Abstract
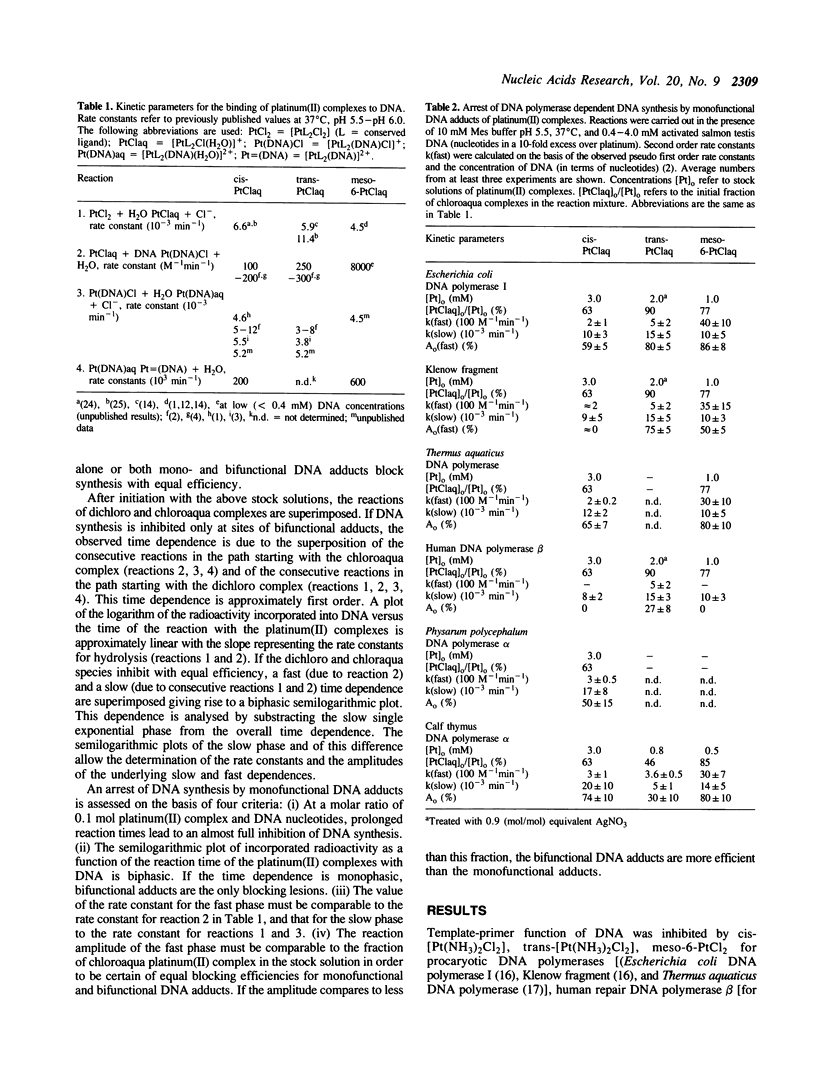
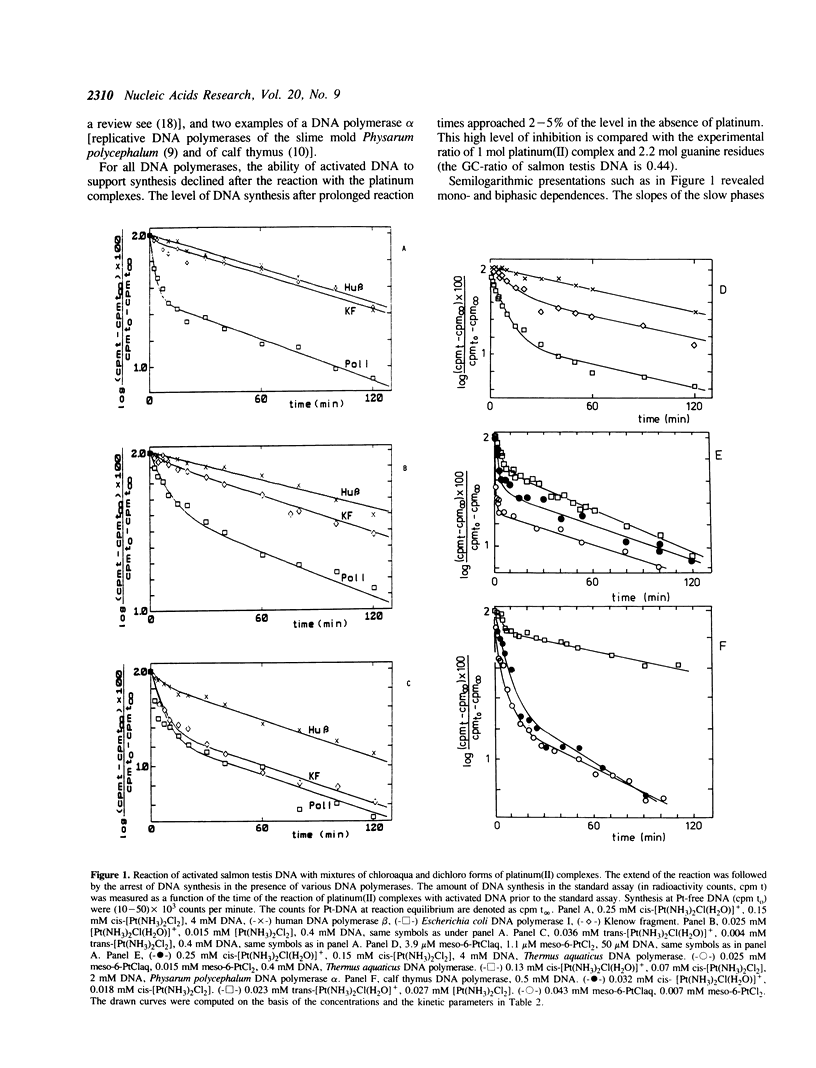
The question of whether monofunctional DNA platinum(II) adducts block synthesis of DNA by purified DNA polymerases of different types and origin has been investigated by comparing the time dependence of synthesis arrest and of DNA adduct formation. Activated salmon testis DNA is used as a suitable substrate for DNA synthesis allowing to probe inhibition by platinum(II) monoadducts for the variety of inherent template-primers. Reaction amplitudes are related to defined mixtures of dichloro and chloroaqua platinum(II) complexes. It is found that (i) all investigated DNA polymerases seem arrested (100% efficiency) at bifunctional DNA adducts. (ii) human DNA polymerase beta bypasses most of the monofunctional lesions of the three platinum(II) complexes investigated. (iii) Klenow fragment is blocked by monoadducts with increasing efficiency in the order cis-diamminechloroaquaplatinum(II) (0%) less than meso-[1,2-bis(2,6- dichloro-4-hydroxyphenyl)ethylenediamine] chloroaquaplatinum(II) (50%) less than trans-diamminechloro-aquaplatinum(II) (75%). (iv) Escherichia coli DNA polymerase I, Thermus aquaticus DNA polymerase, Physarum polycephalum DNA polymerase alpha, and calf thymus DNA polymerase alpha appear to be arrested by monoadducts. According to these examples, blocking efficiencies depend on the cis/trans-stereogeometry of fixation of the carrier ligands at platinum(II) residues, on the size/chemical nature of the platin(II) carrier ligand and on the type/origin of DNA polymerase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbotts J., SenGupta D. N., Zmudzka B., Widen S. G., Notario V., Wilson S. H. Expression of human DNA polymerase beta in Escherichia coli and characterization of the recombinant enzyme. Biochemistry. 1988 Feb 9;27(3):901–909. doi: 10.1021/bi00403a010. [DOI] [PubMed] [Google Scholar]
- Bernges F., Dörner G., Holler E. Escherichia coli DNA polymerase I: inherent exonuclease activities differentiate between monofunctional and bifunctional adducts of DNA and cis- or trans-diamminedichloroplatinum(II). An exonuclease investigation of the kinetics of the adduct formation. Eur J Biochem. 1990 Aug 17;191(3):743–753. doi: 10.1111/j.1432-1033.1990.tb19183.x. [DOI] [PubMed] [Google Scholar]
- Bernges F., Holler E. Effects of coordination of diammineplatinum(II) with DNA on the activities of Escherichia coli DNA polymerase I. Biochemistry. 1988 Aug 23;27(17):6398–6402. doi: 10.1021/bi00417a031. [DOI] [PubMed] [Google Scholar]
- Bernges F., Holler E. The reaction of platinum(II) complexes with DNA. Kinetics of intrastrand crosslink formation in vitro. Nucleic Acids Res. 1991 Apr 11;19(7):1483–1489. doi: 10.1093/nar/19.7.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien A., Edgar D. B., Trela J. M. Deoxyribonucleic acid polymerase from the extreme thermophile Thermus aquaticus. J Bacteriol. 1976 Sep;127(3):1550–1557. doi: 10.1128/jb.127.3.1550-1557.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiger-Bernays W. J., Essigmann J. M., Lippard S. J. Effect of the antitumor drug cis-diamminedichloroplatinum(II) and related platinum complexes on eukaryotic DNA replication. Biochemistry. 1990 Sep 11;29(36):8461–8466. doi: 10.1021/bi00488a037. [DOI] [PubMed] [Google Scholar]
- Hoffmann J. S., Johnson N. P., Villani G. Conversion of monofunctional DNA adducts of cis-diamminedichloroplatinum (II) to bifunctional lesions. Effect on the in vitro replication of single-stranded DNA by Escherichia coli DNA polymerase I and eukaryotic DNA polymerases alpha. J Biol Chem. 1989 Sep 5;264(25):15130–15135. [PubMed] [Google Scholar]
- Hollis L. S., Amundsen A. R., Stern E. W. Chemical and biological properties of a new series of cis-diammineplatinum(II) antitumor agents containing three nitrogen donors: cis-[Pt(NH3)2(N-donor)Cl]+. J Med Chem. 1989 Jan;32(1):128–136. doi: 10.1021/jm00121a024. [DOI] [PubMed] [Google Scholar]
- Hollis L. S., Sundquist W. I., Burstyn J. N., Heiger-Bernays W. J., Bellon S. F., Ahmed K. J., Amundsen A. R., Stern E. W., Lippard S. J. Mechanistic studies of a novel class of trisubstituted platinum(II) antitumor agents. Cancer Res. 1991 Apr 1;51(7):1866–1875. [PubMed] [Google Scholar]
- Johnson N. P., Hoeschele J. D., Rahn R. O. Kinetic analysis of the in vitro binding of radioactive cis- and trans-dichlorodiammineplatinum(II) to DNA. Chem Biol Interact. 1980 May;30(2):151–169. doi: 10.1016/0009-2797(80)90122-2. [DOI] [PubMed] [Google Scholar]
- Kashani-Sabet M., Lu Y., Leong L., Haedicke K., Scanlon K. J. Differential oncogene amplification in tumor cells from a patient treated with cisplatin and 5-fluorouracil. Eur J Cancer. 1990 Mar;26(3):383–390. doi: 10.1016/0277-5379(90)90238-o. [DOI] [PubMed] [Google Scholar]
- Nasheuer H. P., Grosse F. DNA polymerase alpha-primase from calf thymus. Determination of the polypeptide responsible for primase activity. J Biol Chem. 1988 Jun 25;263(18):8981–8988. [PubMed] [Google Scholar]
- Pinto A. L., Lippard S. J. Sequence-dependent termination of in vitro DNA synthesis by cis- and trans-diamminedichloroplatinum (II). Proc Natl Acad Sci U S A. 1985 Jul;82(14):4616–4619. doi: 10.1073/pnas.82.14.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller W., Reisner H., Holler E. Kinetic investigation of the DNA platination reaction: evidence for a transient adduct between deoxyribonucleic acid and cis-platinum(II). Biochemistry. 1987 Feb 10;26(3):943–950. doi: 10.1021/bi00377a039. [DOI] [PubMed] [Google Scholar]
- Segal E., Le Pecq J. B. Role of ligand exchange processes in the reaction kinetics of the antitumor drug cis-diamminedichloroplatinum(II) with its targets. Cancer Res. 1985 Feb;45(2):492–498. [PubMed] [Google Scholar]
- Weber C., Fischer H., Holler E. Purification and characterization of DNA polymerase alpha from plasmodia of Physarum polycephalum. Eur J Biochem. 1988 Sep 1;176(1):199–206. doi: 10.1111/j.1432-1033.1988.tb14269.x. [DOI] [PubMed] [Google Scholar]


