 Scx-null cochlear whole mounts (Fig. 1K–N) demonstrated GFP in inner hair cells and interdental cells (Fig. 1K, N) in a pattern indistinguishable from Scx-GFP expression (compare Fig. 1K–N to G–J). Moreover, the Scx-null organ of Corti was properly patterned with one row of inner and three rows of outer hair cells which displayed apical, phalloidin-positive (phalloidin+) stereociliary bundles, and normal planar polarity (Fig. 1L–N). These data suggest that Scx is not required for gross morphogenesis and patterning of the organ of Corti.
Hearing loss in Scx null mice is associated with middle ear infection
Molecular defects in interdental cells, hair cells, or supporting cells in the organ of Corti are associated with auditory dysfunction (Zwaenepoel et al. 2002; Zhu and Zhao 2010). Since Scx is expressed in all of these cell types, we measured auditory thresholds in Scx-null and control Scx-GFP mice. The average ABR thresholds (± standard deviation [SD]) of Scx-null mice (n = 26 ears from 13 mice) are statistically significantly elevated (t > 2.02, p < 0.001) at 4, 8, 16, and 32 kHz compared to Scx-GFP mice (n = 20 ears from 10 mice; Fig. 2A).
We were surprised to find, however, that the hearing loss in Scx-null mice was heterogeneous with some mice presenting elevated thresholds and others with normal thresholds. The ears from nine of the 13 Scx-null mice whose brainstem audiometric responses were included in the ABR average in Figure 2A were separated into two subpopulations postmortem defined by the absence or presence of pathological evidence of middle ear infection. Viscous white or yellow fluid in the middle ear cavity that blurred visual identification of the malleus when viewed laterally (i.e., from the external acoustic meatus; see Fig. 3A) was considered a sign of middle ear infection. Tightly packed clusters of methylene blue-stained lymphocytes were detected in plastic thin sections from the middle ear of a representative Scx-null mouse with pathological signs of middle ear infection (Fig. 2C, D). Lymphocytic infiltration into the middle ear space was not detected in Scx-null mice with visually clear middle ears or in control Scx-GFP mice (data not shown). We compared the average ABR thresholds ± SD of Scx-null mice with middle ear infection (n = 4 ears from four mice) to Scx-null mice without middle ear infection (n = 5 ears from five mice; Fig. 2B). The ABR thresholds of Scx-null mice with infection are statistically significantly elevated at three of the four frequencies tested (t test, probability values indicated on the graph in Fig. 2B) compared to Scx-null mice without middle ear infection (Fig. 2B, red line, n = 5 ears). In addition, the ABR thresholds of Scx-null mice without infection are not statistically significantly different from control Scx-GFP mice (Fig. 2B; black line, n = 26 ears) at all frequencies tested (t > 2.07, p > 0.12). These data suggest that the hearing loss observed in a subset of Scx-null mice correlates with middle ear infection or otitis media.
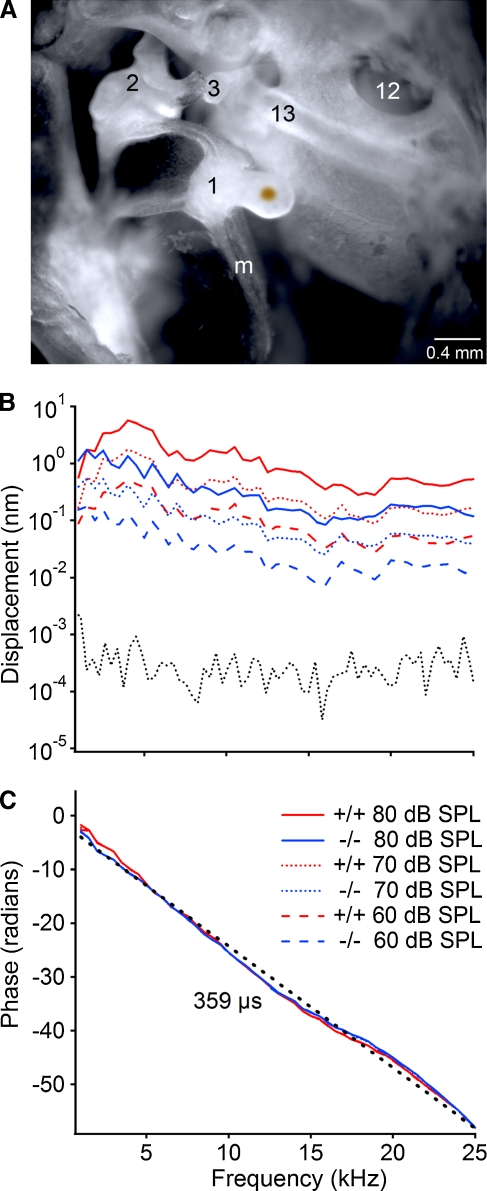
FIG. 3.
Ossicle movements are dampened in Scx-null mice with otitis media. A Brightfield image of the clear, adult mouse middle ear demonstrating placement of the gold-coated glass bead on the medial face of the malleal head for laser interferometric quantitation of malleal movement. Inflammatory cells and fluid were partially cleared from the middle ear cavity to permit placement of the bead in Scx-null mice with otitis media, suggesting that the laser interferometric estimates are likely an underestimation of the decrease in malleal vibration. 1 malleus; 2 incus; 3 stapes; 13 stapedial artery; 12 round window; m manubrium of the malleus. B The vibration magnitude of the malleus in nanometers is plotted as a function of frequency in kHz at 60, 70, and 80 dB SPL. Color key applies to panels B and C; +/+ is Scx-GFP; −/− is Scx-null. Data from a representative Scx-GFP and Scx-null mouse are shown. The dotted gray line is the measured noise floor for the laser interferometric measurements. C The phase in radians is plotted as a function of frequency at 80 dB SPL. The slope of the linear regression line (dotted black line) indicates the group delay (~359 μs). The solid red and blue lines are the observed phase/frequency curves from the Scx-GFP and Scx-null mice at 80 dB SPL.
Scx-null mice with otitis media display conductive hearing loss
To estimate the contribution of otitis media to the elevated auditory thresholds in affected Scx-null mice, we measured the mechanical movements of the malleus in response to sound of varying frequency and intensity by laser interferometry (Fig. 3A). The noise floor of the interferometric analysis was in the picometer range (Fig. 3B, dotted gray line), two orders of magnitude below the magnitude of the measured ossicle movements. The group delay (~359 μs) measures the time elapsed for the sound stimulus to travel from the speaker to the malleus and this value is similar for both Scx-GFP (+/+) and Scx-null (−/−) mice at all frequencies tested (Fig. 3C). The vibration magnitudes in Scx-null mice with otitis media (Fig. 3B, blue lines) are ~5 nm (~10 dB) below those of the Scx-GFP mouse (Fig. 3B, red lines) at corresponding sound pressure levels indicating increased impedance of the middle ear. Malleus movement decreased slightly with frequency at all sound intensities tested. Thus, the ~10 dB decrease of sound transmission due to middle ear infection in affected Scx-null mice can account for the elevated ABR thresholds observed (Fig. 2). This observation, along with normal ABR thresholds in Scx-null mice without infection, strongly suggests that the hearing loss in affected Scx-null mice is conductive in nature and caused by the cellular infiltrate and viscous fluid associated with otitis media.
Scx-GFP is a robust reporter for middle ear tendons
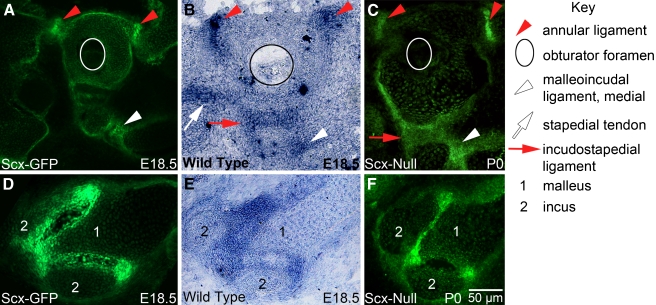
The acoustic reflex in mammals reduces transmission of loud sound to the cochlea thereby protecting the sensory epithelium from damage (Mukerji et al. 2010). The reflex is mediated by contraction of the stapedius and tensor tympani muscles which insert into the stapes and malleus by their respective tendons (Fig. 4A). Scx was previously shown to be expressed in differentiated tendon cells called tenocytes in all tendons of the axial and appendicular skeleton (Pryce et al. 2007). We, therefore, analyzed the expression of Scx in the mature middle ear of Scx-GFP mice to determine if Scx is also a marker for middle ear tendons. GFP expression is present near the stapes in the Scx-GFP middle ear in lateral view (Fig. 4B, arrow). The GFP expression appears in a feathered distribution that coalesces onto the posterior arch of the stapes in ventral view of the intact middle ear (Fig. 4D, arrow). Confocal analysis of the stapes excised from the middle ear and immunostained with the muscle marker myosin 32 (Myo32) confirms Scx expression in the stapedius tendon which overlaps with the stapedius muscle (Fig. 4E).
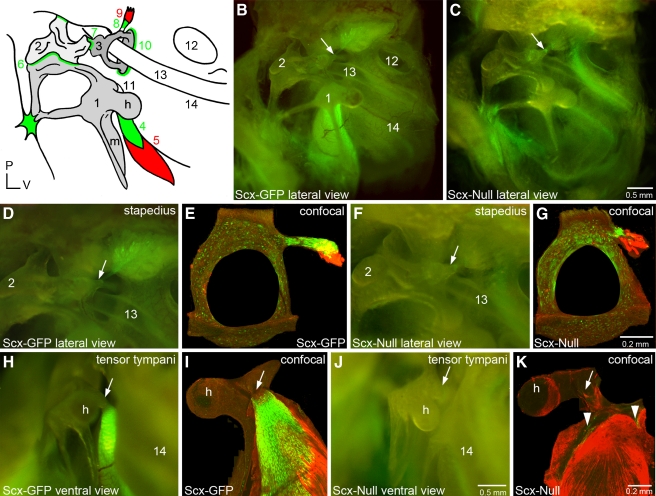
FIG. 4.
Scx is required for middle ear tendon differentiation. A The mammalian middle ear consists of three delicate bones and associated ligaments and tendons. The manubrium (m) of the malleus (1) is inserted into the tympanic membrane or ear drum (see also Fig. 2C, arrow). The incus (2) articulates with the malleus and the stapes (3) at the malleoincudal (6) and incudostapedial (7) joints, respectively, where ligaments provide structural integrity. The footplate of the stapes rests on the oval window of the cochlea where it is stabilized by the annular ligament (10). The tensor tympani muscle (5) inserts into a tubercle (11) adjacent to the head (h) of the malleus through the tensor tympani tendon (4). The stapedius muscle (9) inserts into a tubercle on the posterior arch of the stapes through the stapedial tendon (8). The round window of the cochlea (12), the stapedial artery (13), and the bony labyrinth at the level of the middle turn of the cochlea (14) are prominent anatomical landmarks in this lateral view. All middle ear tendons and ligaments express Scx and green shading schematically summarizes Scx-GFP expression. A patch of Scx-GFP-expressing tissue is localized to the arms of the malleus where they associate with the dorsal wall of the middle ear cavity. A small focus of Scx-GFP-expressing tissue is also present between an arm of the incus and the posterior wall of the middle ear cavity. P posterior; V ventral. These numerical and alphabetic labels are used in subsequent panels (B, C) Lateral view of the Scx-GFP (B) and Scx-null (C) middle ears imaged in combination bright field/epifluorescence (BF/EPI) mode (see Materials and methods). The stapedial tendon insertion point (arrow) on the tubercle of the posterior arch of the stapes is indicated. The tensor tympani tendon located behind the malleus (1) robustly expresses Scx-GFP in wild type (B) but not the null (C) middle ear. Scx-GFP is not detectable in the middle ear mucosa. Scx-GFP expression in the cochlea is detectable through the bony labyrinth in the wild type (14) and null inner ears. Scale bar in (C) applies to (B). (D, E) The stapedial tendon insertion into the tubercle on the posterior arch of the stapes in the Scx-GFP middle ear imaged by BF/EPI (D) and laser confocal (E) microscopy. The tenocytes in the stapes tendon robustly express GFP that is detectable in the whole mount image as a feathered array of green fluorescence terminating at the posterior arch of the stapes (D, arrow). The excised stapes was dissected from its associations with the oval window and incus prior to immunohistochemical detection of Myo32 and confocal analysis. The stapes tendon insertion into the tubercle and its association with a fragment of the Myo32+ stapedius muscle is shown in (E). The posterior arch of the stapes in (E) was fractured during excision or tissue processing (F, G) Scx-GFP expression is weakly detectable in the BF/EPI (F) and laser confocal (G) images of the stapes of Scx-null mice. The stapedius is inserted into the stapes by a GFP+ tendon (F, arrow and G; see also C, arrow). The stapedial tendon in Scx-null mice is dramatically shorter than the tendon in the wild type Scx-GFP middle ear (compare GFP+ tendon in E and G). The foreshortened stapedius tendon is associated with a fragment of the Myo32+ stapedius muscle in the Scx-null middle ear (G). Scale bar in G applies to (E). (H, I) The tensor tympani tendon insertion into the tubercle of the malleus in the Scx-GFP middle ear imaged by BF/EPI (H) and laser confocal (I) microscopy. The tenocytes in the tensor tympani robustly express Scx-GFP (H), and it is this intense expression that is detectable behind the malleus in the lateral view of the Scx-GFP middle ear in panel B. The matrix that anchors the tendon to the tubercle is GFP negative (H, arrow). The excised malleus was dissected from its associations with the ear drum and incus, and the bony arms were trimmed prior to immunohistochemical detection of Myo32 and confocal analysis. Myo32 expression in the tensor tympani (I; red) demonstrates the extensive association of the GFP+ tendon with the muscle and the absence of GFP expression proximal to the insertion point on the tubercle (I, arrow). (J, K) Scx-GFP expression is weakly detectable in BF/EPI (F) and laser confocal (G) images of the malleus in Scx-null mice. Confocal analysis detects a small complement of diffusely arrayed, weakly GFP+ tenocytes (K, arrowheads) associated with the Myo32+ tensor tympani. The tensor tympani inserts into the malleus by a foreshortened, stubby cord of matrix that is GFP negative (J, arrow and Fig. 6). The reduced expression of Scx-GFP in the Scx-null tensor tympani tendon accounts for the inability to detect GFP expression behind the malleus in the BF/EPI image (J) and in the lateral view of the Scx-null middle ear (C). Scale bar in (J) applies to (D, F, H). Scale bar in (K) applies to (I).
Intense GFP expression is also present behind the body of the malleus in an arc that traces the angle of the manubrium in the Scx-GFP middle ear in lateral view (Fig. 4B). The GFP expression localizes to the surface of the tensor tympani muscle in ventral view of the intact middle ear (Fig. 4H). Confocal analysis of the excised malleus confirms Scx-GFP expression in the Myo32-negative tensor tympani tendon (Fig. 4I). GFP is absent from the terminal portion of the tendon at the insertion into the malleus (Fig. 4H, I, arrows). In summary, the expression data suggest that Scx-GFP is a robust reporter for middle ear tendons.
Scx-GFP accurately reports Scx mRNA expression in tenocytes
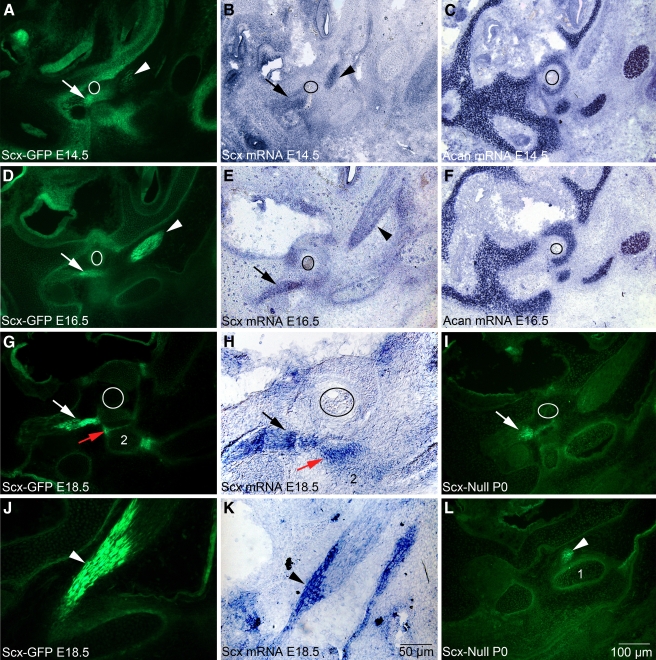
To determine whether Scx-GFP accurately reports Scx mRNA expression in tenocytes of tendons, we performed in situ hybridization in cryostat sections of the middle ear at E18.5 when the tendons are inserted into their target ossicles and display advanced morphogenetic development. Since the full-length Scx mRNA probe also detects the Scx-GFP mRNA (Pryce et al. 2007), we performed our analyses in wild-type mice. We then compared mRNA expression in wild-type middle ears to that in age-matched Scx-GFP middle ears. The stapedius tendon and tensor tympani tendon express Scx-GFP robustly proximal to their respective insertion sites (Fig. 5G, J) consistent with the whole mount images previously shown. Scx-GFP (Fig. 5G, J) and Scx mRNA (Fig. 5H, K) are robustly expressed in tenocytes in both the stapedius tendon (compare Fig. 5G, H, white and black arrows) and the tensor tympani tendon (compare Fig. 5J, K, arrowheads) at E18.5.
FIG. 5.
Scx-GFP fluorescence accurately reports Scx mRNA expression in middle ear tendons and the incudostapedial ligament. White and black arrows indicate the developing stapedius tendon, and arrowheads indicate the tensor tympani tendon in all panels. A, D, G, J, I, LScx-GFP expression at E14.5 (A), E16.5 (D), E18.5 (G, J), and P0 (I, L) in cryostat sections of the Scx-GFP middle ear. C, FAcan expression in chondrocytes of the nascent stapes at E14.5 (C) and E16.5 (F) and chondrocytes in the developing cartilaginous capsule of the inner ear. The circle outlines the obturator foramen of the stapes through which the stapedial artery passes. Acan provides an anatomical framework to interpret Scx-GFP and mRNA expression in (A, B, D, E). H, KScx-mRNA expression in the stapedius tendon (H, black arrow) and tensor tympani tendon (K, arrowhead) at E18.5. The narrow band of Scx-GFP expression (G, red arrow; 2, incus) in the incudostapedial ligament correlates with Scx mRNA expression (H, red arrow). Scale bar in (K) applies to (H, J); scale bar in (L) applies to all other panels.
Scx-GFP accurately reports Scx mRNA expression in tendon progenitors
Scx was also previously shown to be expressed in tendon progenitor cells in all developing tendons of the axial and appendicular skeleton (Schweitzer et al. 2001; Murchison et al. 2007; Pryce et al. 2007). To identify the early stages in the formation of middle ear tendons, we analyzed Scx-GFP and Scx mRNA expression at E14.5 and E16.5 when middle ear tendon progenitors condense proximal to their bony insertion sites and tenocytes become incorporated into the elongating tendon. The Acan probe (Sandell et al. 1991) was used as a convenient marker for chondrocytes that facilitated identification of the developing ossicles and cartilaginous capsule of the cochlea (Hwang and Wu 2008) (Fig. 5C, F). Tendon progenitors that give rise to the stapedius and tensor tympani tendons are condensed into GFP+ clusters in close proximity to their target ossicles at E14.5 (Fig. 5A–C) and begin incorporation into the growing long axis of the tendon by E16.5 (Fig. 5D–F). Scx mRNA expression in differentiating tenocytes closely matches Scx-GFP distribution at E14.5 and E16.5 (compare Fig. 5A, B, D, E). We conclude that Scx-GFP accurately reports Scx-mRNA expression in both tendon progenitor cells and differentiated tenocytes of the middle ear.
Scx is required for middle ear tendon formation
Scx-null mice display a complex range of tendon phenotypes ranging from poorly differentiated tendons to the complete absence of some tendons (Murchison et al. 2007). To determine if Scx is required for middle ear tendon formation, we analyzed the Scx-null mouse carrying the Scx-GFP reporter allele (Murchison et al. 2007). A small focus of GFP expression is present near the head of the stapes in the Scx-null middle ear in lateral view (Fig. 4C, arrow). GFP is weakly detected near the posterior arch of the stapes in the ventral view of the intact middle ear (Fig. 4F, arrow). Confocal analysis of the excised stapes immunostained with Myo32 confirms expression in the foreshortened, GFP+ stapedius tendon that associates with a fragment of the Myo32+ stapedius muscle (Fig. 4G). In summary, GFP+ tenocytes are present in the stapedius tendon in Scx-null mice, the tendon is dramatically shorter than the wild-type tendon, and the stapedius is anchored to the stapes.
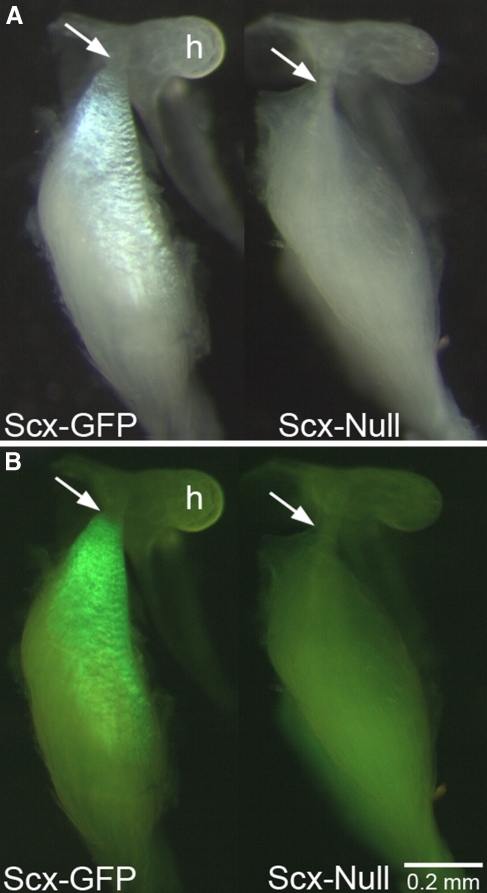
In contrast to the stapedius tendon phenotype, GFP expression is absent from the malleal territory in the Scx-null middle ear in lateral view (Fig. 4C) and Scx-GFP in the basal and middle turns of the cochlea is apparent (Fig. 4C). GFP is only weakly detected above background in the ventral view of the malleal territory (Fig. 4J, arrow). Confocal analysis of the dissected malleus immunostained with Myo32 confirms the absence of organized Scx-GFP expression in the tensor tympani territory with only diffuse GFP+ puncta associated with the Myo32+ tensor tympani muscle (Fig. 4K, arrowheads). To determine if the tensor tympani tendon forms in Scx-null mice, we analyzed the malleus excised from the middle ear with the tensor tympani muscle by combined BF/EPI microscopy (Fig. 6). In bright field, the tensor tympani tendon appears as a residual band of connective tissue that anchors the tensor tympani to the malleus (Fig. 6A, right, arrow). In contrast, the pearly white collagen 1 matrix of the Scx-GFP tensor tympani extends over a large surface area of the muscle (Fig. 6A, left). Epifluorescence microscopy fails to detect GFP expression above background in the Scx-null tensor tympani tendon (Fig. 6B, right), while GFP is robustly expressed in the Scx-GFP tensor tympani (Fig. 6B, left). In summary, despite the presence of only a few GFP+ tenocytes in the tensor tympani tendon in Scx-null mice and the severe reduction of the collagen 1 matrix, a residual tendon matrix that anchors the tensor tympani to the malleus is formed in Scx-null mice.
FIG. 6.
Scx is required for tensor tympani tendon differentiation. Bright field (A) and BF/EPI (B) images of the Scx-GFP (left) and Scx-null (right) tensor tympani and malleus. (A, left) Collagen 1 in the Scx-GFP tensor tympani tendon reflects bright field illumination creating a pearly white appearance. The tendinous insertion into the malleal tubercle is indicated by the arrow. The Scx-null tensor tympani does not possess the collagen 1 reflective surface but the muscle inserts into the malleal tubercle (A, right, arrow). GFP+ tenocytes are present throughout the Scx-GFP tensor tympani tendon, though the terminal insertion is GFP negative (B, left, arrow). No obvious GFP+ tenocytes are detected in the whole mount preparation of the Scx-null tensor tympani (B, right; see also Fig. 4, K, arrowheads). Scale bar in (B) applies to (A).
To gain further insight into the Scx-null tendon phenotype, we analyzed cryostat sections of the P0 middle ear, a time point in wild-type mice by which tenocytes are incorporated into the long axis of the stapedius and tensor tympani tendons. In the Scx-null mutants, the GFP+ tenocytes in the stapedius and tensor tympani are present at P0, though they are not patterned in a linear array coincident with the long axis of the tendon (Fig. 5I, L) as they are by E18.5 in Scx-GFP tendons (Fig. 5G, J). The Scx-null tenocytes are localized adjacent to their bony insertion sites and produce a sufficiently stable extracellular matrix to anchor muscle to bone (Figs. 4G and 6). The Scx-null tendon phenotype is consistent with failed differentiation in which tenocytes are not incorporated into the growing tendon and reduced tendon matrix is produced.
Scx is expressed in middle ear ligaments
Scx is also a marker for ligaments in the axial and appendicular skeleton (Murchison et al. 2007; Pryce et al. 2007). We investigated whether ligaments in the middle ear also express Scx. The middle ear contains three articulating joints associated with three ligaments: the stapes footplate with the oval window (annular ligament); the stapes with the incus (incudostapedial ligament); and the incus with the malleus (malleoincudal ligament; Fig. 4A). We detected Scx-GFP and Scx mRNA expression in the annular ligament (Fig. 7A, B, red arrowheads); in the medial (Fig. 7A, B, white arrowheads) and lateral (Fig. 7D, E) regions of the malleoincudal ligament; and in the incudostapedial ligament (Fig. 5G, H, red arrow). Middle ear ligaments in Scx-null mice appear to be intact (Fig. 7C, F), consistent with the observation of the normal ABR thresholds in Scx-null mice without otitis media.
FIG. 7.
Scx is expressed in the annular, incudostapedial, malleoincudal ligaments. GFP is expressed in the ligaments of the Scx-GFP-reporter mouse (A, D) and Scx mRNA is expressed in the ligaments of wild-type mice (B, E) at E18.5. GFP is expressed in ligaments of the Scx-null middle ear (C, F) at P0 (the Scx-null mouse carries the Scx-GFP reporter allele). The annular ligament (A, B, red arrowheads) secures the stapes footplate to the oval window of the cochlea. The malleoincudal ligament lines the V-shaped articulating surface between the malleus and incus (see Fig. 4A, number 6) and is presented in full cross section in (D, E; 1 malleus; 2 incus). The medial extent of the malleoincudal joint is indicated by the white arrowhead in (A, B). Scx mRNA expression in the incudostapedial ligament is indicated by the red arrow in (B). Stapedial tendon mRNA expression is indicated by the white arrow in (B) and serves as an anatomical reference. The annular (C, red arrowheads), incudostapedial (C, red arrow), and malleoincudal (C, white arrowhead and F) ligaments express Scx-GFP in the P0 Scx-null mutant. The white and black circles (A–C) outline the obturator foramen of the stapes. See the Key to facilitate comparisons. The scale bar in (F) applies to all panels.
Discussion
Our data demonstrate Scx expression in the sensory epithelium of the mammalian cochlea in functionally diverse cell types. Scx is expressed in interdental cells of the spiral limbus that produce otoanchorin which is defective in nonsyndromic, autosomal recessive deafness, DFNB22 (Zwaenepoel et al. 2002). Scx is found in mechanically sensitive hair cells that transduce sound energy into electrical signals that subserve hearing (Hudspeth 1997). In addition, Scx is present in Claudius cells which, along with other supporting cell types, play a role in cochlear ion homeostasis essential for mechanotransduction of sensory hair cells (Zhu and Zhao 2010). Given the functionally critical cell types in which Scx is expressed, it was surprising to observe a heterogeneous auditory phenotype in Scx-null mice that could be explained without postulating a sensorineural etiology: otitis media was observed in ~50% of mutants evaluated. Laser interferometric measurement of malleal movement in the Scx-null mouse with otitis media demonstrated decreased vibration of the ossicular chain that can account for the elevated auditory thresholds observed. Moreover, normal auditory thresholds in Scx-null mice without otitis media suggest that Scx is not required for auditory function. However, functional redundancy cannot formally be ruled out as Paraxis (Burgess et al. 1995), another member of the small Twist family of transcription factors that is required for somite formation and musculoskeletal patterning (Burgess et al. 1996), and Hand1 and Hand2, members of the small Hand subfamily of bHLH transcription factors (Atchley and Fitch 1997; Firulli 2003) that regulate cardiac development (McFadden et al. 2005), have significant sequence similarity to Scx and their expression has not been adequately defined in the mouse inner ear.
The etiology of otitis media in Scx-null mice is unknown. Scx mutants are viable, but they display dramatic musculoskeletal defects including dorsiflexion of the forepaws, inability to reorient efficiently in response to rapidly induced postural changes, and the inability to move the tail (Murchison et al. 2007). Otitis media is more common in mice that are less physically robust and may be related to a commensurate alteration in the immune response. However, it is intriguing to consider Scx-mediated mechanisms for the pathogenesis of otitis media. Scx is expressed in all force bearing and anchoring tendons in the body and the musculoskeletal defects in mutant mice are attributed to failed tendon differentiation (Murchison et al. 2007). The cranial, thoracic, and abdominal musculatures are precisely coordinated during suckling and swallowing. It is conceivable that even minor alterations in tendon-mediated force transmission during synchronous muscle contractions in Scx-null mice could produce extraesophageal reflux into the nasopharynx and Eustachian tube, predisposing the animal to middle ear infection. Extraesophageal reflux is a proposed mechanism by which children develop otitis media (Hall-Stoodley et al. 2006; O'Reilly et al. 2008), the most common infection in children for which antibiotics are prescribed (Coker et al. 2010). Alternatively, the musculature responsible for active dilation of the Eustachian tube (the tensor veli palatini and levator veli palatini) may not exert sufficient force to modulate the ductal valve to equalize middle ear pressures and allow fluid drainage from the middle ear mucosa to the nasopharynx. Future evaluation of the pathogenesis of otitis media in a Scx conditional null that lacks cranial tendons in the absence of confounding musculoskeletal defects in the axial and appendicular skeleton could be informative in determining the etiology of otitis media. Established mouse models for otitis media include genetic manipulation of the immune system, inoculation of the middle ear with infectious agents or their molecular by-products, and mutants with craniofacial defects (MacArthur and Trune 2006; Ryan et al. 2006; Trune and Zheng 2009). A genetically defined mouse model of otitis media with altered muscular control of the Eustachian tube may be a valuable tool to refine the role of the musculoskeletal system in otitis media pathogenesis and for testing appropriate therapeutic interventions.
We have shown that Scx labels middle ear tendon progenitors at E14.5 and differentiated tenocytes at E16.5–18.5. These observations are consistent with Scx expression in tendon lineages in the axial and appendicular skeleton (Cserjesi et al. 1995; Schweitzer et al. 2001). The Scx-GFP reporter will greatly facilitate analyses of tendon development in defined mouse mutants that affect middle ear formation. Furthermore, in the mature middle ear, Scx-GFP effectively illuminates the middle ear cavity with intense green fluorescence that backlights the tympanic membrane and precisely defines the ligamentous connections along the length of the ossicular chain, also highlighting the incal and malleal associations with the walls of the middle ear cavity. We anticipate that the Scx-GFP reporter will emerge as a powerful tool to advance knowledge of middle ear tendon and muscle development and enable novel imaging strategies to refine our understanding of the biomechanical function of the mouse middle ear.
The stapedius tendon in Scx-null mice displays GFP+ tenocytes, is reduced in length, and secures the stapedius muscle to the stapes. The presence of a reduced stapedius tendon that is functionally competent to establish muscle insertion is similar to the phenotype of two force-bearing tendons, the extensor digiti quinti and the extensor carpi ulnaris, in the Scx-null forelimb (Murchison et al. 2007). These extensors display GFP+ tenocytes but the tendons fail to grow to their normal size. Cell proliferation and cell death appear normal in Scx-null forelimb tendons and are not thought to play a role in establishing the reduced tendon phenotype. Rather, failed incorporation of tendon progenitors into the elongating tendon is proposed to explain the reduced, poorly organized matrix that is produced (Murchison et al. 2007). Consistent with this explanation, condensed progenitors adjacent to the posterior arch of the stapes are present at P0 in the Scx-null middle ear, though very few tenocytes are incorporated into the tendon proper as indicated by Scx-GFP labeling of tenocytes and by the reduced tendon matrix produced. We conclude that Scx activity is required for stapedius tendon differentiation and, as in one class of force-bearing tendons of the limb, acts to enable incorporation of condensed tendon progenitors into the growing tendon.
By contrast, the tensor tympani tendon in Scx-null mice presents very few GFP+ tenocytes, and they are dispersed, though the tensor tympani muscle is inserted into the malleus. The paucity of GFP+ tenocytes in the Scx-null tensor tympani tendon is remarkably similar to the phenotype detected in the “intermuscular tendons” that connect muscle to muscle in the thorax and abdomen. For instance, Scx-GFP+ cells are not detected in the subscapularis tendon, the rectus abdominus tendon, or the middle tendon of the diaphragm in Scx-nulls (Murchison et al. 2007). However, a functional connective tissue matrix is produced in each of these muscles. Likewise, the reduced tensor tympani tendon in Scx-null mice is sufficient to enable muscle insertion into the malleus, though the physiological impact on the middle ear muscle reflex has not been assessed. In addition, expression of the tendon matrix proteins tensacin C and collagen XII is not affected in the middle tendon of the Scx-null diaphragm, though collagen 1 is drastically reduced (Murchison et al. 2007). Similarly, the tensor tympani tendon lacks the characteristic pearly white appearance contributed by longitudinally arrayed, parallel collagen 1 fibers that are so clearly evident in the Scx-GFP tensor tympani, indicating altered tendon matrix biosynthesis. We conclude that Scx activity, as in “intermuscular tendons,” is required for appropriate tendon matrix biosynthesis.
The expression of Scx-GFP in the developing stapedius and tensor tympani tendons of Scx-null embryos labels tendon progenitors and differentiating tenocytes, indicating that, consistent with its role in the development of other tendons, Scx is not required for middle ear tendon progenitor specification. We have shown, rather, that Scx is required for middle ear tendon differentiation, though the underlying molecular mechanism remains elusive. In forelimb tendons of Scx-null embryos, collagen XIV (Gelse et al. 2003) and tenomodulin (Brandau et al. 2001) expression are lost, and collagen I levels are decreased (Murchison et al. 2007). Moreover, direct regulation of collagen I enhancers has been demonstrated (Lejard et al. 2007; Espira et al. 2009). Collagen XIV regulates the diameter of collagen fibrils (Young et al. 2002), tenomodulin subtly influences tendon proliferation (Docheva et al. 2005), and collagen I is the major structural protein in the collagen matrix of tendons (Orgel et al. 2006). The misregulation of these proteins in Scx-nulls is not thought to account for Scx function in tenocyte differentiation and for the range of phenotypes observed in the tendons of mutants (Murchison et al. 2007). No other genes known to be specifically expressed in tendons were altered in Scx-nulls (Murchison et al. 2007). The identification of transcriptional regulators of Scx as well as those genes regulated by Scx activity will be necessary to understand the transcriptional control of tendon formation.
The middle ear muscle reflex in humans acts on the stapedius and tensor tympani muscles to increase impedance (Mukerji et al. 2010). The stapedius contracts to limit movement of the stapes footplate on the oval window of the cochlea and the tensor tympani contracts and pulls the tympanic membrane inward. The resulting increased impedance of the middle ear reduces transmission of potentially damaging loud sound from the external ear canal to the inner ear. The Scx-null middle ear tendons are reduced in size and have altered matrix properties. We postulate that Scx-null mice may display an abnormal middle ear muscle reflex in response to chronic, intense suprathreshold sound stimuli and may exhibit enhanced susceptibility to noise-induced hearing loss. Middle ear muscle reflex testing in the Scx-null mouse is not the ideal model system given the systemic musculoskeletal defects and postural weakness. A conditional mouse model with Scx deleted in cranial tissues would be far more efficacious to evaluate the mechanical contribution of the middle ear tendons and musculature to the acoustic reflex. In addition, a mouse predisposed to noise-induced hearing loss may serve as a useful model to discern the mechanisms by which loud sound damages sensory hair cells and to develop interventions to effectively protect auditory function.
Acknowledgements
We thank Doris K. Wu (National Institute on Deafness and other Communication Disorders [NIDCD]) for providing the Acan and Atoh1 in situ hybridization probes; Dennis R. Trune and Beth J. Kempton for expert guidance with ABRs and GMA histochemistry; Brian A. Pryce and Spencer S. Watson for assistance with Scx mouse breeding; Jonathan Jungwirth for technical assistance with tissue dissection; and Patreece Suen for help with in situ hybridization. This work was supported by grants from the NIDCD and the National Institute of Arthritis and Musculoskeletal and Skin Diseases: DC R01 008595 and DC R01 008595-03S2 (to JB); P30 DC005983; R01 DC04554 (to TR); and R01 AR055640 (to RS).
Footnotes
Lingyan Wang and Chris S. Bresee contributed equally to this work.
Contributor Information
Lingyan Wang, Email: wangli@ohsu.edu.
Chris S. Bresee, Email: breseec@u.northwestern.edu
Han Jiang, Email: jiangha@ohsu.edu.
Wenxuan He, Email: hewen@ohsu.edu.
Tianying Ren, Email: rent@ohsu.edu.
Ronen Schweitzer, Email: RSC@shcc.org.
John V. Brigande, Phone: +1-503-4942933, FAX: +1-503-4945656, Email: brigande@ohsu.edu
References
- Atchley WR, Fitch WM. A natural classification of the basic helix-loop-helix class of transcription factors. Proc Natl Acad Sci U S A. 1997;94:5172–5176. doi: 10.1073/pnas.94.10.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandau O, Meindl A, Fassler R, Aszodi A. A novel gene, tendin, is strongly expressed in tendons and ligaments and shows high homology with chondromodulin-I. Dev Dyn. 2001;221:72–80. doi: 10.1002/dvdy.1126. [DOI] [PubMed] [Google Scholar]
- Brown D, Wagner D, Li X, Richardson JA, Olson EN. Dual role of the basic helix-loop-helix transcription factor scleraxis in mesoderm formation and chondrogenesis during mouse embryogenesis. Development. 1999;126:4317–4329. doi: 10.1242/dev.126.19.4317. [DOI] [PubMed] [Google Scholar]
- Burgess R, Cserjesi P, Ligon KL, Olson EN. Paraxis: a basic helix-loop-helix protein expressed in paraxial mesoderm and developing somites. Dev Biol. 1995;168:296–306. doi: 10.1006/dbio.1995.1081. [DOI] [PubMed] [Google Scholar]
- Burgess R, Rawls A, Brown D, Bradley A, Olson EN. Requirement of the paraxis gene for somite formation and musculoskeletal patterning. Nature. 1996;384:570–573. doi: 10.1038/384570a0. [DOI] [PubMed] [Google Scholar]
- Coker TR, Chan LS, Newberry SJ, Limbos MA, Suttorp MJ, Shekelle PG, Takata GS. Diagnosis, microbial epidemiology, and antibiotic treatment of acute otitis media in children: a systematic review. JAMA. 2010;304:2161–2169. doi: 10.1001/jama.2010.1651. [DOI] [PubMed] [Google Scholar]
- Cserjesi P, Brown D, Ligon KL, Lyons GE, Copeland NG, Gilbert DJ, Jenkins NA, Olson EN. Scleraxis: a basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development. 1995;121:1099–1110. doi: 10.1242/dev.121.4.1099. [DOI] [PubMed] [Google Scholar]
- Docheva D, Hunziker EB, Fassler R, Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol. 2005;25:699–705. doi: 10.1128/MCB.25.2.699-705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espira L, Lamoureux L, Jones SC, Gerard RD, Dixon IM, Czubryt MP. The basic helix-loop-helix transcription factor scleraxis regulates fibroblast collagen synthesis. J Mol Cell Cardiol. 2009;47:188–195. doi: 10.1016/j.yjmcc.2009.03.024. [DOI] [PubMed] [Google Scholar]
- Firulli AB. A HANDful of questions: the molecular biology of the heart and neural crest derivatives (HAND)-subclass of basic helix-loop-helix transcription factors. Gene. 2003;312:27–40. doi: 10.1016/S0378-1119(03)00669-3. [DOI] [PubMed] [Google Scholar]
- Franco HL, Casasnovas J, Rodriguez-Medina JR, Cadilla CL. Redundant or separate entities?–roles of Twist1 and Twist2 as molecular switches during gene transcription. Nucleic Acids Res. 2010 doi: 10.1093/nar/gkq890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelse K, Poschl E, Aigner T. Collagens–structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Gillespie PG, Muller U. Mechanotransduction by hair cells: models, molecules, and mechanisms. Cell. 2009;139:33–44. doi: 10.1016/j.cell.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Stoodley L, Hu FZ, Gieseke A, Nistico L, Nguyen D, Hayes J, Forbes M, Greenberg DP, Dice B, Burrows A, Wackym PA, Stoodley P, Post JC, Ehrlich GD, Kerschner JE. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296:202–211. doi: 10.1001/jama.296.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Fridberger A, Porsov E, Grosh K, Ren T. Reverse wave propagation in the cochlea. Proc Natl Acad Sci U S A. 2008;105:2729–2733. doi: 10.1073/pnas.0708103105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms AW, Johnson JE. Progenitors of dorsal commissural interneurons are defined by MATH1 expression. Development. 1998;125:919–928. doi: 10.1242/dev.125.5.919. [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ. How hearing happens. Neuron. 1997;19:947–950. doi: 10.1016/S0896-6273(00)80385-2. [DOI] [PubMed] [Google Scholar]
- Hwang CH, Wu DK. Noggin heterozygous mice: an animal model for congenital conductive hearing loss in humans. Hum Mol Genet. 2008;17:844–853. doi: 10.1093/hmg/ddm356. [DOI] [PubMed] [Google Scholar]
- Kelley MW, Driver EC, Puligilla C. Regulation of cell fate and patterning in the developing mammalian cochlea. Curr Opin Otolaryngol Head Neck Surg. 2009;17:381–387. doi: 10.1097/MOO.0b013e3283303347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejard V, Brideau G, Blais F, Salingcarnboriboon R, Wagner G, Roehrl MH, Noda M, Duprez D, Houillier P, Rossert J. Scleraxis and NFATc regulate the expression of the pro-alpha1(I) collagen gene in tendon fibroblasts. J Biol Chem. 2007;282:17665–17675. doi: 10.1074/jbc.M610113200. [DOI] [PubMed] [Google Scholar]
- MacArthur CJ, Trune DR. Mouse models of otitis media. Curr Opin Otolaryngol Head Neck Surg. 2006;14:341–346. doi: 10.1097/01.moo.0000244193.97301.d7. [DOI] [PubMed] [Google Scholar]
- Mallo M. Formation of the middle ear: recent progress on the developmental and molecular mechanisms. Dev Biol. 2001;231:410–419. doi: 10.1006/dbio.2001.0154. [DOI] [PubMed] [Google Scholar]
- Mallo M. Formation of the outer and middle ear, molecular mechanisms. Curr Top Dev Biol. 2003;57:85–113. doi: 10.1016/S0070-2153(03)57003-X. [DOI] [PubMed] [Google Scholar]
- McFadden DG, Barbosa AC, Richardson JA, Schneider MD, Srivastava D, Olson EN. The Hand1 and Hand2 transcription factors regulate expansion of the embryonic cardiac ventricles in a gene dosage-dependent manner. Development. 2005;132:189–201. doi: 10.1242/dev.01562. [DOI] [PubMed] [Google Scholar]
- Mitchell C, Kempton JB, Creedon T, Trune D. Rapid acquisition of auditory brainstem responses with multiple frequency and intensity tone-bursts. Hear Res. 1996;99:38–46. doi: 10.1016/S0378-5955(96)00081-0. [DOI] [PubMed] [Google Scholar]
- Mukerji S, Windsor AM, Lee DJ. Auditory brainstem circuits that mediate the middle ear muscle reflex. Trends Amplif. 2010;14:170–191. doi: 10.1177/1084713810381771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, Schweitzer R. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007;134:2697–2708. doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC, He Z, Bloedon E, Papsin B, Lundy L, Bolling L, Soundar S, Cook S, Reilly JS, Schmidt R, Deutsch ES, Barth P, Mehta DI. The role of extraesophageal reflux in otitis media in infants and children. Laryngoscope. 2008;118:1–9. doi: 10.1097/MLG.0b013e31817924a3. [DOI] [PubMed] [Google Scholar]
- Orgel JP, Irving TC, Miller A, Wess TJ. Microfibrillar structure of type I collagen in situ. Proc Natl Acad Sci U S A. 2006;103:9001–9005. doi: 10.1073/pnas.0502718103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce BA, Brent AE, Murchison ND, Tabin CJ, Schweitzer R. Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Dev Dyn. 2007;236:1677–1682. doi: 10.1002/dvdy.21179. [DOI] [PubMed] [Google Scholar]
- Ren T. Reverse propagation of sound in the gerbil cochlea. Nat Neurosci. 2004;7:333–334. doi: 10.1038/nn1216. [DOI] [PubMed] [Google Scholar]
- Ryan AF, Ebmeyer J, Furukawa M, Pak K, Melhus A, Wasserman SI, Chung WH. Mouse models of induced otitis media. Brain Res. 2006;1091:3–8. doi: 10.1016/j.brainres.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Sandell LJ, Morris N, Robbins JR, Goldring MB. Alternatively spliced type II procollagen mRNAs define distinct populations of cells during vertebral development: differential expression of the amino-propeptide. J Cell Biol. 1991;114:1307–1319. doi: 10.1083/jcb.114.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–3866. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- Trune DR, Zheng QY. Mouse models for human otitis media. Brain Res. 2009;1277:90–103. doi: 10.1016/j.brainres.2009.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young BB, Zhang G, Koch M, Birk DE. The roles of types XII and XIV collagen in fibrillogenesis and matrix assembly in the developing cornea. J Cell Biochem. 2002;87:208–220. doi: 10.1002/jcb.10290. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Zhao HB. ATP-mediated potassium recycling in the cochlear supporting cells. Purinergic Signal. 2010;6:221–229. doi: 10.1007/s11302-010-9184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaenepoel I, Mustapha M, Leibovici M, Verpy E, Goodyear R, Liu XZ, Nouaille S, Nance WE, Kanaan M, Avraham KB, Tekaia F, Loiselet J, Lathrop M, Richardson G, Petit C. Otoancorin, an inner ear protein restricted to the interface between the apical surface of sensory epithelia and their overlying acellular gels, is defective in autosomal recessive deafness DFNB22. Proc Natl Acad Sci U S A. 2002;99:6240–6245. doi: 10.1073/pnas.082515999. [DOI] [PMC free article] [PubMed] [Google Scholar]