Abstract
Objective
Patients with medial compartment knee osteoarthritis (OA) adopt an abnormal gait pattern, and often develop frontal plane laxity at the knee. The purpose of this study was to quantify the extent of frontal plane knee joint laxity in patients with medial knee OA and genu varum and to assess the effect of joint laxity on knee joint kinetics, kinematics and muscle activity during gait.
Design
Twelve subjects with genu varum and medial compartment knee osteoarthritis (OA group) and twelve age-matched uninjured subjects underwent stress radiography to determine the presence and magnitude of frontal plane laxity. All subjects also went through gait analysis with surface electromyography of the medial and lateral quadriceps, hamstrings, and gastrocnemius to calculate knee joint kinematics and kinetics and co-contraction levels during gait.
Results
The OA group showed significantly greater knee instability (p= 0.002), medial joint laxity (p = 0.001), greater medial quadriceps-medial gastrocnemius (VMMG) co-contraction (p = 0.043), and greater knee adduction moments (p = 0.019) than the control group. Medial joint laxity contributed significantly to the variance in both VMMG and the knee adduction moment during early stance.
Conclusion
The presence of medial laxity in patients with knee OA is likely contributing to the altered gait patterns observed in those with medial knee OA. Greater medial co-contraction and knee adduction moments bodes poorly for the long term integrity of the articular cartilage, suggesting that medial joint laxity should be a focus of interventions aimed at slowing the progression of disease in individuals with medial compartment knee OA.
Keywords: Knee osteoarthritis, Gait, Laxity, Co-contraction
Introduction
Symptomatic knee OA is more common within the medial tibiofemoral compartment than within the lateral compartment (1–3). A primary factor influencing the development of medial compartment joint damage is frontal plane malalignment (4). Genu varum has been associated with the deterioration of the articular cartilage in the knee’s medial tibiofemoral compartment both to experimentally induce OA and as a risk factor for OA in a patient cohort (4, 5).
The high incidence of medial compartment knee OA may be attributable to both anatomical and mechanical factors. Anatomically, the medial compartment has thinner articular cartilage than the lateral compartment (6) and receives less protection from the medial meniscus (7). Mechanically, functional activities such as gait and stair climbing oblige the medial compartment to bear greater loads than the lateral compartment (8). The relatively high medial compartment load is due to the fact that the line of force acting at the foot passes medial to the knee joint center during gait (9, 10). The presence of genu varum alters the forces at the knee in so the line of force shifts farther medially from the knee joint center intensifying the already high medial compartment load and creating a medial joint reaction force that is nearly three and a half times that of the lateral compartment (10). The larger medial load has been correlated with the high external knee adduction moments that are found in patient’s with medial compartment knee OA (8, 11) and may contribute to the progressive destruction of the medial compartment’s articular cartilage (10). The larger knee adduction moment has also been positively correlated with static frontal plane alignment (12–16).
High adduction moments contribute to degenerative changes through greater compression of the medial side of the joint (17), and have been proposed to induce lateral joint laxity through chronic stretching (18). The suspected insufficiency of lateral restraints has caused some to postulate that peak pressures will further increase in the medial compartment because lateral laxity allows the lateral femoral condyle to “lift off” of the tibial plateau. Lateral condylar liftoff theoretically concentrates the stress on the medial aspect of the joint and further increases the knee’s adduction moment (10, 18).
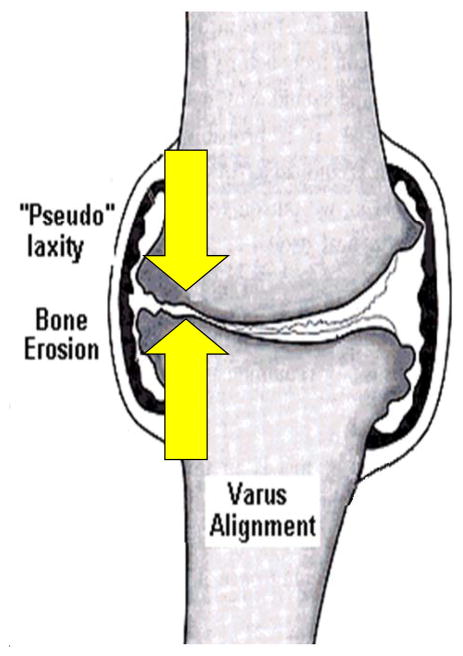
Several authors have confirmed the presence of overall frontal plane joint laxity in patients with knee OA (19, 20), although none have established the location of the laxity as identification of the neutral point of the knee in this population is problematic. As the large medial compartment load contributes to the progressive erosion of the medial compartment’s articular cartilage, the medial soft tissue structures may become involved as well. As the cartilage is worn away, the subchondral bone surfaces draw nearer to each other, reducing the distance between the medial compartment’s ligament insertions (19, 21, 22). Joint laxity could lead to episodes of instability that have been reported recently (23). Recurrent episodes of instability may lead to further degenerative changes within the joint (Figure 1). In animal models, inducing knee instability is used as a model for generating osteoarthritic changes (24).
Figure 1.
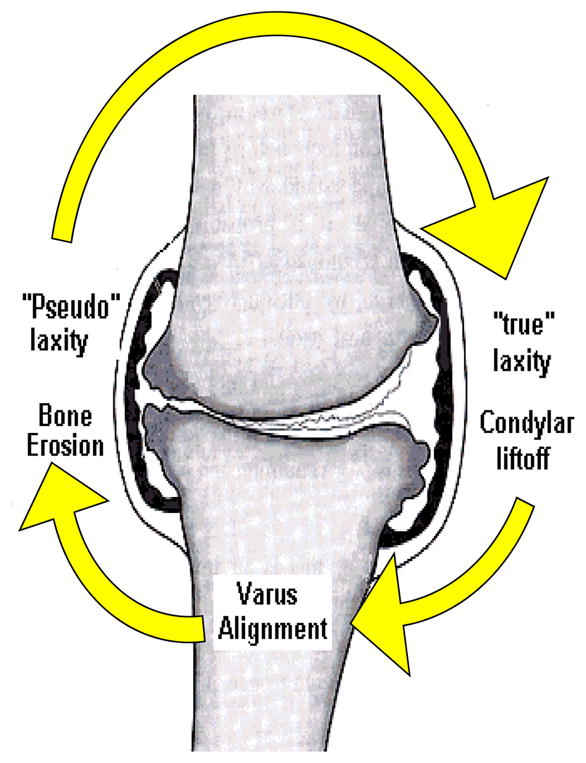
Diagram of the proposed influence of varus alignment on joint laxity. Both medial and lateral joint laxity are thought to develop leading to lateral “condylar liftoff”, increasing joint stress on the medial compartment exacerbating the degenerative process and continuing the cycle. Figure modified, with permission, from an original published by the Arthritis Research Campaign, www.arc.org.uk
Andriacchi proposed that in the presence of lateral joint laxity, the adduction moment must be balanced by a lateral passive (via gravity) and/or active (via muscles) force (18). As lateral muscle co-contraction increases joint compression increases, theoretically limits lateral condylar liftoff. In walking, muscular co-contraction in those with knee OA could likewise contribute to the characteristic knee stiffening strategy, marked by reduced knee flexion excursion that is often observed in these subjects (25). If the frontal plane laxity were localized to the medial compartment, then a patient with medial compartment knee OA would require a method of minimizing the medial laxity, in order to avoid instability. Increasing medial muscle activity, or allowing a greater medial load (via a further increased adduction moment), however, would result in even greater medial compression, exacerbate joint pain, and lead to further destruction of the articular cartilage. Indetifying the location and extent of the frontal plane laxity, which has been reported by others (19, 20), and determine how patients with medial compartment knee OA and genu varum act to avoid instability and redistribute the joint load. Is important
The purpose of this study was to quantify frontal plane knee joint laxity in patients with medial knee OA and genu varum and to assess the laxity’s effects on knee joint kinetics, kinematics and muscle activity during gait. It was hypothesized that subjects with medial compartment knee OA would have greater medial and lateral frontal plane laxity and more knee instability than healthy age matched control subjects. It was also hypothesized that laxity would result in a greater knee adduction moment as well as higher levels of muscular co-contraction, resulting in a characteristic knee stiffening strategy manifested by reduced knee flexion excursion during the loading phase of gait.
Methods
Subjects
Twelve patients (6 females, 6 males ranging in age from 39 to 64 years old, mean = 50.3, SD = 7.4) with symptomatic, medial compartment knee osteoarthritis and genu varum (OA group) were referred for testing by an orthopedic surgeon. Each subject was being treated by the surgeon for complaints of knee pain, and had been scheduled for an opening wedge, high tibial osteotomy. The diagnosis of OA was made from the clinical history, a physical examination, and radiographic changes observed from a standing postero-anterior radiograph with the knees flexed to 30° (31). From these standing radiographs, measurements of joint space width were measured to the nearest 0.1mm at the narrowest location of both the medial and lateral compartments using calipers. The radiographs of all subjects in the OA group showed definite joint space narrowing in the medial compartment (medial compartment: 1.99 ± 0.84 mm of joint space; lateral compartment: 5.9±1.2 mm of joint space). Assessment of skeletal alignment was made from a weight-bearing radiograph that contained the entire lower extremity, from the hip joints to the feet (32). The OA group had a weight-bearing line of 23.1±10.0%. Subjects who had torn knee ligaments, lateral compartment or patellofemoral osteoarthritis, other orthopedic problems or neurological damage in either lower extremity were excluded from the study. In addition, a Body Mass Index (BMI) of 40 or greater was an exclusion criterion. A control group of twelve age- and gender-matched healthy subjects (6 females, 6 males ranging in age from 40 to 62 years old, mean = 49.5, SD = 6.1) with no evidence of knee OA was recruited to undergo identical testing to the OA group. The control group had a weight bearing line of 46±8.6% and had 4.9±1.0 mm of joint space in the medial compartment and 5.9±1.3 mm in the lateral compartment (31). All subjects were informed of the purpose of the study and signed informed consent forms approved by the IRB prior to testing.
Measurement of Joint Laxity
Measurements of joint laxity were made from stress radiographs taken with subjects lying supine on the x-ray table with the knee flexed to 20° (Figure 2). The posterior aspect of the knee was resting on a foam bolster placed directly on top of the film cassette. The x-ray tube was centered 101.6 cm (40 inches) above the knee joint and care was taken to ensure that the leg was rotated to a position in which the patella was facing anteriorly. Subjects were instructed to relax the leg muscles. A TELOS (Austin & Associates, Fallston, MD) stress device was used to apply a 15daN (33lbs) force to generate both a varus and then a valgus force (33). An electronic digital display provided a measure of the force exerted against the knee joint line for reproducibility. The varus stress compressed the articular surfaces of the medial compartment while stretching the lateral soft tissue structures. In contrast, the valgus stress compressed the lateral compartment, but separated the articular surfaces of the medial compartment. Joint space measurements were made at the narrowest location of both the medial and lateral compartments to the nearest 0.1 mm for both the varus and valgus stresses using calipers. To avoid the issue of estimating a neutral point, joint laxity was operationally defined as joint space width at maximum opening minus joint space width measured with the joint maximally closed. Medial joint laxity was calculated as the measurement of the medial joint space during a valgus stress minus the medial joint space during a varus stress when the medial joint surfaces were approximated. Similarly, lateral laxity was calculated as the lateral joint space during a varus stress minus the lateral joint space during a valgus stress when the lateral joint surfaces were approximated (34). To ensure the validity of our measurements and avoid issues related to magnification, an independent observer measured a known distance (one of the beams on the TELOS device) on each radiograph. These measurements were then used to scale the measures of joint space narrowing to avoid any potential magnification on the film. The reliability of our measurements was performed by repeated testing on eight subjects with no history of knee injury. This procedure generated an ICC (3, 1) of 0.95 for lateral laxity measurements and 0.97 for medial laxity measurements.
Figure 2.
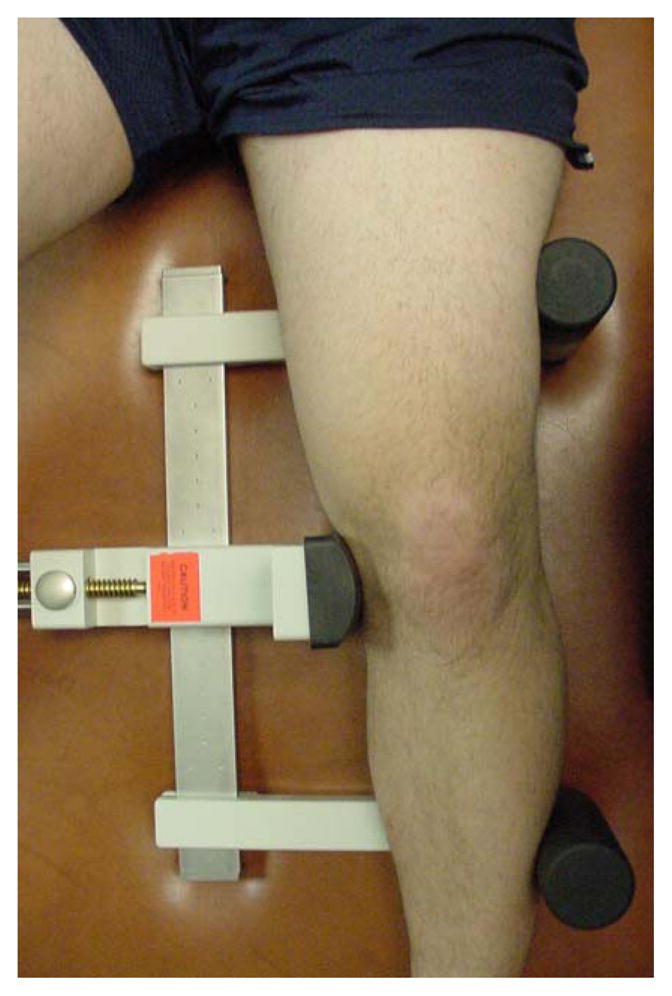
Setup for a stress radiograph. The knee is flexed to 20°, and placed in the TELOS device. For the valgus stress (shown), a consistent 15dN force is applied to the lateral aspect of the knee at the joint line. For the varus stress (not shown), the force is applied to the medial aspect of the joint.
Assessment of Knee Joint Function and Instability
All subjects were asked to rate knee function using the Knee Outcome Survey-Activities of Daily Living Scale (KOS-ADLS) (35). Responses are taken from a 6 point scale. Instability was assessed using the response to the following question: “To what degree does giving way, buckling or shifting of the knee affect your level of daily activity?” taken from the KOS-ADLS. Reliability and responsiveness of the questionnaire and this particular question for assessing knee function and instability in OA have been assessed and reported by others (23, 35, 36).
Motion Analysis
All subjects underwent gait analysis with surface electromyography for measurement of knee joint angles and moments, and muscle activation. The motions of the lower extremity segments were tracked with a six camera VICON 512 passive motion analysis system (Oxford Metrics, UK) collecting at 120Hz. The subjects walked across a 6-component force plate (Bertec Corp, Worthington, OH), placed flush with the floor in the middle of the volume to collect ground reaction force data. Ground reaction force data were collected at 1920 Hz and were synchronized with the VICON system for simultaneous collection. Limb segments were identified by 25mm, retro reflective markers placed over the bilateral iliac crests, greater trochanters, lateral femoral condyles, lateral malleolus, and the heads of the 5th metatarsals to indicate the ends of the segments and to identify appropriate joint centers. Rigid thermoplastic shells, each with four markers firmly affixed, were attached to the posterolateral aspect of the thigh and shank and covered with an elastic wrap to minimize movement between the shell and the bone. An additional shell with a triad of markers was placed on the posterior pelvis, and two additional markers were placed on the posterior heel counter of the subject’s shoe to track the foot’s coordinate system along with the marker placed over the 5th metatarsal head.
Electromyography (EMG) was recorded at 1920Hz using a 16-channel system (Motion Lab Systems, Baton Rouge, LA) interfaced with the VICON for simultaneous recording. Active surface electrodes, with a center to center distance of 0.8 inches and a disk diameter of 0.47 inches, were taped over the mid-muscle belly of the vastus lateralis, vastus medialis, medial and lateral hamstrings, and the medial and lateral heads of the gastrocnemius (37). Elastic bands (SuperWrapTM, Fabrifoam, Inc., Exton, PA) were wrapped over the electrodes to minimize movement. Subjects were positioned on a padded plinth to isometrically test the quadriceps, hamstrings, and gastrocnemius, (38) for verification of electrode placement. A baseline resting signal, as well as a signal from the maximum isometric test, were recorded for each muscle for normalization.
Each subject performed practice walking trials across a 10 meter walkway until a consistent velocity was achieved as measured by two photoelectric cells placed 286.5 cm apart. Subjects were free to choose the gait velocity. Trials were collected until the subject achieved ten trials in which the speed did not vary more than ±5% from that of the practice trials and only the test foot struck the force plate without targeting. Marker trajectory data were filtered with a 6Hz low pass filter, and force plate data were filtered using a low pass filter with a cutoff frequency of 40Hz. Sagittal plane hip, knee and ankle joint angles were calculated using rigid body analysis with Euler angles, and joint moments were calculated using an inverse dynamics approach (Move3D, NIH Biomechanics Laboratory, Bethesda, MD) and normalized to body mass and height. The variables included the knee flexion excursion from initial contact to peak knee flexion and the peak external knee adduction moment during early stance.
EMG data were bandpass filtered from 20–1000Hz in the hardware prior to sampling at 1920Hz. Custom written software filtered the signals using a 350Hz low pass Butterworth filter. Following full wave rectification, a linear envelope of the signal was created using a phase corrected 8th order Butterworth filter with a low pass cutoff frequency of 20Hz. This linear envelope was normalized to the maximum signal obtained during the MVIC trials and was used for calculation of the co-contraction index. Co-contraction is defined as the simultaneous activation of antagonist muscles and was calculated according to the following equation:
This equation allows for the quantification of co-contraction between two muscles during a given time frame. This equation was calculated for both the medial and lateral sides of the joint for the following muscle groups: quadriceps-hamstrings (VLLH and VMMH) and the quadriceps-gastrocnemius (VLLG and VMMG) at each point between 100msec prior to initial contact to the time of peak knee adduction moment. This time interval was normalized to 100 data points and the resulting values were integrated to arrive at a single representative value of co-contraction between the two muscles.
Statistical Analysis
Statistical analysis was performed using SPSS (version 11.0, Chicago, IL). Group data for joint laxity were compared using independent samples t-tests. As walking velocity influences gait parameters, (39) velocity was used as a covariate in one way ANCOVA to test group differences in the knee flexion excursion and knee adduction moment. A two-way ANOVA (medial/lateral X muscle group) was used to determine group differences in co-contraction values. Tukey post hoc tests were used. A linear regression analysis was used to examine the relationship between joint laxity, muscular activation patterns and gait variables. Significance was set at α = 0.05.
Results
The OA group had 5.1 ± 1.5 mm of laxity in the medial compartment. This was significantly greater than the control groups laxity of 3.2 ± 1.0 mm (p = 0.001). No difference in joint laxity was measured in the lateral compartment, however, as the OA group had 3.6 ± 1.6mm of joint laxity, and the control group had 4.3 ± 1.3 mm of lateral compartment laxity (p = 0.239). Subjects in the OA group (50.2 +/− 14.7) had significantly more impaired knee function than the control group (99.5 +/− 1.0) as measured by the KOS-ADLS (p < 0.000). No subject in the control group (N = 12) complained of instability, while 9 of 12 subjects in the OA group reported the presence of instability. Of the 9 subjects in the OA group who reported instability, 7 of these subjects complained that instability affected the ability to perform activities of daily living (see Table 1).
Table.
| Number | Percentage | |
|---|---|---|
| I do not have giving way, buckling, or shifting of the knee | 3 | 25 |
| I have the symptom but it does not affect my activity | 2 | 17 |
| The symptom affects my activity slightly | 3 | 25 |
| The symptom affects my activity moderately | 2 | 17 |
| The symptom affects my activity severely | 1 | 8 |
| The symptom prevents me from all daily activity | 1 | 8 |
| Total | 12 | 100 |
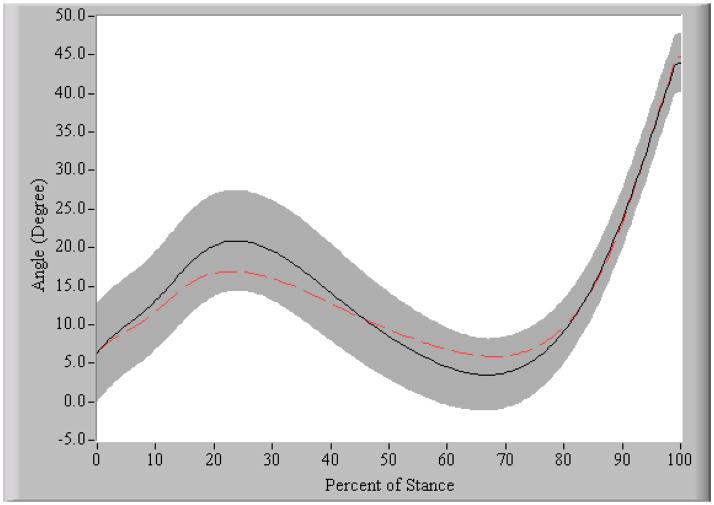
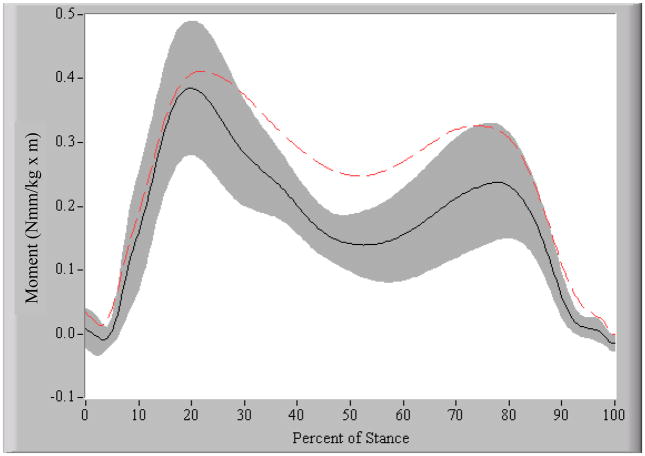
The OA group walked at an average velocity of 1.4 ± 0.1 m/sec and the control group walked at 1.5 ± 0.2 m/sec (p = 0.056). During the weight acceptance portion of stance, the OA group had a knee flexion excursion of 10.3 ± 4.6°, which was significantly less than the 15.3 ± 4.1° excursion of the healthy control subjects (p = 0.010) (see Figure 3). After accounting for differences in walking velocity, the peak knee adduction moment was significantly higher (p = 0.019) in the OA group (0.391 ± 0.075 Nmm/kg·m) compared to the control group (0.351 ± 0.102 Nmm/kg·m) (see Figure 4).
Figure 3. Knee flexion angle during stance phase of gait.
Positive values indicate flexion. The black solid line is the mean and the shaded region is ∀ 1SD of the control group. The dotted line represents the mean of the OA group. The OA subjects demonstrated a reduced knee flexion excursion from initial contact to peak knee flexion, during weight acceptance.
Figure 4. External knee adduction moment during stance phase of gait.
The black solid line is the mean and the shaded region is ∀ 1SD of the control group. The dotted line represents the mean of the OA group. The OA group had a greater peak external knee adduction moment compared to the control group.
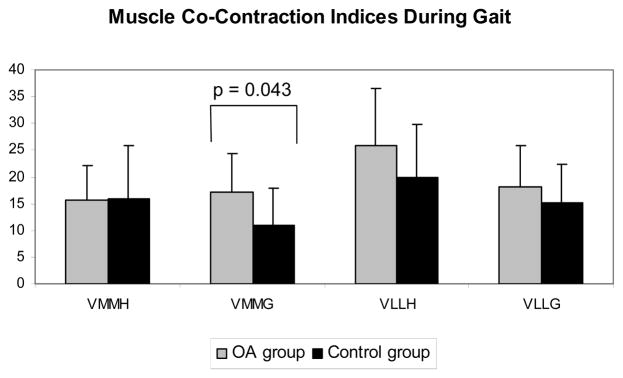
An examination of co-contraction indices during gait (see Figure 5), revealed a significant main effect for side (medial vs. lateral) (p = 0.001). Both the OA group (p = 0.017) and the healthy control group (p = 0.029) had greater lateral co-contraction indices than medial co-contraction. Examining differences between the groups revealed that on the medial side of the joint, the OA subjects had a significantly greater VMMG co-contraction index (17.2 ± 7.3) compared to the control subjects, which was 10.9 ± 7.2 (p = 0.043). No difference were observed between groups for the VMMH (OA group = 15.6 ± 6.6; control group = 16.0 ± 9.9; p = 0.908), the VLLH (OA group = 25.7 ± 10.8; control group = 20.0 ± 9.8; p = 0.184), or the VLLG muscle groups (OA group = 18.0 ± 7.8; control group = 15.2 ± 7.2; p = 0.370).
Figure 5. Muscle co-contraction during gait.
Co-contraction values calculated from 100msec prior to initial contact through peak knee adduction moment for VMMH (vastus medialis-medial hamstrings), VMMG (vastus medialis-medial gastrocnemius), VLLH (vastus lateralis-lateral hamstrings), and VLLG (vastus lateralis-lateral gastrocnemius). Values represent mean and standard deviation. The OA group is in gray and the control group is in black.
Medial laxity accounted for a significant portion of the variability in both the VMMG co-contraction values (r2 = 0.208, p = 0.029) and the knee adduction moment (r2 = 0.213, p = 0.027) for the entire subject pool.
Discussion
The hypothesis that patients with medial knee OA and genu varum would have greater frontal plane laxity and instability than an age matched control group without OA was supported by the data. Interestingly, excessive laxity was observed only on the medial side of the joint and was accompanied by greater medial muscle (vastus medialis and medial gastrocnemius) co-contraction. The medial location of the excessive frontal plane laxity is likely contributing to the cycle of articular cartilage degeneration, joint malalignment, and altered joint loading (Figure 6).
Figure 6.
Revised theory of the influence of genu varum on joint laxity. Frontal plane laxity is located on the medial side. Greater co-contraction and medial joint load exacerbate medial compartment degeneration, increasing joint laxity, and propagating a cycle of medial articular cartilage destruction. Figure modified, with permission, from an original published by the Arthritis Research Campaign, www.arc.org.uk
It has been theorized that lateral laxity develops in response to an inability to minimize lateral tensile forces, which in turn creates larger medial joint compressive loads. This would also presumably diminish the lateral joint loads as the lateral femoral condyle lifts off the tibial plateau, however even in a group of subjects with LCL insufficiency, no reduction in lateral loading was observed (40). Lateral laxity was not observed in our sample, bringing the concept of lateral condylar liftoff during gait into question (10, 18, 40). Although we found greater adduction moments in the OA group, which is consistent with the findings of other authors (8, 11), it was not associated with the presence of greater lateral laxity. The OA group’s large adduction moment was also present despite the large magnitude of lateral muscle co-contraction. In fact, both groups had larger lateral co-contraction than the medial side which may represent the normal mechanism for controlling the knee adduction moment during stance. We have confirmed that there is indeed a great deal of lateral muscle co-contraction, in both the OA and control groups, which may help to redistribute the load laterally as others have speculated previously (10, 18). In the presence of larger adduction moments, however, patients with knee OA did not increase the lateral co-contraction to attempt to compensate for the large medial load. This may be due to the fact that the frontal plane laxity in individuals with medial knee OA appears to be localized to the medial side of the joint, and the relatively larger medial load (adduction moment) serves to stabilize the medial side of the joint through increased compression. The presence of greater medial muscle co-contraction in the OA group therefore appears to be a response to further stabilize the medial side of the knee joint and control excessive medial laxity. The control of medial laxity through increased medial co-contraction, while allowing a large adduction moment, seems perplexing and counter-intuitive, as this strategy should theoretically increase the compressive forces across the joint, exacerbating the joint pain. It may, however, reflect an inability of the subjects with knee OA and medial joint laxity to control knee instability by any other means.
The fact that the OA group’s frontal plane laxity is localized to the medial compartment presents a complicated scenario for preventing instability. In our sample, joint stability was provided by high medial muscle co-contraction and a large adduction moment, which likely results in a higher joint reaction force and could provide insight into the pathogenesis of the degenerative process in patients with genu varum and medial compartment knee OA. The OA group needs to control the excessive medial laxity in order to avoid instability, or risk further articular cartilage damage from the resulting episodes of instability. The process of preventing joint instability itself, however, is capable of causing damage to the medial compartment’s articular cartilage. Therefore, it appears that once medial joint laxity develops from the medial articular cartilage degeneration, all methods of preventing instability (i.e. increased joint load or muscle co-contraction) lead to greater joint damage, propagating the destructive cycle. Theoretically, this could explain, in part, the higher incidence of knee osteoarthritis in patients with previous medial meniscectomy and MCL tears compared to the general population. Meniscectomy results in increased varus-valgus laxity at the knee (29, 41), and typically leads to the development of knee OA (42).
The presence of frontal plane laxity in patients with medial knee osteoarthritis has been established previously (19, 20, 33). These authors however were unable to pinpoint the location (medial and/or lateral joint) of the laxity because of an inability to determine a neutral position. The use of stress films allowed us to overcome this problem, providing us with valuable information regarding the location of the frontal plane laxity. This study provides insight into the rationale for the characteristic stiffening gait pattern that is adopted in patients with medial knee OA. It also provides the impetus for further research into interventions to minimize frontal plane laxity that develops at the knee as articular cartilage is progressively eroded. Although joint laxity is clearly not the only variable that contributes to the observed gait pattern, it should be addressed by whatever intervention is being planned (surgical, or otherwise) for the patient with painful medial compartment knee OA. We feel that a failure to eliminate this frontal plane laxity could cause the continuation of detrimental movement patterns, and thus, subsequent progression of the painful OA process.
Acknowledgments
The authors would like to thank William Newcomb, MD for his valuable assistance with subject recruitment, Laura C. Schmitt, MPT for her assistance with data collections, and Laurie Andrews, RTR for assistance with the stress radiographs.
Funding provided by the National Institute of Health (1P20RR016458, 2T32HD007490), Foundation for Physical Therapy (PODS II), American College of Sports Medicine (Doctoral Student Research Grant), and EBI Medical, LP
Abbreviations
- OA
Osteoarthritis
- VMMH
Vastus medialis-medial hamstrings co-contraction
- VMMG
Vastus medialis-medial gastrocnemius co-contraction
- VLLH
Vastus lateralis-lateral hamstrings co-contraction
- VLLG
Vastus lateralis-lateral gastrocnemius co-contraction
- KOS-ADLS
Knee Outcome Survey-Activities of Daily Living Scale
Contributor Information
Michael D. Lewek, Doctoral student in the Biomechanics and Movement Science Program, Department of Physical Therapy, University of Delaware, Newark, DE 19716 when this study is completed. He is currently a postdoctoral fellow at the Rehabilitation Institute of Chicago.
Katherine S. Rudolph, Assistant Professor in the Department of Physical Therapy and Biomechanics and Movement Science Program, University of Delaware, Newark, DE 19716.
Lynn Snyder-Mackler, Professor in the Department of Physical Therapy and Biomechanics and Movement Science Program, University of Delaware, Newark, DE 19716.
References
- 1.Ledingham J, Regan M, Jones A, Doherty M. Radiographic patterns and associations of osteoarthritis of the knee in patients referred to hospital. Ann Rheum Dis. 1993;52(7):520–6. doi: 10.1136/ard.52.7.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dearborn JT, Eakin CL, Skinner HB. Medial compartment arthrosis of the knee. Am J Orthop. 1996;25(1):18–26. [PubMed] [Google Scholar]
- 3.Bartel DL. Unicompartmental arthritis: biomechanics and treatment alternatives. Instr Course Lect. 1992;41:73–6. [PubMed] [Google Scholar]
- 4.Wu DD, Burr DB, Boyd RD, Radin EL. Bone and cartilage changes following experimental varus or valgus tibial angulation. J Orthop Res. 1990;8(4):572–85. doi: 10.1002/jor.1100080414. [DOI] [PubMed] [Google Scholar]
- 5.Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. Jama. 2001;286(2):188–95. doi: 10.1001/jama.286.2.188. [DOI] [PubMed] [Google Scholar]
- 6.Cicuttini FM, Wluka AE, Wang Y, Davis SR, Hankin J, Ebeling P. Compartment differences in knee cartilage volume in healthy adults. J Rheumatol. 2002;29(3):554–6. [PubMed] [Google Scholar]
- 7.Riegger-Krugh C, Gerhart TN, Powers WR, Hayes WC. Tibiofemoral contact pressures in degenerative joint disease. Clin Orthop. 1998;(348):233–45. [PubMed] [Google Scholar]
- 8.Hurwitz DE, Ryals AB, Case JP, Block JA, Andriacchi TP. The knee adduction moment during gait in subjects with knee osteoarthritis is more closely correlated with static alignment than radiographic disease severity, toe out angle and pain. J Orthop Res. 2002;20(1):101–7. doi: 10.1016/S0736-0266(01)00081-X. [DOI] [PubMed] [Google Scholar]
- 9.Johnson F, Leitl S, Waugh W. The distribution of load across the knee. A comparison of static and dynamic measurements. J Bone Joint Surg Br. 1980;62(3):346–9. doi: 10.1302/0301-620X.62B3.7410467. [DOI] [PubMed] [Google Scholar]
- 10.Schipplein OD, Andriacchi TP. Interaction between active and passive knee stabilizers during level walking. J Orthop Res. 1991;9(1):113–9. doi: 10.1002/jor.1100090114. [DOI] [PubMed] [Google Scholar]
- 11.Baliunas AJ, Hurwitz DE, Ryals AB, Karrar A, Case JP, Block JA, et al. Increased knee joint loads during walking are present in subjects with knee osteoarthritis. Osteoarthritis Cartilage. 2002;10(7):573–9. doi: 10.1053/joca.2002.0797. [DOI] [PubMed] [Google Scholar]
- 12.Hilding MB, Lanshammar H, Ryd L. A relationship between dynamic and static assessments of knee joint load. Gait analysis and radiography before and after knee replacement in 45 patients. Acta Orthop Scand. 1995;66(4):317–20. doi: 10.3109/17453679508995552. [DOI] [PubMed] [Google Scholar]
- 13.Wada M, Maezawa Y, Baba H, Shimada S, Sasaki S, Nose Y. Relationships among bone mineral densities, static alignment and dynamic load in patients with medial compartment knee osteoarthritis. Rheumatology (Oxford) 2001;40(5):499–505. doi: 10.1093/rheumatology/40.5.499. [DOI] [PubMed] [Google Scholar]
- 14.Wada M, Imura S, Nagatani K, Baba H, Shimada S, Sasaki S. Relationship between gait and clinical results after high tibial osteotomy. Clin Orthop. 1998;(354):180–8. doi: 10.1097/00003086-199809000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Andrews M, Noyes FR, Hewett TE, Andriacchi TP. Lower limb alignment and foot angle are related to stance phase knee adduction in normal subjects: a critical analysis of the reliability of gait analysis data. J Orthop Res. 1996;14(2):289–95. doi: 10.1002/jor.1100140218. [DOI] [PubMed] [Google Scholar]
- 16.Catani F, Marcacci M, Benedetti MG, Leardini A, Battistini A, Iacono F, et al. The influence of clinical and biomechanical factors on the results of valgus high tibial osteotomy. Chir Organi Mov. 1998;83(3):249–62. [PubMed] [Google Scholar]
- 17.Sharma L, Hurwitz DE, Thonar EJ, Sum JA, Lenz ME, Dunlop DD, et al. Knee adduction moment, serum hyaluronan level, and disease severity in medial tibiofemoral osteoarthritis. Arthritis Rheum. 1998;41(7):1233–40. doi: 10.1002/1529-0131(199807)41:7<1233::AID-ART14>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 18.Andriacchi TP. Dynamics of knee malalignment. Orthop Clin North Am. 1994;25(3):395–403. [PubMed] [Google Scholar]
- 19.Wada M, Imura S, Baba H, Shimada S. Knee laxity in patients with osteoarthritis and rheumatoid arthritis. Br J Rheumatol. 1996;35(6):560–3. doi: 10.1093/rheumatology/35.6.560. [DOI] [PubMed] [Google Scholar]
- 20.Sharma L, Lou C, Felson DT, Dunlop DD, Kirwan-Mellis G, Hayes KW, et al. Laxity in healthy and osteoarthritic knees [see comments] Arthritis Rheum. 1999;42(5):861–70. doi: 10.1002/1529-0131(199905)42:5<861::AID-ANR4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 21.Sharma L, Hayes KW, Felson DT, Buchanan TS, Kirwan-Mellis G, Lou C, et al. Does laxity alter the relationship between strength and physical function in knee osteoarthritis? Arthritis Rheum. 1999;42(1):25–32. doi: 10.1002/1529-0131(199901)42:1<25::AID-ANR3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 22.Cameron JC, Saha S. Management of medial collateral ligament laxity. Orthop Clin North Am. 1994;25(3):527–32. [PubMed] [Google Scholar]
- 23.Fitzgerald GK, Piva SR, Irrgang JJ. Complaints of Joint Instability in Knee Osteoarthritis: Its Prevalence and Relationship to Physical Function. Arthritis Care and Research. doi: 10.1002/art.20825. (In Press) [DOI] [PubMed] [Google Scholar]
- 24.Lukoschek M, Boyd RD, Schaffler MB, Burr DB, Radin EL. Comparison of joint degeneration models. Surgical instability and repetitive impulsive loading. Acta Orthop Scand. 1986;57(4):349–53. doi: 10.3109/17453678608994409. [DOI] [PubMed] [Google Scholar]
- 25.Ouellet D, Moffet H. Locomotor deficits before and two months after knee arthroplasty. Arthritis Rheum. 2002;47(5):484–93. doi: 10.1002/art.10652. [DOI] [PubMed] [Google Scholar]
- 26.Olmstead TG, Wevers HW, Bryant JT, Gouw GJ. Effect of muscular activity on valgus/varus laxity and stiffness of the knee. J Biomech. 1986;19(8):565–77. doi: 10.1016/0021-9290(86)90162-4. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Wang G. Dynamic and static control of the human knee joint in abduction- adduction. J Biomech. 2001;34(9):1107–15. doi: 10.1016/s0021-9290(01)00080-x. [DOI] [PubMed] [Google Scholar]
- 28.Markolf KL, Graff-Radford A, Amstutz HC. In vivo knee stability. A quantitative assessment using an instrumented clinical testing apparatus. J Bone Joint Surg Am. 1978;60(5):664–74. [PubMed] [Google Scholar]
- 29.Markolf KL, Bargar WL, Shoemaker SC, Amstutz HC. The role of joint load in knee stability. J Bone Joint Surg Am. 1981;63(4):570–85. [PubMed] [Google Scholar]
- 30.Bullough PG, Walker PS. The distribution of load through the knee joint and its possible significance to the observed patterns of articular cartilage breakdown. Bull Hosp Joint Dis. 1976;37(2):110–23. [PubMed] [Google Scholar]
- 31.Piperno M, Hellio Le Graverand MP, Conrozier T, Bochu M, Mathieu P, Vignon E. Quantitative evaluation of joint space width in femorotibial osteoarthritis: comparison of three radiographic views. Osteoarthritis Cartilage. 1998;6(4):252–9. doi: 10.1053/joca.1998.0118. [DOI] [PubMed] [Google Scholar]
- 32.Dugdale TW, Noyes FR, Styer D. Preoperative planning for high tibial osteotomy. The effect of lateral tibiofemoral separation and tibiofemoral length. Clin Orthop. 1992;(274):248–64. [PubMed] [Google Scholar]
- 33.Tallroth K, Lindholm TS. Stress radiographs in the evaluation of degenerative femorotibial joint disease. Skeletal Radiol. 1987;16(8):617–20. doi: 10.1007/BF00357109. [DOI] [PubMed] [Google Scholar]
- 34.Moore TM, Meyers MH, Harvey JP., Jr Collateral ligament laxity of the knee. Long-term comparison between plateau fractures and normal. J Bone Joint Surg Am. 1976;58(5):594–8. [PubMed] [Google Scholar]
- 35.Irrgang JJ, Snyder-Mackler L, Wainner RS, Fu FH, Harner CD. Development of a patient-reported measure of function of the knee. J Bone Joint Surg Am. 1998;80(8):1132–45. doi: 10.2106/00004623-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Marx RG, Jones EC, Allen AA, Altchek DW, O'Brien SJ, Rodeo SA, et al. Reliability, validity, and responsiveness of four knee outcome scales for athletic patients. J Bone Joint Surg Am. 2001;83-A(10):1459–69. doi: 10.2106/00004623-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Perroto A. Anatomical Guide for the Electromyographer: The Limbs and Trunk. 3. Springfield; IL: 1994. pp. 152–207. [Google Scholar]
- 38.Kendall FP, McCreary EK, Provance PG. Muscle Testing and Function. 4. Philadelphia, PA: Williams and Wilkins; 1993. pp. 145–59. [Google Scholar]
- 39.Holden JP, Chou G, Stanhope SJ. Changes in knee joint function over a wide range of walking speeds. Clin Biomech (Bristol, Avon) 1997;12(6):375–82. doi: 10.1016/s0268-0033(97)00020-x. [DOI] [PubMed] [Google Scholar]
- 40.Noyes FR, Schipplein OD, Andriacchi TP, Saddemi SR, Weise M. The anterior cruciate ligament-deficient knee with varus alignment. An analysis of gait adaptations and dynamic joint loadings. Am J Sports Med. 1992;20(6):707–16. doi: 10.1177/036354659202000612. [DOI] [PubMed] [Google Scholar]
- 41.Markolf KL, Mensch JS, Amstutz HC. Stiffness and laxity of the knee--the contributions of the supporting structures. A quantitative in vitro study. J Bone Joint Surg Am. 1976;58(5):583–94. [PubMed] [Google Scholar]
- 42.Kannus P, Jarvinen M. Osteoarthrosis in a knee joint due to chronic posttraumatic insufficiency of the medial collateral ligament. Nine-year follow-up. Clin Rheumatol. 1988;7(2):200–7. doi: 10.1007/BF02204455. [DOI] [PubMed] [Google Scholar]